Abstract
Background
The nucleus accumbens (NAc) has been implicated in the neurochemical effects of ethanol (EtOH). Evidence suggests that repeated EtOH exposures and chronic EtOH drinking increase dopamine (DA) neurotransmission in the NAc due, in part, to a reduction in D2 autoreceptor function. The objectives of the current study were to evaluate the effects of a single EtOH pretreatment and repeated EtOH pretreatments on DA neurotransmission and D2 autoreceptor function in the NAc of Wistar rats.
Methods
Experiment 1 examined D2 receptor function after a single intraperitoneal (i.p.) injection or repeated i.p. injections of 0.0, 0.5, 1.0, or 2.0 g/kg EtOH to female Wistar rats. Single EtOH pretreatment groups received 1 daily i.p. injection of 0.9% NaCl (saline) for 4 days, followed by 1 day of saline or EtOH administration; repeated EtOH pretreatment groups received 5 days of saline or EtOH injections. Reverse microdialysis experiments were conducted to determine the effects of local perfusion with the D2-like receptor antagonist (−)sulpiride (SUL; 100 uM), on extracellular DA levels in the NAc. Experiment 2 evaluated if pretreatment with a single, moderate (1.0 g/kg) dose of EtOH would alter levels and clearance of extracellular DA in the NAc, as measured by no-net-flux (NNF) microdialysis. Subjects were divided into the EtOH-naïve and the single EtOH pretreated groups from Experiment 1.
Results
Experiment 1: Changes in extracellular DA levels induced with SUL perfusion were altered by the EtOH dose (p < 0.001), but not the number of EtOH pretreatments (p > 0.05). Post-hoc analyses indicated that groups pretreated with single or repeated 1.0 g/kg EtOH showed significantly attenuated DA response to SUL, compared with all other groups (p < 0.001). Experiment 2: Multiple linear regression analyses yielded significantly (p < 0.05) higher extracellular DA concentrations in the NAc of rats receiving EtOH pretreatment, compared with their EtOH-naïve counterparts (3.96 ± 0.42 nM and 3.25 ± 0.23 nM, respectively). Extraction fractions were not significantly different between the 2 groups.
Conclusions
The present results indicate that a single EtOH pretreatment at a moderate dose can increase DA neurotransmission in the NAc due, in part, to reduced D2 autoreceptor function.
Keywords: Microdialysis, Ethanol, Dopamine, Nucleus Accumbens, D2 Autoreceptor
The mesolimbic dopamine (DA) circuit is an important substrate mediating appetitive or reward-related activities, including consumption of ethanol (EtOH) and other drugs of abuse (Di Chiara et al., 2004; Gonzales et al., 2004; Koob et al., 1998; Spanagel and Weiss, 1999). EtOH’s interactions with the mesolimbic DA system have been well investigated in order to elucidate the neurochemical correlates of alcohol abuse disorders. Research investigating the projections from DA cell bodies in the ventral tegmental area to terminals in the nucleus accumbens (NAc) has generated substantial support that this mesolimbic DA system mediates at least some of the adaptive effects of EtOH, as well as other drugs of abuse, that may underlie addictive properties of these drugs (see Pierce and Kumaresan, 2006 for review).
Activation of the mesolimbic DA system by EtOH is evidenced by significant increases in extracellular DA levels in the NAc following operant oral self-administration of EtOH using in vivo microdialysis (Gonzales and Weiss, 1998; Melendez et al., 2002; Weiss et al., 1993, 1996). Similar increases in extracellular DA have been reported following local (Wozniak et al., 1991; Yoshimoto et al., 1992a) and systemic (Imperato and DiChiara, 1986; Smith and Weiss, 1999; Yoshimoto et al., 1992a) exposure to EtOH. Neuroadaptive effects of EtOH on the mesolimbic DA system have also been reported by a number of investigators. Smith and Weiss (1999), for example, utilized no-net-flux microdialysis (Justice, 1993; Parsons and Justice, 1992) and found alterations in DA levels in the NAc core subregion following repeated (5) 1.0 g/ kg intraperitoneal (i.p.) EtOH pretreatments. They observed that outbred Wistar and selectively bred alcohol-preferring (P) rats exhibited similar increases in basal DA levels following EtOH exposure. Moreover, these results were observed at least 15 hours after the last injection, indicating that the effects of EtOH pretreatment persisted after EtOH cleared the system (Smith and Weiss, 1999). Analyses of extraction fractions in these animals indicated that the observed neuroadaptions did not result from alterations in NAc DA clearance (Smith and Weiss, 1999).
Many previous reports support the hypothesis that the observed elevations in NAc DA levels following EtOH exposure are not mediated by changes in clearance (e.g., Budygin et al., 2005; Engleman et al., 2003; Jones et al., 2006; Ramachandra et al., 2007). Contradictory results, however, have been reported in High Alcohol Drinking replicate line-1 (HAD-1) rats (Carroll et al., 2006), as well as in P rats, following periadolescent EtOH exposure (Sahr et al., 2004).
Several methods have been employed to evaluate the manner in which EtOH experience alters DA neurotransmission in the NAc. One approach has been to investigate potential modifications in DA D2 autoreceptor regulation, following EtOH exposure. PET scan research has indicated that human alcoholics exhibit a diminished number of DA D2 receptors, but not transporters, in the striatum compared with nonalcoholics (Volkow et al., 1996). Further, this population of receptors has been found to be significantly greater in nonalcoholic family members of alcoholics, compared with those family negative for alcoholism, suggesting that the receptors may present a protective mechanism against the condition (Volkow et al., 2006).
Prolonged EtOH drinking alters DA D2 receptor functioning in the NAc of P rats under both 1-hr limited (Engleman et al., 2003) and continuous (Thielen et al., 2004) EtOH access paradigms, as well as following EtOH deprivation (Thielen et al., 2004). Both of these studies reported a diminished response to DA D2-like receptor antagonist, (−)sulpiride (SUL), following EtOH experience. In addition, Levy and colleagues (1991) reported that intra-NAc SUL injections led to increases in EtOH intake in P rats. These studies are suggestive of a reciprocal relationship between EtOH drinking and DA D2 receptor function. Further, the studies by Engleman and colleagues (2003) and Thielen and colleagues (2004) indicate that chronic and/ or repeated EtOH exposure may alter the mesolimbic DA system via functional changes to DA D2 receptors in the NAc, and likely through associated changes in DA regulatory mechanisms.
The previous studies examined changes in neurobiological measures after repeated or chronic exposure to EtOH. However, few studies have assessed the effects of brief EtOH administration on subsequent D2 autoreceptor function. Research in this area would be informative in characterizing the onset of vulnerability to alcoholism, particularly following the first experience with ethanol. The current study evaluated the effects of brief EtOH exposure on DA neurotransmission in the NAc. Experiment 1 was undertaken to determine whether systemic EtOH pretreatment would alter DA D2 receptor function, in a dose- and experience-dependent manner. Previous evidence (Engleman et al., 2003; Thielen et al., 2004) indicated that EtOH pretreatment attenuated SUL-induced increases in extracellular DA in the NAc. Further, the current study tested the hypothesis that repeated systemic EtOH exposures (similar to those employed by Smith and Weiss, 1999) would have a greater effect to diminish DA D2 receptor response to SUL than would a single exposure.
Experiment 2 was performed to quantify the basal extracellular concentration and extraction fraction of NAc DA, following a single 1.0 g/ kg EtOH treatment. Previous reports by Smith and Weiss (1999) indicated repeated i.p. exposure to 1.0 g/ kg EtOH would alter DA neurotransmission, however, it was unclear whether a single EtOH exposure would be sufficient to produce similar results. This experiment tested the hypothesis that a single, moderate exposure to EtOH would alter DA neurotransmission, but not clearance, in the NAc.
METHODS
Subjects
Adult female Wistar rats, (250 to 300 g; Harlan Inc., Indianapolis, IN) were single housed on a 12 hours light cycle (lights on at 0700 hours). Female subjects were used for all experiments, as they preserve body weight and head size better than do males. Any effects of the estrous cycle on the response of the DA system to EtOH would have been minimized because experiments were conducted across several days at the different EtOH doses. All animals were EtOH-naïve at the start of the experiment. Food and water were available ad libitum throughout the experiment, except during the microdialysis phases. All research protocols were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee, and experiments were conducted in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Procedures
Stereotaxic Surgery and Probe Insertion
Animals were positioned in the stereotaxic apparatus and anesthetized with 2% isofluorane in air. They were implanted unilaterally with an 18-gauge guide cannula (Plastics One, Roanoke, VA) aimed 4.0 mm above the floor of the right hemispheric NAc (AP +1.7, ML +2.2, DV −4.3, 10.0° offset from vertical; Paxinos and Watson, 1998). Cannulae were secured to the skull with cranioplastic cement.
Following recovery from surgery (minimum: 5 days), animals were briefly anesthetized with 2% isofluorane, and implanted with loop-style microdialysis probes (Kohl et al., 1998). Care was taken to ensure that the loop was in a rostral/ caudal orientation to maximize exposure of the microdialysis membrane to the NAc. Probes had a 4.0 mm loop of active dialysis surface (molecular weight cutoff: 13.0 kD, Spectrum Laboratories, Rancho Dominguez, CA), extending 2.0 mm beyond the cannulae, into the NAc. Distance between ascending and descending segments of active microdialysis membrane did not exceed 1.0 mm. The implanted probes were permanently cemented into place with cranioplastic cement 48 hours prior to microdialysis.
Experiment 1: SUL Microdialysis
Treatment Groups
Two days following cannulation surgery, animals were assigned randomly to treatment groups and began a 5 consecutive day regimen of 15% w/ v EtOH solution (in sterile saline), or a volumetric equivalent of saline, by i.p. injection. Groups were assigned by EtOH pretreatment dose (0.0, 0.5, 1.0, or 2.0 g/kg), as well as the number of EtOH pretreatments (0, 1, or 5) received at the assigned dose. Treatment regimens were defined as follows: (1) 1 control group received 5 days of saline and 1 control group had no injection pretreatment; because these groups did not differ significantly, they were collapsed for analyses; (2) single EtOH pretreatment groups received 4 days of saline treatment, followed by 1 day of EtOH administration; and (3) repeated EtOH groups received 5 days of EtOH treatment. For sample sizes, see Table 1.
Table 1.
Summary of Sample Sizes Within Each Group of Experiment 1, Basal Dopamine Levels (Nm), Sulpiride-Induced DA Change From Baseline, and Probe Distribution Between Nucleus Accumbens Shell and Core
| EtOH pretreatments | EtOH dose (g/kg) | Basal DA (nM) | DA (% change during SUL) | n (core) | n (shell) | n (core/shell ) | N (total) |
|---|---|---|---|---|---|---|---|
| 0 | 0.0 | 1.26 ± 0.21 | 165 ± 5 | 7 | 9 | 7 | 23* |
| 1 | 0.5 | 1.26 ± 0.31 | 164 ± 7 | 2 | 7 | 3 | 12 |
| 1 | 1.0 | 1.66 ± 0.33 | 124 ± 6 | 3 | 6 | 1 | 10 |
| 1 | 2.0 | 1.20 ± 0.39 | 153 ± 12 | 3 | 4 | 0 | 7 |
| 5 | 0.5 | 1.61 ± 0.37 | 170 ± 13 | 4 | 3 | 2 | 9 |
| 5 | 1.0 | 1.41 ± 0.30 | 125 ± 5 | 4 | 5 | 3 | 12 |
| 5 | 2.0 | 0.84 ± 0.37 | 166 ± 9 | 5 | 2 | 1 | 8 |
Columns 5 to 8 indicate the sample size for each microdialysis group in Experiment 1, in which the probe was verified in the core and/or shell of the nucleus accumbens. Basal extracellular dopamine levels (mean ± SEM; nM) are presented in column 3. Percentages of increase from basal DA levels following sulpiride perfusion are presented in column 4.
Nonpretreated and saline-pretreated control groups (0.5, 1.0, 2.0 ml/kg) were collapsed, as no statistical differences were observed (p = 0.418).
SUL Reverse Microdialysis
Sixteen to twenty hours following the final injection, animals were placed in Plexiglas tubs (25 × 44 × 38 cm; W × L × H) for reverse microdialysis, with local perfusion to the NAc. Probes were perfused using a Harvard micro-infusion pump (Harvard Bioscience, Inc., Holliston, MA), at a flow rate of 1.0 μl/ minute.
Rats were initially perfused with artificial cerebrospinal fluid (aCSF; 140.0 mM NaCl, 3.0 mM KCl, 2.5 mM CaCl2, 1.0 mM MgCl2, 2.0 mM Na2PO4, and 0.2 mM ascorbate; Sigma-Aldrich Co., St Louis, MO; pH 7.4, filtered for impurities; Thielen et al., 2004). Prior to dialysate sample collection, animals underwent a 70-minute uninterrupted collection period of aCSF perfusion to stabilize basal DA levels, then aCSF was perfused for at least an additional 80 minutes, with sample collection every 20 minutes. Next, animals were perfused with aCSF containing 100 μM SUL (Sigma-Aldrich Co.), at the same flow rate for 80 minutes. This concentration was chosen based upon results from previous studies employing reverse microdialysis of SUL in Wistar and P rats (Engleman et al., 2003; Kohl et al., 1998; Thielen et al., 2004). All rats subsequently received 140 minutes aCSF.
Histologies
Following microdialysis, animals were killed with CO2 inhalation. Probes were perfused with methylene blue dye (Sigma), and brains were immediately extracted and frozen at −20°C for sectioning. Brains were sliced in 40-μm sections on a cryostat (Leica Microsystems Inc., Bannockburn, IL), and probe locations were verified according to the Paxinos and Watson (1998) rat brain atlas. An inclusion criterion was defined such that at least 60% of the active probe membrane was within the NAc. Data from animals not fulfilling the inclusion criteria were excluded from the study.
Dialysate DA Analysis
Each dialysate sample was collected in 5.0 μl of 0.1 N perchloric acid to minimize oxidation of DA. Samples were immediately frozen on dry ice, then stored at −70°C until analysis. Samples were assayed for DA content by high performance liquid chromatography with an electrochemical detector (HPLC-EC). DA was detected via a 6.0 mm glassy carbon electrode (+720 mV potential, 0.5 nA/ V sensitivity setting), coupled to an amperometric detector (EG&G Princeton Applied Sciences, Model 400), as previously described (Kohl et al., 1998; Yoshimoto et al., 1992a). Samples were introduced into a 10.0 μl sample loop (Valco Instruments Co. Inc., Houston, TX), and injected onto an analytical column (Spherisorb C18, 3.0 μm OD, 2.0 × 150.0 mm; Keystone Scientific, Bellafonte, PA). The HPLC mobile phase consisted of 9.0 g/ l sodium phosphate, 35.0 mg/ l 1-octanesulfonic acid, 190 mg/ l ethylene diamine tetraacetic acid, 8.0 ml/ l tetrahydrofuran, and 10% acetonitrile; pH adjusted to 3.0 with 85% phosphoric acid. The flow rate on the delivery system was set to 0.20 ml/ min (Model 582; ESA Inc., Chelmsford, MA). ChromPerfect (version 4.4.23; Justice Innovations Inc., Palo Alto, CA), a specialized computer program for HPLC data collection, was utilized to analyze detector output. DA levels were determined via comparison with a standard DA curve. The detection limit for DA on this system was approximately 0.1 nM.
Data Analysis
All data analyses were performed using statistics software (SPSS version 14, SPSS Inc., Chicago, IL). Basal DA values (nM) were established by averaging values for the last 2 DA determinations collected prior to initiating SUL perfusion. Analyses of the effects of EtOH pretreatment on basal extracellular NAc DA concentrations were performed using a one-way ANOVA.
Basal DA levels were operationally defined as 100%. Dialysate DA concentrations were normalized by expressing them as a percentage of change from basal values, to correct for variability between subjects and probe recovery. Mean increases in DA levels during SUL perfusion were compared between treatment groups. The effects of perfusing SUL to the NAc on extracellular DA as a function of number of EtOH pre-exposures, EtOH dose, and sampling time point were analyzed by a mixed ANOVA with repeated measures for time (data shown in Fig. 3). All analyses were followed by a Tukey HSD post hoc test. Data from the first 2 collections after initiating SUL perfusion were not included in analyses, as this transitional period did not reflect a full effect of the compound. Data from the time point immediately following cessation of SUL was included in analyses. p-Values were set at a statistical significance level of less than 0.05.
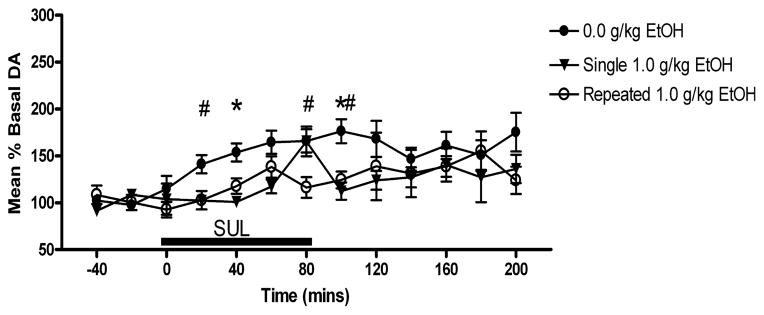
Fig. 3.
Time course for 100 μM SUL-induced increases in extracellular DA in the NAc. Subjects receiving 1.0 g/kg EtOH pretreatment showed attenuated DA responses to SUL perfusion. #Differences between repeated ethanol exposure subjects and controls. *Differences between single ethanol exposure subjects and controls (p < 0.05).
Experiment 2: DA No-net-flux
All protocols for Experiment 2 were as described for Experiment 1, unless otherwise noted.
Treatment Groups
Animals were assigned randomly to 1 of 2 groups. In order to maintain consistency with the previous protocol in Experiment 1, and to compare the results of the 2 studies, all subjects received 1 i.p. injection daily, for 5 consecutive days. Groups were comprised of a control group, which received 5 consecutive days of 1.0 ml/ kg sterile saline (n = 5), and an EtOH-pretreated group, which received 4 days of saline, followed by 1 day 1.0 g/ kg EtOH in saline administration (n = 5).
DA No-net-flux Microdialysis
The day following completion of the assigned treatments, subjects were perfused at 0.5 μl/ min with aCSF for 120 minutes to establish basal extracellular DA levels. Next, aCSF was perfused for an additional 60 minutes for determination of DA levels. Three DA concentrations (5.0, 10.0, and 20.0 nM) were then perfused for 100 minutes each, in random order. Animals were once again perfused with aCSF for 120 minutes to re-establish basal extracellular DA levels.
Data Analysis
Quantitative levels and in vivo extraction fractions for DA were analyzed via multiple linear regression modeling software (GraphPad Prizm, version 4.02, San Diego, CA). The net exchange of DA between the probe and extracellular space was expressed as the difference of the DA concentration perfused into the probe [DA]in and the DA concentration recovered in the samples [DA]out. The data were plotted as [DA]in − [DA]out, as a function of [DA]in. Quantitative DA levels were determined by the x-intercept of the regression line. Extraction fraction was determined by the slope of the regression line. Comparisons of slopes and intercepts were performed by analysis of covariance (Zar, 1984).
RESULTS
Experiment 1: SUL Reverse Microdialysis
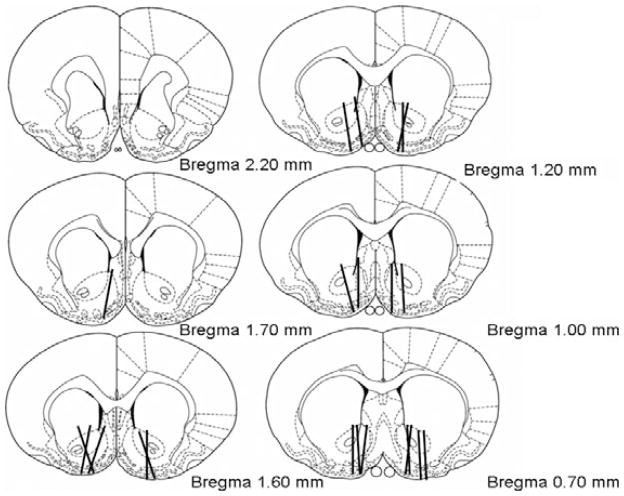
Figure 1 shows representative probe placements for all SUL-treated groups. For distribution of core and/or shell placements in each group, see Table 1. Only animals with at least 60% of the probe within the NAc were included in this study.
Fig. 1.
Representative nucleus accumbens (NAc) microdialysis probe placements. Lines indicate active regions of the probes, which were verified with methylene blue dye. Experiment 1: Placements on the left depict probes for (−)sulpiride (SUL)-treated animals. See Table 1 for divisions of core/shell placements × treatment group. Experiment 2: Placements in the right hemisphere show NAc DA no-net-flux probe placements. Placements in the right hemisphere are for illustrative purposes only, and do not represent the side on which microdialysis was performed. Most placements overlapped both the core and shell although a few probes were mainly in the shell.
Comparison of basal extracellular DA levels prior to SUL perfusion did not yield significant differences among groups, either for number of EtOH pretreatments, F(2,73) = 0.085, p > 0.05, or dose of EtOH pretreatment, F(3,73) = 0.123, p > 0.05. For basal levels, see Table 1.
Mean increases in extracellular NAc DA levels during SUL perfusion are shown in Fig. 2 and in Table 1. The time-course microdialysis data comparing the effects of local SUL perfusion on DA overflow in P-rats with differing EtOH pretreatments were analyzed by a mixed ANOVA with main effects of number of treatments (1 day vs. 5 days of EtOH administration) and EtOH dose (0, 0.5, 1.0, or 2.0 g/ kg) with repeated measures on time.
Fig. 2.
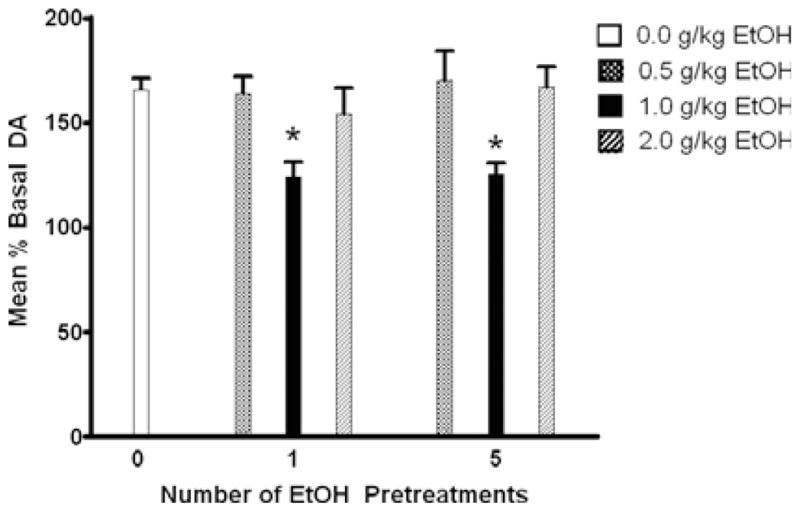
Mean increases of extracellular DA above baseline in the NAc during 100 μM SUL perfusion for each EtOH pretreatment group. Subjects receiving 1.0 g/kg EtOH pretreatment displayed a significant attenuation of SUL effects, when compared with those receiving *0.0, 0.5, or 2.0 g/kg EtOH pretreatment (p < 0.05).
Results of the mixed ANOVA showed a significant main effect of dose [F(3,233) = 17.828; p < 0.001] but not days of pretreatment [F(2,233) = 1.319; p > 0.05], or time [F(3,110) = 1.176; p > 0.05]. A pair-wise post-hoc analysis of the dose effect indicated that the 1.0 g/ kg EtOH pretreatment dose resulted in a significantly reduced effect of SUL on extracellular DA levels (p < 0.001) compared with all other pretreatment doses. Figure 3 shows the time course effects for SUL (in percent of basal extracellular DA levels) in subjects receiving 1.0 g/ kg EtOH pretreatment (both single and repeated) compared with EtOH-naïve subjects.
Univariate analyses of maximal SUL-induced DA increases showed no effects of number of EtOH pretreatments. The administered dose of EtOH, however, yielded significant differences in maximal DA increase. The maximal SUL effect in groups treated with 1.0 g/ kg EtOH (158 ± 10%) was lower than in those treated with 0.0 (206 ± 10%; p < 0.01) or 0.5 (201 ± 11%; p < 0.05) g/kg. No differences in maximal SUL-induced DA levels were seen between 1.0 and 2.0 (196 ± 13%) g/ kg EtOH-pretreatment groups (p > 0.05).
Experiment 2: DA No-net-flux
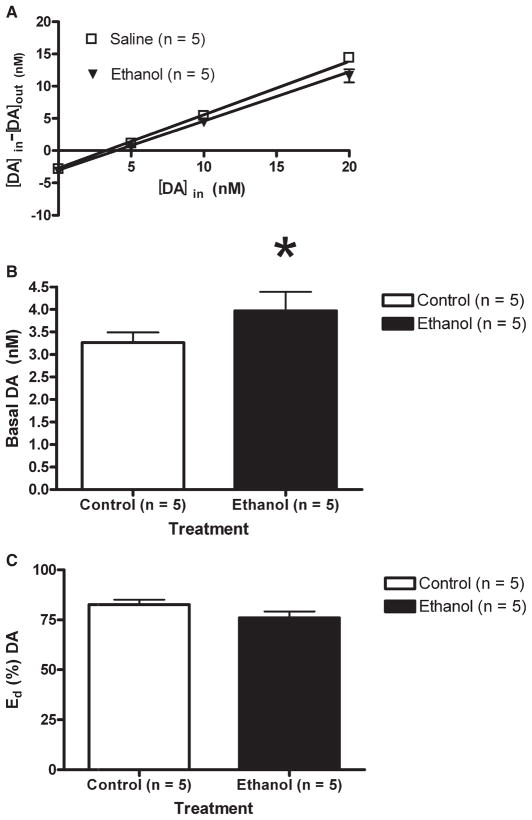
Probe placements for all no-net-flux subjects are illustrated in Fig. 1. Most placements included portions of both the shell and core regions of the NAc. Multiple linear regression analyses of the [DA]in plotted against the [DA]in − [DA]out were conducted for both the EtOH-naïve and EtOH-treated rats, with the x-intercepts indicating the basal extracellular DA concentration and the slope of the regression lines revealing the extraction fractions (Fig. 4A). The results indicated that subjects receiving EtOH pretreatment displayed significantly greater basal DA levels in the NAc (3.96 ± 0.42 nM), compared with EtOH-naïve animals (3.25 ± 0.23 nM), F(1,147) = 4.80; p < 0.05 (Fig. 4B). Figure 4C compares the extraction fractions of EtOH-naïve and EtOH-exposed subjects. The results suggest that extraction fractions were not different between the 2 groups (82 ± 2% vs. 76 ± 3%, respectively), F(1,146) = 2.03; p > 0.05.
Fig. 4.
(A) Multiple linear regression analyses for EtOH-naïve (square) and EtOH-exposed (triangle) animals are expressed as the concentration of DA perfused into the probe minus the concentration of the DA found in dialysate, [DA]in − [DA]out (nM; mean ± SEM) as a function of the known concentration of DA perfused into the probe, [DA]in. X-intercepts indicate the quantitative basal extracellular DA concentrations. The slopes of the regression lines reveal the extraction fractions. (B) Basal extracellular DA (nM) concentration. Non-EtOH-treated animals received 5 daily injections of saline (white). EtOH-treated subjects received 4 saline pretreatments, followed by a single dose of 1.0 g/kg EtOH (black). The results indicated that 24 hours after the injection, subjects receiving EtOH pretreatment displayed significantly greater basal DA levels in the NAc (3.96 ± 0.42 nM), compared with EtOH-naïve animals (3.25 ± 0.23 nM), F(1,147) = 4.80; *p = 0.02. (C) The percent extraction fraction (mean ± SEM) for EtOH-naïve (white) and EtOH-exposed (black) subjects. The results suggest that extraction fractions were not significantly different between EtOH-naive and EtOH-exposed groups (82 ± 2% vs. 76 ± 3%, respectively), F(1,146) = 2.03; p = 0.15.
DISCUSSION
The current study examined the effects of a single EtOH pretreatment and repeated EtOH pretreatments on mesolimbic DA system function in female Wistar rats. The present results support the hypothesis that brief EtOH exposure alters DA regulatory mechanisms, as well as basal extracellular DA levels, in the NAc. Experiment 1 showed that SUL perfusion increased extracellular DA levels in the NAc to 164 ± 5% of basal levels in EtOH-naïve subjects, and that a single or repeated 1.0 g/ kg systemic EtOH pretreatment attenuated SUL-induced elevations in extracellular DA levels to 125 ± 8% and 122 ± 7%, respectively, of basal DA levels (Fig. 2; Table 1). These findings are consistent with previous self-administration data, which suggested that moderate EtOH drinking attenuates the extracellular DA response to 100 μM SUL in P rats (Engleman et al., 2003; Thielen et al., 2004).
A transient increase in extracellular DA levels was seen in the single 1.0 g/ kg EtOH pretreated group, after 80 minutes of SUL perfusion. This increase could reflect a cumulative effect of SUL over the entire perfusion period. Given the results from the control group, however, which show a clear gradual, time dependent elevation followed by a gradual return toward baseline, as is typical for a response to local application of drugs, the sporadic elevation and return to baseline suggests that this effect is a chance phenomenon.
Moderate EtOH doses (e.g., 1.0 g/ kg) often have been shown to increase extracellular DA concentrations (Campbell et al., 1996; Imperato and DiChiara, 1986; Kohl et al., 1998; Smith and Weiss, 1999; Yim et al., 1998; Yoshimoto et al., 1992a,b). According to Engleman and colleagues (2000, 2003), P rats given scheduled access to EtOH drink approximately 1.0 g/ kg—the same dose at which the current study exhibits its significant effects. In addition, Rodd and colleagues (2004b) showed that male Wistar rats would intracranially self-administer EtOH into the ventral tegmental area at moderate (100 to 300 mg%) doses, but not at low (0 to 75 mg%) or high (400 mg%) doses. These studies suggest that moderate levels of EtOH may provide the greater reinforcing properties than either high or low doses of EtOH.
In the current study, neither the single nor the repeated 0.5 g/ kg EtOH-pretreated groups displayed significant alterations in SUL-induced increases in DA (162 ± 7% and 172 ± 9% of basal DA levels, respectively), compared with controls (Fig. 2), suggesting that this dose may be too low to produce significant neuronal alterations in the NAc of Wistar rats.
In the present study, groups receiving single or repeated 2.0 g/ kg EtOH pretreatment did not show attenuation of DA response to SUL (155 ± 9 and 172 ± 8, respectively). This dose of EtOH may alter mechanisms that are protective against or compensate for DA D2 neuroadaptations. EtOH exhibits nonlinear dose-related effects (Calabrese and Baldwin, 2003). For example, higher doses appear to be behaviorally sedating, while lower doses are behaviorally activating (see Pohorecky, 1977 for review). In several areas, including social interaction (see Spear and Varlinskaya, 2005 for review), locomotor behavior (Rodd et al., 2004a), intracranial EtOH self-administration (Rodd et al., 2004b), and neurochemical responses (Howard et al., 2008; Mereu et al., 1984), EtOH does not yield a simple linear dose–response curve. The mechanisms underlying these nonlinear effects of EtOH remain unclear. It is difficult to reconcile the finding that a 1.0 g/ kg dose of EtOH reduces D2 autoreceptor function, whereas a 2.0 g/ kg dose does not. Both doses increase DA release with the higher dose producing a greater and more prolonged effect (Yoshimoto et al., 1992a,b). However, the majority of previous work focused on the direct effects of EtOH rather than its adaptive effects. The effect of a single administration of 1.0 g/kg EtOH to reduce the function of the D2 autoreceptor in the NAc may be due to a combination of a prolonged increased release of DA and a separate pharmacological action of EtOH. It is possible that differences observed in the current study between the 1.0 and 2.0 g/ kg EtOH doses may be due to differential DA D2 receptor availability.
The reduced function of the D2 autoreceptors following 1.0 g/ kg EtOH could result from post-translational modification and/ or internalization of the autoreceptors. For example, in vitro evidence suggests that increases in DA levels, such as those caused by acute EtOH exposure, may lead to internalization of D2 receptors (Goggi et al., 2007). The higher EtOH exposure may lead to disordered properties of neuronal membranes. Evidence suggests that EtOH exposure may alter cellular membrane composition and permeability in a concentration-dependent manner (Patra et al., 2006). SUL is a lipophilic agent, capable of crossing neuronal membranes to bind to intracellular D2 receptors (Sun et al., 2003). It is possible that alterations in membrane fluidity with the higher EtOH dose could facilitate SUL’s ability to bind to D2 autoreceptors, which could offset any actions produced by the lower EtOH dose.
Previous studies with repeated (Smith and Weiss, 1999) and acute (Yim and Gonzales, 2000) administration of 1.0 g/ kg EtOH reported no significant effect on DA clearance, although others have shown that EtOH may enhance DA transporter activity (Carroll et al., 2006; Mayfield et al., 2001; Wang et al., 1997). It is possible that the intermittent administration of 2.0 g/kg EtOH pretreatment employed in the current study increased NAc DA clearance, thereby protecting against alterations to D2 autoreceptors.
In conclusion, the apparent lack of effect of the 2.0 g/ kg dose to produce a similar effect as the 1.0 g/ kg dose suggests that the higher EtOH levels may be altering post-translational processes, membrane properties, and/ or gene expression that prevent changes in D2 autoreceptor function. Future research is needed to characterize these complex actions of EtOH.
In contradiction to the initial hypothesis of this study, there were no apparent differences in extracellular DA levels between groups treated with repeated EtOH exposure compared with a single EtOH exposure. While DA response to local SUL application in the group treated with 1.0 g/ kg repeated EtOH was significantly lower than in controls, no further attenuation of SUL-induced DA levels was seen, compared with the single 1.0 g/kg EtOH-treated group. One possible explanation for this finding is that a single exposure to EtOH is sufficient to obtain maximal effects on DA D2 receptors. In support of this suggestion, maximal SUL-induced DA increases following EtOH pretreatment were similar following single or repeated exposure to EtOH.
Extracellular DA levels had not returned to baseline levels 120 minutes following cessation of SUL perfusion (Fig. 3). It is possible that SUL had not completely diffused from the extracellular space in the time measured, and continued to bind competitively to D2 receptors. Kohl and colleagues (1998) perfused SUL to the NAc for 60 minutes, following which time, levels returned to baseline. The current study, however, perfused SUL for 80 minutes for all groups. This increase in perfusion time may lead to larger accumulation of SUL levels in the areas surrounding the probes compared with the study by Kohl and colleagues (1998), requiring an extended period to clear the SUL and for a return to pre-perfusion D2 autoreceptor function.
Another possibility for this continued elevation in DA levels following SUL perfusion is that DA was incapable of displacing SUL at these higher concentrations. SUL binds with high affinity to D2 receptors (pKI = 7.6, Kuroki et al., 1999; Seeman and Van Tol, 1995) and may not dissociate from the receptor sufficiently to diminish extracellular DA levels in the time allowed in the current experiment. Engleman and colleagues (2003) previously found that 100 μM is the optimal concentration for SUL-induced increases in extracellular NAc DA levels. Similar to the current study, however, Engleman and colleagues (2003) did not report that extracellular DA levels at 100 μM concentration returned to baseline following cessation of SUL perfusion. Future studies investigating the dose-related effects of SUL may provide useful information to explain the continued elevation of extracellular DA levels after termination of SUL perfusion.
The current findings are in agreement with previous studies reporting desensitization or down-regulation of DA D2 receptors, following EtOH exposure. Rommelspacher and colleagues (1992) demonstrated that 2 weeks of EtOH exposure, followed by a 24-hr withdrawal period, led to a diminishment in the density of DA D2 receptors in Wistar rats. In addition, acute and prolonged exposure to EtOH diminished striatal binding of [3H]-Spiroperidol in Wistar males (Reggiani et al., 1980). Lograno and colleagues (1993), however, reported no such effects in caudate putamen DA D2 receptors in subjects receiving prolonged EtOH treatment. These findings suggest that the effects of EtOH on the number of DA D2 receptors may be dependent upon the substrate assayed, the particular regimen of EtOH administration, or other factors, such as the technique used to evaluate DA D2 receptor function. Supplemental experiments that specifically evaluate NAc DA receptor population and sensitivity would be useful additions to the current study. These studies would offer more information regarding any desensitization or down-regulation in DA D2 receptors, following EtOH exposure.
As a DA D2-like receptor antagonist, SUL may bind to both pre- and postsynaptic receptors. Kohl and colleagues (1998) reported that perfusion of SUL to the ventral tegmental area increased extracellular DA in the ventral tegmental area and in the NAc. This provided evidence of a negative feedback loop between the NAc terminal fields of DA axonal projections and the ventral tegmental DA cell bodies, which is consistent with the suggestion that the effects of SUL may occur both pre-synaptically and postsynaptically.
No differences in basal extracellular DA levels were observed between EtOH-naïve and EtOH-experienced groups using traditional reverse microdialysis (Table 1). This finding is consistent with several previous traditional microdialysis studies measuring DA levels following EtOH experience (Engleman et al., 2003; Katner et al., 1996; Thielen et al., 2004; Weiss et al., 1993). Also in agreement with the current results, Rougé-Pont and colleagues (2002) found that wild-type and DA D2 receptor knock-out mice exhibited no differences in basal striatal DA levels. The lack of basal differences presently reported using reverse microdialysis, coupled with findings of significant differences between EtOH-naïve and EtOH-experienced rats using quantitative no-net-flux microdialysis (Smith and Weiss, 1999; Thielen et al., 2004; present findings) suggest that quantitative measures may be necessary to find small differences in basal DA levels after such treatments. One caveat is that basal differences may not have been detected during reverse microdialysis because of variability in probe recoveries, which also increases the variability in DA levels under reverse microdialysis conditions.
Experiment 2 employed no-net-flux microdialysis to examine the effects of a single, moderate (1.0 g/kg) dose of EtOH on NAc DA neurotransmission in the Wistar rat. The results indicated a significant increase in basal extracellular DA levels in subjects that had been pretreated with 1.0 g/ kg EtOH, compared with those receiving saline (Fig. 4). These findings are consistent with the results of Experiment 1, showing a significant diminishment in the DA response to SUL perfusion following a single moderate EtOH pre-treatment. Taken together, these results indicate that the reduced D2 receptor function evidenced in the first experiment may result in increased DA neurotransmission.
Thielen and colleagues (2004) also found basal increases in DA following prolonged EtOH exposure via drinking by P rats, as well as following EtOH deprivation. The findings are similar to the current study, in that basal DA alterations in the EtOH-experienced rats were observed when employing no-net-flux microdialysis, but not during basal conditions with traditional (flux) microdialysis. The increase in quantitative extracellular DA levels reported in the current study following EtOH pretreatment also is in agreement with reports by Smith and Weiss (1999). These researchers reported alterations in the mesolimbic DA system following repeated (5) 1.0 g/ kg EtOH treatments. The present study suggests that these changes may occur following a briefer exposure to EtOH than that previously described.
Consistent with previous reports (Budygin et al., 2005; Engleman et al., 2003; Jones et al., 2006; Ramachandra et al., 2007; Smith and Weiss, 1999; Yim and Gonzales, 2000), the current study suggests that the effects of EtOH on the mesolimbic DA system are not mediated by alterations in DA clearance (Fig. 4). In contrast, Carroll and colleagues (2006) reported that prolonged EtOH access alters ex vivo DA uptake in the NAc of HAD-1 rats. The different findings between the current study and Carroll and colleagues (2006) might be a result of several methodological differences, including the rat lines used in the studies, the ethanol exposure protocols, or the experimental assays that were performed.
There are several methods other than extraction fraction which could be employed to analyze DA transport. Similar to the current experiment, studies employing fast-scan cyclic voltammetry (e.g., Budygin et al., 2001; Jones et al., 2006; Mathews et al., 2006) and binding (e.g., Russell et al., 1988) have reported no effect of EtOH on DA uptake in the NAc or striatum. There are limitations with any technique employed to analyze uptake. According to Cosford and colleagues (1996), however, extraction fraction has been shown to be a useful measurement of clearance, as it does not depend upon neuronal release or metabolism as its method of analysis. Parsons and Justice (1994) and Justice (1993) have reviewed the use of quantitative microdialysis as a method for ascertaining transmitter clearance. They concluded that quantitative microdialysis is one efficacious manner of evaluating DA clearance. More recently, Chefer and colleagues (2006) reported similar findings, following an evaluation of the validity of using quantitative microdialysis to analyze DA clearance in the mouse NAc.
The present study did not distinguish between NAc shell and core, but sub-regional comparisons should be made in the future. Several studies have reported regional differences within the NAc, including those investigating substrates related to reward, motivation, and learning (Fenu et al., 2001; Phillips et al., 2003; Smith-Roe and Kelley, 2000; Tzschentke, 1998). The shell and core have been established to possess disparate afferent and efferent projections (Usuda and Koichi, 1998). These sub-regions appear to exhibit functional heterogeneity in several areas, including in DA D2 receptor number (McBride et al., 1993, 1997) and response to drugs of abuse (McKittrick and Abercrombie, 2007).
The present study utilized only female subjects. Female subjects were used because they preserve body and head size better than do males. Effects of the estrous cycle on the response of the DA system to EtOH likely would have been minimized because experiments were conducted across several days, at the different EtOH concentrations. Replication of the current experiment should be performed in male subjects to establish whether these findings will generalize across sex.
A further consideration of the current study is that the use of a higher (2.5 mM vs. 1.2 mM) concentration of CaCl2 in the aCSF than that which is typically reported. The concentration used in the present study was chosen to coincide with previous experiments (Thielen et al., 2004). While this higher concentration of CaCl2 potentially could affect transmitter release and basal DA levels in the current experiment, the present data do not show an elevation in extracellular DA levels, as compared with a previous report using lower CaCl2 concentrations (Smith and Weiss, 1999).
In conclusion, the current study suggests that a single, moderate dose of EtOH is sufficient to reduce DA D2 receptor function, and increase DA neurotransmission, in the NAc of Wistar rats. This finding offers new information regarding neuradaptations in the mesolimbic reward system following the first experience with EtOH. These results could offer valuable information regarding the time course of alcohol drinking acquisition. In addition, the current study shows that a moderate exposure to EtOH is sufficient to prime a cascade of neurobiological changes that may increase vulnerability to alcohol abuse or alcoholism.
Acknowledgments
This research was supported by grants AA10717, AA10721, AA11261, and AA07462.
References
- Budygin EA, Mathews TA, Lapa GB, Jones SR. Local effects of acute ethanol on dopamine neurotransmission in the ventral striatum in C57BL/6 mice. Eur J Pharmacol. 2005;523:40–45. doi: 10.1016/j.ejphar.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Wightman RM, Jones SR. Terminal effects of ethanol on dopamine dynamics in rat nucleus accumbens: an in vitro voltammetric study. Synapse. 2001;42:77–79. doi: 10.1002/syn.1101. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Ethanol and hormesis. Crit Rev Toxicol. 2003;33:407–424. doi: 10.1080/713611043. [DOI] [PubMed] [Google Scholar]
- Campbell AD, Kohl RR, McBride WJ. Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol. 1996;13:569–574. doi: 10.1016/s0741-8329(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Carroll MR, Rodd ZA, Murphy JM, Simon JR. Chronic ethanol consumption increases dopamine uptake in the nucleus accumbens of high alcohol drinking rats. Alcohol. 2006;40:103–109. doi: 10.1016/j.alcohol.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Zapata A, Shippenberg TS, Bungay PM. Quantitative no-net-flux microdialysis permits detection of increases and decreases in dopamine uptake in mouse nucleus accumbens. J Neurosci Methods. 2006;155:187–193. doi: 10.1016/j.jneumeth.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Cosford RJ, Vinson AP, Kukoyi S, Justice JB., Jr Quantitative microdialysis of serotonin and norepinephrine: pharmacological influences on in vivo extraction fraction. J Neurosci Methods. 1996;68:39–47. doi: 10.1016/0165-0270(96)00057-x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Engleman EA, McBride WJ, Li TK, Lumeng L, Murphy JM. Ethanol drinking experience attenuates (−)sulpiride-induced increases in extracellular dopamine levels in the nucleus accumbens of Alcohol-Preferring (P) rats. Alcohol Clin Exp Res. 2003;27:424–431. doi: 10.1097/01.ALC.0000056618.57931.A5. [DOI] [PubMed] [Google Scholar]
- Engleman EA, McBride WJ, Wilber AA, Shaikh SR, Eha RD, Lumeng L, Li TK, Murphy JM. Reverse microdialysis of a dopamine uptake inhibitor in the nucleus accumbens of alcohol-preferring rats: effects on dialysate dopamine levels and ethanol intake. Alcohol Clin Exp Res. 2000;24:795–801. [PubMed] [Google Scholar]
- Fenu S, Bassareo V, Di Chiara G. A role for dopamine D1 receptors of the nucleus accumbens shell in conditioned taste aversion learning. J Neurosci. 2001;21:6897–6904. doi: 10.1523/JNEUROSCI.21-17-06897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggi JL, Sardini A, Egerton A, Strange PG, Grasby PM. Agonist-dependent internalization of D2 receptors: imaging quantification by confocal microscopy. Synapse. 2007;61:231–241. doi: 10.1002/syn.20360. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard EC, Schier CJ, Wetzel JS, Duvauchelle CL, Gonzales RA. The shell of the nucleus accumbens has a higher dopamine response compared with the core after non-contingent intravenous ethanol administration. Neuroscience. 2008;154:1042–1053. doi: 10.1016/j.neuroscience.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, DiChiara G. Preferential stimulation of dopamine release in the n. accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;238:219–228. [PubMed] [Google Scholar]
- Jones SR, Mathews TA, Budygin EA. Effect of moderate ethanol dose on dopamine uptake in rat nucleus accumbens in vivo. Synapse. 2006;60:251–255. doi: 10.1002/syn.20294. [DOI] [PubMed] [Google Scholar]
- Justice JB. Quantitative microdialysis of neurotransmitters. J Neurosci Methods. 1993;48:263–276. doi: 10.1016/0165-0270(93)90097-b. [DOI] [PubMed] [Google Scholar]
- Katner SN, Kerr TM, Weiss F. Ethanol anticipation enhances dopamine efflux in the nucleus accumbens of alcohol-preferring (P) but not Wistar rats. Behav Pharmacol. 1996;7:669–674. [PubMed] [Google Scholar]
- Kohl RR, Katner JS, Chernet E, McBride WJ. Ethanol and negative feedback regulation of mesolimbic dopamine release in rats. Psychopharmacology (Berl) 1998;139:79–85. doi: 10.1007/s002130050692. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Meltzer HY, Ichikawa J. Effects of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. J Pharm Exp Therapeutics. 1999;288:774–781. [PubMed] [Google Scholar]
- Levy AD, Murphy JM, McBride WJ, Lumeng L, Li TK. Microinjection of sulpiride into the nucleus accumbens increases ethanol drinking in alcohol-preferring (P) rats. Alcohol Alcohol Suppl. 1991;1:417–420. [PubMed] [Google Scholar]
- Lograno DE, Matteo F, Trabucchi M, Govoni S, Cagiano R, Lacomba C, Cuomo V. Effects of chronic ethanol intake at a low dose on the rat brain dopaminergic system. Alcohol. 1993;10:45–49. doi: 10.1016/0741-8329(93)90052-p. [DOI] [PubMed] [Google Scholar]
- Mathews TA, John CE, Lapa GB, Budygin EA, Jones SR. No role of the dopamine transporter in acute ethanol effects on striatal dopamine dynamics. Synapse. 2006;60:288–294. doi: 10.1002/syn.20301. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Maiya R, Keller D, Zahniser NR. Ethanol potentiates the function of the human dopamine transporter expressed in Xenopus oocytes. J Neurochem. 2001;79:1070–1079. doi: 10.1046/j.1471-4159.2001.00656.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Chernet E, Dyr W, Lumeng L, Li T-K. Densities of dopamine D2 receptors are reduced in CNS regions of alcohol-preferring P-rats. Alcohol. 1993;10:387–390. doi: 10.1016/0741-8329(93)90025-j. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Chernet E, Russell RN, Chamberlain JK, Lumeng L, Li TK. Regional CNS densities of serotonin and dopamine receptors in high alcohol-drinking (HAD) and low alcohol-drinking (LAD) rats. Alcohol. 1997;14:603–609. doi: 10.1016/s0741-8329(97)00072-4. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Abercrombie ED. Catecholamine mapping within nucleus accumbens: differences in basal and amphetamine-stimulated efflux of norepinephrine and dopamine in shell and core. J Neurochem. 2007;100:1247–1256. doi: 10.1111/j.1471-4159.2006.04300.x. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, Engleman EA, Li T-K, McBride WJ, Murphy JM. Microdialysis of dopamine in the nucleus accumbens of alcohol preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res. 2002;26:318–325. [PubMed] [Google Scholar]
- Mereu G, Fadda F, Gessa GL. Ethanol stimulates the firing rate of nigral dopaminergic neurons in unanesthetized rats. Brain Res. 1984;292:63–69. doi: 10.1016/0006-8993(84)90890-4. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Parsons LH, Justice JB. Extracellular concentration and in vivo recovery of dopamine in the nucleus accumbens using microdialysis. J Neurochem. 1992;58:212–218. doi: 10.1111/j.1471-4159.1992.tb09298.x. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr Quantitative approaches to in vivo brain microdialysis. Crit Rev Neurobiol. 1994;8:189–220. [PubMed] [Google Scholar]
- Patra M, Salonen E, Terama E, Vattulainen I, Faller R, Lee BW, Holopainen J, Karttunen M. Under the influence of alcohol: the effect of ethanol and methanol on lipid bilayers. Biophys J. 2006;90:1121–1135. doi: 10.1529/biophysj.105.062364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Setzu E, Hitchcott PK. Facilitation of appetitive Pavlovian conditioning by d-amphetamine in the shell, but not the core, of the nucleus accumbens. Behav Neurosci. 2003;117:675–684. doi: 10.1037/0735-7044.117.4.675. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Biphasic action of ethanol. Biobehav Rev. 1977;1:231–240. [Google Scholar]
- Ramachandra V, Phuc S, Franco AC, Gonzales RA. Ethanol preference is inversely correlated with ethanol-induced dopamine release in 2 substrains of C57BL/6 mice. Alcohol Clin Exp Res. 2007;31:1669–1676. doi: 10.1111/j.1530-0277.2007.00463.x. [DOI] [PubMed] [Google Scholar]
- Reggiani A, Barbaccia ML, Spano PF, Trabucchi M. Dopamine metabolism and receptor function after acute and chronic ethanol. J Neurochem. 1980;35:34–37. doi: 10.1111/j.1471-4159.1980.tb12486.x. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McKinzie DL, Webster AA, Murphy JM, Lumeng L, Li TK, McBride WJ. Low-dose stimulatory effects of ethanol during adolescence in rat lines selectively bred for high alcohol intake. Alcohol Clin Exp Res. 2004a;28:535–543. doi: 10.1097/01.alc.0000122107.08417.d0. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004b;24:1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelspacher H, Raeder C, Kaulen P, Bruning G. Adaptive changes of dopamine-D2 receptors in rat brain following ethanol withdrawal: a quantitative autoradiographic investigation. Alcohol. 1992;9:355–362. doi: 10.1016/0741-8329(92)90032-6. [DOI] [PubMed] [Google Scholar]
- Rougé-Pont F, Usiello A, Menoit-Marand M, Gonon F, Piazza P, Borrelli E. Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors. J Neurosci. 2002;22:3293–3301. doi: 10.1523/JNEUROSCI.22-08-03293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell VA, Lamm MC, Taljaard JJ. Effect of ethanol on [3H]dopamine release in rat nucleus accumbens and striatal slices. Neurochem Res. 1988;13:487–492. doi: 10.1007/BF01268885. [DOI] [PubMed] [Google Scholar]
- Sahr AE, Thielen RJ, Lumeng L, Li TK, McBride WJ. Long-lasting alterations of the mesolimbic dopamine system after periadolescent ethanol drinking by alcohol-preferring rats. Alcohol Clin Exp Res. 2004;28:702–711. doi: 10.1097/01.alc.0000125344.79677.1c. [DOI] [PubMed] [Google Scholar]
- Seeman P, Van Tol HH. Deriving the therapeutic concentrations for clozapine and haloperidol: the apparent dissociation constant of a neuroleptic at the dopamine D2 or D4 receptor varies with the affinity of the competing radioligand. Eur J Pharmacol. 1995;291:59–66. doi: 10.1016/0922-4106(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Smith AD, Weiss F. Ethanol exposure differentially alters central monoamine neurotransmission in alcohol-preferring versus – nonpreferring rats. J Pharmacol Exp Ther. 1999;288:1223–1228. [PubMed] [Google Scholar]
- Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci. 2000;20:7737–7742. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Low dose effects in psychopharmacology: ontogenetic considerations. Nonlinearity Biol, Toxicol, Med. 2005;3:97–111. doi: 10.2201/nonlin.003.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Ginovart N, Ko F, Seeman P, Kapur S. In vivo evidence for dopamine-mediated internalization of D2-receptors after amphetamine: differential findings with [3H]raclopride versus [3H]spiperone. Mol Pharmacol. 2003;63:456–462. doi: 10.1124/mol.63.2.456. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, Li T-K, McBride WJ. Ethanol drinking and deprivation alter dopaminergic and serotonergic function in the nucleus accumbens of alcohol-preferring rats. J Pharmacol Exp Ther. 2004;309:216–225. doi: 10.1124/jpet.103.059790. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Usuda I, Koichi T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D, Thanos PK. High levels of dopamine D2 receptors in unaffected members of alcoholic families. Arch Gen Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Palmer MR, Cline EJ, Gerhardt GA. Effects of ethanol on striatal dopamine overflow and clearance: an in vivo electrochemical study. Alcohol. 1997;14:593–601. doi: 10.1016/s0741-8329(97)00054-2. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytiä P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak KM, Pert A, Mele A, Linnoila M. Focal application of alcohols elevates extracellular dopamine in rat brain: a microdialysis study. Brain Res. 1991;540:31–40. doi: 10.1016/0006-8993(91)90489-i. [DOI] [PubMed] [Google Scholar]
- Yim HJ, Gonzales RA. Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol. 2000;22:107–115. doi: 10.1016/s0741-8329(00)00121-x. [DOI] [PubMed] [Google Scholar]
- Yim HJ, Schallert T, Randall PK, Gonzales RA. Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol Clin Exp Res. 1998;22:367–374. [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Ethanol enhances the release of dopamine and serotonin in the nucleus accumbens of HAD and LAD lines of rats. Alcohol Clin Exp Res. 1992a;16:781–785. doi: 10.1111/j.1530-0277.1992.tb00678.x. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992b;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. 2. Prentice Hall; New Jersey: 1984. [Google Scholar]