Abstract
Background
Adipocytokines may mediate the association between adiposity and lethal prostate cancer outcomes.
Methods
In the Physicians’ Health Study, we prospectively examined the association of prediagnostic plasma concentrations of adiponectin and leptin with risk of developing incident prostate cancer (654 case diagnosed 1982-2000 and 644 age-matched controls) and, among cases, risk of dying from prostate cancer by 2007.
Results
Adiponectin concentrations were not associated with risk of overall prostate cancer. However, men with higher adiponectin concentrations had lower risk of developing high grade or lethal cancer (metastatic or fatal disease). The relative risk (95% confidence interval) comparing the highest to the lowest quintile (Q5 vs. Q1) was 0.25 (0.07-0.87; Ptrend=0.02) for lethal cancer. Among all the cases, higher adiponectin concentrations predicted lower prostate cancer-specific mortality (hazard ratio, HR Q5 vs. Q1=0.39; 0.17-0.85; Ptrend=0.02), independent of body mass index (BMI), plasma C-peptide (a marker of insulin secretion), leptin, clinical stage and tumor grade. This inverse association was apparent mainly among men whose BMI ≥25 kg/m2 (HR Q5 vs. Q1=0.10; 0.01-0.78; Ptrend=0.02), but not among men of normal weight (Ptrend=0.51). Although the correlation of leptin concentrations with BMI (r=0.58, P <0.001) was stronger than that of adiponectin (r=−0.17, P<0.001), leptin was unrelated to prostate cancer risk or mortality.
Conclusion
Higher prediagnostic adiponectin (but not leptin) concentrations predispose men to a lower risk of developing high grade prostate cancer and a lower risk of subsequently dying from the cancer, suggesting a mechanistic link between obesity and poor PCa outcome.
Keywords: Adiponectin, obesity, prostate cancer, risk, survival
INTRODUCTION
Adiposity has been consistently associated with increased risk of biochemical progression of prostate cancer, metastasis, and fatal outcomes (1, 2), but the underlying mechanisms are poorly understood. We and others suggested that these effects may be mediated by adipocytokines such as adiponectin and leptin.(3-6) Adiponectin is produced solely by adipose tissue, is abundantly present in the plasma, and is inversely related to the degree of adiposity.(7) It activates the AMP-activated protein kinase (AMPK), stimulates fatty acid oxidation, improves insulin sensitivity and glucose metabolism (8), acts as a direct endogenous inhibitor of inflammation and angiogenesis (9-11), and reduces the invasiveness of breast cancer cells.(12) In contrast, circulating concentrations of leptin are closely and directly related to adiposity, and leptin’s biological effects are generally opposite to those of adiponectin.(13) Both adiponectin and leptin receptor isoforms are expressed in androgen-dependent and androgen-independent prostate cancer cell lines and human prostate cancer tumor tissues.(14-16) In addition, adiponectin inhibits both androgen-dependent and androgen-independent prostate cancer cell growth in vitro at physiological concentrations(17), whereas leptin stimulates cell proliferation specifically in androgen-independent DU145 and PC-3 prostate cancer cells but not in androgen-dependent LNCaP-FGC cells.(14)
Epidemiological data regarding circulating adiponectin and leptin and risk of prostate cancer are limited and inconsistent. Significantly lower concentrations of plasma adiponectin were found among prostate cancer patients compared to healthy men (4) and among prostate cancer patients, those with higher grade tumor (Gleason score ≥ 8) or advanced clinical stage (extraprostatic cancer) tended to have lower adiponectin concentrations (3). In a recent small prospective study, however, Baillargeon et al. found no significant relationship between prediagnostic concentrations of adiponectin and overall prostate cancer risk (n = 125 cases).(18) Stattin et al. reported a significant association between plasma leptin concentrations and increased risk of prostate cancer in the prospective Northern Sweden Health and Disease Cohort.(19) However, in a subsequent study of a different cohort, the same research group found no association of leptin with prostate cancer.(20) Neither the study by Baillargeon et al. nor three other retrospective studies found any significant associations for leptin.(5, 18, 21) Because of the weak or absent link between BMI and risk of overall incident prostate cancer, such null findings might be expected. However, the role of these adipocytokines in association with prostate cancer metastasis and lethal outcomes has not yet been evaluated. Given the close link between obesity and prostate cancer mortality, we hypothesized that these adipocyte-derived cytokines could be involved in prostate cancer progression and the development of lethal outcome.
We therefore examined the association of baseline circulating concentrations of adiponectin and leptin with future risk of developing incident prostate cancer, especially lethal cancer, in a case-control study nested within the Physicians’ Health Study. Among the cases diagnosed with prostate cancer during the follow-up, we further assessed whether their prediagnostic plasma concentrations of adiponectin and leptin predicted their subsequent risk of dying from the cancer.
MATERIALS AND METHODS
Study Population
The Physicians’ Health Study was a randomized trial of aspirin and β-carotene among 22,071 healthy U.S. male physicians ages 40-84 years that began in 1982 (22), with continuing follow-up. The research protocol was approved by the Human Subjects Committee at Brigham and Women’s Hospital, and all participants gave written informed consent.
Men were excluded at baseline if they had a history of myocardial infarction, stroke, transient ischemic attack, unstable angina, or cancer (except for nonmelanoma skin cancer) or current renal or liver disease, peptic ulcer, gout, or use of platelet-active agents, vitamin A, or β-carotene supplements. Study participants provided baseline information via self-administrated questionnaires, including height, weight, and presence of diabetes. Before randomization, 14,916 men (68%) provided a blood sample in 1982. Additional questionnaires were mailed at 6 and 12 months and annually thereafter to obtain medical information. Study investigators, unaware of the questionnaire or assay data, verified the reports of prostate cancer by participants and reviewed medical records and pathological reports to determine the tumor Gleason score (Gleason 2-6, 7 or 8-10) and clinical stage using the TNM classification system (T1/T2N0M0, T3/T4N0M0, and TxN1/M1) at diagnosis. Causes of death are determined by the End Points Committee using all available information from medical records, death certificates, and the participant’s family. Follow-up for morbidity and mortality up to 2007 is 97% complete. Because the associations of adiponectin with risks of metastatic cancer at diagnosis (TxN1/M1, n= 22) and fatal prostate cancer (n=96) during subsequent follow-up were similar and the sample size of each of these groups was relatively small, we report results for “lethal prostate cancer” for these categories combined.
Cases for the current study were drawn from participants who provided blood specimens at baseline and were later diagnosed with prostate cancer during 1982-2000. For each case, one control was selected at random from those who provided blood, had not had a prostatectomy, and had not reported a diagnosis of prostate cancer at the time the diagnosis was reported by the case subject. Controls were individually matched to cases byage (±1 year when feasible, or up to ±5 years for older men).
Laboratory Assessment for Adiponectin
Plasma adiponectin and leptin concentrations were measured by competitive radioimmunoassay (Linco Research, St. Charles, MO). We previously demonstrated that the body mass index (BMI)-adjusted intraclass correlation between two adiponectin measurements over a 1-year period was high (r = 0.84, 95% CI 0.65-0.94) (23), indicating that adiponectin measured from a single blood sample is reasonably accurate and stable over a long period and is not substantially affected by transport conditions. Samples for cases and matched controls were analyzed together, in random order, with the case status unknown to the laboratory personnel. Adiponectin concentrations were successfully measured for 620 men diagnosed with prostate cancer between 1982 and 2000 and 599 controls. The median intraindividual coefficient of variation for blinded duplicate quality-control samples was 11%. Leptin concentrations were available for 649 prostate cancer patients and 635 controls. The median intraindividual coefficient of variation for blinded duplicate quality-control samples was 5.0%. Of these, we had previously assayed PSA concentrations at baseline for 416 cases and 402 controls.
Statistical Analysis
We compared baseline characteristics between cases and controls. We used nonparametric Wilcoxon rank sum test to assess differences in plasma concentrations of adiponectin, leptin, and PSA, because data for these tests were not normally distributed, and generalized linear models for all other continuous variables. We examined the association of circulating adiponectin and leptin concentrations with risk of overall prostate cancer using conditional logistic regression models and calculated odds ratios (OR) and 95% confidence intervals (CI) comparing quintiles, as defined by the distribution among controls, with the lowest quintile as the reference. We then separately performed analyses according to clinical stage (T1/T2N0M0, T3/T4N0M0, and TxN1/M1) and grade (Gleason 2-6, 7 or 8-10) at diagnosis, and for those with metastases or who died from prostate cancer (lethal prostate cancer). In these subgroup analyses, we excluded 14 men with unknown grade and 63 men with unknown stage.
To assess adiponectin or leptin (in quintiles) in relation to prostate cancer mortality among all the cases, we estimated hazard ratios (HRs) and 95% CIs using Cox proportional hazard models. All tests for trend for the plasma adiponectin or leptin concentration were conducted using median concentrations of quintiles among controls. We further conducted stratified analyses by duration of follow-up (diagnosed before or after 1990, i.e., pre- or post-PSA era).
We further investigated whether the associations of adiponectin or leptin with prostate cancer are independent of BMI, plasma concentrations of C-peptide (reflecting insulin production), and the major components of the insulin-like growth factor system and whether they are modified by age or BMI. To test for the statistical significance of potential interactions of BMI with adiponectin or leptin, or the interaction between the two biomarkers, we analyzed models with and without the cross-product term and conducted the likelihood ratio test. To assess whether any associations between plasma adiponectin or leptin concentrations and risk of prostate cancer may be influenced by latent disease at baseline, we conducted secondary analyses after excluding cases (for the nested case-control analysis) or all deaths (for the prostate-specific mortality analysis) that occurred during the first five years of follow-up after the blood collection. We also tested whether the associations of adiponectin or leptin with prostate cancer were modified by the β-carotene and the aspirin treatments in the randomized trial. All statistics were calculated by SAS (version 9.1.3; SAS Institute Inc, Cary, NC), with a two-sided significance level of 0.05.
RESULTS
Baseline characteristics among cases and controls are presented in Table 1. Among controls, baseline plasma adiponectin concentrations were inversely correlated with BMI (age-adjusted Spearman correlation r = −0.17, P =0.0001), plasma concentrations of C-peptide (r = −0.23, P<0.0001), leptin (r =−0.13, P = 0.0016), and IGFBP-3 (r = −0.11, P = 0.01), but not with IGF-I. The correlations of plasma leptin concentrations with BMI (r = 0.58, P <0.0001) and C-peptide (r = 0.34, P<0.0001) were much stronger than those of adiponectin, although in the opposite direction. Leptin concentrations were not correlated to IGFs.
Table 1.
Baseline characteristics among prostate cancer (PCa) cases and controls
| Cases (n = 654) | Controls (n = 644) | |
|---|---|---|
| Age at study enrollment (yr) ab | 59.0 ± 8.2 | 58.6 ± 7.9 |
| Diabetes, N (%) | 10 (1.5) | 6 (0.9) |
| Smoking status, N (%) a | ||
| Current smoker | 58 (8.9) | 54 (8.4) |
| Past smoker | 279 (42.7) | 276 (42.9) |
| Age at diagnosis (yr) b | 68.6 ± 7.2 | -- |
| Interval from baseline to diagnosis (yr) | 9.8 (0.1-17.8) | -- |
| BMI (kg/m2) b | 24.6 ± 2.4 | 24.7 ± 2.5 |
| BMI 25-29.9, N (%) | 244 (37.3) | 245 (38.0) |
| BMI 30+, N (%) | 19 (2.9) | 19 (3.0) |
| Baseline PSA c (ng/mL), median (10th–90th percentile) |
2.7 (1.0-14.4) | 1.1 (0.4-3.5) |
| PSA >= 4, N (%) | 158 (38.0) | 33 (8.2) |
| PSA >= 10, N (%) | 64 (15.4) | 3 (0.8) |
| PSA c at diagnosis (ng/mL), median (10th–90th percentile) |
8.7 (4.0 – 35.4) | -- |
| PSA >= 4, N (%) | 390 (90.1) | -- |
| PSA >= 10, N (%) | 188 (43.4) | -- |
| Tumor grade at diagnosis, N (%) | -- | |
| Gleason 2-6/well-differentiated | 240 (37.9) | -- |
| Gleason 7/moderately-differentiated | 266 (42.0) | -- |
| Gleason 8-10/poorly-differentiated | 127 (20.1) | -- |
| Unknown | 21 | |
| Clinical stage at diagnosis d, N (%) | -- | |
| T1/T2N0M0 | 445 (75.3) | -- |
| T3/T4N0M0 | 112 (19.0) | -- |
| TxN11 | 34 (5.7) | |
| Unknown | 63 | |
| Metastases or PCa-specific death by 2007 | 118 (18.0) | -- |
| Plasma concentration e, median (10th – 90th) | ||
| Adiponectin (mg/L) | 6.2 (2.6-12.7) | 6.4 (2.8-13.1) |
| Leptin (μg/L) | 5.5 (2.4-13.4) | 5.5 (2.3-14.1) |
Matching factors.
Mean ± standard deviation.
Baseline PSA: available for 416 cases and 402 controls; PSA at diagnosis: available for 433 cases.
American Joint Committee on Cancer (AJCC) TNM classification system.
Adiponectin concentrations were available for 620 cases and 599 controls; leptin concentrations were available for 649 cases and 635 controls
We first examined the association of plasma adiponectin concentrations and risk of incident prostate cancer using conditional logistic regression analysis and found no association for overall risk. However, men with higher adiponectin concentrations had lower risk of developing high grade tumors (Gleason score ≥ 8) or lethal cancer. Comparing the highest quintile (Q5) to the lowest quintile (Q1) of plasma adiponectin concentrations, the relative risks (RR Q5 vs. Q1, 95% confidence interval, CI) were 0.49 (95% CI: 0.20-1.22; Ptrend = 0.25) for high grade tumors and 0.25 (95% CI: 0.07-0.87; Ptrend = 0.02) for lethal prostate cancer (Table 2). The association for high grade disease became stronger after adjustment for BMI and plasma concentrations of C-peptide (RR Q5 vs. Q1 = 0.23, 95% CI: 0.06-0.83; Ptrend = 0.08). However, adjustment for BMI and C-peptide concentrations significantly attenuated the association for lethal prostate cancer (RR Q5 vs. Q1 = 0.61, 95% CI: 0.12-2.99; Ptrend = 0.44), suggesting that adiponectin serves in part as a mediator of the link between obesity and lethal prostate cancer. Plasma adiponectin concentrations were not significantly associated with risk of nonlethal prostate cancer (data not shown). Exclusion of cases diagnosed during the first 5 years of follow-up did not materially change the results for lethal prostate cancer except to broaden the confidence interval, reflecting the decreased statistical power (RR Q5 vs. Q1 = 0.35, 95% CI: 0.09-1.36; Ptrend = 0.07).
Table 2.
Relative risk (RR) and 95% confidence interval (CI) of prostate cancer (PCa) according to quintile of baseline concentrations of plasma adiponectin and leptina
| Quintile categories according to baseline plasma adiponectin concentration | ||||||
|---|---|---|---|---|---|---|
| 1 (ref.) | 2 | 3 | 4 | 5 | P,trend | |
| Adiponectin | ||||||
| Median concentration (range, mg/L) b |
2.8 (0.3 – 3.8) |
4.7 (3.9 – 5.5) |
6.4 (5.6 – 7.2) |
8.6 (7.3 – 10.4) |
13.1 (10.5 – 31.9) |
|
| Overall PCa (599 cases and 599 controls) | ||||||
| No. of cases/controls | 131/119 | 116/120 | 116/120 | 140/120 | 96/120 | |
| Simple model | 1.00 | 0.86 (0.59-1.26) |
0.85 (0.58-1.25) |
1.04 (0.73-1.49) |
0.69 (0.47-1.03) |
0.18 |
| Simple model + BMI + C-peptide | 1.00 | 0.82 (0.53-1.27) |
1.00 (0.66-1.53) |
1.13 (0.75-1.69) |
0.73 (0.46-1.14) |
0.38 |
| High grade PCa c (115 cases and 115 controls) | ||||||
| No. of cases/controls | 27/20 | 21/18 | 20/31 | 30/22 | 17/24 | |
| Simple model | 1.00 | 0.83 (0.32-2.11) |
0.47 (0.20-1.10) |
0.95 (0.42-2.16) |
0.49 (0.20-1.22) |
0.25 |
| Simple model + BMI + C-peptide | 1.00 | 0.29 (0.08-1.06) |
0.23 (0.07-0.72) |
0.37 (0.12-1.16) |
0.23 (0.06-0.83) |
0.08 |
| Lethal PCa c (117 cases and 117 controls) | ||||||
| No. of cases/controls | 29/23 | 26/23 | 24/20 | 28/32 | 10/19 | |
| Simple model | 1.00 | 0.69 (0.27-1.76) |
0.70 (0.24-2.03) |
0.53 (0.21-1.32) |
0.25 (0.07-0.87) |
0.02 |
| Simple model + BMI + C-peptide | 1.00 | 0.77 (0.26-2.26) |
0.97 (0.26-3.53) |
0.69 (0.24-1.98) |
0.61 (0.12-2.99) |
0.44 |
| Leptin | ||||||
| Median concentration (range, μg/L) b |
2.3 (0.8 – 3.2) |
3.9 (3.3 – 4.6) |
5.5 (4.7– 6.5) |
8.0 (6.6 – 10.0) |
14.1 (10.1 – 50.6) |
|
| Overall PCa (635 cases and 635 controls) | ||||||
| No. of cases/controls | 121/127 | 128/127 | 129/127 | 131/127 | 126/127 | |
| Simple model | 1.00 | 1.06 (0.74-1.52) |
1.07 (0.75-1.54) |
1.09 (0.76-1.56) |
1.05 (0.73-1.51) |
0.90 |
| Simple model + BMI + C-peptide | 1.00 | 1.00 (0.67-1.49) |
1.07 (0.70-1.64) |
1.10 (0.71-1.71) |
1.06 (0.65-1.72) |
0.8 |
| High grade PCa c (124 cases and 124 controls) | ||||||
| No. of cases/controls | 23/26 | 24/28 | 20/27 | 29/23 | 28/20 | |
| Simple model | 1.00 | 1.02 (0.46-2.22) |
0.83 (0.37-1.85) |
1.58 (0.68-3.68) |
1.74 (0.76-4.00) |
0.12 |
| Simple model + BMI + C-peptide | 1.00 | 0.76 (0.30-1.89) |
0.52 (0.19-1.46) |
1.04 (0.36-3.02) |
1.29 (0.44-3.80) |
0.34 |
| Lethal PCa c (121 cases and 121 controls) | ||||||
| No. of cases/controls | 24/26 | 26/30 | 25/24 | 23/25 | 23/16 | |
| Simple model | 1.00 | 0.96 (0.43-2.14) |
1.22 (0.54-2.77) |
0.99 (0.44-2.26) |
1.69 (0.67-4.23) |
0.24 |
| Simple model + BMI + C-peptide | 1.00 | 0.66 (0.25-1.74) |
0.58 (0.19-1.79) |
0.41 (0.12-1.45) |
0.94 (0.25-3.51) |
0.81 |
All analyses were performed using conditional logistic regression models adjusting for matching factors (age and smoking status at baseline); p-values for trend were calculated using median of quintiles as a continuous variable. Clinical stage for cases was determined on the basis of the TNM staging; high-grade PCa included men with poorly differentiated or Gleason 8-10 tumor; cases who had unknown stage and grade (and matched controls) were excluded from subgroup analyses.
Medians and range calculated among control subjects only.
Metastatic/Fatal PCa included patients with stage D PCa at diagnosis and those who developed metastases or death due to PCa during the follow-up.
In contrast to adiponectin, prediagnostic plasma leptin concentrations were not associated with risk of incident prostate cancer (lethal or nonlethal), with or without adjustment for BMI and C-peptide concentration (Table 2). We found no significant interactions between BMI and adiponectin or leptin or between the two biomarkers in association with risk of incident prostate cancer.
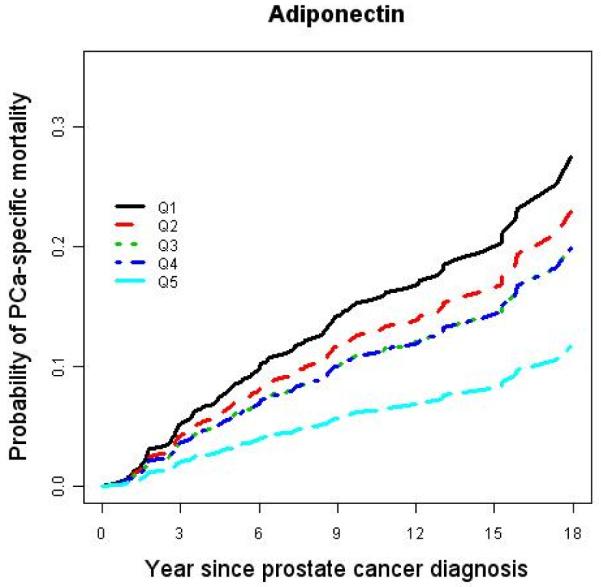
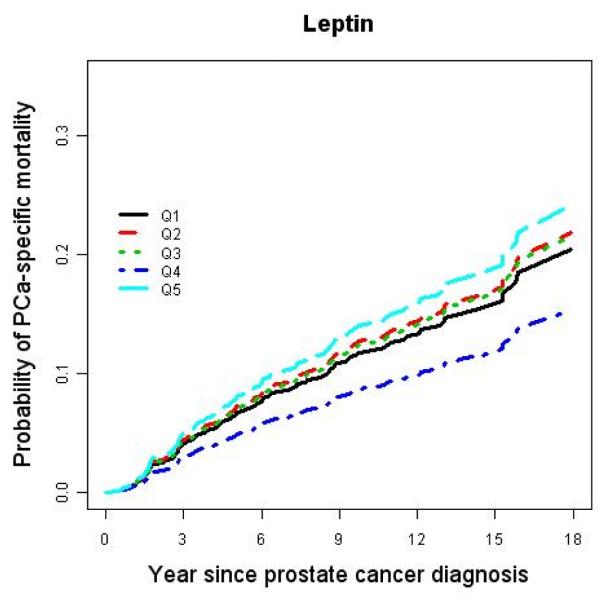
Among the men diagnosed with prostate cancer during the 25 years of follow-up, men in the highest quintile of adiponectin were less than half as likely to develop lethal disease after diagnosis (9%) than those whose adiponectin was in the lowest quintile (21%) (Table 3). Using a Cox proportional hazard regression model, we found a significant inverse dose-response relationship between prediagnostic concentrations of adiponectin and prostate cancer-specific mortality (Figure 1). In a multivariate model, the proportional hazard ratio (HR) Q5 vs. Q1 was 0.35 (95% CI: 0.14-0.89, Ptrend = 0.03) after controlling for age at diagnosis, baseline BMI, plasma C-peptide concentrations at baseline, clinical stage, and tumor grade at diagnosis (Table 4). The association remained similar after excluding men who died of any cause during the first five years after the baseline blood collection. In contrast, plasma leptin concentrations did not predict prostate cancer-specific mortality (Table 4).
Table 3.
Disease characteristics by plasma concentration quintiles (1, 3 and 5) of adiponectin (or leptin) among prostate cancer (PCa) cases a
| Characteristic | Quintile 1 | Quintile 3 | Quintile 5 | P c |
|---|---|---|---|---|
| Adiponectin (mg/L), median (range) | 2.7 (0.2-3.8) | 6.3 (5.5-7.2) | 13.3 (10.5-26.3) | -- |
| N | 133 | 118 | 100 | -- |
| Metastases or PCa-specific death by 2007, N (%) |
28 (21.1) | 20 (17.0) | 9 (9.0) | 0.13 |
| BMI (kg/m2) b | 25.3 ± 2.5 | 24.7 ± 2.7 | 24.1 ± 2.3 | <0.0001 |
| Baseline PSA (ng/mL), median (10th–90th percentile) |
2.7 (1.0-14.4) | 2.5 (0.8-13.0) | 2.5 (0.8-8.6) | 0.37 |
| PSA at diagnosis (ng/mL), median (10th–90th percentile) |
8.0 (3.5-28.4) | 9.0 (4.1-50.0) | 8.0 (4.0-31.0) | 0.49 |
| Tumor grade at diagnosis d, N (%) | ||||
| Gleason 2-6/well-differentiated | 41 (31.8) | 44 (38.3) | 41 (42.3) | |
| Gleason 7/moderately-differentiated | 61 (47.3) | 50 (43.5) | 38 (39.2) | 0.81 |
| Gleason 8-10/poorly-differentiated | 27 (20.9) | 21 (18.2) | 18 (18.5) | |
| Clinical stage at diagnosis d, N (%) | ||||
| T1/T2N0M0 | 92 (76.0) | 82 (76.6) | 69 (77.5) | |
| T3/T4N0M0 | 24 (19.8) | 16 (15.0) | 17 (19.1) | 0.42 |
| TxN1/M1 | 5 (4.2) | 9 (8.4) | 3 (3.4) | |
|
| ||||
| Leptin (μg/L), median (range) | 2.4 (1.0-3.1) | 5.5 (4.6-6.5) | 13.4 (10.3- 119.2) |
-- |
| N | 125 | 132 | 128 | |
| Metastases and/or died of PCa by 2007, N (%) |
23 (18.4) | 24 (18.2) | 22 (17.2) | 0.83 |
| BMI (kg/m2) b | 23.2 (21.1- 25.1) |
24.3 (22.4- 26.4) |
26.4 (23.5-29.8) | <0.0001 |
| Baseline PSA (ng/mL), median (10th–90th percentile) |
2.5 (0.8-11.5) | 2.5 (0.9-17.6) | 2.4 (1.0-15.3) | 0.80 |
| PSA at diagnosis (ng/mL), median (10th–90th percentile) |
9.6 (3.4-35.4) | 7.5 (3.6-28.0) | 8.4 (4.5-28.9) | 0.28 |
| Tumor grade at diagnosis d, N (%) | ||||
| Gleason 2-6/well-differentiated | 45 (37.2) | 48 (38.1) | 43 (34.4) | |
| Gleason 7/moderately-differentiated | 51 (42.1) | 58 (46.0) | 54 (43.2) | 0.83 |
| Gleason 8-10/poorly-differentiated | 25 (20.7) | 20 (15.9) | 28 (22.4) | |
| Clinical stage at diagnosis d, N (%) | ||||
| T1/T2N0M0 | 86 (76.1) | 89 (73.6) | 90 (77.6) | 0.90 |
| T3/T4N0M0 | 20 (17.7) | 23 (19.0) | 20 (17.2) | |
| TxN1/M1 | 7 (6.2) | 9 (7.4) | 6 (5.2) | |
Quintile categories was defined based on the distribution among controls.
Mean ± standard deviation.
P-values calculated using generalized linear regression for BMI and nonparametric techniques for plasma concentrations of PSA at baseline and at diagnosis.
American Joint Committee on Cancer (AJCC) TNM classification system. Cases with unknown clinical stage (or grade/Gleason score) were excluded.
Figure 1.
Age-adjusted cumulative probability of prostate cancer-specific mortality by quintile of plasma adiponectin and leptin.
Table 4.
Proportional hazard ratio (HR) of prostate cancer (PCa)-specific mortality among prostate cancer cases according to baseline prediagnostic plasma adiponectin and leptin concentrations a
| HR (95% CI) by quintile | ||||||
|---|---|---|---|---|---|---|
| 1 (ref.) | 2 | 3 | 4 | 5 | P,trend | |
| Adiponectin (90 PCa deaths/530 censored) | ||||||
| No. of PCa deaths /Censored | 26/107 | 19/101 | 16/102 | 21/128 | 8/92 | |
| Simple model | 1.00 | 0.81 (0.45-1.47) |
0.69 (0.37-1.30) |
0.69 (0.39-1.23) |
0.39 (0.17-0.85) |
0.02 |
| Simple model + BMI b | 1.00 | 0.83 (0.46-1.49) |
0.73 (0.39-1.36) |
0.76 (0.42-1.37) |
0.42 (0.19-0.92) |
0.03 |
| Simple model + BMI, C-peptide b | 1.00 | 0.87 (0.45-1.65) |
0.69 (0.35-1.36) |
0.87 (0.46-1.62) |
0.36 (0.14-0.90) |
0.04 |
| Simple model + BMI, C-peptide, stage, grade |
1.00 | 0.97 (0.50-1.88) |
0.58 (0.28-1.17) |
0.79 (0.40-1.53) |
0.35 (0.14-0.89) |
0.03 |
|
| ||||||
|
| ||||||
| Leptin (94 PCa deaths/555 censored) | ||||||
| No. of PCa deaths/Censored | 19/106 | 20/111 | 19/113 | 14/119 | 22/106 | |
| Simple model | 1 | 1.08 (0.58-2.03) | 1.06(0.56-2.02) | 0.73 (0.36-1.47) | 1.21 (0.65-2.24) | 0.68 |
| Simple model + BMI b | 1 | 1.03 (0.55-1.94) | 0.94 (0.49-1.80) | 0.59 (0.28-1.22) | 0.82 (0.40-1.68) | 0.47 |
| Simple model + BMI, C-peptide b | 1 | 0.97 (0.49-1.93) | 0.91 (0.46-1.82) | 0.57 (0.26-1.24) | 0.71 (0.32-1.58) | 0.32 |
| Simple model + BMI, C-peptide, stage, grade |
1 | 1.10 (0.54-2.22) | 0.90 (0.43-1.87) | 0.46 (0.20-1.09) | 0.66 (0.28-1.53) | 0.24 |
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval; BMI, body mass index.
Quintiles were categorized on the basis of concentrations among matched controls, as for the nested case-controls study (see Table 2). All the survival analyses were performed using Cox proportional hazard model adjusting for age at diagnosis (<65, 65-74.9, >=75 years); p-values for trend were calculated using the median values of quintiles as a continuous variable.
BMI (kg/m2), continuous; plasma C-peptide in quartiles.
We found that the inverse trend between prediagnostic adiponectin concentrations and progression to fatal prostate cancer after diagnosis was apparent mainly among men who were overweight or obese (BMI ≥25 kg/m2); in that group, the HRs from the lowest to the highest quintile were 1.00 (reference), 0.56, 0.71, 0.72, and 0.10 (95% CI 0.01-0.78, Ptrend = 0.02). Among those of normal weight (BMI <25 kg/m2), adiponectin concentrations were not significantly associated with prostate cancer mortality (HRQ1-5 = 1.00 (reference), 1.44, 0.87, 0.95, and 0.86, 95% CI 0.31-2.38, Ptrend = 0.51). A formal test for interaction yielded a p-value of 0.08. We observed no interactions between plasma adiponectin and C-peptide concentrations, or with the β-carotene, or aspirin treatments of the trial. There were no interactions of leptin with any of these factors.
DISCUSSION
Both adiponectin and leptin have been suggested as potential biological links between obesity and prostate cancer but little epidemiological data are available for their roles in fatal prostate cancer.(24-26) In this prospective nested case-control study of U.S. physicians, prediagnostic concentrations of adiponectin and leptin were not associated with risk of developing overall prostate cancer. However, men with higher (top 20%) prediagnostic plasma concentrations of adiponectin were at lower risk for a Gleason ≥ 8 tumor and had a markedly significant 75% lower risk of developing lethal prostate cancer (metastatic or fatal cancer) than men with low (bottom 20%) plasma adiponectin concentrations. Our prospective observations are in line with previous clinical observations that prostate cancer patients with Gleason score ≥ 8 or extraprostatic cancer had lower adiponectin concentrations (3). The inverse association of adiponectin with lethal prostate cancer was attenuated after controlling for baseline BMI and C-peptide concentrations suggesting that adiponectin serves in part as a mediator of the link between obesity and lethal prostate cancer. Moreover, among men diagnosed with prostate cancer, those with higher prediagnostic plasma adiponectin concentrations had a significant 65% reduced prostate-specific mortality compared to those with lower concentrations, independent of baseline BMI, C-peptide, Gleason grade, and clinical stage.
In contrast to adiponectin, circulating concentrations of leptin were unrelated to risk of developing lethal prostate cancer or to prostate cancer-specific mortality. These findings were somewhat unexpected because leptin is much more strongly correlated to BMI (r=0.58) than adiponectin (r=−0.17) in this study population. Among three prospective and three case-control published studies (5, 18-21), however, only one reported a significant positive association with incident prostate cancer.(19) Our findings together with these data suggest that circulating concentrations of leptin are unlikely to be related to prostate cancer risk or progression. Our parallel evaluation of the two major adipocytokines for both risk of developing prostate cancer and subsequent risk of dying from the cancer suggests a possible role of circulating adiponectin (but not leptin) as an inverse mediator of the effects of obesity in tumor progression.
Several lines of evidence support a biological link between adiponectin and prostate cancer tumorigenesis. First, adiponectin stimulates phosphorylation and activation of the AMPK pathway (8), an evolutionarily conserved sensor of cellular energy status that plays a crucial role in systemic energy balance. Activation of AMPK also inhibits growth of androgen-independent (DU145, PC3) and androgen-sensitive (LNCaP) prostate cancer cell lines by 90%.(27) Adiponectin at physiological concentrations inhibits both androgen-dependent and androgen-independent prostate cancer cell growth. (17) Second, adiponectin inhibits inflammation by suppressing the phagocytic activity of mature macrophages, their lipopolysaccharide-induced production of tumor necrosis factor alpha (TNF-α), and the activity of Toll-like receptor (TLR)-4, all of which have been linked to prostate cancer.(9, 10, 28) Third, adiponectin inhibits in vitro and in vivo cell proliferation and induces apoptosis in vitro.(10, 11) Finally, adiponectin has inhibitory effects on metastatic prostate cancer cell lines from three distinct sites: brain (DU145), bone (PC-3), and lymph nodes (LNCAP-FGC)(17), and inhibits in vivo primary tumor growth by decreasing neovascularization.(11) These data, together with recent evidence that adiponectin reduces the invasiveness of breast cancer cells (12) and our current findings, suggest that adiponectin may act as a direct endogenous inhibitor of metastasis and may thereby prolong cancer survival.
The significant inverse trend between prediagnostic adiponectin concentrations and prostate cancer-specific mortality was apparent mainly among men with BMI ≥25 kg/m2. This observation is consistent with our previous finding of a strong positive association of plasma C-peptide with prostate cancer mortality primarily in men with BMI ≥25 kg/m2. (2)The obesity-linked up-regulation of insulin production and down-regulation of adiponectin and, as a consequence, down-regulation of AMPK, is a plausible mechanism whereby obesity directly or indirectly influences prostate cancer progression.
These observations support the hypothesis that certain obesity-related metabolic factors such as hyperinsulinemia favor aggressive neoplastic behavior, whereas adiponectin counteracts these obesity-related adverse effects (2). Our prospective data further support the notion that tumors can start developing metastasis very early, i.e., before they may even become clinically detactable (29) and that aggressive neoplastic behavior could be manipulated by systemic metabolic factors. These concepts may aid in refinement of prostate cancer risk prediction (especially for lethal outcomes) by including BMI (30) and related biomarkers such as C-peptide and adiponectin in the clinical nomogram to improve performance. The concepts may also help to identify novel cancer therapeutic and prevention strategies. Recent in vitro data demonstrated that adiponectin has the characteristics of an AMPK-dependent growth inhibitor that is deficient in obesity, and this may contribute to the adverse effects of obesity on neoplastic disease (31). Furthermore, metformin, a widely used antidiabetic drug, activates AMPK, inhibits prostate cancer cell growth (31), and lowers overall cancer risk and mortality among diabetic patients (32) and is associated with lower risk of prostate cancer (33). Together, these data suggest that metformin, as an insulin-lowering drug, and adiponectin, as an endogenous insulin-sensitivity regulator, may be of value in attenuating the adverse effects of obesity on prostate cancer prognosis via the common AMPK pathway.
Our prospective study design reduces the potential for bias due to the influence of an existing tumor (or its treatment) on adiponectin concentrations. Moreover, the associations persisted after we excluded cases (or deaths) that occurred during the first five years after blood collection, further suggesting that these results are likely unaffected by underlying disease (reverse causation). Our study was limited by the use of a single adiponectin measurement from the baseline sample, which may not have fully characterized long-term status. However, repeated adiponectin measurements analyzed from samples collected over a 1-year period showed a high correlation of 0.7-0.8.(23) In addition, blood samples for cases and matched controls were collected, processed, and stored similarly and assayed together in matched pairs to assure comparability. Any random within-person variation would tend to be associated with underestimates of the true associations.
In conclusion, our prospective data provide strong evidence that higher prediagnostic concentrations of adiponectin, but not leptin, are associated with a lower risk of developing high grade or lethal prostate cancer and predispose prostate cancer patients to a lower cancer-specific mortality, independent of clinical stage and Gleason grade. Our findings provide new evidence for a biological link between obesity and fatal prostate cancer development and also may help to guide the development of novel cancer therapeutic strategies targeting the AMPK pathway, especially among overweight prostate cancer patients. Circulating and adipose tissue adiponectin concentrations can be increased, whereas insulin (and C-peptide) concentrations can be reduced by weight loss (34), dietary modifications (35), caloric restriction (36), physical exercise (37, 38), or antidiabetic therapy.(39, 40) These observations further indicate the importance of maintaining a healthy body weight and lifestyle to reduce risk of developing a clinically significant high grade tumor or lethal prostate cancer.
Acknowledgments
We thank Ms. Haiyan Zhang and Daad Abraham for their dedicated technical assistance. We are also indebted to the 22,071 dedicated and committed participants randomized into the Physicians’ Health Study starting in 1982.
Funding/Support: The project was supported in part by grants from the Prostate Cancer Foundation, the National Institutes of Health (CA42182, CA58684, CA90598, CA 34944, and CA 40360), and the Department of Defense (DoD) United States Army Medical Research and Materiel Command Congressionally Directed Medical Research Programs (CDMRP) (PC030095).
Abbreviations
- AMPK
AmP-activated protein kinase
- BMI
Body mass index
- PSA
Prostate cancer specific androgen
- OR
Odds ratio
- CI
Confidence intervals
- HR
Hazard ratio
- Q5
Highest quintile
- Q1
Lowest Quintile
- TNF-α
Tumor necrosis factor alpha
- TLR-4
Toll-like receptor
Footnotes
Conflict of Interest: The authors state no conflict of interest.
Financial Disclosures: None reported.
Publisher's Disclaimer: Clinical Chemistry Disclaimer: “This is an un-copyedited authored manuscript copyrighted by The American Association for Clinical Chemistry (AACC). This may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 107, Title 17, U.S. Code) without permission of the copyright owner, AACC. The AACC disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The final publisher-authenticated version of the article will be made available at http://www.clinchem.org 12 months after its publication in Clinical Chemistry.”
REFERENCES
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9:1039–47. doi: 10.1016/S1470-2045(08)70235-3. PMCID: PMC2651222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, Bedir S. Prostate cancer and adiponectin. Urology. 2005;65:1168–72. doi: 10.1016/j.urology.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 4.Michalakis K, Williams CJ, Mitsiades N, Blakeman J, Balafouta-Tselenis S, Giannopoulos A, Mantzoros CS. Serum adiponectin concentrations and tissue expression of adiponectin receptors are reduced in patients with prostate cancer: a case control study. Cancer Epidemiol Biomarkers Prev. 2007;16:308–13. doi: 10.1158/1055-9965.EPI-06-0621. [DOI] [PubMed] [Google Scholar]
- 5.Freedland SJ, Sokoll LJ, Platz EA, Mangold LA, Bruzek DJ, Mohr P, et al. Association between serum adiponectin, and pathological stage and grade in men undergoing radical prostatectomy. J Urol. 2005;174:1266–70. doi: 10.1097/01.ju.0000173093.89897.97. [DOI] [PubMed] [Google Scholar]
- 6.Mistry T, Digby JE, Desai KM, Randeva HS. Leptin and adiponectin interact in the regulation of prostate cancer cell growth via modulation of p53 and bcl-2 expression. BJU Int. 2008;101:1317–22. doi: 10.1111/j.1464-410X.2008.07512.x. PMC Journal - In Process. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T, et al. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005;579:6821–6. doi: 10.1016/j.febslet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–32. [PubMed] [Google Scholar]
- 11.Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101:2476–81. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KY, Baek A, Hwang JE, Choi YA, Jeong J, Lee MS, et al. Adiponectin-activated AMPK stimulates dephosphorylation of AKT through protein phosphatase 2A activation. Cancer Res. 2009;69:4018–26. doi: 10.1158/0008-5472.CAN-08-2641. PMC Journal - In Process. [DOI] [PubMed] [Google Scholar]
- 13.Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967:379–88. doi: 10.1111/j.1749-6632.2002.tb04293.x. [DOI] [PubMed] [Google Scholar]
- 14.Onuma M, Bub JD, Rummel TL, Iwamoto Y. Prostate cancer cell-adipocyte interaction: leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. J Biol Chem. 2003;278:42660–7. doi: 10.1074/jbc.M304984200. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki T, Bub JD, Uzuki M, Iwamoto Y. Adiponectin activates c-Jun NH2-terminal kinase and inhibits signal transducer and activator of transcription 3. Biochem Biophys Res Commun. 2005;333:79–87. doi: 10.1016/j.bbrc.2005.05.076. [DOI] [PubMed] [Google Scholar]
- 16.Mistry T, Digby JE, Chen J, Desai KM, Randeva HS. The regulation of adiponectin receptors in human prostate cancer cell lines. Biochem Biophys Res Commun. 2006;348:832–8. doi: 10.1016/j.bbrc.2006.07.139. [DOI] [PubMed] [Google Scholar]
- 17.Bub JD, Miyazaki T, Iwamoto Y. Adiponectin as a growth inhibitor in prostate cancer cells. Biochem Biophys Res Commun. 2006;340:1158–66. doi: 10.1016/j.bbrc.2005.12.103. [DOI] [PubMed] [Google Scholar]
- 18.Baillargeon J, Platz EA, Rose DP, Pollock BH, Ankerst DP, Haffner S, et al. Obesity, adipokines, and prostate cancer in a prospective population-based study. Cancer Epidemiol Biomarkers Prev. 2006;15:1331–5. doi: 10.1158/1055-9965.EPI-06-0082. [DOI] [PubMed] [Google Scholar]
- 19.Stattin P, Soderberg S, Hallmans G, Bylund A, Kaaks R, Stenman UH, et al. Leptin is associated with increased prostate cancer risk: a nested case-referent study. J Clin Endocrinol Metab. 2001;86:1341–5. doi: 10.1210/jcem.86.3.7328. [DOI] [PubMed] [Google Scholar]
- 20.Stattin P, Kaaks R, Johansson R, Gislefoss R, Soderberg S, Alfthan H, et al. Plasma leptin is not associated with prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2003;12:474–5. [PubMed] [Google Scholar]
- 21.Hsing AW, Chua S, Jr., Gao YT, Gentzschein E, Chang L, Deng J, Stanczyk FZ. Prostate cancer risk and serum levels of insulin and leptin: a population-based study. J Natl Cancer Inst. 2001;93:783–9. doi: 10.1093/jnci/93.10.783. [DOI] [PubMed] [Google Scholar]
- 22.Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 23.Pischon T, Hotamisligil GS, Rimm EB. Adiponectin: stability in plasma over 36 hours and within-person variation over 1 year. Clin Chem. 2003;49:650–2. doi: 10.1373/49.4.650. [DOI] [PubMed] [Google Scholar]
- 24.Buschemeyer WC, 3rd, Freedland SJ. Obesity and prostate cancer: epidemiology and clinical implications. Eur Urol. 2007;52:331–43. doi: 10.1016/j.eururo.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 25.Hsing AW, Sakoda LC, Chua S., Jr. Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr. 2007;86:843–57. doi: 10.1093/ajcn/86.3.843S. [DOI] [PubMed] [Google Scholar]
- 26.Mistry T, Digby JE, Desai KM, Randeva HS. Obesity and prostate cancer: a role for adipokines. Eur Urol. 2007;52:46–53. doi: 10.1016/j.eururo.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 27.Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321:161–7. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 28.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–9. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 29.Dattoli G, Guiot C, Delsanto PP, Ottaviani PL, Pagnutti S, Deisboeck TS. Cancer metabolism and the dynamics of metastasis. J Theor Biol. 2009;256:305–10. doi: 10.1016/j.jtbi.2008.10.008. PMC Journal - In Process. [DOI] [PubMed] [Google Scholar]
- 30.Strom SS, Wang X, Pettaway CA, Logothetis CJ, Yamamura Y, Do KA, et al. Obesity, weight gain, and risk of biochemical failure among prostate cancer patients following prostatectomy. Clin Cancer Res. 2005;11:6889–94. doi: 10.1158/1078-0432.CCR-04-1977. [DOI] [PubMed] [Google Scholar]
- 31.Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Phila Pa) 2008;1:369–75. doi: 10.1158/1940-6207.CAPR-08-0081. PMC Journal - In Process. [DOI] [PubMed] [Google Scholar]
- 32.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–5. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control. 2009 doi: 10.1007/s10552-009-9407-y. PMC Journal - In Process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan DC, Watts GF, Ng TW, Yamashita S, Barrett PH. Effect of weight loss on markers of triglyceride-rich lipoprotein metabolism in the metabolic syndrome. Eur J Clin Invest. 2008;38:743–51. doi: 10.1111/j.1365-2362.2008.02019.x. PMC Journal - In Process. [DOI] [PubMed] [Google Scholar]
- 35.Duda MK, O’Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, et al. Dietary supplementation with omega-3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc Res. 2007;76:303–10. doi: 10.1016/j.cardiores.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fargnoli JL, Fung TT, Olenczuk DM, Chamberland JP, Hu FB, Mantzoros CS. Adherence to healthy eating patterns is associated with higher circulating total and high-molecular-weight adiponectin and lower resistin concentrations in women from the Nurses’ Health Study. Am J Clin Nutr. 2008;88:1213–24. doi: 10.3945/ajcn.2008.26480. PMC Journal - In Process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberbach A, Tonjes A, Kloting N, Fasshauer M, Kratzsch J, Busse MW, et al. Effect of a 4 week physical training program on plasma concentrations of inflammatory markers in patients with abnormal glucose tolerance. Eur J Endocrinol. 2006;154:577–85. doi: 10.1530/eje.1.02127. [DOI] [PubMed] [Google Scholar]
- 38.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 39.Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–74. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 40.Phillips SA, Ciaraldi TP, Kong AP, Bandukwala R, Aroda V, Carter L, et al. Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes. 2003;52:667–74. doi: 10.2337/diabetes.52.3.667. [DOI] [PubMed] [Google Scholar]