Abstract
The N-methyl-D-aspartate receptor (NMDAR) is a Ca2+-permeable glutamate receptor mediating many neuronal functions under normal and pathological conditions. Ca2+-influx via NMDARs activates diverse intracellular targets, including Ca2+-dependent protease calpain. Biochemical studies suggest that NR2A and NR2B subunits of NMDARs are substrates of calpain. Our physiological data showed that calpain, activated by prolonged NMDA treatment (100 µM, 5 min) of cultured cortical neurons, irreversibly decreased the whole-cell currents mediated by extrasynaptic NMDARs. Animals exposed to transient forebrain ischemia, a condition that activates calpain, exhibited the reduced NMDAR current density and the lower full-length NR2A/B level in a calpain-dependent manner. Disruption of the association between NMDARs and the scaffolding protein PSD-95 facilitated the calpain regulation of synaptic NMDAR responses and NR2 cleavage in cortical slices, while inhibition of calcineurin activity blocked the calpain effect on NMDAR currents and NR2 cleavage. Calpain-cleaved NR2B subunits were removed from the cell surface. Moreover, cell viability assays showed that calpain, by targeting NMDARs, provided a negative feedback to dampen neuronal excitability in excitotoxic conditions. These data suggest that calpain activation suppresses NMDAR function via proteolytic cleavage of NR2 subunits in vitro and in vivo, and the susceptibility of NMDARs to calpain cleavage is controlled by PSD-95 and calcineurin.
NMDAR, the Ca2+-permeable glutamate receptor channel, is implicated in diverse neuronal functions ranging from development to synaptic plasticity to excitotoxicity (Dingledine et al., 1999). Over-activation of NMDARs induces excessive Ca2+ entry, which can activate the Ca2+-dependent protease calpain in cortical and hippocampal neurons (Hewitt et al., 1998; Adamec et al., 1998). Calpain catalyzes the proteolysis of a wide array of protein targets including those involved in cytoskeleton remodeling, signal transduction, apoptosis and necrosis, cell differentiation, vesicular trafficking and synaptic transmission (Perrin and Huttenlocher, 2002; Goll et al., 2003; Yuen et al., 2007a). Disturbances of the calpain system have been associated with a number of pathological conditions such as ischemia, stroke, Alzheimer’s disease and Huntington’s disease (Saito et al., 1993; Patrick et al., 1999; Gafni and Ellerby, 2002; Rami 2003; Amadoro et al., 2006). Thus, modifying calpain-mediated cleavage has been proposed as one potential approach to treat these disorders (Huang and Wang, 2001; Carragher 2006).
Previous biochemical studies suggest that calpain cleaves the C-terminal region of NMDA receptor NR2A and NR2B subunits (Guttmann et al., 2001; 2002). Since the C-termini of NMDAR subunits contain structural domains required for association with scaffolding proteins, signaling molecules, and cytoskeletal proteins (Wenthold et al., 2003), the calpain-induced truncation of NR2 subunits is expected to have a significant impact on NMDAR surface expression and function in neurons. Indeed, transgenic mice with deleted NMDAR C-terminus show impaired NMDAR subcellular localization and synaptic plasticity (Sprengel et al., 1998; Steigerwald et al., 2000). However, the physiological consequence of calpain-mediated cleavage of NMDARs and the mechanism that controls the efficiency of this cleavage are unclear. Here, we show that activation of calpain, induced by over-stimulation of NMDARs in vitro or by transient focal cerebral ischemia in vivo, produces a sustained down-regulation of NMDAR currents, which is accompanied by the reduced level of full-length NR2 subunits, in cortical pyramidal neurons. Moreover, the susceptibility of NMDARs to calpain cleavage is controlled by two molecules. One is the scaffolding protein PSD-95, which protects synaptic NMDARs from being proteolyzed by calpain. The other is the major Ca2+-dependent protein phosphatase calcineurin, which provides a “gate” to enable the calpain regulation of NMDA receptors. The downregulation of NMDAR function by calpain provides a negative feedback to dampen neuronal excitability in excitotoxic conditions like ischemia and neurodegenerative diseases. By decreasing or increasing calpain-mediated cleavage of NMDARs, PSD-95 and calcineurin could be especially critical for neurons to control excessive excitability.
Materials and Methods
Primary culture
All experiments were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of the State University of New York at Buffalo. Rat cortical cultures were prepared with procedures similar to what we used previously (Wang et al., 2003; Yuen et al., 2005). Briefly, frontal cortex was dissected from 18 d rat embryos and cells were dissociated using trypsin and trituration through a Pasteur pipette. Cells were plated on coverslips coated with poly-L-lysine in Dulbecco’s Modified Eagle medium (DMEM) with 10% fetal calf serum at a density of 2.5 × 105 cells/cm2. When cells attached to the coverslip within 24 hr, the media was changed to Neurobasal with B27 supplement. Cytosine arabinoside (AraC, 5 µM) was added at DIV3 to stop glia proliferation. Neurons were maintained for 10–15 days before being used for electrophysiological recording or immunocytochemical staining.
Whole-cell recording
Acutely dissociated cortical neurons from 3–4 weeks old rats were prepared as described previously (Yuen et al. 2005). In brief, rats were anaesthetized with halothane vapour before decapitation. Brain slices (300 µm) were incubated in a NaHCO3-buffered saline bubbled with 95% O2. The frontal cortical areas were dissected and digested in an oxygenated chamber consisted of papain (0.4 mg/ml; Calbiochem) for 40 min at room temperature. Following washing, the tissue was mechanically dissociated with a graded series of fire-polished Pasteur pipettes. The isolated cells were then dispersed into a 35mm Lux Petri dish positioned on the stage of a Nikon inverted microscope. Whole-cell recording of NMDAR channel currents used standard voltage-clamp techniques (Wang et al., 2003; Yuen et al., 2005). The internal solution contained (in mM): 180 N-methyl-D-glucamine (NMG), 4 MgCl2, 40 HEPES, 0.5 BAPTA, 12 phosphocreatine, 3 Na2ATP, and 0.5 Na2GTP, pH 7.2–7.3, 265–270 mosM. The external solution contained (in mM): 127 NaCl, 20 CsCl, 1 CaCl2, 10 HEPES, 5 BaCl2, 12 glucose, 0.001 TTX, and glycine 0.02, pH 7.3–7.4, 300–305 mosM. Recordings were obtained with an Axon Instruments 200B amplifier that was controlled and monitored by an IBM PC running pClamp 8 with a DigiData 1320 series interface (Axon instruments). Following seal rupture, series resistance (4–10 MΩ) was compensated (70–90%). NMDAR-mediated current was evoked by application of NMDA (100 µM) for 2 s every 30 s in neurons held at −60 mV. During prolonged NMDA treatment, NMDA (100 µM) was continuously applied for 5 min in solution containing 2 mM CaCl2 and 20 µM glycine. Drugs were delivered with a ‘sewer pipe’ system. The array of drug capillaries (ca. 150 µm i.d.) was positioned a few hundred microns from the cell under examine. Solution changes were controlled by the SF-77B fast-step solution stimulus delivery device (Warner Instruments). Data were analyzed with Clampfit (Axon instruments) and Kaleidagraph (Albeck Software).
Electrophysiological recordings in slices
The whole-cell voltage-clamp technique was used to measure NMDAR-EPSC in cortical slices (Wang et al., 2003; Yuen et al., 2005). The slice (300 µm) was incubated with artificial CSF (ACSF) containing bicuculline (10 µM) and CNQX (20 µM). The internal solution contained (in mM): 130 Cs-methanesulfonate, 10 CsCl, 4 NaCl, 1 MgCl2, 10 HEPES, 5 EGTA, 2.2 QX-314, 12 phosphocreatine, 5 MgATP, 0.5 Na2GTP, pH 7.2–7.3, 265–270 mosM. Neurons were visualized with a 40 × water-immersion lens and illuminated with near infrared IR light. All recordings were performed using a Multiclamp 700A amplifier. Tight seals (2–10 GΩ) were generated by applying negative pressure. Additional suction was applied to disrupt the membrane and obtain the whole-cell configuration. EPSCs were evoked by stimulating the neighboring cortical neurons with a bipolar tungsten electrode (FHC, Inc.) located at a few hundred micrometers away from the neuron under recording. Before stimulation, neurons were held at −70 mV and then depolarized to +60 mV for 3 s to fully relieve the voltage-dependent Mg2+ block of NMDARs (Hestrin et al., 1990).
Reagents such as calpain inhibitor III, ALLN (Ac-Leu-Leu-Nle-H), cyclosporine A, FK506, microsystin, okadaic acid (OA) and KN-93 (Calbiochem) were made up as concentrated stocks in water or DMSO and stored at −20°C. Stocks were thawed and diluted immediately prior to use. The sequence of TAT-fused NR2CT peptide is “YGRKKRRQRRRKLSSIEDV”, and the scrambled control peptide is “YGRKKRRQRRR SSKLIDVES”.
Western blotting
After treatment, slices were homogenized in boiling 1% SDS, followed by centrifugation (13,000 × g, 20 min). The supernatant fractions were subjected to 7.5% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The blots were blocked with 5% nonfat dry milk for 1 hr at room temperature, followed by incubation with various primary antibodies including α-spectrin (Chemicon, Temecula, CA), NR2A (C-terminal, aa 1265–1464, Upstate Biotechnology, Lake Placid, NY), NR2B (C-terminal, last 20 aa, Upstate), NR1 (C-terminal, aa 660–811, Chemicon), NR2B (N-terminal, Zymed), GABAAR β subunits (Upstate) or GABAAR β subunits (Chemicon). After incubation with horseradish peroxidase-conjugated secondary antibodies (Sigma-Aldrich), the blots were exposed to the enhanced chemiluminescence substrate (Amersham Biosciences). Quantitation was obtained from densitometric measurements of immunoreactive bands on films.
Biochemical Measurement of Surface Receptors
The surface NMDA receptors were detected as described previously (Wang et al., 2003). Briefly, after treatment, cortical slices were incubated with ACSF containing 1 mg/ml Sulfo-NHS-LC-Biotin (Pierce Chemical Co., Rockford, IL) for 40 min on ice. The slices were then rinsed three times in TBS to quench the biotin reaction, followed by homogenization in 300 µl of modified RIPA buffer (1% Triton X-100, 0.1% SDS, 0.5% deoxycholic acid, 50 mM NaPO4, 150 mM NaCl, 2 mM EDTA, 50 mM NaF, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM PMSF, and 1 mg/ml leupeptin). The homogenates were centrifuged at 14,000 × g for 30 min at 4°C. 15 µg of protein were removed to measure total protein. For surface protein, 150 µg of protein were incubated with 100 µl 50% Neutravidin Agarose (Pierce Chemical Co.) for 2 hr at 4°C, and bound proteins were resuspended in 25 µl of SDS sample buffer and boiled. Quantitative Western blots were performed on both total and biotinylated (surface) proteins using antibodies against the N-terminus of NR2B (1:500, Zymed) and an antibody against GABAAR β subunits (1:500, Chemicon).
Small interfering RNA
To suppress the endogenous µ-calpain expression, we transfected cortical cultures with the small interfering RNA (siRNA, Santa Cruz, CA). We used a pool of 3 siRNA oligonucleotides that target 3 distinct sites of calpain regulatory subunit including 5’-CACGUAGUCAUUACUCUA-3’, 5’-ACUAUCGGUAGCCAUGAA-3’ and 5’-UACCCAGCUUCCCAAUCA-3’. The siRNAs were cotransfected with EGFP into cultured cortical neurons (DIV 8) using the Lipofectamine 2000 method. Recording was performed on DIV 10–11.
Co-immunoprecipitation
After treatment, each slice was collected and homogenized in 1 ml lysis buffer (50 mM Tris, 1% deoxycholic acid, 10 mM EDTA, 10 mM EGTA, 1 mM PMSF, and 1 mg/ml leupeptin). Lysates were ultracentrifuged (200,000 × g) at 4°C for 60 min. Supernatant fractions were incubated with an anti-PSD95 antibody (Affinity BioReagents, 1:100) for 2 hr at 4°C, followed by incubation with 50 µl of protein A/G plus agarose (Santa Cruz Biotechnology) for 2 hr at 4°C. Immunoprecipitates were washed for three times with PBS, then boiled in 2x SDS loading buffer for 5 min, and separated on 7.5% SDS-polyacrylamide gels. Western blotting experiments were performed with antibodies against NR2A (Upstate), NR2B (Upstate) and PSD-95 antibody (Affinity BioReagents).
Ischemia model
Ischemic procedures were performed in 4–6 month-old male Mongolian gerbils as described previously (Yuen et al., 2007a). Animals were anesthetized by i.p. injection of pentobarbital (5mg/100g body weight, Abbott Laboratories) before surgery. A midline ventral incision was made in the neck and bilateral carotid arteries were occluded using non-transmatic aneurysm clips. After 10 min of occlusion, the clips were removed. Four hours later animals were anesthetized by inhaling halothane vapor and decapitated. Brains were sliced for electrophysiological and biochemical experiments. Sham-operated animals were under the same surgical procedures except that the common carotid arteries were not occluded.
Immunocytochemistry
Neuronal viability was evaluated with co-staining of MAP2 (to label survival neurons) and propidium iodide (PI, to label apoptotic neurons). Cortical cultures (DIV 14) were treated with NMDA (100 µM, 10 min), and returned to regular culture media. In some experiments, TAT-NR2C peptide (10 µM) and/or calpain inhibitor III (20 µM) were added 30 min before NMDA treatment. Twenty-four hours later, cells were fixed with 4% paraformaldehyde for 30 min and permeabilized with 0.2% Triton X-100 for 10 min. After 1 hr incubation in 5% BSA to block non-specific staining, cells were incubated with anti-MAP2 (1:1000, Chemicon) for 1 hr at room temperature. After washing, cells were incubated in an FITC-conjugated secondary antibody (1:200, Invitrogen) for 2 hr at room temperature. After three washes in PBS, neurons were exposed to PI (4 µg/ml, Sigma) for 10 min at room temperature. After washing, coverslips were mounted on slides with VECTASHIELD mounting media (Vector Laboratories). The number of MAP2+ neurons (survival neurons) and neurons showing shrunk and condensed nucleus in PI staining (apoptotic neurons) were counted and compared to control (untreated cultures). Each specimen was imaged under identical conditions and analyzed using identical parameters.
Results
Activation of calpain, induced by prolonged NMDA treatment or transient ischemia, suppresses NMDAR-mediated currents in cortical pyramidal neurons
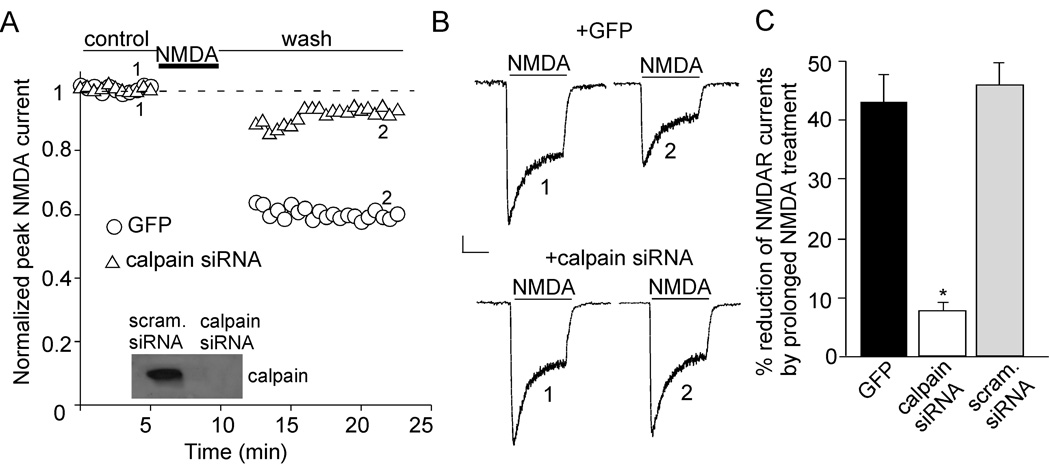
To test the impact of calpain on NMDAR functions, we transfected an siRNA against the calpain regulatory subunit (Yuen et al., 2007b), and examined the effect of calpain on NMDAR-mediated currents in cultured cortical pyramidal neurons (DIV10–11). Transfection of calpain siRNA specifically and effectively suppressed the expression of calpain (Figure 1A, inset). Application of short NMDA pulses (100 µM, 2 sec every 30 sec) evoked stable inward currents (Wu et al., 2005). Since most synaptic NMDARs are located at spines of distal dendrites, the whole-cell NMDA-elicited currents are primarily mediated by extrasynaptic NMDARs located at soma and proximal dendrites. As shown in Figure 1A and 1B, a prolonged NMDA application (100 µM, 5 min) produced a persistent depression of NMDAR currents in neurons transfected with GFP (43.3 ± 4.4%, n = 5, Figure 1C) or a scrambled siRNA (46.0 ± 3.8%, n = 4, Figure 1C). However, this effect was almost abolished in neurons transfected with calpain siRNA (Figure 1A and 1B, 7.7 ± 1.4%, n = 6, Figure 1C). The basal NMDAR current was not altered by calpain siRNA transfection (control: 35.5 ± 1.4 pA/pF, n = 9; calpain siRNA: 34.2 ± 1.6 pA/pF, n = 6). These data indicate that calpain mediates the reducing effect on NMDAR currents by prolonged NMDA treatment. Consistent with our previous finding (Yuen et al., 2007a), GABAAR current kept stable following a prolonged NMDA treatment (100 µM, 5–10 min), suggesting that neurons remained healthy during the electrophysiological experimental period.
Figure 1. Prolonged NMDA treatment reduces NMDAR-mediated currents via calpain activation in cultured cortical pyramidal neurons.
A, Plot of normalized peak NMDAR currents (INMDA) with a prolonged NMDA application (100 µM, 5 min) in GFP-positive neurons transfected with or without calpain siRNA. INMDA was elicited by NMDA pulses (100 µM, 2 sec). Inset: Immunoblot of calpain regulatory subunit in cultured cortical neurons transfected with calpain siRNA or a scrambled siRNA. B, Representative current traces taken from the records used to construct A (at time points denoted by numbers). Scale bars: 100 pA, 1 sec. C, Cumulative data (mean ± SEM) showing the percentage reduction of NMDAR currents by prolonged NMDA treatment in GFP-positive cells transfected without or with calpain siRNA or a scrambled siRNA. *: p < 0.001. ANOVA.
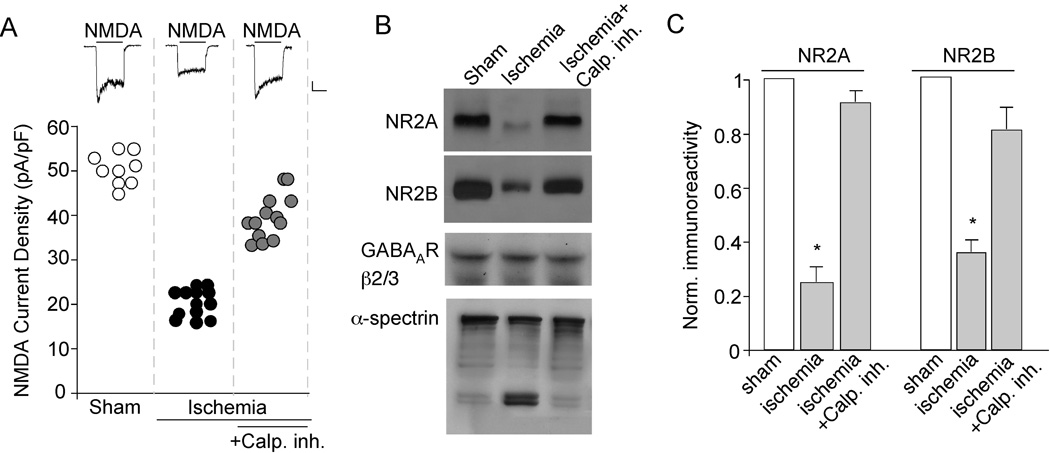
To test the physiological relevance of the effect of calpain on NMDA receptors induced by prolonged NMDA treatment in vitro, we examined whether cerebral ischemia can indeed activate calpain and thus cause the down-regulation of NMDA receptors in vivo. To do this, we recorded NMDA (100 µM)-elicited currents in cortical pyramidal neurons acutely isolated from gerbils exposed to transient ischemic insults. As shown in Figure 2A, the NMDAR current density was significantly smaller in neurons from ischemic animals (20.2 ± 2.2 pA/pF, n = 13, p < 0.001, ANOVA), compared to those from sham-operated animals (48.2 ± 1.3 pA/pF, n = 9). In contrast, the GABAAR current density was not affected (sham: 60.2 ± 4.8 pA/pF, n = 7; ischemia: 59.4 ± 2.1 pA/pF, n = 6). Moreover, the cortical neurons from ischemic animals injected with calpain inhibitor III (3 mg/kg, i.p. at 5 min after the onset of ischemia) showed substantial restoration of NMDAR current density (41.3 ± 1.4 pA/pF, n = 13, p < 0.001, ANOVA, compared to ischemic animals). These data suggest that functional NMDA receptors are selectively down-regulated after forebrain ischemia, and inhibition of calpain blocks the ischemia-induced suppression of NMDAR currents.
Figure 2. Transient forebrain ischemia reduces NMDAR current density via calpain activation.
A, Scatterplot depicting the NMDAR current density in cortical pyramidal neurons acutely dissociated from sham-operated vs. ischemic animals injected with or without calpain inhibitor III (3 mg/kg). Inset: Representative current traces (evoked by 100 µM NMDA) taken from various experimental groups. Scale bars: 100 pA, 1 sec. B, Western blot analysis of NR2A and NR2B (detected with C-terminal antibodies), GABAAR β2/3 and α-spectrin in cortical slices from sham-operated vs. ischemic animals with or without calpain inhibitor III. C, Quantitive analysis (means ± SEM) showing the levels of NR2A and NR2B in cortical slices from sham-operated vs. ischemic animals with or without calpain inhibitor III. *: p < 0.001, ANOVA.
Next, we measured the level of NMDAR subunits in cortical slices from ischemic animals. Full-length NR2A and NR2B proteins were detected with antibodies directed against their C-terminal regions. As shown in Figure 2B and 2C, the levels of full-length NR2A and NR2B subunits were substantially lower in animals exposed to ischemic insults compared to sham-operated animals (NR2A: 25 ± 5% of control; NR2B: 35 ± 4% of control, n = 4). Injection of calpain inhibitor III in ischemic animals prevented the proteolytic processing of NR2A and NR2B subunits (NR2A: 91 ± 4% of control; NR2B: 81 ± 8% of control, n = 4). The level of GABAAR β2/3 subunits remained unchanged in ischemic animals, suggesting that the significant decrease in full-length NR2A/B is not a general effect of neuronal death. α-spectrin, an indicator of calpain activation, was cleaved into two fragments in ischemic animals, confirming that calpain was strongly activated after transient forebrain ischemia. Taken together, these results suggest that, similar to prolonged NMDA treatment in vitro (Wu et al., 2005), forebrain ischemia in vivo leads to calpain proteolysis of NMDAR subunits.
The anchoring protein PSD-95 controls calpain regulation of synaptic NMDA receptors
Previous studies have suggested that NMDAR membrane stability is regulated by its interaction with the scaffolding protein PSD-95 (Roche et al., 2001; Prybylowski et al., 2005). We next examined whether the binding between PSD-95 and NMDARs could influence the effect of calpain on synaptic NMDAR responses. To disrupt preformed NMDAR/PSD-95 complexes, we applied the peptide NR2CT derived from NR2B C-terminal residues (Aarts et al., 2002, KLSSIESDV, conserved at NR2A C-term except for 2 aa), which contains the binding region for PSD-95 (Kornau et al., 1995). This peptide was fused with the protein transduction domain of the human immunodeficiency virus (HIV) TAT protein (YGRKKRRQRRR, Schwarze et al., 1999), which rendered it cell-permeant. As shown in Figure 3A and 3B, treatment of cortical slices with TAT-NR2CT peptide (25 µM, 30 min) significantly reduced PSD-95/NR2A and PSD-95/NR2B interactions.
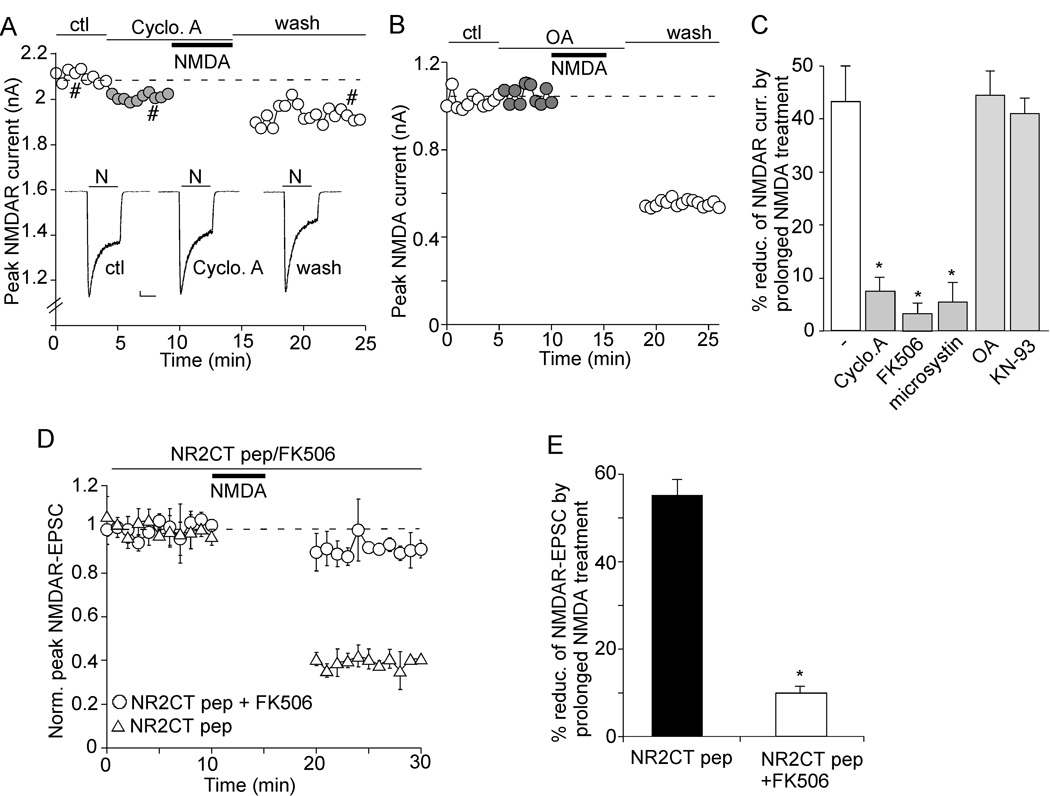
Figure 3. Disruption of the PSD-95/NMDAR interaction facilitates calpain regulation of NMDAR-EPSC.
A, Effect of TAT-NR2CT peptide (25 µM, 30 min treatment) on the interaction of NMDA receptors with PSD-95. A scrambled peptide was used as a control. After treatment, cell lysates from cortical slices were immunoprecipitated with anti-PSD-95 and Western blotted with anti-NR2A, anti-NR2B or PSD-95. B, Bar graphs showing levels of NR2A and NR2B bound to PSD-95 in the absence or presence of TAT-NR2CT peptide or a scrambled peptide. *: p < 0.001, ANOVA. C, Plot of normalized peak NMDAR-EPSC showing the effect of prolonged NMDA treatment (100 µM, 5 min) in neurons dialyzed with or without TAT-NR2CT peptide (10 µM) in the absence or presence of calpain inhibitor ALLN (25 µM). Inset: Representative traces (average of 3 trials) taken from the recordings used to construct C (at time points denoted by numbers). Scale bars: 100 pA, 100 ms. D, Cumulative data (mean ± SEM) summarizing the percentage reduction of NMDAR-EPSC amplitude by prolonged NMDA treatment under different conditions. *: p < 0.001, ANOVA.
To examine the impact of calpain on synaptic NMDA receptors, we measured NMDAR-EPSC in cortical slices. In contrast to whole-cell currents primarily mediated by extrasynaptic NMDA receptors in cultured or dissociated neurons, prolonged NMDA (100 µM, 5 min or 10 min) treatment did not induce a sustained reduction of NMDAR-EPSC (measured at 20 min after washing off NMDA, compared to the pre-NMDA control baseline) (Figure 3C, 2.5 ± 2.9%, n = 8, Figure 3D). Only a transient reduction of NMDAR-EPSC was observed with prolonged NMDA treatment (not illustrated in Figure 3C). To test whether PSD-95 protects synaptic NMDARs from being cleaved by calpain, we dialyzed neurons with the TAT-NR2CT peptide to disrupt PSD-95/NR2 binding. Dialysis with TAT-NR2CT peptide (10 µM) induced a decline of NMDAR-EPSC (Figure 3C, 24.8 ± 4.3%, n = 7), which may be caused by the internalization of NMDARs due to the loss of PSD-95 binding (Roche et al., 2001; Prybylowski et al., 2005). After the current had reached a steady state in the presence of TAT-NR2CT peptide, a prolonged NMDA treatment (100 µM, 5 min) induced a marked reduction of NMDAR-EPSC (Figure 3C, 56.0 ± 5.9%, n = 6, Figure 3D). This effect was significantly blocked by bath application of the selective calpain inhibitor ALLN (25 µM, Figure 3C, 6.2 ± 3.1%, n = 5, Figure 3D). It suggests that the suppression of NMDAR-EPSC by prolonged NMDA treatment in the presence of TAT-NR2CT peptide is mediated by calpain activation.
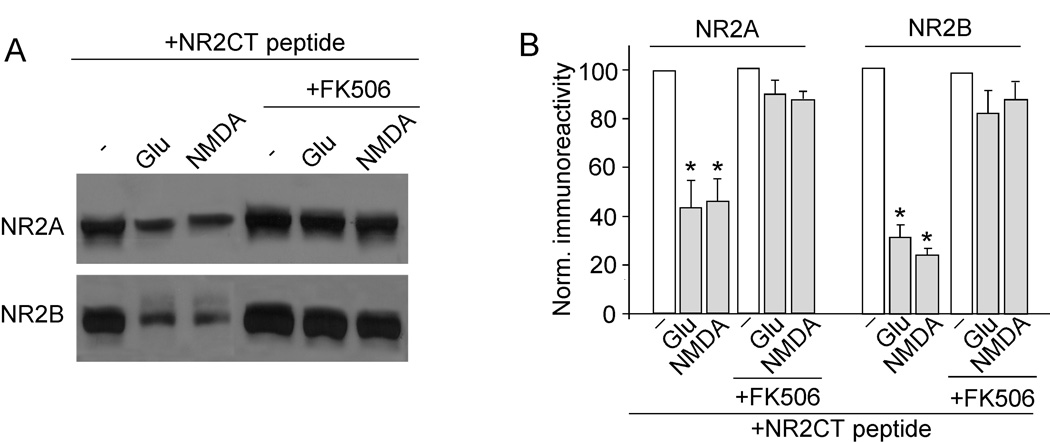
To test whether prolonged NMDA treatment reduces NMDAR-EPSC by cleaving NMDARs when they are no longer associated with PSD-95, we detected the level of NR2A and NR2B subunits in cortical slices treated with or without TAT-NR2CT peptide (10 µM, 30 min). As shown in Figure 4A and 4B, prolonged NMDA (100 µM, 5 min) or glutamate (500 µM, 5 min) treatment significantly reduced the level of full-length (uncleaved) NR2A (glutamate: 43.0 ± 7% of control; NMDA: 53.0 ± 6% of control, n = 4) and NR2B (glutamate: 23.0 ± 10% of control; NMDA: 18.0 ± 8% of control, n = 4) only in slices treated with TAT-NR2CT peptide. It suggests that dissociating NMDARs from PSD-95 promotes calpain-mediated NMDAR cleavage.
Figure 4. Calpain cleavage of NR2A and NR2B subunits requires dissociation with PSD-95, and cleaved NMDARs are removed from the surface.
A, Immunoblots of NR2A, NR2B and NR1 subunits (detected with C-terminal antibodies) in lysates of cortical slices following prolonged glutamate (500 µM, 5 min) or NMDA (100 µM, 5 min) treatment in the absence or presence of TAT-NR2CT peptide (10 µM, added 30 min before glutamate/NMDA treatment). Cells were collected after 10 min of washing following glutamate/NMDA treatment. B, Quantitive analysis (means ± SEM) showing the levels of NR2A and NR2B with glutamate or NMDA treatment in cortical slices in the absence or presence of TAT-NR2CT peptide. *: p < 0.001, ANOVA. C, Immunoblots of the total and surface NR2B subunit in lysates of cortical slices treated with glutamate (500 µM, 10 min) in the absence or presence of TAT-NR2CT peptide (10 µM, 30 min). Cells were collected after 10 min of washing. NR2B was detected with an antibody against the extracellular N-terminal, which labeled both the cleaved and uncleaved subunit. The total and surface GABAAR β subunits were also measured as a control. Similar results were obtained from four experiments. D, Quantitive analysis (means ± SEM) showing the level of cleaved NR2B fragment (115KDa) in total lysate or cell surface with glutamate treatment in cortical slices in the absence or presence of TAT-NR2CT peptide. #: p < 0.05, *: p < 0.01, ANOVA.
For calpain-cleaved NMDA receptors, one possibility is that they remain on the surface but become less functional. Alternatively, they get removed from the surface. To test this, we performed biotinylation experiments to measure the level of surface NMDARs in cortical slices. Surface proteins were first labeled with sulfo-NHS-LC-biotin, and then biotinylated surface proteins were separated from non-labeled intracellular proteins by reaction with Neutravidin beads. Surface and total proteins were subjected to eletrophoresis and probed with an antibody against the N-terminal domain of NR2B, which labeled both cleaved (truncated) and non-cleaved (full-length) fragments. As shown in Figure 4C and 4D, prolonged glutamate treatment (500 µM, 10 min) slightly increased the level of cleaved NR2B fragment (115 KDa) in the total protein lysate (168 ± 27% of control, n = 4, p < 0.05, ANOVA), and this effect was significantly potentiated in the presence of TAT-NR2CT peptide (309 ± 49% of control, n = 4, p < 0.01, ANOVA). However, this truncated form of NR2B (115 KDa) was largely undetectable in the cell surface with or without TAT-NR2CT peptide (Glu: 101 ± 9% of control; Glu+NR2CT peptide: 105 ± 17% of control, n = 4). To prove that biotin labeling is restricted to surface proteins, we have re-probed the blots with a control internal protein, actin. No actin was detected in the biotinylated fraction (data not shown). Our results suggest that calpain-cleaved NR2B subunits were removed from the plasma membrane.
Activation of calpain exerts a protective effect against NMDA-induced excitotoxicity
Since excessive [Ca2+]i elevation by overstimulation of NMDARs can cause excitotoxic neuronal death, the calpain-mediated down-regulation of NMDAR function may provide a neuroprotective effect against NMDAR-mediated excitotoxicity. To test this, we measured neuronal viability in cortical cultures (DIV 14) treated with NMDA (100 µM, 10 min). Neurons were washed several times after NMDA treatment and kept in regular culture media. Twenty-four hours later, cultures were collected for immunocytochemical experiments. Surviving neurons were detected using the dentritic marker MAP2, while apoptotic cell death was indicated by shrunk and condensed nucleus in propidium iodide (PI) staining (Ankarcrona et al, 1995; Bonfoco et al, 1995). As shown in Figure 5A and 5B, NMDA treatment induced remarkable apoptosis in cortical pyramidal neurons, as indicated by significantly decreased number of MAP2+ neurons (27.6 ± 1.6 % survival, Figure 5F) and significantly increased number of cells with shrunk and condensed nucleus in PI staining (control: 4.4 ± 1.1% apoptosis; NMDA-treated: 70.4 ± 4.5%, apoptosis, Figure 5G). Note that NMDA-induced condensed nucleus PI staining only occurred in MAP2- neurons, but not in MAP2+ neurons, suggesting that the MAP2+ neurons were indeed healthy cells that remained alive. In the presence of calpain inhibitor III (20 µM, added 30 min prior to NMDA), NMDA treatment resulted in less cell survival and more severe neuronal death (Figure 5C, 4.5 ± 0.6% survival, Figure 5F; 95.5 ± 1.0% apoptosis, Figure 5G). There was no change of cell viability in cultures treated with calpain inhibitor III alone (99.2 ± 7% survival), suggesting that calpain inhibitor III itself is not toxic to neurons. When adding calpain inhibitor III simultaneously with NMDA, it was still able to potentiate the NMDA-induced neuronal death (7.7 ± 1.3% survival). These results suggest that calpain has a neuroprotective effect against NMDAR-mediated excitotoxicity.
Figure 5. Disruption of NR2/PSD-95 reduces NMDA-induced cell death via calpain activation.
A–E, Immunocytochemical images showing the co-staining of MAP2 (green) and propidium iodide (PI, a nuclear marker, red). Cortical cultures (DIV 14) were treated with NMDA (100 µM, 10 min) in the absence or presence of calpain inhibitor III (20 µM, added 30 min prior to NMDA treatment) or/and TAT-NR2CT peptide (10 µM, added 30 min prior to NMDA treatment). Neurons were collected 24 hrs later for staining. Surviving neurons are positive for MAP2 staining. Apoptotic neuronal death was indicated by shrunk and condensed nucleus in PI staining. F, G, Cumulative data (mean ± SEM) showing the percentage of surviving neurons (F) or neuronal death (G) under various treatments. Data were summarized from 5–7 experiments with each condition. *: p < 0.001, ANOVA.
Pretreatment of cortical cultures with TAT-NR2CT peptide (10 µM, added 30 min prior to NMDA treatment) significantly promoted cell survival and attenuated NMDA-induced excitotoxicity (Figure 5D), as indicated by more living neurons (59.2 ± 3.7% survival, Figure 5F) and less neuronal death (42.4 ± 1.9% apoptosis, Figure 5G). However, this protective effect of TAT-NR2CT peptide was abrogated by addition of calpain inhibitor III (Figure 5E, 15.0 ± 1.3% survival, Figure 5F; 79.8 ± 4.0% apoptosis, Figure 5G). It suggests that TAT-NR2CT peptide protects neurons against NMDA-induced excitotoxicity through a mechanism dependent on calpain activation.
Calcineurin activity affects the calpain regulation of NMDA receptors
Since the calpain cleavage of many substrates is affected by their phosphorylation state (Bi et al., 1998a; 2000; Rong et al., 2001; Yuen et al., 2007b), we tested whether manipulating the activity of various kinases or phosphotases alters the susceptibility of NMDARs to calpain regulation. Previous studies have shown that calcineurin (i.e. protein phosphatase 2B, PP2B), the major Ca2+-dependent phosphatase in neurons, is activated by calpain cleavage and mediates Ca2+-triggered cell death (Kim et al., 2002; Wu et al., 2004), thus we first examined the role of calcineurin in calpain cleavage of NMDARs. Two agents that specifically inhibit calcineurin via distinct mechanisms, cyclosporine A and FK506, were used. As shown in Figure 6A, in the presence of cyclosporine A (20 µM), the reducing effect on NMDAR currents by prolonged NMDA treatment (100 µM, 5 min) was significantly diminished (7.5 ± 2.5%, n = 6, Figure 6C). Cyclosporine A itself did not alter basal NMDA currents (Figure 6A). After the calpain-mediated reduction of NMDAR currents was established, subsequent application of cyclosporine A failed to reverse the effect (data not shown). It suggests that inhibiting calcineurin activity has minimal impact on basal NMDAR currents, but preventing calpain from regulating NMDARs. Consistently, the calpain effect was largely blocked by application of FK506 (10 µM, 3.2 ± 1.9%, n = 5, Figure 6C) or dialysis with the nonspecific phosphatase inhibitor microsystin (5 µM, 5.3 ± 3.7%, n = 5, Figure 6C). In contrast, the calpain effect remained intact in the presence of PP1/2A inhibitor okadaic acid (1 µM, Figure 6B, 44.6 ± 4.5%, n = 5, Figure 6C), or CaMKII inhibitor KN-93 (20 µM, 41.0 ± 2.9%, n = 5, Figure 6C).
Figure 6. Inhibition of calcineurin attenuates the effect of prolonged NMDA treatment on NMDAR currents.
A, B, Plot of INMDA with a prolonged NMDA treatment (100 µM, 5 min) in the presence of cyclosporine A (20 µM, calcineurin inhibitor, A) or okadaic acid (1 µM, PP1/2A inhibitor, B) in acutely dissociated cortical pyramidal neurons. C, Cumulative data (mean ± SEM) summarizing the percentage reduction of NMDAR currents by prolonged NMDA treatment with different agents that affect calcineurin, PP1/2A or CaMKII. *: p < 0.001, ANOVA. D, Plot of normalized peak NMDAR-EPSC with a prolonged NMDA treatment (100 µM, 5 min) in cells injected with TAT-NR2CT peptide (10 µM) in the presence or absence of FK506 (5 µM). Inset: Representative traces taken from the recordings at indicated times. Scale bars: 100 pA, 100 ms. E, Cumulative data (mean ± SEM) showing the percentage reduction of NMDAR-EPSC by prolonged NMDA treatment in the presence of TAT-NR2CT peptide with or without FK506. *: p < 0.001, ANOVA.
We also verified the involvement of calcineurin in calpain regulation of synaptic NMDA responses. As shown in Figure 6D and 6E, prolonged NMDA treatment (100 µM, 5 min) led to the suppression of NMDAR-EPSC in neurons dialyzed with TAT-NR2CT peptide (10 µM, 55.1 ± 4%, n = 6), but it failed to do so in neurons co-injected with FK506 (10 µM, 9.6 ± 1.6%, n = 5). These data suggest that calcineurin activity is specifically required for calpain regulation of NMDAR currents.
Next, we measured the calpain cleavage of NR2A and NR2B subunits (detected with C-term antibodies) in cortical slices treated with calcineurin inhibitors. Slices were incubated with TAT-NR2CT peptide (10 µM, 30 min pretreatment) to disrupt NMDAR/PSD-95 association and therefore enable calpain cleavage of NR2 subunits. As shown in Figure 7A and 7B, prolonged glutamate treatment (500 µM, 5 min) markedly reduced the level of full-length NR2A (53.0 ± 2% of control, n = 4) and NR2B (59.0 ± 2% of control, n = 4). However, application of FK506 (10 µM, 10 min pretreatment) significantly blocked its effect on NR2A (93.0 ± 4% of control, n = 4) and NR2B (83.0 ± 10% of control, n = 4). These results indicate that the calpain-mediated proteolysis of NR2 subunits is controlled by calcineurin activity.
Figure 7. Calpain cleavage of NR2A and NR2B subunits requires calcineurin activity.
A, Western blot analysis of NR2A and NR2B (detected with C-terminal antibodies) in lysates of cortical slices following glutamate (500 µM, 5 min) or NMDA (100 µM, 5 min) treatment in the absence or presence of FK506 (5 µM, added 10 min before glutamate/NMDA treatment). Note that slices were incubated with TAT-NR2CT peptide (10 µM) throughout the experiments. Slices were collected after 10 min of washing. B, Quantitive analysis (mean ± SEM) showing the levels of NR2A and NR2B with glutamate or NMDA treatment in cortical slices in the absence or presence of FK506. *: p < 0.001, ANOVA.
Discussion
Elevation of calcium via NMDAR stimulation during sustained synaptic activity can lead to the activation of the calcium-dependent cysteine protease calpain. By exhibiting broad substrate specificity, calpain influences diverse cellular functions including gene expression (Abe and Takeichi, 2007), excitotoxic neuronal death (Bano et al., 2005; Xu et al., 2007), neurodegeneration (Saito et al., 1993; Gafni and Ellerby, 2002), synaptic plasticity and memory formation (Hawasli et al., 2007; Shimizu et al., 2007). Although NR2 subunits of NMDARs have been identified as calpain substrates in vitro and in heterologous systems via biochemical assays (Bi et al., 1998b; Guttmann et al., 2001; 2002; Simpkins et al., 2003), the in vivo occurrence and physiological consequence of the calpain-mediated cleavage of NMDARs in neurons are still unclear. Our previous study provides electrophysiological and biochemical evidence indicating that activation of calpain in NMDA-treated cortical cultures induces the proteolysis of both NR2A and NR2B subunits and the suppression of NMDAR-mediated ionic currents (Wu et al., 2005). In this study, using siRNA to knockdown calpain specifically instead of using pharmacological agents that lack specificity among cysteine proteases and other proteolytic enzymes, we further demonstrate that calpain, activated by NMDA exposure (100 µM, 5 min) to cortical cultures, suppresses NMDAR function. Consistent with the in vitro finding, we also demonstrate that calpain, activated by transient focal cerebral ischemia in vivo, causes the down-regulation of NMDAR current density, which is accompanied by proteolysis of NR2A and NR2B subunits. These results suggest that calpain activation is not necessarily detrimental, and it provides a negative feedback to dampen NMDAR-mediated excitotoxicity. Consistent with this notion, it has been shown recently that calpain activation following an initial NMDA exposure cleaves CRMPs (collapsing response mediator proteins), which leads to the decreased amount of surface NR2B subunit and increased resistance of cortical neurons to subsequent NMDA exposure (Bretin et al., 2006).
In contrast to the impact of calpain on whole-cell NMDA-evoked current that is primarily mediated by extrasynaptic NMDA receptors, prolonged NMDA exposure (100 µM, 5 min) did not affect the NMDAR-EPSC mediated by synaptic NMDA receptors (NMDAR-EPSC at 20 min after washing off NMDA were compared to the pre-NMDA control level), suggesting that some protein at postsynaptic sites protects synaptic NMDARs from being cleaved by calpain. Biochemical studies show that co-expression of PSD-95 with NMDA receptors in cell lines blocks the calpain cleavage of NMDARs (Dong et al., 2004), however it is unclear whether PSD-95 affects the susceptibility of NMDA receptors to calpain cleavage during synaptic transmission and excitotoxicity. In this study, we demonstrate that injection of NR2CT peptide to disrupt the NR2/PSD-95 binding facilitates calpain-mediated reduction of NMDAR-EPSC. Moreover, the expression level of full-length NR2A and NR2B is reduced by NMDA-activated calpain only in cortical slices treated with TAT-NR2CT peptide. These results suggest that PSD-95-bound NMDARs are resistant to calpain cleavage, and disruption of the NMDAR/PSD-95 association enables calpain to cleave NMDARs more effectively. Since the association between NMDARs and PSD-95 is decreased following transient global ischemia (Takagi et al., 2000), it explains the strong cleavage of NR2A and NR2B by calpain in cortical slices from ischemic animals that we have observed in this study. A previous study suggests that TAT-NR2CT peptide prevents ischemia-induced cell death potentially via nitric oxide synthase (Aarts et al., 2002). Our data have confirmed the protective role of TAT-NR2CT peptide, but suggested an alternative mechanism underlying the neuroprotection. TAT-NR2CT peptide, by perturbing the NMDAR/PSD-95 interaction, facilitates calpain-mediated down-regulation of synaptic NMDA responses, leading to the protection against NMDAR excitotoxicity.
Our previous study has shown that the down-regulation of NMDAR current induced by prolonged NMDA treatment (100 µM, 5 min) is dependent on Ca2+ and calpain (Wu et al., 2005). Other studies using brief applications of NMDA (5–10 sec) have found Ca2+-independent but internalization-dependent down-regulation of NMDAR current (Vissel et al., 2001; Li et al., 2002), which may be because calpain is not activated under these conditions, since calpain activation requires µM [Ca2+]i (Glading et al., 2002; Goll et al., 2003). Another reason for not seeing the calpain-mediated proteolysis of NMDARs in previous studies by others is the use of protease inhibitor leupeptin (Vissel et al., 2001) or high concentration of Ca2+ chelator BAPTA (Nong et al., 2003) in the recording electrodes, which blocks the effect of activated calpain, if there is any.
Using an antibody against N-terminal (NT) NR2B, we found that calpain-cleaved NR2B subunits were removed from the plasma membrane. Because of the lack of a reliable antibody against NT-NR2A, we cannot exactly localize the cleaved NR2A using biochemical assays. A previous study with a nonspecific NT-NR2A/B antibody has shown that the calapin-cleaved NR2 fragment (115 kDa) can be detected at the cell surface (Simpkins et al., 2003), however the identity of the surface fragment is unknown. Since NR2A and NR2B have distinct endocytic motifs and endocytic sorting, with NR2B undergoing more robust endocytosis than NR2A (Lavezzari et al., 2004), it is likely that C-terminal (CT)-cleaved NR2B is more easily to be removed from the surface than cleaved NR2A. Moreover, surface biotinylation assays cannot tell whether the increased NR2 fragment on the surface represents the total or partial population of cleaved NR2. It is possible that only a portion of cleaved NR2A remains at the cell surface. Using electrophysiological recordings of functional NMDA receptors at synapses, we found that calpain cleavage reduced NMDAR-EPSC (mediated by synaptic NR2A and NR2B) by 55–60% (when PSD-95 binding was disrupted). Because only ~30% NMDAR-EPSC is mediated by synaptic NR2B (Liu et al., 2004), it suggests that synaptic NR2A, at least in part, is also removed from plasma membrane after calpain cleavage. Interestingly, a recent study (von Engelhardt et al. 2007) shows that cortical cultures with CT-truncated NR2A have significantly reduced (40–65%) synaptic NMDAR-mediated charge component. It supports our speculation that a large portion of calpain-truncated NR2A is likely removed from the membrane.
Recent studies have found that NR2A and NR2B subunits have differential roles in mediating excitotoxic neuronal death (Liu et al., 2007, Engelhardt et al. 2007). While it is agreed that NR2B underlies the cell death in young cultures (DIV14), the role of NR2A is not very clear. Engelhardt et al. has reported that NR2A contributes to excitotoxicity in older cultures (DIV21), but has a neuroprotective aspect at submaximal NMDA concentration. On the other hand, Liu et al. has reported that NR2A promotes neuronal survival in vitro (DIV11–14 cultures) and in vivo (ischemia model). Our data indicate that in cortical cultures (DIV14), calpain down-regulates NMDAR ionic currents (primarily mediated by NR2B), suggesting a neuroprotective role of calpain in immature synapses. On the other hand, because of the opposing action of NR2B and NR2A in mediating cell death and survival, calpain down-regulation of NMDAR-EPSC (mediated by NR2A and NR2B) in acute slices (from 3–4 weeks old rats) could have complicated consequences in terms of excitotoxicity depending on subunit dominance and NMDA concentrations.
In addition to NMDAR subtypes, it has been found that activation of synaptic vs. extrasynaptic NMDARs exerts opposing actions on excitotoxicity (Hardingham et al. 2002). Synaptic NMDAR triggers phosphorylation of CREB, induces BDNF expression and promotes neuronal survival against ischemic insults. Conversely, extrasynpatic NMDAR antagonizes synaptic NMDARs by stimulating CREB shut-off signaling, which overrides the neuroprotective effect induced by synaptic NMDARs and consequently leads to neuronal death (Hardingham et al. 2002). Our data suggest that the calpain effect on NMDARs also depends on the synaptic localization of NMDARs. Calpain readily reduces whole-cell NMDAR currents (mainly mediated by extrasynaptic NMDARs), while it fails to modulate NMDAR-EPSC (mediated by synaptic NMDARs) unless the NMDAR-PSD-95 interaction is disrupted. This suggests that, under normal conditions, calpain preferentially down-regulates extrasynaptic NMDARs, therefore providing a neuroprotective mechanism by removing CREB shut-off and cell death pathways.
NMDAR functions are influenced by multiple protein kinases and phosphotases, including CaMKII (Leonard et al., 1999; Wang et al., 2003), Src kinase (Yu et al., 1997; Salter and Kalia, 2004) and calcineurin (Lieberman and Mody, 1994). The putative sites of calpain-mediated truncation of NR2A subunit are at C-terminal residues 1279 and 1330 (Bi et al., 2000; Guttmann et al., 2001), which are adjacent to the phosphorylation sites by CaMKII (S1303), Src (Y1281) and Fyn (Y1336) kinases (Wenthold et al., 2003). Several reports show that tyrosine phosphorylation of NR2 subunits modifies their susceptibility to calpain cleavage (Bi et al., 2000; Rong et al., 2001; Wu et al., 2007), however it is unknown whether serine/threonine phosphorylation and dephosphorylation of NMDARs have any effect on their sensitivity to calpain cleavage. It has been shown that calcineurin, the Ca2+ -dependent protein phosphatase, is activated during neuroexcitotoxicity by calpain cleavage of itself (Wu et al., 2004) or its inhibitor cain/cabin1 (Kim et al., 2002). Calcineurin shortens NMDAR channel opening time (Lieberman and Mody, 1994) and enhances NMDAR desensitization (Krupp et al., 2002), presumably by changing the phosphorylation state of NMDA receptors. Here we demonstrate that calcineurin also regulates NMDAR function via facilitating calpain-mediated proteolysis of NMDAR subunits. Thus, in addition to altering electrophysiological properties of NMDARs directly, calcineurin also indirectly modifies NMDAR function through calpain.
Comparing to calpain-mediated regulation of AMPARs that we have reported recently (Yuen et al., 2007a, b), the present study shows that calpain regulates NMDARs in a way that has several similar features. First, calpain, activated by prolonged NMDAR stimulation, induces a substantial reduction of full-length NR2 and GluR1 subunits, which leads to the down-regulation of NMDAR and AMPAR channel currents. Second, in ischemic conditions when calpain is activated, full-length NR2 and GluR1 subunits are reduced, which is accompanied by lower NMDAR and AMPAR current densities. Our present study has also identified two unique factors controlling the sensitivity of NMDAR to calpain cleavage. First, synaptic NMDARs are protected from calpain cleavage by binding to the anchoring protein PSD-95. Second, the sensitivity of NMDARs to calpain regulation is affected by the Ca2+-dependent phosphatase calcineurin, whereas the CaMKII-mediated phosphorylation of GluR1 subunits determines the susceptibility of AMPARs to calpain cleavage (Yuen et al., 2007b).
Taken together, our present study shows that calpain, activated by NMDAR stimulation in vitro or transient ischemia in vivo, suppresses NMDAR function via proteolysis of NR2A and NR2B subunits. Furthermore, this effect can be dynamically regulated by the anchoring protein PSD-95 and the protein phosphatase calcineurin. Given the critical involvement of both calpain and NMDARs in neuronal excitotoxicity, molecules controlling calpain cleavage of NMDARs may provide therapeutics targets for treating excitotoxic disorders (Cui et al., 2007).
Acknowledgements
We would like to thank Xiaoqing Chen and Yong Ren for their technical support.
This work was supported by grants from American Heart Association and National Institute of Health to Z.Y.
Abbreviations
- NMDAR
N-methyl-D-aspartate receptor
- EPSC
excitatory postsynaptic current
- PP2B
protein phosphatase 2B
- CaMKII
Ca2+/camodulin-dependent protein kinase II
- PSD
postsynaptic density
References
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- Abe K, Takeichi M. NMDA-receptor activation induces calpain-mediated beta-catenin cleavages for triggering gene expression. Neuron. 2007;53:387–397. doi: 10.1016/j.neuron.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Adamec E, Beermann ML, Nixon RA. Calpain I activation in rat hippocampal neurons in culture is NMDA receptor selective and not essential for excitotoxic cell death. Brain Res Mol Brain Res. 1998;54:35–48. doi: 10.1016/s0169-328x(97)00304-5. [DOI] [PubMed] [Google Scholar]
- Amadoro G, Ciotti MT, Costanzi M, Cestari V, Calissano P, Canu N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad Sci U S A. 2006;103:2892–2897. doi: 10.1073/pnas.0511065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–285. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Bi R, Bi X, Baudry M. Phosphorylation regulates calpain-mediated truncation of glutamate ionotropic receptors. Brain Res. 1998a;797:154–158. doi: 10.1016/s0006-8993(98)00433-8. [DOI] [PubMed] [Google Scholar]
- Bi R, Rong Y, Bernard A, Khrestchatisky M, Baudry M. Src-mediated tyrosine phosphorylation of NR2 subunits of N-methyl-D-aspartate receptors protects from calpain-mediated truncation of their C-terminal domains. J Biol Chem. 2000;275:26477–26483. doi: 10.1074/jbc.M003763200. [DOI] [PubMed] [Google Scholar]
- Bi X, Rong Y, Chen J, Dang S, Wang Z, Baudry M. Calpain-mediated regulation of NMDA receptor structure and function. rain Res. 1998b;790:245–253. doi: 10.1016/s0006-8993(98)00067-5. [DOI] [PubMed] [Google Scholar]
- Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced respectively by mild and intense insults with NMDA or nitric oxide/superoxide in cortical cell cultures. Proc. Natl. Acad. Sci. USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretin S, Rogemond V, Marin P, Maus M, Torrens Y, Honnorat J, Glowinski J, Prémont J, Gauchy C. Calpain product of WT-CRMP2 reduces the amount of surface NR2B NMDA receptor subunit. J Neurochem. 2006;98:1252–1265. doi: 10.1111/j.1471-4159.2006.03969.x. [DOI] [PubMed] [Google Scholar]
- Carragher NO. Calpain inhibition: a therapeutic strategy targeting multiple disease states. Curr Pharm Des. 2006;12:615–638. doi: 10.2174/138161206775474314. [DOI] [PubMed] [Google Scholar]
- Cui H, Hayashi A, Sun HS, Belmares MP, Cobey C, Phan T, Schweizer J, Salter MW, Wang YT, Tasker RA, Garman D, Rabinowitz J, Lu PS, Tymianski M. PDZ protein interactions underlying NMDA receptor-mediated excitotoxicity and neuroprotection by PSD-95 inhibitors. J Neurosci. 2007;27:9901–9915. doi: 10.1523/JNEUROSCI.1464-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Dong YN, Waxman EA, Lynch DR. Interactions of postsynaptic density-95 and the NMDA receptor 2 subunit control calpain-mediated cleavage of the NMDA receptor. J Neurosci. 2004;24:11035–11045. doi: 10.1523/JNEUROSCI.3722-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni J, Ellerby LM. Calpain activation in Huntington's disease. J Neurosci. 2002;22:4842–4849. doi: 10.1523/JNEUROSCI.22-12-04842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glading A, Lauffenburger DA, Wells A. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/s0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Guttmann RP, Baker DL, Seifert KM, Cohen AS, Coulter DA, Lynch DR. Specific proteolysis of the NR2 subunit at multiple sites by calpain. J Neurochem. 2001;78:1083–1093. doi: 10.1046/j.1471-4159.2001.00493.x. [DOI] [PubMed] [Google Scholar]
- Guttmann RP, Sokol S, Baker DL, Simpkins KL, Dong Y, Lynch DR. Proteolysis of the N-methyl-d-aspartate receptor by calpain in situ. J Pharmacol Exp Ther. 2002;302:1023–1030. doi: 10.1124/jpet.102.036962. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, Greengard P, Powell CM, Cooper DC, Bibb JA. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S, Nicoll RA, Perkel DJ, Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt KE, Lesiuk HJ, Tauskela JS, Morley P, Durkin JP. Selective coupling of mu-calpain activation with the NMDA receptor is independent of translocation and autolysis in primary cortical neurons. J Neurosci Res. 1998;54:223–232. doi: 10.1002/(SICI)1097-4547(19981015)54:2<223::AID-JNR10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wang KK. The calpain family and human disease. Trends Mol Med. 2001;7:355–362. doi: 10.1016/s1471-4914(01)02049-4. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Jo DG, Hong GS, Kim BJ, Lai M, Cho DH, Kim KW, Bandyopadhyay A, Hong YM, Kim DH, Cho C, Liu JO, Snyder SH, Jung YK. Calpain-dependent cleavage of cain/cabin1 activates calcineurin to mediate calcium-triggered cell death. Proc Natl Acad Sci U S A. 2002;99:9870–9875. doi: 10.1073/pnas.152336999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737-–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Calcineurin acts via the C-terminus of NR2A to modulate desensitization of NMDA receptors. Neuropharmacology. 2002;42:593–602. doi: 10.1016/s0028-3908(02)00031-x. [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1999;96:3239–3244. doi: 10.1073/pnas.96.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Chen N, Luo T, Otsu Y, Murphy TH, Raymond LA. Differential regulation of synaptic and extrasynaptic NMDA receptors. Nature Neuroscience. 2002;5:833–834. doi: 10.1038/nn912. [DOI] [PubMed] [Google Scholar]
- Lieberman DN, Mody I. Regulation of NMDA channel function by endogenous Ca(2+)-dependent phosphatase. Nature. 1994;369:235–239. doi: 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW. Glycine binding primes NMDA receptor internalization. Nature. 2003;422:302–307. doi: 10.1038/nature01497. [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Perrin BJ, Huttenlocher A. Calpain. Int J Biochem Cell Biol. 2002;34:722–725. doi: 10.1016/s1357-2725(02)00009-2. [DOI] [PubMed] [Google Scholar]
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rami A. Ischemic neuronal death in the rat hippocampus: the calpain-calpastatin-caspase hypothesis. Neurobiol Dis. 2003;13:75–88. doi: 10.1016/s0969-9961(03)00018-4. [DOI] [PubMed] [Google Scholar]
- Rao A, Kim E, Sheng M, Craig AM. Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J Neurosci. 1998;18:1217–1229. doi: 10.1523/JNEUROSCI.18-04-01217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Rong Y, Lu X, Bernard A, Khrestchatisky M, Baudry M. Tyrosine phosphorylation of ionotropic glutamate receptors by Fyn or Src differentially modulates their susceptibility to calpain and enhances their binding to spectrin and PSD-95. J Neurochem. 2001;79:382–390. doi: 10.1046/j.1471-4159.2001.00565.x. [DOI] [PubMed] [Google Scholar]
- Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci U S A. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Phan T, Mansuy IM, Storm DR. Proteolytic degradation of SCOP in the hippocampus contributes to activation of MAP kinase and memory. Cell. 2007;128:1219–1229. doi: 10.1016/j.cell.2006.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins KL, Guttmann RP, Dong Y, Chen Z, Sokol S, Neumar RW, Lynch DR. Selective activation induced cleavage of the NR2B subunit by calpain. J Neurosci. 2003;23:11322–11331. doi: 10.1523/JNEUROSCI.23-36-11322.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A, Hvalby O, Jensen V, Paulsen O, Andersen P, Kim JJ, Thompson RF, Sun W, Webster LC, Grant SG, Eilers J, Konnerth A, Li J, McNamara JO, Seeburg PH. Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell. 1998;92:279–289. doi: 10.1016/s0092-8674(00)80921-6. [DOI] [PubMed] [Google Scholar]
- Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Kohr G. C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci. 2000;20:4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi N, Logan R, Teves L, Wallace MC, Gurd JW. Altered interaction between PSD-95 and the NMDA receptor following transient global ischemia. J Neurochem. 2000;74:169–178. doi: 10.1046/j.1471-4159.2000.0740169.x. [DOI] [PubMed] [Google Scholar]
- Vissel B, Krupp JJ, Heinemann SF, Westbrook GL. A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat Neurosci. 2001;4:587–596. doi: 10.1038/88404. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Coserea I, Pawlak V, Fuchs EC, Köhr G, Seeburg PH, Monyer H. Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA receptors. Neuropharmacology. 2007;53:10–17. doi: 10.1016/j.neuropharm.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhong P, Gu Z, Yan Z. Regulation of NMDA receptors by dopamine D4 signaling in prefrontal cortex. J. Neurosci. 2003;23:9852–9861. doi: 10.1523/JNEUROSCI.23-30-09852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003;43:335–358. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- Wu HY, Hsu FC, Gleichman AJ, Baconguis I, Coulter DA, Lynch DR. Fyn-mediated phosphorylation of NR2B Tyr-1336 controls calpain-mediated NR2B cleavage in neurons and heterologous systems. J Biol Chem. 2007;282:20075–20087. doi: 10.1074/jbc.M700624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Tomizawa K, Oda Y, Wei FY, Lu YF, Matsushita M, Li ST, Moriwaki A, Matsui H. Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. J Biol Chem. 2004;279:4929–4940. doi: 10.1074/jbc.M309767200. [DOI] [PubMed] [Google Scholar]
- Wu HY, Yuen EY, Lu YF, Matsushita M, Matsui H, Yan Z, Tomizawa K. Regulation of N-methyl-D-aspartate receptors by calpain in cortical neurons. J Biol Chem. 2005;280:21588–21593. doi: 10.1074/jbc.M501603200. [DOI] [PubMed] [Google Scholar]
- Xu W, Wong TP, Chery N, Gaertner T, Wang YT, Baudry M. Calpain-mediated mGluR1alpha truncation: a key step in excitotoxicity. Neuron. 2007;53:399–412. doi: 10.1016/j.neuron.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Yu XM, Askalan R, Keil GJ, Salter MW. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005;25:5488–5501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Gu Z, Yan Z. Calpain regulation of AMPA receptor channels in cortical pyramidal neurons. J Physiol. 2007a;580:241–254. doi: 10.1113/jphysiol.2006.122754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Yan Z. The phosphorylation state of GluR1 subunits determines the susceptibility of AMPA receptors to calpain cleavage. J. Biol. Chem. 2007b;282:16434–16440. doi: 10.1074/jbc.M701283200. [DOI] [PubMed] [Google Scholar]