Abstract
Optimal immunity to microorganisms depends upon the regulated death of clonally expanded effector cells and the survival of a cohort of cells that become memory cells. After activation of naïve T cells, CD44, a widely expressed receptor for extracellular matrix components, is up-regulated. High levels of CD44 remain on memory cells and despite its wide usage as a ‘memory marker,’ its function is unknown. Here we report that CD44 is essential for the generation of memory Th1 cells by promoting effector cell survival. This dependency is not found in Th2, Th17, or CD8 cells despite similar levels of CD44 and the absence of splice variants in all subsets. CD44 limits Fas-mediated death in Th1 cells and its ligation engages the PI3K/Akt signaling pathway that regulates cell survival. The difference in CD44-regulated apoptosis resistance in T cell subpopulations has important implications in a broad spectrum of diseases.
In becoming memory cells, T cells undergo stages of dramatic expansion and contraction that depend upon regulated cell death, and thereafter are maintained by survival signals from the environment. Survival of T cells during a response can be profoundly affected by the availability of co-stimulatory molecules and cytokines that modulate engagement of death pathways (Krammer et al., 2007). Once a response subsides, common gamma chain cytokines, such as interleukin (IL)-7 and IL-15, are essential to the homeostatic control of T cell memory (Boyman et al., 2007). However, as predominantly mobile populations, both effector and memory T cells have the potential to receive additional signals through adhesive interactions with the extracellular matrix (ECM) or other cells (Gilmore, 2005). CD44 is an adhesion molecule that is expressed by most cells and mediates binding to the ECM and other cells via its only known in vivo ligand, the glycosaminoglycan, hyaluronic acid (HA) (Ponta et al., 2003). CD44 expression is up-regulated on naive T cells after activation via the T cell receptor (TCR) and high levels are maintained indefinitely on memory cells (Pure and Cuff, 2001). As a consequence, elevated expression of CD44 is generally used to identify antigen-experienced T cells.
CD44 is associated with cell migration and together with HA has been implicated in numerous biologic processes that are regulated by migrating cells (Ponta et al., 2003). The function of CD44 differs for different cell types and additional roles in the regulation of proliferation and apoptosis have been described (Hauptschein et al., 2005; Pure and Assoian, 2009). CD44 is the product of a single gene that gives rise to a family of HA-binding molecules by alternative exon RNA splicing (Lynch, 2004). In addition to the non-variant or standard form of CD44, at least 5 isoforms are generated through translation of various combinations of 10 variable exons, which are inserted into a single site in the membrane proximal region of the extracellular domain (Figure S6B). Additional cell-type-specific post-translational modifications of CD44 include differences in glycosylation (Camp et al., 1991). The variable forms of CD44 contribute to functional variations that allow for diverse interactions of cells with their environments through a variety of signaling events, which are not yet fully defined and can vary in different cell types (Pure and Assoian, 2009).
Whereas CD44 has the potential to participate in several processes associated with immune responses, the physiological functions of CD44 in T cells in vivo remain ill-defined. It has been established that T cells bind HA, and that either HA-binding or TCR-signaling can augment the adhesive function and expression of CD44 (DeGrendele et al., 1997; Lesley et al., 1994). CD44 together with VLA-4 (α4 integrin) can regulate T cell migration into sites of inflammation (Nandi et al., 2004) and the association of these receptors correlates with enhanced T cell motility and survival after TCR stimulation in vitro (Marhaba et al., 2006). The binding of CD44 expressed on T cells to HA on the surface of dendritic cells (DC) can promote cell clustering (Do et al., 2004) that can be blocked by HA inhibitors (Mummert et al., 2002). Although ligation of CD44 does not elicit proliferation of T cells, it can activate the TCR-associated src family kinases, Lck and Fyn. This suggests that induction of signaling events by CD44 impacts the T cell response (Rozsnyay, 1999), including that to TCR engagement (Foger et al., 2000). CD44 has been associated with both resistance (Marhaba et al., 2006; Naor et al., 2007; Wittig et al., 2000) and susceptibility (McKallip et al., 2002; Nakano et al., 2007) of activated T cells to apoptosis suggesting that it participates in the control of expansion. However, while CD44 is broadly connected with the regulation of T cell responses, distinguishing direct roles in vivo has remained elusive, prompting us to study its function in CD4 T cells.
Using a murine model of influenza virus infection in which a Th1 cell response is induced in CD4 cells, we show that memory in CD4 cells fails to develop in the absence of CD44 engagement. Although the development of effectors appears to proceed normally without CD44, CD4 cells fail to survive due to apoptosis that engages caspase-8, suggesting the involvement of extrinsic death-receptor signaling. Unexpectedly, Th1 cells, but not Th2, Th17, or activated CD8 cells show a CD44 requirement for survival and resistance to apoptosis induced by Fas-engagement in vitro, which correlates with higher levels of Fas expression but not with differences in CD44. Further, ligation of CD44 in vivo enhances Th1 cell accumulation, and in vitro engages the Akt signaling pathway, which can promote survival of activated CD4 (Jones et al., 2002; Varadhachary et al., 2001). The results indicate that CD44 maintains Th1 cells through active control, which could be mediated by physical contacts with HA in the ECM or on other cells. The results support the concept that other subsets of T cells are less susceptible to death in part because of inherent differences in Fas-expression (Varadhachary et al., 1999; Zhang et al., 1997). This differential regulation may permit strategies for immunotherapeutic targeting of Th1 cells in pathological responses to infections and autoimmune diseases.
RESULTS
Loss of CD4 cell memory in the absence of CD44
To investigate the role of CD44 in the development of immunity, we used an influenza model in which viral clearance from the lung epithelium depends upon a local T cell response. In an initial comparison of wild type (WT) and CD44-deficient (CD44−/−) mice, we found that CD4 and CD8 lymphocyte subsets are normally represented in CD44−/− mice, as previously reported (Schmits et al., 1997), because of additional compensatory HA binding receptors (Naor et al., 2007) (Figure S1A). Moreover, we did not find differences in expression of several adhesion receptors, including CD62L, the integrins CD11a and CD49, CD45RB, and CD69 on CD4 cells from 6 month-old animals (Figure S1B). Since CD44 is expressed by multiple cell types, the role of CD44 in CD4 cells was directly assessed using WT and CD44−/− mice crossed to OT-II TCR transgenic (Tg) mice whose CD4 cells recognize a peptide of ovalbumin (OVAII or OVA323–339) (Barnden et al., 1998). Naive WT and CD44−/− Tg CD4 cells marked by expression of the Vβ5 chain of the TCR and by the allelic variants of Ly5.1 (CD45.1) or Thy1.1 (CD90.1), respectively, were isolated by negative selection from the lymphoid tissues of 6 week-old donors. The WT and CD44−/− cells were co-injected in equal numbers into normal C57BL/6 mice (Thy1.2, Ly5.2) to provide an internal control for the response. The cells were titered to the lowest number needed for a consistently detectable response (3×105), where all cells became engaged as measured by division that was detected by CFSE dilution. The recipients were then infected with the WSN virus expressing the OVAII peptide (WSN-OVAII) (Chapman et al., 2005). CD4 memory development was assessed by challenging recipients 3 weeks later with the recombinant influenza virus, HKx31 (H3N2), that also expressed the OVAII peptide (Thomas et al., 2006). The kinetics of cell expansion was evaluated as measured by the recovery of donor cells.
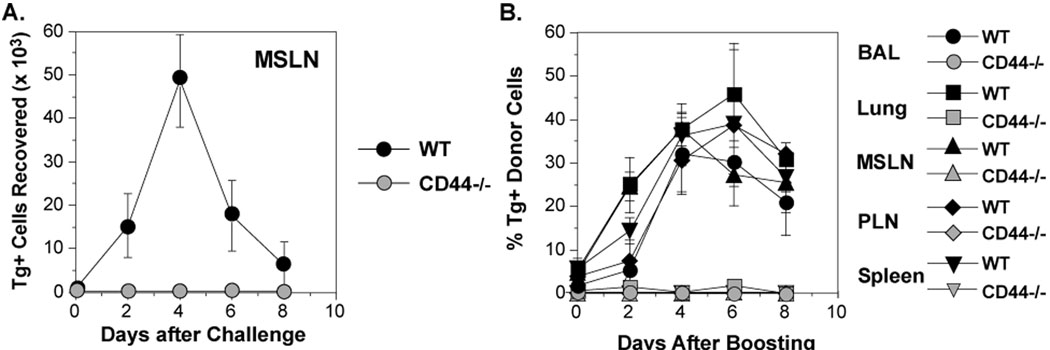
At the time of challenge, the frequencies of donor cells were very low. As anticipated, a memory response was observed from WT OT-II donor cells in the draining mediastinal lymph nodes (MSLN) as indicated by a > 50 fold increase in the number of Tg+ CD4 cells on day 4, the peak of the response (Figure 1A). Dramatic increases in the frequencies of WT OT-II cells were also observed in the airways (retrieved by bronchoalveolar lavage, BAL) and lungs, as well as other lymphoid tissues (peripheral lymph nodes, PLN, and spleen) (Figure 1B). However, CD4 cells from the CD44−/− OT-II donors were undetectable in the MSLN (Figure 1A) and were only present in very low numbers systemically (Figure 1B). The data suggest that expression of CD44 on CD4 cells might be necessary for either the appropriate development of memory cells or their capacity for expansion.
Figure 1. Requirement for CD44 in the generation of memory responses in CD4 cells.
CFSE-labeled OT-II cells from WT (Ly5.1) and CD44 −/− (Thy1.1) mice were co-transferred (3×105 each) into C57BL/6 recipients (Ly5.2, Thy1.2) that were then infected with WSN-OVAII. After 22 days, the recipients were challenged with HKx31-OVAII. A) The recovery of Tg+ WT and CD44−/− cells in the MSLN from individual animals. B) The percentage Tg+ cells in the Vβ5+, CD4+ population from BAL, lung, MSLN, PLN and spleen (Mean ± SEM, n = 3–4/group).
Unimpaired induction of CD4 cell responses in the absence of CD44
CD44 can regulate T cell migration via interactions with vascular endothelium through HA, which acts to initiate extravasation into tissue (DeGrendele et al., 1997; Nandi et al., 2004). Therefore, the absence of CD44 could generally affect the trafficking of CD44−/− CD4 cells. Thus, we evaluated the recovery of naive CD44−/− and WT CD4 cells with time after transfer to unimmunized hosts. The presence of comparable numbers of naïve and WT cells (Figure S2A) in the lymphoid compartment and no differences in their distribution (data not shown) suggest normal homeostatic regulation and migration. Since inflammation could affect CD4 cell trafficking and since the cells from CD44−/− mice display the normal responses to TCR activation in vitro (Schmits et al., 1997), we tested if the failure of CD44−/− CD4 cells to generate a memory effector population could be attributed to defects in trafficking of naive or effector cells after influenza virus infection. Homing of naive or in vitro-activated OT-II effector CD4 cells to either lymphoid or non-lymphoid tissues was unimpaired by the absence of CD44 irrespective of whether the recipients were naive or were infected several days previously (Figure S2B, C). The results indicate that although CD44 can regulate homing of CD4 cells (DeGrendele et al., 1997), this function is not essential and/or is replaceable by other adhesion receptors.
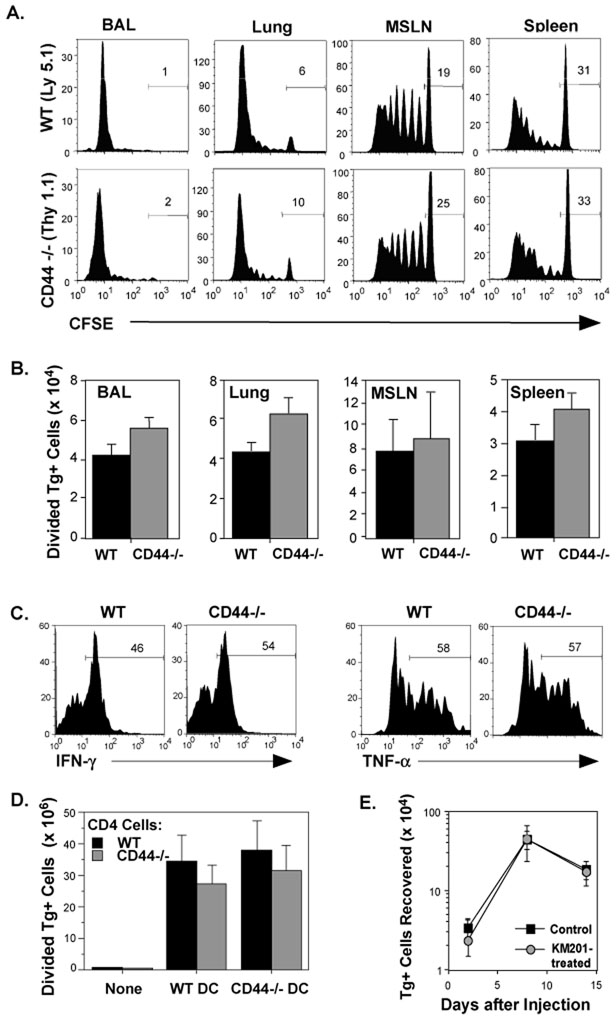
Although differences in the responses of CD44−/− and WT OT-II cells to TCR stimulation with peptide and antigen-presenting cells (APC) were not observed in vitro, it is possible that CD44 contributes to the initial priming or responses of CD4 cells in vivo. Therefore, the division of adoptively transferred WT and CD44−/− Tg+ cells was analyzed by CFSE dilution at the peak of the response to WSN-OVAII. Here we used a higher dose of Tg+ cells to observe potential differences in the kinetics of division. The data in Figure 2 show similar kinetics of division in WT and CD44−/− cells (A) and distribution of cells in the lymphoid tissues and lungs as measured by recovery (B). This suggest that CD44 is not essential for either the induction of a CD4 cell response to influenza virus or for the migration of CD4 cells. Furthermore, both populations exhibited a similar capacity to develop effector function as measured by secretion of IFN-γ and TNF-α (Figure 2C). Additionally, we directly confirmed that expression of CD44 was not required on either CD4 cells or DCs to initiate a primary response in vivo, as shown by the induction of comparable numbers of dividing WT and CD44−/− cells after immunization with OVA peptide-pulsed WT or CD44−/− splenic DCs (Figure 2D). To further investigate whether blocking CD44 during Th1 cell priming could predispose the cells to death, OT-II cells were treated with the anti-CD44 adhesion-blocking mAb, KM201 (Zheng et al., 1995), during in vitro culture with APC and peptide under Th1 polarizing conditions. Cell recoveries at day 4 were similar to the isotype control-treated cultures, and exposure to KM201 did not negatively affect the ability of Th1 cells to persist (Figure 2E). The results show that initial activation, expansion, and effector development proceed normally in the CD44−/− CD4 cells.
Figure 2. CD44-independence of CD4 cell priming.
C57BL/6 mice were injected with CFSE-labeled WT and CD44−/− OT-II cells (1.5×106 each) and infected with WSN-OVAII. A) After 8 days, division of Tg+ cells was analyzed by CFSE. The marker on each histogram shows the fraction of undivided cells. B) The average recoveries of Tg+ donor cells that underwent 1 or more divisions on day 8 (Mean ± SEM, n = 3–4/group). C) IFN-γ and TNF-α production by WT and CD44−/− cells after overnight re-stimulation by OVAII peptide with splenic APC. D) CFSE-labeled WT and CD44−/− OT-II cells were co-injected into C57BL/6 recipients as for (A) together with 2×105 CD11c+, OVAII peptide-pulsed DCs from either WT or CD44−/− C57B/6 mice. Recovery of donor CD4 cells that had undergone 1 or more divisions in the spleen 4 days later (Mean ± SEM, n = 4/group). E) WT OT-II Th1 cells were generated with APC and OVAII peptide in the presence of the blocking anti-CD44 mAb, KM201, or control IgG. The cells were then injected into separate groups of C57BL/6 recipients (2×106/mouse). The donor Tg+ cells recovered in the pooled lymph node (LN) and spleens of mice are shown at the indicated times after injection (Mean ± SEM, n = 3–4/group).
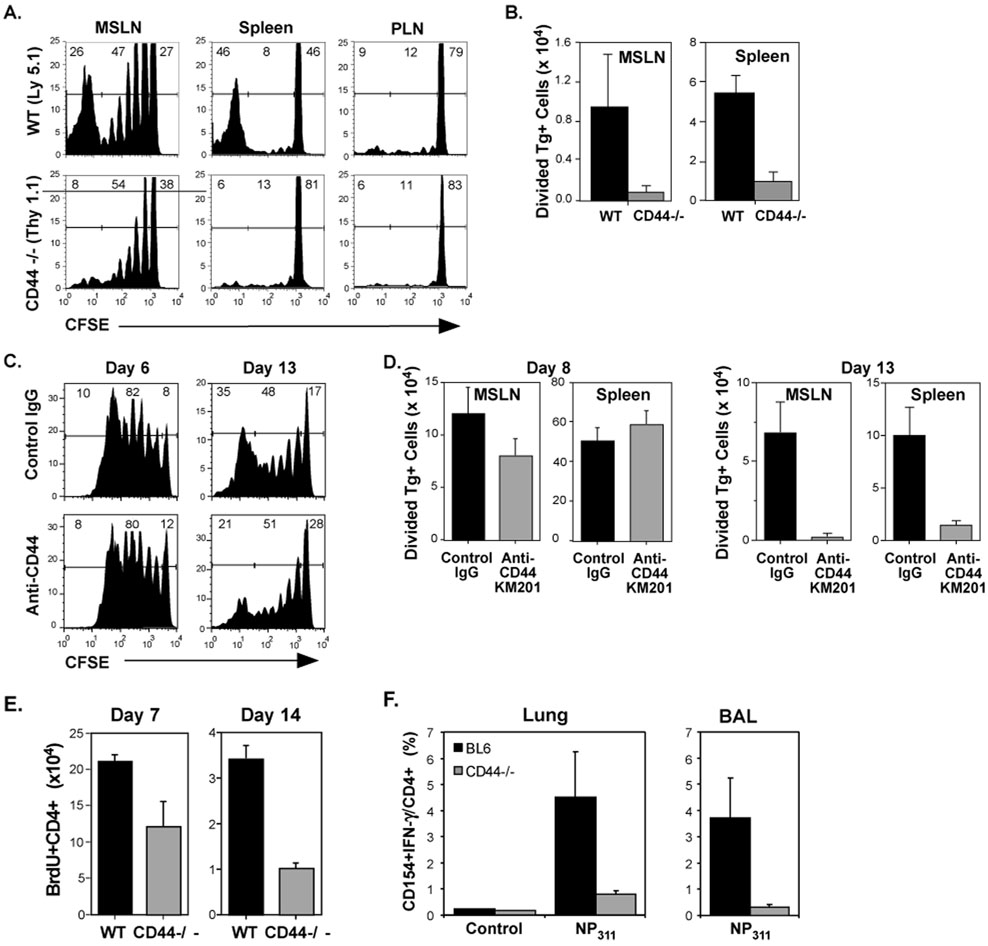
These findings suggest that the mechanisms which limit the development of memory in CD4 cells could become manifest at the later stages of the primary response. Therefore, cell division was analyzed at 13 days after infection. Divided CD44−/− CD4 cells failed to accumulate in the lymphoid tissues and were not detected in the lungs (Figure 3A,B). In order to prevent ligand-binding by CD44, the KM201 mAb was administered, beginning at the time of WT cell transfer and infection. This did not affect the recovery of divided cells on day 6, but the accumulation of responding CD4 cells on day 13 was inhibited when compared to mice that received control IgG (Figure 3C, D). This outcome is unlikely to be due to cytotoxic effects, since KM201 and several other anti-CD44 reagents did not elicit complement-mediated killing of activated OT-II cells (data not shown). The results suggest that CD44 participates in mechanisms that promote the survival of activated CD4 cells.
Figure 3. Loss of CD4 effectors in the absence of CD44 engagement.
C57BL/6 recipients were injected with CFSE-labeled WT and CD44−/− OT-II cells (1.5×106 each) and infected with WSN-OVAII. On day 13 after infection, division (A) and recovery (B) of Tg+ cells was determined as for Figure 2 in the MSLN and spleen. C–D) Recipients of CFSE-labeled WT OT-II cells were injected with either KM201 anti-CD44 mAb or control IgG at the time of cell transfer and infection with WSN-OVAII influenza virus and 3 more times at 3 day intervals. C) The division of the donor cells on days 6 and 13. D) Recovery of donor CD4 cells that had undergone 1 or more divisions in the MSLN and spleen 8 and 13 days after infection. E–F) WT and CD44−/− mice were infected with PR8 influenza virus. E) The mice were treated with BrdU for 7 days before sampling. The recovery of BrdU+ CD4 cells is shown. F) On day 21 after infection, the virus-specific CD4 response was assessed by intracellular staining of cells from the lungs and BALs after overnight culture with anti-CD28 in the presence or absence of NP311 peptide. . Shown are the percentages of CD154+IFN-γ+ virus-specific CD4 cells in the lung. B, D–F, Mean + SEM, n=3–4/group.
Since the model used involves transfer of a relatively large number of Tg+ CD4 cells which might affect regulation, the responses of endogenous CD4 cells from the lungs and MSLN of WT and CD44−/− mice were assessed (Schmits et al., 1997) after infection with influenza virus using BrdU uptake (Figure 3E). The results showed that fewer divided CD4 cells were present in the MSLN in CD44−/− mice compared to WT mice on day 14. In addition, the lungs and BAL contained fewer virus-specific CD44−/− CD4 cells than WT CD4 cells on day 21 (Figure 3F).
Responding CD44−/− CD4 cells die by apoptosis
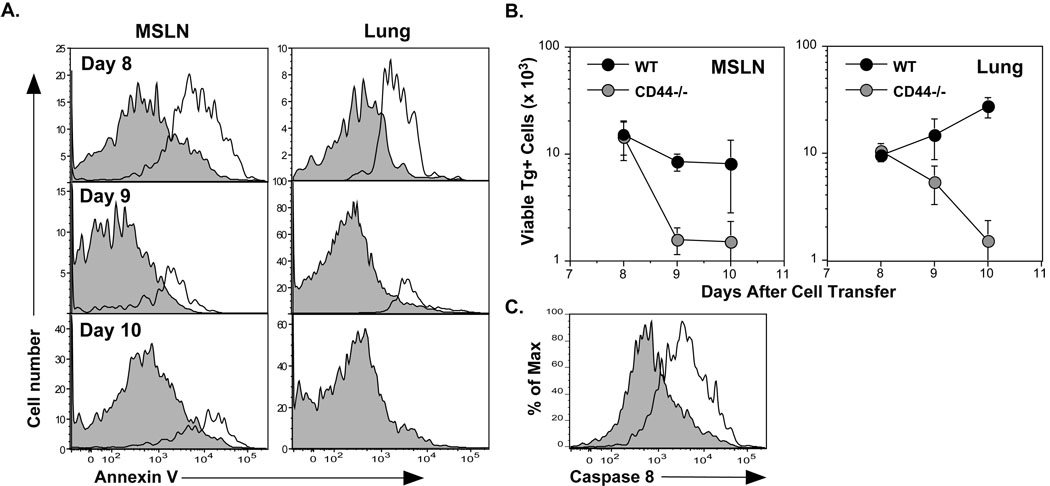
To assess if apoptosis was evident in CD44−/− CD4 cells during expansion after infection, Annexin V-binding was examined in cells from the MSLN and lungs (Figure 4A). Apoptotic cells were observed on day 8, followed by the disappearance of responding cells on day 9 (Figure 4B). Since CD44 has been associated with resistance to Fas-mediated cell death in various cell lines and tumor cells (Hauptschein et al., 2005; Mielgo et al., 2007), the activation of intracellular caspase 8 in CD44−/− CD4 cells was quantified. Activation characterizes the induction of extrinsic apoptotic death (Kim et al., 2006) and occurs as a consequence of the assembly of the death-inducing signaling complex (DISC). On day 7 after adoptive transfer, activated caspase 8 was markedly increased in CD44−/− cells compared to WT cells from the MSLN (Figure 4C). Although changes in Bcl-2 family members correlate with the loss of CD44 and cell death in various cell types in vitro (Marhaba et al., 2003), no altered expression of the pro-apoptotic members, Bim and BNIP-3, or the anti-apoptotic members, Bcl-2 and Bcl-xl, was detected (Figure S3).. The results support the hypothesis that death receptors participate in the mechanism of CD44−/− CD4 cell death in vivo and suggest a previously unidentified role of CD44 in maintaining CD4 effector cells engaged in an immune response.
Figure 4. Induction of apoptosis in responding CD4 cells deficient in CD44.
C57BL/6 recipients were given WT and CD44−/− OT-II cells (1.5×106 each) and infected with WSN-OVAII. A) Apoptosis was assessed by binding of Annexin V and exclusion of 7AAD by WT Tg+ cells (shaded histograms) and CD44−/− Tg+ cells (open histograms) in the indicated tissues. B) Viable recoveries of WT and CD44−/− donor Tg+ cells in the MSLN and lungs (Mean ± SEM, n = 5/group). C) Caspase 8 activation was assessed using a fluorophore-modified substrate with dispersed MSLN cells from recipients of WT and CD44−/− CD4 cells on day 7 after infection. The fluorescence induced by activated caspase 8 for WT Tg+ cells (shaded histogram) and CD44−/− Tg+ cells (open histogram) is shown in 7AAD- Tg+ population. The results are representative of those from 6 recipients.
CD44 can regulate CD4 cell survival in the absence of antigen
After influenza virus infection, antigen presentation to CD4 cells is prolonged (Jelley-Gibbs et al., 2005), raising the possibility that TCR-mediated AICD occurs in the absence of CD44. We therefore asked whether death of CD44−/− CD4 cells occurred in the absence of overt signaling by antigen. To this end, activated effectors were generated from WT and CD44−/− OT-II cells in culture. Both populations underwent comparable expansion and were similarly activated as measured by size (data not shown). Although WT and CD44−/− effector cells showed similar distribution one day after co-injection into normal recipients, CD44−/− CD4 cells decayed more quickly than WT cells in the lung and spleen (Figure 5A), as well as in the PLN, liver and bone marrow (data not shown).
Figure 5. Impaired survival of activated CD4 in naive recipients in the absence of CD44.
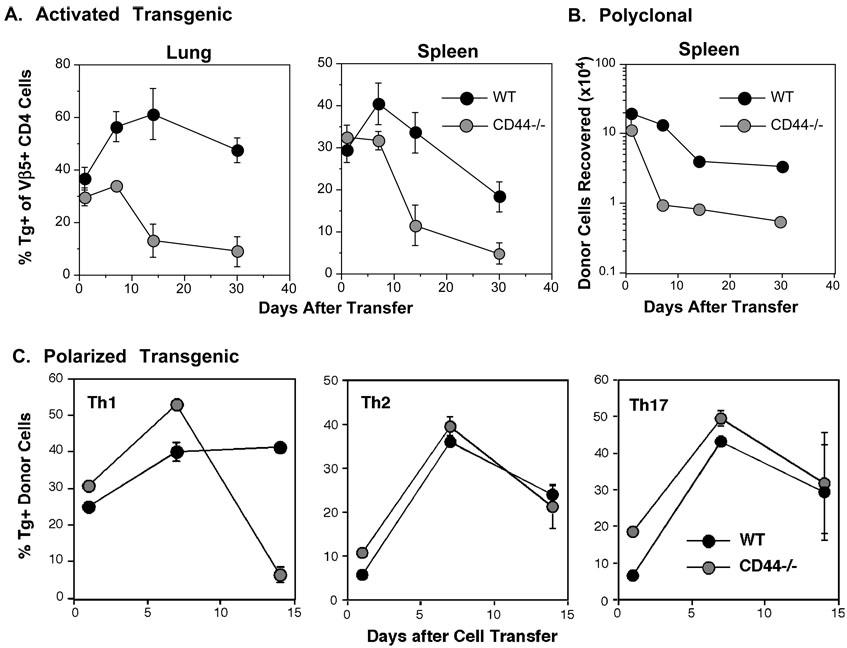
A) WT and CD44−/− OT-II cells were stimulated in vitro with APC and OVA peptide and then co-injected (1.5×106 each) into naive C57BL6 recipients. The frequencies of Tg+ cells, gated on the Vβ5+, CD4+ population in the lungs and spleen at the indicated times after cell transfer are shown (Mean ± SEM, n = 4/group). B) Polyclonal, non-Tg WT CD4 cells (Thy1.1, Ly5.2) and CD44−/− CD4 cells (Thy1.2, Ly5.2) were stimulated with anti-CD3/anti-CD28 and co-injected into Ly5.1, Thy1.2 recipients in a dose of 1.5×106/recipient. The recovery of donor cells in the spleen is shown (Mean ± SEM, n = 4/group). C) Th1, Th2, and Th17 cells were generated from OT-II cells with APC and OVAII peptide. Allelically-marked WT and CD44−/− cells of each of the corresponding subsets were co-injected in a dose of 1.5×106 each into C57BL/6 recipients. Shown are the frequencies of donor cells recovered at the indicated times after cell transfer after gating on the Vβ5+, CD4+ population.
To confirm that impaired survival was not unique to Tg+ cells or the viral response, we examined the decay of CD44−/− effector CD4 cells that were generated from polyclonal naive cells in vitro. Decay of CD44−/− CD4 cells compared to WT cells was pronounced (Figure 5B). The results support the concept that activated CD4 cells that are unable to engage CD44 ligands fail to survive in the absence of CD44.
Since CD4 cells develop into distinct subsets specified by different transcription factors and signature cytokines (Harrington et al., 2006), and the response to influenza virus is Th1 biased, we next asked whether the polarization of effector CD4 cells might affect CD44-dependent regulation of apoptosis. Th1, Th2, and Th17 WT and CD44−/− cells were generated from naive CD4 cells with the appropriate cytokines. The effector phenotypes were confirmed by cytokine analyses, with production of IFN-γ and TNF-α by Th1 cells, IL-17 by Th17 cells, and IL-4 and IL-10 by Th2 cells (Figure S4A). The CD4 cell subpopulations were similarly activated as indicated by the expression of CD11a and CD69 (Figure S4B). WT and CD44−/− effectors of each subset were co-injected into C57BL/6 recipients. The donor cell recoveries at days 1, 7 and 14 revealed that only Th1 cells were affected by the absence of CD44 (Figure 5C). Using Th1 and Th2 WT and CD44−/− cells, no differences were observed in their ability to home to lymphoid tissues (Figure S4C), or to localize in the T cell areas of the spleens (Figure S4D). To determine whether CD44 might play a role in the survival of CD8 cells, TCR Tg+ OVA-specific OT-I or polyclonal CD8 effectors were generated from WT and CD44−/− mice. In contrast to Th1 cells, the decay of CD8 cells in unimmunized recipients was not altered by the absence of CD44 (Figure S5). Thus, CD44 regulates Th1 CD4 cells distinctly and plays a non-redundant role in the regulation of the Th1 response and ultimately their development into memory cells.
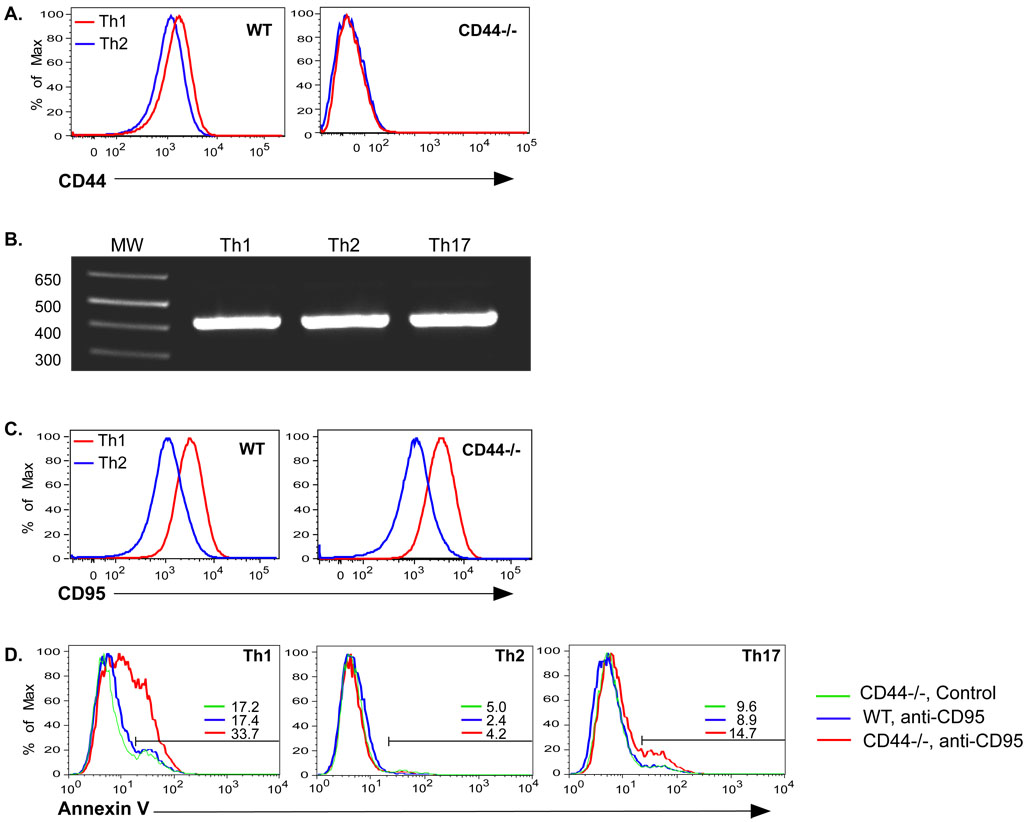
The differential requirements of the CD4 cell subsets could not be attributed to CD44 expression, as this was equal between Th1 and Th2 cells (Figure 6A). Previous studies established that Th1 cells differ from Th2 cells with respect to regulation of apoptosis (Hur et al., 2007; Toscano et al., 2007; Varadhachary et al., 1997; Zhang et al., 1997) and differential expression of CD44 isoforms has previously been associated with protection against death of various cell lines, including T cell lines (Mielgo et al., 2006). However no splice variants were observed in Th1 and Th2 cells as only the standard form of CD44 was detected (Figure 6B, S6B). In addition, no major differences in glycosylation that would lead to differences in the sizes of CD44 on Th1 vs. Th2 were observed (Figure S6A).
Figure 6. CD44s regulates Fas-mediated death in Th1 cells.
A) Th1 and Th2 cells were generated from WT and CD44−/− OT-II Thy1.1 cells with OVAII peptide and APC and tested for expression of CD44. B) Th1, Th2, and Th17 cells were generated with WT OT-II cells. RNA was isolated and tested for the presence of CD44 splice variants by RT-PCR using primers for the constant regions that flank the variant region. CD44 standard is 428bp in size. C) Expression of Fas (CD95) on Th1 and Th2 cells. D) Th1, Th2, and Th17 cells were generated from WT and CD44−/− CD4 cells and re-cultured overnight in the presence or absence of plate bound anti-Fas mAb. The number after the colored bars indicate the percentages of cells undergoing apoptosis as indicated by binding of Annexin V and exclusion of 7AAD.
CD44 has been associated with regulation of Fas-mediated cell death (Hauptschein et al., 2005; Mielgo et al., 2007). We therefore compared expression of Fas and CD44 on Th1 and Th2 cells. Th1 cells expressed much higher levels of Fas than Th2 cells, a difference that was also evident with CD44−/− Th1 and Th2 cells (Figure 6C). Furthermore, incubation of in vitro-polarized CD4 cells with anti-Fas elicited apoptosis in CD44−/− Th1 cells, but not Th2 or Th17 cells (Figure 6D). This difference in Th1 cell susceptibility to apoptosis was detectable by 3 hrs after Fas-ligation (Fig. S7). Thus, CD44 and Fas could interact to limit death of Th1 cells by affecting extrinsic death receptor engagement or signaling. However, we were unable to detect a physical association of these molecules in the membranes of Th1, Th2 or Th17 cells by immunoprecipitation or fluorescence microscopy (data not shown).
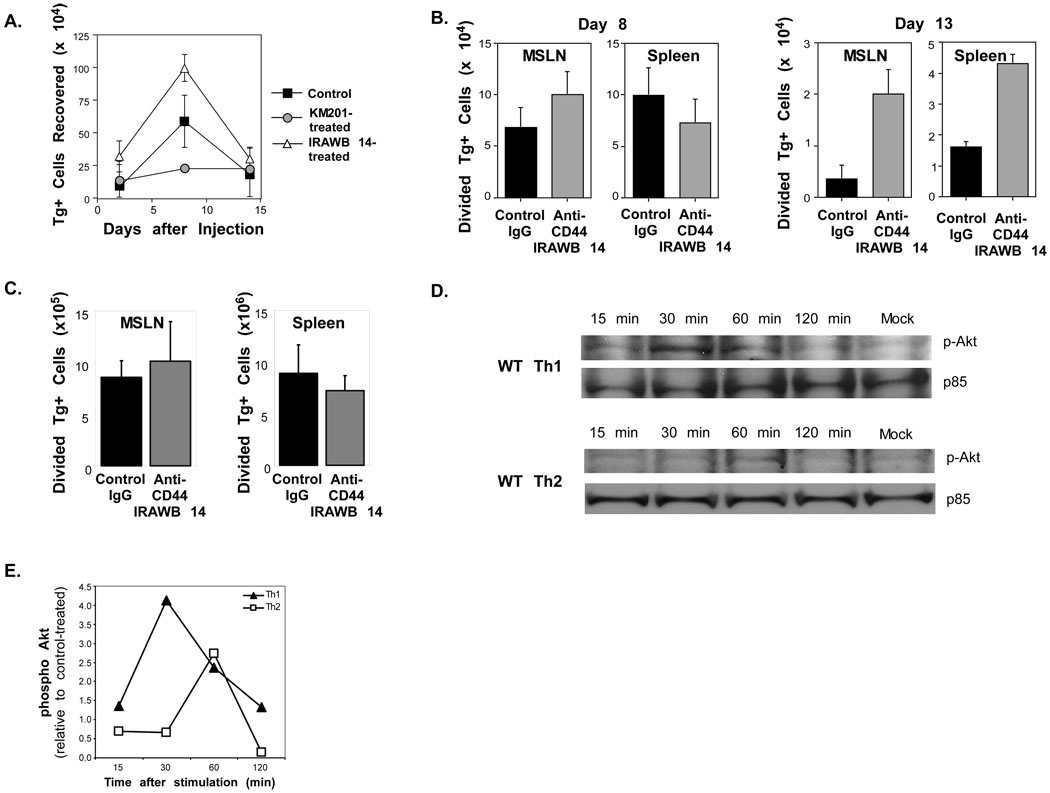
We next sought to determine if signals that might contribute to CD4 cell survival could be initiated by CD44. Therefore, we examined the recoveries of Th1 effectors after transfer to unimmunized recipients that were treated with the agonist anti-CD44 antibody IRAWB 14 antibody (Lesley et al., 1993), or with the blocking anti-CD44 antibody, KM201. Compared to the IgG control, greater or reduced persistence of Th1 cells was observed after treatment with IRAWB 14 or KM201, respectively (Figure 7A). Thus, for Th1 cells, inhibiting CD44 binding to its ligand inhibits survival, whereas signaling through CD44 enhances survival, implying a direct effect of CD44 engagement.
Figure 7. Requirement for CD44 signaling in Th1 CD44−/−.
A) WT OT-II Th1 cells were generated with APC and OVAII peptide. The cells were then injected into C57BL/6 recipients (1.5×106/mouse) and treated with control IgG, KM201, or IRAWB 14 on the day of cell transfer, and 3 more times at 3 day intervals. The donor Tg+ cells recovered in the pooled LN and spleens are shown at the indicated times after injection. B) C57BL/6 recipients of 1.5×106 CFSE-labeled WT OT-II cells were injected with either IRAWB 14 or control IgG at the time of cell transfer and infection with WSN-OVAII influenza virus. The antibodies were administered 3 more times at 3-day intervals. The recoveries of donor CD4 cells that had undergone 1 or more divisions in the MSLN and spleen were measured 8 and 13 days later. C). C57BL/6 recipients of 1.5×106 CFSE-labeled WT OT-II cells were injected with either IRAWB 14 or control IgG 8 days after infection with WSN-OVAII influenza virus. The recoveries of donor CD4 cells that had undergone 1 or more divisions in the MSLN and spleen were measured at 10 days after infection. A–C, Mean ± SEM, n = 3–4/group. D, E) Th1 and Th2 cells were generated from WT and CD44−/− C57BL/6 CD4 cells by stimulation with plate-bound anti-CD3 and anti-CD28. After resting for 1 day in rIL-7 and a further day without, the cells were cultured for the indicated times with plate-bound IRAWB 14 mAb. D) Phospho-Akt was detected by western blots and compared to the p85 subunit of PI3K as a loading control. E) Densitometry of phospho Akt on western blot data in C. Results are represented as a ratio between band densities for IRAWB 14- and unstimulated control cells and are corrected for loading differences.
To test this prediction in an immune response, mice were treated with IRAWB 14 or control IgG at the time of transfer of WT OT-II cells and infection with the influenza virus. After IRAWB 14-treatment there was a greater recovery of CD4 cells engaged in the response by division as compared to the controls on day 13 after infection (Figure 7B). The results suggest that ligation of CD44 can promote the accumulation of CD4 cells either by effects on survival, expansion, or both. However, IRAWB 14 did not promote proliferation of in vitro activated WT OT-II cells (data not shown), and other studies support the concept that ligation of CD44 without TCR signaling does not promote division of T cells (Marhaba et al., 2006). Thus, the data suggest that CD44 participates in maintaining survival of effector cells engaged in the response to influenza virus. To determine if CD44 was required during the expansion vs contraction phase of the CD4 cell response to influenza virus, IRAWB 14 or control Ig treatment were initiated on day 8 after WT OT-II cell transfer and infection of the recipient animals. No differences in the recoveries of OT-II cells were observed under these conditions on day 10 of the response (Figure 7C). These data imply that CD44-regulated survival signals are engaged during CD4 cell expansion.
Ligation of CD44 can lead to activation of the PI3K/Akt pathway in some cell types (Klingbeil et al., 2009) and can inhibit Fas-mediated CD4 cell death (Varadhachary et al., 2001) by interfering with DISC assembly (Jones et al., 2002). To determine if ligation of CD44 might differentially signal in T cell subsets, Th1 and Th2 cells were generated from WT OT-II cells. After resting for 2 days, the cells were cultured with plate-bound IRAWB 14 or with the control IgG antibody. Phosphorylated Akt was measured as a down-stream readout of PI3K activation. Phosphorylated Akt was induced in Th1 cells with peak expression of 30 min after ligation of CD44, whereas this response was lower in Th2 cells and was not observed until 60 min (Figure 7D,E). To confirm the CD44-dependence of PI3K induction, Akt phosphorylation in WT Th1 cells was compared to CD44−/− Th1 cells in response to IRAWB 14. PI3K was engaged only in the WT Th1 cells and expression could be reduced with the PI3K inhibitor, Ly294002 (Figure S8). The results support the concept that CD44 engagement elicits signaling that promotes survival in Th1 cells, which may be crucial in this subset because of the high expression of Fas and the associated greater susceptibility to apoptosis by this pathway.
Discussion
In this study, we identified a critical function of CD44 in the regulation of memory generation in Th1 CD4 cells. Despite potential roles in migration and interactions with DC, CD44 was not required for the initial induction of a primary immune responses in vivo, or for the localization of naive or effector cells in either lymphoid or non-lymphoid tissues. This is probably because of redundancies in adhesion receptor usage that enable T cells to bypass its contribution and/or the ability of other HA binding receptors to perform these functions. However, CD44 plays a non-redundant role in regulating the survival of CD4 effector cells in the influenza model, which is dominated by a Th1 cell response. Without engagement of CD44, effector cells that have progressed through several rounds of division die by apoptosis, whereas agonist signaling via CD44 during the expansion phase can lead to enhanced in vivo accumulation of effector cells. Thus, the generation of a memory population in Th1 cells most likely depends upon engagement of CD44 on responding effectors during the primary response. We show that CD44 ligation can activate the PI3K/Akt signaling pathway in Th1 cells. The mechanism by which CD44 activates PI3K remains to be explored, but could be due to constitutive association with the src family kinases, Lck and Fyn (Rozsnyay, 1999), or to associations with β1 integrins that mediate the survival response (Lee et al., 2008; Marhaba et al., 2006; Nandi et al., 2004).
Th1 cells may uniquely require this survival signal through CD44 because of elevated Fas levels and an inherent ability to rapidly assemble the DISC in response to Fas trimerization (Varadhachary et al., 1999). Thus, we suggest that without engagement of CD44, the response to Fas-ligation cannot be overcome. Such a mechanism may not be necessary in Th2 cells, and possibly other subsets of T cells, because of overall lower levels of Fas expression in addition to a greater capacity to engage PI3K/Akt in response to TCR signaling or co-stimulation (Varadhachary et al., 1999). It is of significance that activation of PI3K/Akt can block DISC formation by preventing the association of FADD and recruitment of pro-caspase 8 in CD4 cells (Jones et al., 2002) and our studies lead us to favor this mechanism for regulation of Th1 cell survival.
Although CD44 can mediate resistance of tumor cells to apoptosis by death receptor ligation via FasL/Fas, DR5/TRAIL, and TNFR1/TNF-α by interfering with DISC assembly through the physical association of Fas and CD44 (Hauptschein et al., 2005), this interaction occurs through variant isoforms. Isoforms that include variants v6 and v9 are in close proximity with Fas in the membranes of transfected Jurkat cells and thereby prevent Fas trimerization (Mielgo et al., 2006). However, the lack of CD44 isoforms on CD4 cells activated in vivo after influenza virus infection (data not shown) or on Th1, Th2, or Th17 cells generated in vitro, which differ in their susceptibility to Fas-mediated death in the absence of CD44, further argues against sequestration of Fas as the only mechanism that accounts for a selective function of CD44 in Th1 cells. The lack of CD44 isoforms on CD4 cell subsets also argues against a mechanism whereby osteopontin-binding to CD44 variants containing v7 leads to activation of NF-κB and prevents mitochondrial death controlled by the transcription factor Foxo3a, a regulator of Bim (Hur et al., 2007). Indeed, we did not detect changes of either pro- or anti-apoptotic Bcl-2 family proteins in WT compared to CD44−/− CD4 cells. Although differences in glycosylation of Th2 cells has been reported to account for resistance to cell death compared to Th1 and Th17 cells (Toscano et al., 2007), the mechanism involves protection from binding of galectin-1, and we did not observe differences in the molecular weight of CD44 from Th1 and Th2 cells that would suggest significant differences in glycosylation. Since CD44 is up-regulated on activated and memory CD8 cells, we did not anticipate differences in their regulation that would suggest independence from CD44-mediated survival signals. However, there are many differences in the regulation of CD4 and CD8 cells, including in the programming to develop into memory cells after the initiation of a response (Kaech and Ahmed, 2001; van Stipdonk et al., 2003). Our data support the concept that internal signaling differences rather than external molecular variation account for the differences in regulation by CD44 on T cells.
A role for CD44 in regulating survival of CD4 cells engaged in an immune response in vivo has not been previously examined directly. However, protection from TCR-mediated AICD by CD44 has been suggested by in vitro studies of in vivo primed cells (Marhaba et al., 2003). The results described herein, which show normal priming of CD4 cells in vivo irrespective of the presence of CD44 was on T cells or DCs, support the concept that engagement of CD44 in vivo is required for Th1 cells only after activation. Our results suggest that survival signals are transmitted in Th1 cells during the expansion phase of the effector response to influenza virus, which is profoundly compromised in the absence of CD44 or when adhesion binding of CD44 is blocked
A CD44-dependent survival mechanism remained operative in activated Th1 cells that were withdrawn from overt Ag stimulation by transfer into naive recipients. This result suggests that TCR signaling in the context of an effector response and the production of pro-inflammatory cytokines both of which can augment CD44-binding of HA (Marhaba et al., 2003), are not necessary for the function of CD44 in promoting apoptosis resistance. Indeed, in the absence of an immune response, agonist engagement of CD44 in vivo promoted enhanced accumulation of CD4 cells. Our previous studies indicate that maintaining effector survival through co-stimulation can be key to the generation of robust memory in CD4 cells (Linton et al., 2003; Linton et al., 2000). In this regard, we propose that CD44 can be viewed as an ECM-dependent, Th1 cell-specific ‘co-stimulatory’ molecule that sustains effector cell response through survival and thereby supports the development of memory.
The homeostasis of many cell types is regulated by contact dependence, and signals from the ECM can be crucial to prevent cells from undergoing anoikis, or programmed cell death which can be due to intrinsic death resulting in mitochondrial permeabilization, and extrinsic death that is initiated by death receptors (Gilmore, 2005). While the molecular mechanisms that lead to PI3K activation and the downstream targets in Th1 cells remain to be defined, our results support the concept that physical contacts of Th1 cells with HA in the immediate environment regulate processes during which CD44-dependent survival signals are engaged. By promoting optimal survival of effector CD4 cells engaged in an immune response (clonal burst), CD44 provides a previously unknown contribution to the development T cell immunity in vivo.
METHODS
Mice and viruses
CD44−/− mice (Schmits et al., 1997) were bred to B6PL-Thy mice and crossed to OT-I and OT-II TCR Tg mice in the vivarium at the Burnham Institute for Medical Research. These mice were also crossed to B6 Ly5.1 mice. C57BL/6 mice were purchased from Jackson Laboratories. All mice were males between 6–16 weeks of age. The mice were housed under specific pathogen-free conditions according to NIH guidelines and the animal review committee at our institution.
All influenza viruses were grown in chicken eggs (10 days of embryonation) and titered using MDCK cells for plaque forming units (pfu) (Jelley-Gibbs et al., 2005). Infective doses elicited an optimal T cell response and were given i.n. in 30 µl. The WT influenza A viruses Puerto Rico/8/34 (PR8, H1N1) was given in a dose of 12.5 pfu. The engineered influenza A viruses, WSN-OVAII (H1N1) (Chapman et al., 2005) and HKx31-OVAII (H3N2) (Thomas et al., 2006) that express the OVA323–339 peptide recognized by OT-II CD4 cells were given in doses of 1250 pfu and 112 pfu, respectively.
Antibodies for Cell Surface and Intracellular Proteins
Anti-Thy1.1 (CD90.1, OX-7) and -Ly5.1 (CD45.1, A20) were from Biolegend. The following antibodies were from eBiosciences: -Bcl2 (10C4), -Bcl-xl (2H12), and -Bnip3 (polyclonal rabbit), -IFN-γ (XMG1.2) and -TNF-α (MP6xt22). Anti-vβ5 (MR9) and anti-Fas (CD95, Jo2) were from BD Biosciences. Anti-BimS (14A8) was from Millipore. Cell lines producing the adhesion blocking anti-CD44 mAb, KM201, and the agonist/activating anti-CD44 mAb, IRAWB 14, were obtained from Dr. Paul Kincade (Zheng et al., 1995). These mAb, anti-CD3 (145-2C11), and anti-CD28 (37.51) were produced and purified by BioLegend. Rat IgG was used as the control (Jackson ImmunoResearch).
Cell Preparation
CD4 and CD8 cells were isolated from pooled spleen and lymph nodes by magnetic sorting (Imag, BD Biosciences) using the manufacturer’s protocol. To generate effector cells, naive T cells were cultured at 106/ml for 4 days with immobilized anti-CD3 (10 µg/ml) and anti-CD28 (5 µg/ml), and from TCR Tg mice using C57BL/6 splenic APC (2×106/ml), and 5µM OVA peptides (257–264, OT-I and 323–339, OT-II, Sigma-Genosys) (Harbertson et al., 2002). CD4 subsets were polarized in the presence of rIL-2 (10 ng/ml) with anti-CD3 and anti-CD28, or with APC and peptide for 4 days. Th1 cells were elicited with rIL-12 (5 ng/ml), and anti-IL-4 (11b11, 10 µg/ml) and Th2 cells with rIL-4 (10 ng/ml) and anti-IFN-γ (XMG1.2, 10 µg/ml). For Th17 cells, we used rIL-1b (10 ng/ml), rIL-6 (20 ng/ml), rTGF-β1 (1 ng/ml), TNF-α (10 ng/ml) and 10 µg/ml each of anti-IL-4 and anti-IFN-γ. Splenic DC were enriched by centrifugation using 13.5% (w/v) histodenz (Sigma) (McLellan et al., 1995).
Adoptive transfer and detection of T cells
T cells were injected i.v. into recipients in doses of 0.3–3.0×106 using donor and host combinations that differed by expression of Thy1 or Ly5. WT and CD44−/− cells were co-injected in equal ratios. DC pulsed for 2 hr with 10 µg/ml OVA peptide were injected in a dose of 1×105/recipient at the time of T cell transfer. After sacrifice, cells in the airways of recipient mice were collected by BAL. Cell suspensions of perfused lungs were obtained by digestion with collagenase D (Roche) at 10 mg/ml for 60 min at 37°C. Flow cytometry was used to detect Tg+ CD4 cells in these tissues, and in LN and spleen cells after fluorescent staining for CD4, Thy1.1, Ly5.1 and vβ5 (BD Biosciences). Viable lymphocyte recovery was determined by flow cytometry using propidium iodide-uptake.
T cell responses
CFSE or BrdU labeling (Harbertson et al., 2002; Linton et al., 2003) were used to assess cell division. Intracellular staining was used to detect cytokine secretion by donor cells after overnight culture of lymphocyte suspensions from the MSLN with splenic APC and OVAII peptide (Harbertson et al., 2002; Linton et al., 2003). After surface staining, the cells were permeabilized (BD Biosciences) and stained with anti-IFN-γ and -TNF-α. Annexin V and 7-Amino-actinomycin D (7AAD) staining were used to distinguish dead from dying cells by flow cytometry. To test caspase 8 activity, freshly isolated MSLN cells were incubated with CaspaLux 8-L1D2 (OncoImmunin, Inc) according to the manufacturer’s protocol. Fas-mediated cell death was induced with plate-bound anti-Fas (10 µg/ml). In vitro treatment of CD4 cells with anti-CD44 mAb or Rat IgG was done as soluble or plate bound (10µg/ml) as indicated in the text.
Anti-CD44 Treatment In Vivo
KM201, IRAWB 14, or Rat IgG were administered in a dose of 200 µg/mouse on the day of cell transfer and 3 times thereafter spaced by 3-day intervals or 1 mg/mouse 8 days after cell transfer.
PCR for CD44 isoforms
OTII CD4 T cells were enriched and polarized as described above and RNA isolated (RNeasy, Qiagen) . Primers spanning the CD44 isoform region (forward Exon 5 primer: CATCAGTCACAGACCTACCCAATTCC and reverse exon 16 primer CCAAGATGATGAGCCATTCTGGAATC) (Figure S6B) were used to distinguish isoforms by RT-PCR (GeneAmp, Applied Biosystems). Reverse transcriptase was for 60min at 42°C using random hexamers and reverse primer, followed by PCR with the following conditions 94°C 2min, 64°C 30s, 72°C 1min30s (35cycles).
Western Blots for Akt
CD4+ cells from WT or CD44−/− mice were stimulated with plate-bound anti-CD3 plus anti-CD28 (5 µg/ml each) under Th1 or Th2 conditions for 4 days (see above), followed by 2 days of resting in RPMI culture medium with or without rIL-7 for the first 24 hr. To crosslink CD44, 106 cells were plated on IRAWB 14- or rat IgG-coated plates, incubated at 37°C for 15, 30, 60 or 120 minutes. Following stimulation, cell lysate proteins were resolved through 4–12% PAGE and transferred to nitrocellulose membranes. The membranes were probed and re-probed with Abs to phospho-Akt and p85 subunit of PI3K (Cell Signaling Technology), respectively. Densitometry on blots was performed using ImageJ (NIH).
Supplementary Material
Acknowledgements
The authors thank R. Padrick, K. Falls and T. Brondstetter for technical assistance, and E. Virts for advice on the RT-PCR for CD44 variants. This work was supported by grants from NIH (AI061615 and AI046530) to L.M.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnden MJ, Allison J, Heath WR, R CF. Defective TCR expression in transgenic mice constructed using cDNA based a- and b- chain genes under the control of heterologous regulatory elements. Immunol. Cell. Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr. Opin. Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Camp RL, Kraus TA, Pure E. Variations in the cytoskeletal interaction and posttranslational modification of the CD44 homing receptor in macrophages. J Cell Biol. 1991;115:1283–1292. doi: 10.1083/jcb.115.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman TJ, Castrucci MR, Padrick RC, Bradley LM, Topham DJ. Antigen-specific and non-specific CD4+ T cell recruitment and proliferation during influenza infection. Virology. 2005;340:296–306. doi: 10.1016/j.virol.2005.06.023. [DOI] [PubMed] [Google Scholar]
- DeGrendele HC, Kosfiszer M, Estess P, Siegelman MH. CD44 activation and associated primary adhesion is inducible via T cell receptor stimulation. J. Immunol. 1997;159:2549–2553. [PubMed] [Google Scholar]
- Do Y, Nagarkatti PS, Nagarkatti M. Role of CD44 and hyaluronic acid (HA) in activation of alloreactive and antigen-specific T cells by bone marrow-derived dendritic cells. J. Immunother. 2004;27:1–12. doi: 10.1097/00002371-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Foger N, Marhaba R, Zoller M. CD44 supports T cell proliferation and apoptosis by apposition of protein kinases. Eur. J. Immunol. 2000;30:2888–2899. doi: 10.1002/1521-4141(200010)30:10<2888::AID-IMMU2888>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Gilmore AP. Anoikis. Cell Death Differ. 2005;12 Suppl 2:1473–1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- Harbertson J, Biederman E, Bennett KE, Kondrack RM, Bradley LM. Withdrawal of stimulation may initiate the transition of effector to memory CD4 cells. J. Immunol. 2002;168:1095–1102. doi: 10.4049/jimmunol.168.3.1095. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr. Opin. Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Hauptschein RS, Sloan KE, Torella C, Moezzifard R, Giel-Moloney M, Zehetmeier C, Unger C, Ilag LL, Jay DG. Functional proteomic screen identifies a modulating role for CD44 in death receptor-mediated apoptosis. Cancer Res. 2005;65:1887–1896. doi: 10.1158/0008-5472.CAN-04-3571. [DOI] [PubMed] [Google Scholar]
- Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat. Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J. Exp. Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RG, Elford AR, Parsons MJ, Wu L, Krawczyk CM, Yeh WC, Hakem R, Rottapel R, Woodgett JR, Ohashi PS. CD28-dependent activation of protein kinase B/Akt blocks Fas-mediated apoptosis by preventing death-inducing signaling complex assembly. J Exp Med. 2002;196:335–348. doi: 10.1084/jem.20020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: Initial antigen encounter triggers a developmental program in naive cells. Nature Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Emi M, Tanabe K, Murakami S, Uchida Y, Arihiro K. Regulation and interplay of apoptotic and non-apoptotic cell death. J. Pathol. 2006;208:319–326. doi: 10.1002/path.1885. [DOI] [PubMed] [Google Scholar]
- Klingbeil P, Marhaba R, Jung T, Kirmse R, Ludwig T, Zoller M. CD44 variant isoforms promote metastasis formation by a tumor cell-matrix cross-talk that supports adhesion and apoptosis resistance. Mol Cancer Res. 2009;7:168–179. doi: 10.1158/1541-7786.MCR-08-0207. [DOI] [PubMed] [Google Scholar]
- Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat. Rev. Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- Lesley J, Howes N, Perschl A, Hyman R. Hyaluronan binding function of CD44 is transiently activated on T cells druing an in vivo immune response. J. Exp. Med. 1994;180:383–387. doi: 10.1084/jem.180.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J, Kincade PW, Hyman R. Antibody-induced activation of the hyaluronan receptor function of CD44 requires multivalent binding by antibody. Eur J Immunol. 1993;23:1902–1909. doi: 10.1002/eji.1830230826. [DOI] [PubMed] [Google Scholar]
- Linton P-J, Bartista B, Biederman E, Bradley ES, Harbertson J, Kondrack RM, Padrick RC, Bradley LM. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J. Exp. Med. 2003;197:875–883. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton P-J, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J. Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- Lynch KW. Consequences of regulated pre-mRNA splicing in the immune system. Nat Rev Immunol. 2004;4:931–940. doi: 10.1038/nri1497. [DOI] [PubMed] [Google Scholar]
- Marhaba R, Bourouba M, Zoller M. CD44v7 interferes with activtion-induced cell death by up-regulation of anti-apoptotic gene expression. J. Leukoc. Biol. 2003;74:135–148. doi: 10.1189/jlb.1202615. [DOI] [PubMed] [Google Scholar]
- Marhaba R, Freyschmidt-Paul P, Zoller M. In vivo CD44-CD49d complex formation in autoimmune disease has consequences on T cell activation and apoptosis resistance. Eur. J. Immunol. 2006;36:3017–3032. doi: 10.1002/eji.200636158. [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Do Y, Fisher MT, Robertson JL, Nagarkatti PS, Nagarkatti M. Role of CD44 in activation-induced cell death: CD44-deficient mice exhibit enhanced T cell response to conventional and superantigens. Int. Immunol. 2002;14:1015–1026. doi: 10.1093/intimm/dxf068. [DOI] [PubMed] [Google Scholar]
- McLellan AD, Starling GC, Hart DN. Isolation of human blood dendritic cells by discontinuous Nycodenz gradient centrifugation. J. Immunol. Meth. 1995;184:81–89. doi: 10.1016/0022-1759(95)00077-n. [DOI] [PubMed] [Google Scholar]
- Mielgo A, van Driel M, Bloem A, Landmann L, Gunthert U. A novel antiapoptotic mechanism based on interference of Fas signaling by CD44 variant isoforms. Cell Death Differ. 2006;13:465–477. doi: 10.1038/sj.cdd.4401763. [DOI] [PubMed] [Google Scholar]
- Mummert ME, Mummert D, Edelbaum D, Hui F, Matsue H, Takashima A. Synthesis and surface expression of hyaluronan by dendritic cells and its potential role in antigen presentation. J. Immunol. 2002;169:4322–4331. doi: 10.4049/jimmunol.169.8.4322. [DOI] [PubMed] [Google Scholar]
- Nakano K, Saito K, Mine S, Matsushita S, Tanaka Y. Engagement of CD44 up-regulates Fas Ligand expression on T cells leading to activation-induced cell death. Apoptosis. 2007;12:45–54. doi: 10.1007/s10495-006-0488-8. [DOI] [PubMed] [Google Scholar]
- Nandi A, Estess P, Siegelman M. Bimolecular complex between rolling and firm adhesion receptors required for cell arrest; CD44 association with VLA-4 in T cell extravasation. Immunity. 2004;20:455–465. doi: 10.1016/s1074-7613(04)00077-9. [DOI] [PubMed] [Google Scholar]
- Naor D, Nedvetzki S, Walmsley M, Yayon A, Turley EA, Golan I, Caspi D, Sebban LE, Zick Y, Garin T, et al. CD44 involvement in autoimmune inflammations: the lesson to be learned from CD44-targeting by antibody or from knockout mice. Ann N Y Acad Sci. 2007;1110:233–247. doi: 10.1196/annals.1423.025. [DOI] [PubMed] [Google Scholar]
- Ponta H, Sherman L, Herrlich P. CD44: From adhesion molecules to signaling regulators. Nat. Rev. Molec. Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- Pure E, Assoian RK. Rheostatic signaling by CD44 and hyaluronan. Cell Signal. 2009;21:651–655. doi: 10.1016/j.cellsig.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pure E, Cuff CA. A crucial role for CD44 in inflammation. Trends Molec. Med. 2001;7:213–221. doi: 10.1016/s1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- Rozsnyay Z. Signaling complex formation of CD44 with src-related kinases. Immunol Lett. 1999;68:101–108. doi: 10.1016/s0165-2478(99)00037-1. [DOI] [PubMed] [Google Scholar]
- Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T, Wakeman A, Shahinian A, Catzavelos C, Rak J, et al. CD44 regulates hematopoietic progenitor distribution, granuloma formation and tumorgenicity. Blood. 1997;90:2217. [PubMed] [Google Scholar]
- Thomas PG, Brown SA, Yue W, So J, Webby RJ, Doherty PC. An unexpected antibody response to an engineered influenza virus modifies CD8+ T cell responses. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2764–2769. doi: 10.1073/pnas.0511185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, Rabinovich GA. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat. Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- Varadhachary AS, Edidin M, Hanlon AM, Peter ME, Krammer PH, Salgame P. Phosphatidylinositol 3'-kinase blocks CD95 aggregation and caspase-8 cleavage at the death-inducing signaling complex by modulating lateral diffusion of CD95. J Immunol. 2001;166:6564–6569. doi: 10.4049/jimmunol.166.11.6564. [DOI] [PubMed] [Google Scholar]
- Varadhachary AS, Perdow SN, Hu C, Ramanarayanan M, Salgame P. Differential ability of T cell subsets to undergo activation-induced cell death. Proc Natl Acad Sci U S A. 1997;94:5778–5783. doi: 10.1073/pnas.94.11.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadhachary AS, Peter ME, Perdow SN, Krammer PH, Salgame P. Selective up-regulation of phosphatidylinositol 3'-kinase activity in Th2 cells inhibits caspase-8 cleavage at the death-inducing complex: a mechanism for Th2 resistance from Fas-mediated apoptosis. J Immunol. 1999;163:4772–4779. [PubMed] [Google Scholar]
- Wittig BM, Johansson B, Zoller M, Schwarzler C, Gunthert U. Abrogation of experimental colitis correlates with increased apoptosis in mice deficient for CD44 variant exon 7 (CD44v7) J. Exp. Med. 2000;178:497–507. doi: 10.1084/jem.191.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Brunner T, Carter L, Dutton RW, Rogers P, Bradley LM, Sato T, Reed J, Green D, Swain SL. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2 effectors, undergo rapid Fas/FasL-mediated apoptosis. J. Exp. Med. 1997;185:1837–1849. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Katoh S, He Q, Oritani K, Miyake K, Lesley J, Hyman R, Hamik A, Parkhouse RM, Farr AG, et al. Monoclonal antibodies to CD44 and their influence on hyaluronan recognition. J. Cell Biol. 1995;130:485–495. doi: 10.1083/jcb.130.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.