Abstract
Older adults sometimes show a “positivity effect” in memory, remembering proportionally more positive information than young adults. Using a modified Memory Characteristics, the present study examined whether emotional valence impacts the phenomenological qualities associated with young and older adults’ memories. Aging did not impact the effect of valence on the qualities of high-arousal memories. However, aging sometimes impacted subjective memory for detail of low-arousal memories: In Experiment 2, older adults reported remembering more thoughts, feelings, and temporal order details about positive low-arousal stimuli, while young adults’ ratings for those dimensions were higher for negative low-arousal stimuli. These findings suggest that valence most readily affects the qualities of young and older adults’ emotional memories when those memories are low in arousal.
Many studies have examined whether emotion influences the likelihood that young and older adults remember an item (reviewed by Kensinger, 2008a; Mather, 2006). Much of this literature suggests that older adults show a “positivity effect” in their memories, remembering a larger quantity of positive events than negative ones; by contrast, young adults often show a bias to remember negative information (Mather & Carstensen, 2005). Though these studies have focused on the number of events that young and older adults remember, fewer have compared the phenomenological details that the two age groups remember about emotional events (see D’Argembeau, Comblain & Van Der Linden, 2003; Destun & Kuiper, 1999; and Schaefer & Philippot, 2005 for the studies that have examined this issue in autobiographical memories). But of course, daily experiences contain multiple details or components, any of which can be remembered. For example, if you were fired from your job, there could be many elements of your memory for that experience: you could remember what you had just done prior to entering your boss’ office, you could remember visual details such as the color of your boss’ tie, you could recall the nauseous feeling in your stomach when your boss told you the news, or you might remember your thoughts about your family and your now dismal financial situation.
Understanding how emotion influences the phenomenological qualities of young and older adults’ memories is a particularly important issue, because the hallmark of an emotional memory may be the subjective richness with which it is remembered (Neisser & Harsch, 1992; Rubin & Kozin, 1984; Schmolck, Buffalo, & Squire, 2000). It is not always the case that someone will be more likely to remember an emotional item than a nonemotional item (Sharot, Delgado, & Phelps, 2004; Leiphart, Rosenfeld, & Gabrieli, 1993), and it is not always true that a person will remember an emotional experience accurately (Neisser & Harsch, 1992; Southwick, Morgan III, Nicolaou & Charney, 1997; Talarico & Rubin, 2003). However, it is generally true that when someone remembers an emotional item, he or she will remember it confidently and with subjectively rich detail (Kensinger & Corkin, 2003; Ochsner, 2000; Sharot, et al., 2004; Dougal & Rotello, 2007).
There are at least two factors which are important when examining the effects of emotion on the phenomenological characteristics of memories. First, it is important to distinguish among different phenomenological characteristics. For example, a distinction can be drawn between internal details – such as remembering your thoughts or feelings – and external details – such as remembering the context of an event or its temporal unfolding. In general, emotion may have more of an influence on memory for internal details than for contextual ones (e.g., Schaefer & Philippot, 2005), perhaps because of individuals’ reliance on schema-based reconstruction of emotional memories (e.g., Leventhal & Scherer, 1987) or because of emotion’s tendency to narrow attention onto the most arousing elements of an experience (e.g., Reisberg & Hertel, 2004), which may often be our internal reactions. Second, it is important to distinguish the valence of the remembered item. Valence may impact the phenomenological characteristics of memories, although it is unclear in which direction valence would exert its effect. Some research has suggested that young adults may remember negative information more vividly, confidently, and with more visual detail (Dewhurst & Parry, 2000; Doerksen & Shimamura, 2001; Kensinger & Schacter, 2006; Kensinger, Garoff-Eaton, & Schacter, 2007; Ochsner, 2000) than positive information, perhaps because negative emotion tends to evoke analytical and detail-oriented modes of processes more readily than positive emotion (reviewed by Anderson, 2005; Gasper, 2004; Gasper & Clore, 2002; Reisberg & Hertel, 2004). However, there are some studies (most often those examining retrieval of autobiographical memories) that have come to the opposite conclusion, suggesting that positive emotion yields phenomenologically richer memories than negative emotion (e.g., D’Argembeau et al., 2003; Destun & Kuiper, 1999; Petrican, Moscovitch, & Schimmack, 2008; Schaefer & Phillipot, 2005). A recent study suggested that part of the reason for the contrary findings may have to do with the self-relevance of the material; individuals may be more likely to remember positive experiences with rich detail when those experiences are closely tied to their self concept, but they may be more likely to remember negative experiences with rich detail when those experiences are less personally relevant (D’Argembeau & van der Linden, 2008).
Though consensus has not been reached about which types of subjective details are most readily enhanced by emotion, or about the effects of valence on memory’s phenomenology (see Kensinger, in press; Larson & Steuer, in press; Mather, in press; Hamann, in press for recent discussion of these issues), the literature emphasizes the need to distinguish different types of phenomenological characteristics and to consider emotional valence when examining the nature of emotional memory. Consideration of these factors may be particularly important when trying to understand how aging affects emotional memory. In particular, age differences may be more pronounced when examining emotion’s effects on memory for internal details than when examining emotion’s effects on memory for external ones, because older adults tend to focus more on their internal state, and on how information makes them feel, than on objective event details (reviewed by Kensinger, 2008b). For example, older adults tend to remember how information made them feel better than they remember the information’s content (e.g., Hashtroudi, Johnson, & Chrosniak, 1990). When asked to justify their confidence in a memory, they often do so based upon their memory for emotional reactions (e.g., Comblain et al., 2004), and they do better at remembering affective qualities of information (e.g., whether a name is associated with a “good” or a “bad” person) compared to nonaffective qualities (e.g., whether a name was read by a male or a female voice; Rahhal et al., 2002; May, Rahhal, Berry, & Leighton, 2005). More generally, it has been revealed that with aging, emotion-related goals take on additional importance (Gross et al., 1997; Lawton, Kleban, Rajagopal, & Dean, 1992), and older adults devote more resources toward interpreting and regulating their emotional reactions than do younger adults (reviewed by Carstensen, 1992; Mather, 2006). It would make sense that older adults’ increased focus on affective meaning could yield larger age differences in the phenomenological details tied to internal experience (e.g., thoughts and feelings; and see Comblain, D’Argembeau, & Van der Linden, 2005 for evidence that older adults may be more likely to reappraise emotional memories than young adults) rather than in those details tied to external event context (e.g., sensory detail).
The magnitude of the age differences may also depend upon the valence of the memory. As noted earlier, there have been a number of reports of a “positivity effect” in older adults’ memories, with older adults retaining proportionally more positive information and younger adults retaining proportionally more negative information (Mather & Carstensen, 2005). The “positivity effect” does not always occur (see Murphy & Isaacowitz, 2008), but there has been enough replication of the effect in the literature to suggest that young and older adults process positive and negative information in different ways from one another. In particular, it has been proposed that older adults are motivated to differentially sustain their attention on positive information (Isaacowitz, Wadlinger, Goren, & Wilson, 2006a; 2006b), to process the positive information in a self-relevant fashion (Kensinger & Leclerc, in press), and to regulate their affective processes so as to maximize their positive affect (Mather & Knight, 2005). If true, then it would make sense that the qualities associated with positive and negative memories could also differ across the adult lifespan, and the valence-based differences might be particularly pronounced for those details tied to internal experience.
Though the discussion thus far has emphasized the importance of considering the type of phenomenological detail and the valence of information, another dimension that must be considered is the arousal level of the emotional memory. Arousal clearly contributes to enhancements in emotional memory for many different types of information (Cahill, Prins, Weber, McGaugh, 1994; Cahill & McGaugh, 1990, 1995), and there is evidence that the memory enhancements that occur for arousing information operate via distinct mechanisms (e.g., amygdala modulation of hippocampal activity) from the memory enhancements that occur for low-arousal information (discussed by Cahill et al., 1996; Kensinger, 2004; Talmi & Goshen-Gottstein, 2006). The vast majority of studies examining the effects of valence on memory have included only one arousal level (but see Bradley, Greenwald, Petry, & Lang, 1992 for studies varying arousal as well as valence), and to our knowledge no study has examined whether the effects of valence on the phenomenological qualities of memories differ depending upon the arousal level of the memories. Yet there is reason to believe that arousal may critically impact the magnitude of age-related differences in memory for positive and negative information. In particular, a recent study (Kensinger, 2008b) suggested that the positivity effect may occur more readily for low-arousal stimuli, whereas young and older adults may remember high-arousal stimuli equally well regardless of valence (see also Tomaszczyk, Fernandes, & MacLeod, 2008). Though the reason why the positivity effect might be more pronounced for low arousal stimuli has not yet been determined, one possibility is that low arousal stimuli receive their mnemonic benefit from engagement of elaborative, controlled processes (see Kensinger, 2004; Koenig & Mecklinger, 2008; LaBar, 2007). These may be the same types of processes that support older adults’ positivity effect (see Kensinger & Leclerc, in press; Mather, 2006). By contrast, as noted above, arousing stimuli (regardless of valence) tend to receive mnemonic benefits via distinct mechanisms (amygdala-hippocampal interactions), and these automatic processes may be more stable across the lifespan (see Kensinger & Leclerc, in press for further discussion).
Interestingly, the fact that valence-based differences may be greater for low arousal stimuli than for high arousal stimuli aligns well with a theory dating back to Matlin & Stang (1978), stating that positive and negative stimuli are processed equally well when they are above a certain level of intensity. However, valence-based differences in processing might be more pronounced below that intensity threshold.
The primary goal of the present study, therefore, was to examine how the emotional valence (whether positive or negative) of an emotional item influenced the phenomenological qualities of young and older adults’ memories for that item. Based on the literature outlined above, we hypothesized that valence-based age differences would be present, but that they would be more pronounced for qualities tied to internal sensations (i.e., thoughts and feelings) and that they would be more pronounced when stimuli were low-arousal rather than high-arousal. To address the validity of these hypotheses, we used a modified version of the Memory Characteristics Questionnaire (MCQ; Johnson, Foley, Suengas, & Raye, 1988), to allow participants to rate their memory for a variety of phenomenological qualities: confidence, visual details, thoughts, feelings, temporal order and other associations (see Appendix A).
Experiment 1
Method
Participants
Participants were 26 younger and 26 older adults (See Table 1 for demographic information). Young adults were Boston College students. Older adults were recruited from the Harvard Cooperative Program on Aging, from advertisements placed in newsletters, or from fliers posted in public locations. All participants had normal or corrected-to-normal vision, were native English speakers, and had no history of a neuropsychological or psychiatric disorder. No participant listed taking medications that affect the central nervous system.
Table 1.
Demographic information and test scores for the young and older adults, Mean (Standard Error).
| Age (yrs) | Education (yrs) | Backwards Digit Score | WAIS-III Digit Symbol | FAS Score | Shipley Vocab. | Depression Inventory | Beck Anxiety | Gender | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment 1 | Younger Adults | 19.16 (0.34)* | 13.94 (0.36)* | 8.80 (0.32) | 50.48 (1.59)* | 45.40 (1.54) | 31.92 (0.61)* | 3.68 (0.58)*+ | 7.55 (1.22) | 20 Female, 6 Male |
| Older Adults | 78.20 (0.82)* | 16.07 (0.47)* | 7.46 (0.76) | 29.36 (1.56)* | 51.6 (3.3) | 35.24 (0.60)* | 1.46 (0.40)* | 5.09 (1.17) | 17 Female, 9 Male | |
| Experiment 2 | Younger Adults | 19.64 (0.24)* | 13.79 (0.23)* | 8.79 (0.45)* | 47.38 (1.00)* | 46.92 (2.05) | 31.42 (1.04)* | 3.44 (0.73)* | 6.92 (1.20) | 10 Male, 15 Female |
| Older Adults | 75.6 (1.24)* | 16.35 (0.56)* | 7.54 (0.52)* | 32.04 (1.47)* | 43.0 (3.41) | 35.65 (0.60)* | 0.58 (0.26)* | 3.42 (0.75) | 17 Female, 7 Male |
Note. WAIS-III = Wechsler Adult Intelligence Scale, 3rd Edition (Wechsler, 1997). FAS score refers to the total number of words beginning with the letter “F,” “A,” and “S” produced in 60 seconds.
Indicates a significant difference between older and younger adults, p < .05
Although there was a difference in depression scores between older and younger adults, with older adults receiving lower scores than young adults, no participant in either age group received a high enough score to be considered depressed.
Materials and Procedure
Stimuli included 200 pictures taken from the International Affective Picture Series (Lang, Bradley, & Cuthbert, 1999). Pictures were selected to represent three valence categories based on the IAPS standard normative scores for valence (1, negative; 9, positive): negative (mean valence = 2.93), positive (mean valence = 7.08), and neutral (mean valence = 5.15). Negative and positive items were further subdivided into those that were high-arousal and low-arousal based on the IAPS standard normative scores for valence arousal (1, calming or subduing; 9, exciting or agitating): high-arousal (mean arousal = 5.89) and low-arousal (mean arousal = 3.89). All neutral pictures were low-arousal. So for example, a negative high-arousal picture could be of a snake attacking, a negative low-arousal picture could be of a sad child, a positive high-arousal picture could be of money, a positive low-arousal picture could be of a sunset, and a neutral picture could be of an office. Of the 200 pictures, 40 were positive high-arousal, 40 were positive low-arousal, 40 were negative high-arousal, 40 were negative low-arousal and 40 were neutral. Positive, negative and neutral pictures were matched on visual complexity (normative data taken from a prior investigation; Kensinger & Schacter, 2006) and brightness [as indicated in Adobe Photoshop (Adobe Systems, San Jose, CA; data from Kensinger & Schacter, 2006)], and pictures were specifically selected so that valence and arousal ratings did not differ between young and older adults (as determined via ratings by a separate group of 50 young and 50 older adults gathered for a prior experiment; Leclerc & Kensinger, submitted). The pictures that were included as items on the study list or as unstudied foils on the recognition memory test were counterbalanced across participants.
Cognitive Tests
Before completing the memory test, participants were given a number of paper and pencil cognitive tests (see Table 1). The tests included: The Shipley Vocabulary Test (Shipley, 1986), Wechsler Backwards Digit Span Test (Wechsler, 1997), The Wechsler Adult Intelligence Scale Digit Symbol Test (Wechsler, 1997), The FAS Test of Phonemic fluency (Spreen & Benton, 1977), The Shipley Vocabulary Test (Shipley, 1986), the Depression Inventory (Sheikh & Yesavage, 1986), and the Beck Anxiety Scale (Beck, Epstein, Brown, & Steer, 1988).
Study Procedure
Participants viewed 100 pictures (20 of each emotion type). Each picture was presented for 1.5 seconds and pictures of each emotion type were randomly intermixed with the constraint that no more than 5 pictures from a particular emotion category could be shown in succession. In order to ensure encoding of the pictures, participants were asked to view the picture for the duration of the time that it was on the screen (1.5 seconds). The picture then was removed from the screen and participants indicated with a button press if they would like to “approach” or “back away” from each picture. Once the button press was made, the next picture immediately appeared. No further information was given to the participants on the nature of the task.
Test Procedure
After an approximately 45-min delay, participants were given a surprise recognition memory test. None of the participants indicated on the debriefing forms that they tried to remember the pictures presented at encoding. The recognition memory test included studied pictures intermixed with nonstudied foils. Participants were asked to indicate whether each item was “old” (studied) or “new” (nonstudied). If participants indicated that an item was “old,” they were then asked to complete a series of questions (a modified version of the Memory Characteristics Questionnaire; Johnson et al., 1988), asking them to rate their confidence in their memory, and the amount of thoughts, feelings, other associations1, visual details, and temporal information that they remembered about each item’s presentation (see Appendix). Each characteristic was rated on a 5 point Likert scale. The recognition test was self-paced, such that the next picture (or the next rating scale) occurred immediately following the participant’s button press.
Data Analysis
For the memory recognition data, we first calculated corrected recognition scores by subtracting the false alarm rate from the hit rate; this corrected recognition score was computed separately for each emotional item type (e.g. “old” responses to new negative high-arousal items were subtracted from “old” responses to old negative high-arousal items). We also computed d’ scores (Peterson, Birdsall & Fox, 1954; Swets, 1996), but because these scores yielded the same findings as the corrected recognition scores, we only report the corrected recognition results here.
For the analyses of the memory characteristic scores, we were concerned that older adults might anchor or interpret increases on the rating scales in a different way from young adults, such that their ratings would be scaled in a different way from younger adults. For example, pilot testing revealed that older adults were less likely than young adults to use the extremes of the scale, despite instructions to do so. To circumvent this possible confound, we computed difference scores for each rating in an attempt to standardize young and older adults’ ratings for the emotional items2. These difference scores were created by subtracting a person’s average rating for a neutral item from his or her average rating for each of the four types of emotional items (positive high-arousal, positive low-arousal, negative high-arousal, negative low-arousal). The difference scores were computed separately for each of the memory characteristics. See Table 3 for the ratings data that were used to compute these difference scores. Please note that because these subtractions were done on the participant level, the mean subtraction score may not equal the subtraction of the means from the emotional and neutral categories.
Table 3.
Experiment 1. Memory Characteristics Questionnaire Ratings (from five point scale, Mean (Standard Error)).
| High Arousal | Low Arousal | Neutral | ||||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||
| Younger Adults | Confidence | 4.77 (0.05) | 4.71 (0.05) | 4.62 (0.07) | 4.61 (0.07) | 4.69 (0.05) |
| Visual Details | 4.14 (0.11) | 4.26 (0.12) | 4.09 (0.14) | 4.07 (0.13) | 4.03 (0.15) | |
| Feelings | 3.40 (0.12) | 3.95 (0.10) | 3.50 (0.13) | 3.59 (0.12) | 3.13 (0.16) | |
| Thoughts | 3.22 (0.14) | 3.48 (0.13) | 3.17 (0.15) | 3.15 (0.14) | 2.93 (0.18) | |
| Other Associations | 2.16 (0.19) | 2.22 (0.21) | 2.12 (0.20) | 2.06 (0.19) | 1.96 (0.19) | |
| Order | 2.26 (0.19) | 2.27 (0.21) | 2.25 (0.20) | 2.25 (0.20) | 2.22 (0.21) | |
| Older Adults | Confidence | 4.22 (0.09) | 4.24 (0.29) | 4.09 (0.10) | 4.13 (0.09) | 4.24 (0.08) |
| Visual Details | 3.95 (0.12) | 3.96 (0.09) | 3.85 (0.10) | 3.82 (0.09) | 3.84 (0.12) | |
| Feelings | 2.87 (0.18) | 3.26 (0.16) | 3.01 (0.16) | 3.17 (0.17) | 2.56 (0.16) | |
| Thoughts | 2.47 (0.18) | 2.62 (0.18) | 2.49 (0.18) | 2.59 (0.19) | 2.30 (0.16) | |
| Other Associations | 1.96 (0.19) | 1.98 (0.19) | 1.93 (0.20) | 2.07 (0.21) | 1.78 (0.16) | |
| Order | 2.16 (0.17) | 2.09 (0.16) | 2.05 (0.18) | 2.06 (0.19) | 2.00 (0.17) | |
Ratings were only given when participants indicated that a picture was “old,” because it would not make sense to ask participants to rate their memory for items they claimed they had never seen. Most of our analyses focus on ratings to “hits” (i.e., correct item endorsements); however, “false alarms” (items incorrectly cited as “old”) are also analyzed in the last section of the results.
For both the corrected recognition scores and the phenomenological ratings, the difference scores for high-arousal and low-arousal items were submitted to separate ANOVAs with valence (positive, negative) as within-subject factor and age (young, old) as a between-subject factor.
Results
In the sections below, we first discuss the effects of emotion on recognition memory performance and then describe the effects of emotion on each of the memory characteristics.
Overall Recognition Memory Performance
A valence × age ANOVA revealed no main effects or interactions for the high-arousal items (all p>.25), but revealed an interaction between age and valence for the low-arousal items (F(1,50) = 5.04, p<.05, hp2 = .10), with young adults remembering more positive low-arousal items (t(25) = 2.34, p < .05). and older adults remembering numerically more negative low-arousal items (t < 1.23, p > 0.10).
Because corrected recognition scores subtract the false alarm rate from the hit rate, the valence × age interaction for the low-arousal items could have been due to different effects of valence on young and older adults’ hit rates, false alarm rates, or both rates. To distinguish among these alternatives, we ran separate ANOVAs for the hit rates and for the false alarm rates. An age × valence ANOVA revealed an interaction for the hit rates (F(1,50) = 9.01, p<.01, hp2 = .15), with young adults giving numerically more correct endorsements for positive low-arousal than for negative low-arousal items (t < 1.50, p > 0.10), and with older adults showing the opposite pattern (t(25) = 2.64, p < 0.05). There was no interaction between age and valence for the false alarm rates (p>.25); thus, age affected only the distribution of correct endorsements and did not influence the distribution of incorrect endorsements.
Memory Characteristic Ratings
For “confidence,” a valence × age ANOVA revealed no effects or interactions for either the high-arousal items or for the low-arousal items (all p>.20). Similarly, for “visual details” and for “temporal order,” the ANOVAs revealed no effects or interactions for the high-arousal or the low-arousal items (all p>.2).
For “feelings,” the ANOVA revealed a main effect of valence for the high-arousal items (F(1,50) = 22.90, p<.001, hp2 = 0.31) and for the low-arousal items (F(1,50) = 6.18, p<.05, hp2 = 0.11), with higher ratings given to negative items than to positive items (high arousal: t(51) = 4.82, p < .001; low arousal: t(51) = 3.72, p < .001). For “thoughts,” ANOVA revealed a main effect of valence for the high-arousal items (F(1,50) = 13.70, p<.001, hp2 = 0.22), with negative high-arousal items given higher ratings than positive high-arousal items (t(51) = 3.72, p < .001). There were no significant effects of age or valence for the “thoughts” remembered for low-arousal items (p> 0.15). Critically, neither of these memory characteristics showed an interaction between age and valence; thus, the effects of valence on “thoughts” and “feelings” were comparable in the two age groups.
The only characteristic to show a marginal interaction between age and valence was “other associations.” Though ratings for high-arousal items were unaffected by valence or age (all p> 0.20), ratings for low-arousal items revealed a marginal interaction between age and valence (F(1,50) = 3.78, p< 0.10, hp2 = 0.07): Young adults gave numerically higher ratings for positive low-arousal items (t < 4.00, p > 0.10), while older adults gave numerically higher ratings for negative low-arousal items(t < 0.50, p > 0.10; see Table 3).
Analysis of Hits and False Alarms for Ratings
Because the present study relied on participants’ subjective reports of the qualities of their memory, a potential concern was that participants might not be rating the qualitative nature of their memories but rather the qualitative nature of their current experience (e.g., how many thoughts they had about the stimulus when they saw it presented at retrieval). Although debriefing suggested that participants were complying with the task instructions and rating their actual memory for the various characteristics as opposed to their current reaction, we reasoned that if participants’ ratings reflected their current reactions, then ratings should be fairly similar for false alarms as compared to hits. By contrast, if participants were reporting the qualitative nature of their memories, then their ratings should be higher for hits than for false alarms. An ANOVA was conducted with item type (hit, false alarm), and detail type (6 levels) as within-subject factors and with age (young, older) as a between-subject factor. This analysis revealed a significant effect of item type (F (1,43) = 58.40, p<.001, Hp2 = 0.58) and of detail (F (5,34) = 69.05, p<.001, Hp2 = 0.62). These main effects were qualified by an interaction between item type and age (F(5,43) = 10.52, p<.01, Hp2 = .32). This interaction was due to the fact that though younger and older adults were similar on ratings of false alarms (p > .05), younger adults had significantly higher ratings for hits than older adult hits ratings (t(49) = 2.81, p < .01). The main effects also were qualified by an interaction between item type and detail type (F(5,39) = 19.89, p<.001, Hp2 = .32); post hoc tests revealed that this interaction occurred because although there was a clear difference in ratings between hits and false alarms (with ratings being higher for hits than for false alarms) for most details, this difference was not as great for order and other associations (t(44) < 4) as it was for the other types of details (t(44) > 4.7). The fact that participants’ ratings were systematically higher for hits than for false alarms provides evidence that they were rating the qualities of their memories, rather than the qualities of their current experience, although the fact that this difference was less pronounced for order and other associations may suggest that participants had a harder time making that distinction for those rating scales.
Discussion
As outlined in the introduction, we had hypothesized that age differences in the phenomenological qualities of positive and negative memories would be greater for internal details than for external details and would also be greater when stimuli were low-arousal. These hypotheses were not supported by Experiment 1. Instead, the results revealed little evidence of valence-based age differences in the phenomenological qualities of memories, and in the instances where interactions between age and valence were identified (for corrected recognition of low-arousal items and for the “other associations” remembered about low-arousal items), the interaction revealed better memory or higher ratings for negative low-arousal items than for positive low-arousal items in the older adults. In fact, the results of Experiment 1 revealed striking similarity in the effects of valence on the phenomenological qualities associated with young and older adults’ memories. In both age groups, valence effects were present for ratings of internal details (i.e., thoughts, feelings, and other associations) but not for more contextual dimensions (i.e., visual details, temporal order), and with the exception of other associations (as noted above) negative items were rated as being remembered with more internal details than positive items in both ages. The fact that valence effects were only present for phenomenological memories of internal details would be consistent with the evidence outlined in the introduction to suggest that emotion’s effects may be most pronounced for information tied to the internal processing of an emotional experience (Leventhal & Scherer, 1987; Shaefer & Philippot, 2005). In addition, the fact that negative information is reported as remembered with more of these internal details than positive information is consistent with evidence that negative emotion tends to narrow attention onto the arousing properties of an experience more readily than positive emotion (see Fredrickson, 2004; Gasper & Clore, 2002; Reisberg & Heuer, 2004). The finding that this effect of negative valence was consistent across the two age groups is consistent with a few studies suggesting that negative valence focuses attention similarly in young and older adults (e.g., Mather & Knight, 2005) and can lead to a narrowing of young and older adults’ memories onto those details most directly tied to a person’s arousal response during an experience (e.g., Denburg, Buchanan, Tranel & Adolphs, 2003; Kensinger et al., 2007).
While it would be intriguing if it were true that age had little influence on the effect of valence on the phenomenological qualities of a memory, before settling on that conclusion, we sought to improve upon two aspects of the experimental design. First, there was relatively little variability in ratings across the emotion categories in Experiment 1, which may have made it harder to detect age differences. Second, the retrieval cue (the emotional picture) was the same as the encoded item, which may have made it difficult for participants to tease apart their experience at retrieval from their memory of the item’s presentation during study. In an attempt to create more variability in participant responses, and to distinguish the retrieval cue from the encoded item, Experiment 2 asked participants to view pictures, each preceded by a sentence describing the scene. The sentence was used as the retrieval cue, and participants were asked to rate their memory for the associated picture. We reasoned that this design would add variability by providing instances where participants might remember the sentence but have little memory for the associated picture. The design also would eliminate the overlap between the retrieval cue and the studied picture. Experiment 2 allowed us to examine whether we would replicate the findings of Experiment 1– finding few age differences in the effects of valence on phenomenological ratings – or whether this revised design would allow us to detect age differences that were not manifest in Experiment 1.
Experiment 2
Method
Participants
Participants were 25 younger and 24 older adults (See Table 1 for demographic information). The participants met the same criteria as outlined for Experiment 1, and no participant enrolled in Experiment 2 had participated in Experiment 1.
Design and Materials
The photographs were the same as those in Experiment 1 and the participants completed the same cognitive tasks (See Table 1). Sentences describing each picture also were used (e.g., “This is an angry attack dog trained to bite strangers”). Sentences were taken from those used in Foti & Hajack (2008) and were supplemented with additional sentences created by the authors.
The study design was similar to Experiment 1 except that participants were first presented with a sentence which they were asked to covertly read. Once they had read the sentence, they pressed a button and were shown the corresponding picture for 1.5 seconds. As in Experiment 1, when the picture went away, they indicated if they would like to “approach” or “back away” from the picture. The next picture was shown immediately after participants made their button press. At test, the participants were shown the sentences, and they were asked to rate their memory for the picture presented during study along the same scales as provided in Experiment 1. The recognition test was self-paced, so that the next picture or rating scale appeared as soon as participants had made their button response to the prior item.
Data Analysis
Data were analyzed in the identical fashion to Experiment 1.
Results
In the sections below, we first discuss the effects of emotion on recognition memory performance and then describe the effects of emotion on each of the memory characteristics.
Overall Memory Performance
The valence × age ANOVA conducted on the corrected memory scores (hits-FA) revealed an interaction between age and valence for both the high-arousal items (F(1,44) = 7.73, p< 0.01, hp2 = 0.15), and for the low-arousal items (F(1,44) = 6.75, p< 0.05, hp2 = 0.13). This interaction reflected the fact that older adults showed a greater propensity to remember negative compared to positive items (t(22) = 3.76, p < .001) than did young adults for both high arousal and low arousal items (see Table 2). Subsequent ANOVAs conducted on the hit rates and false alarm rates separately revealed that this interaction resulted from age differences in the hit rates (interaction between age and valence: [F(1,44) = 6.09, p < 0.05, hp2 = 0.12 for the high-arousal items; F(1,44) = 12.24, p < 0.001, hp2 = 0.22 for the low-arousal items]) rather than from age differences in the false alarm rate (interaction: p> 0.20).
Table 2.
Hit rate, false alarm rate and corrected recognition (hit – false alarm) rate as a function of emotion type and age group, mean (standard error).
| High Arousal | Low Arousal | Neutral | |||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||||
| Experiment 1 | Younger Adults | Hit | 0.88 (0.02) | 0.89 (0.02) | 0.88 (0.02) | 0.86 (0.02) | 0.89 (0.02) |
| False Alarms | 0.02 (0.0) | 0.03 (0.01) | 0.02 (0.00) | 0.04 (0.02) | 0.01 (0.01) | ||
| Corrected Recognition | 0.86 (0.02) | 0.86 (0.02) | 0.86 (0.02) | 0.82 (0.02) | 0.88 (0.33) | ||
| Older Adults | Hit | 0.54 (0.04) | 0.63 (0.03) | 0.56 (0.04) | 0.64 (0.03) | 0.59 (0.04) | |
| False Alarm | 0.06 (0.01) | 0.11 (0.02) | 0.07 (0.01) | 0.11 (0.02) | 0.05 (0.02) | ||
| Corrected Recognition | 0.48 (0.04) | 0.53 (0.03) | 0.49 (0.03) | 0.53 (0.03) | 0.54 (0.04) | ||
| High Arousal | Low Arousal | Neutral | |||||
| Positive | Negative | Positive | Negative | ||||
| Experiment 2 | Younger Adults | Hit | 0.83 (0.03) | 0.84 (0.03) | 0.83 (0.03) | 0.80 (0.03) | 0.79 (0.03) |
| False Alarm | 0.03 (0.01) | 0.03 (0.01) | 0.03 (0.01) | 0.03 (0.01) | 0.02 (0.03) | ||
| Corrected Recognition | 0.80 (0.03) | 0.81 (0.03) | 0.79 (0.03) | 0.77 (0.03) | 0.78 (0.03) | ||
| Older Adults | Hit | 0.52 (0.04) | 0.63 (0.04) | 0.59 (0.04) | 0.67 (0.04) | 0.56 (0.04) | |
| False Alarm | 0.05 (0.01) | 0.04 (0.01) | 0.05 (0.01) | 0.05 (0.01) | 0.02 (0.01) | ||
| Corrected Recognition | 0.47 (0.03) | 0.59 (0.04) | 0.54 (0.04) | 0.62 (0.04) | 0.55 (0.04) | ||
Memory Characteristic Ratings
As in Experiment 1, difference scores were computed for each memory characteristic (see Table 4 for the ratings data that were used to compute these difference scores).
Table 4.
Experiment 2. Memory Characteristics Questionnaire Ratings (from five point scale, Mean (Standard Error)).
| High Arousal | Low Arousal | Neutral | ||||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||
| Younger Adults | Confidence | 4.56 (0.07) | 4.60 (0.06) | 4.55 (0.06) | 4.51 (0.08) | 4.62 (0.06) |
| Visual Details | 4.27 (0.11) | 4.24 (0.12) | 4.20 (0.11) | 4.17 (0.10) | 4.23 (0.12) | |
| Feelings | 3.60 (0.14) | 3.96 (0.15) | 3.54 (0.16) | 3.70 (0.17) | 3.24 (0.17) | |
| Thoughts | 3.23 (0.13) | 3.47 (0.13) | 2.18 (0.16) | 2.50 (0.17) | 3.03 (0.16) | |
| Other Associations | 2.15 (0.18) | 2.36 (0.22) | 2.09 (0.19) | 2.16 (0.20) | 2.02 (0.16) | |
| Order | 2.43 (0.16) | 2.56 (0.17) | 2.45 (0.17) | 2.50 (0.17) | 2.42 (0.16) | |
| Older Adults | Confidence | 4.38 (0.10) | 4.45 (0.11) | 4.40 (0.23) | 4.24 (0.12) | 4.42 (0.10) |
| Visual Details | 3.89 (0.13) | 3.88 (0.14) | 3.95 (0.15) | 3.74 (0.14) | 3.66 (0.14) | |
| Feelings | 3.44 (0.16) | 3.52 (0.16) | 3.41 (0.18) | 3.29 (0.19) | 3.07 (0.18) | |
| Thoughts | 3.01 (0.17) | 3.15 (0.20) | 3.11 (0.21) | 2.99 (0.19) | 2.85 (0.18) | |
| Other Associations | 2.11 (0.20) | 2.32 (0.20) | 2.34 (0.24) | 2.29 (0.22) | 2.04 (0.22) | |
| Order | 2.57 (0.19) | 2.86 (0.18) | 2.91 (0.21) | 2.71 (0.19) | 2.63 (0.17) | |
For “confidence,” a valence × age ANOVA revealed no effects or interactions for either the high-arousal items or for the low-arousal items (all p> 0.20). Similarly, for “visual details,” no main effects or interactions were revealed (all p > 0.15).
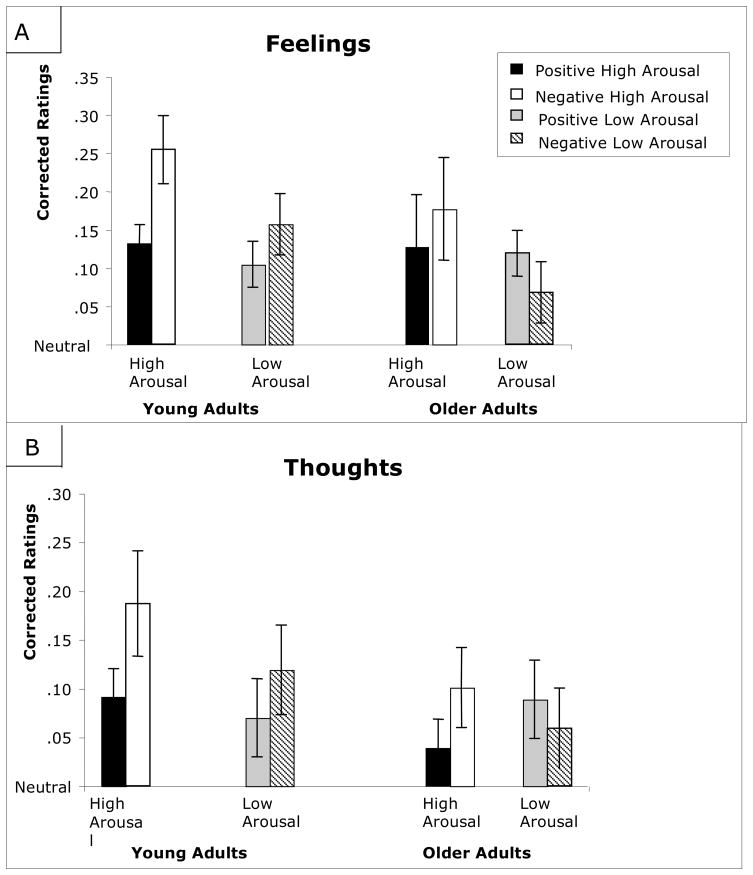
For “feelings,” the ANOVA revealed only a main effect of valence for the high-arousal items (F(1,44) = 14.67, p< 0.001, hp2 = 0.25), with both age groups remembering more feelings for negative high-arousal than for positive high-arousal items (t(51) = 3.76, p < .001). For the low-arousal items, the ANOVA revealed an interaction between age and valence (F(1,44) = 6.03, p< 0.05, hp2 = 0.12), with young adults providing higher ratings for negative low-arousal items (t(22) = 2.86, p < 0.01) and older adults providing higher ratings for positive low-arousal items (t < 1.40, p > 0.10), see Table 4).
For “thoughts,” the ANOVA for high-arousal items revealed only a main effect of valence (F(1,44)=9.44, p< 0.01, hp2 = 0.18), with both age groups giving higher ratings to negative high-arousal items than to positive high-arousal items (t(51) = 3.09, p < 0.01). By contrast, the ANOVA for low-arousal items revealed a marginal interaction between age and valence (F(1,44) = 3.18, p< 0.10, hp2 = 0.07), with young adults giving higher ratings for negative low-arousal items (t(22) = 1.84, p < 0.09), and older adults giving higher ratings for positive low-arousal items (t < 1.0, p > 0.10).
For “temporal order,” an ANOVA revealed only a main effect of valence for the high-arousal items (F(1,44) = 8.43, p< 0.01, hp2 = 0.16), with ratings higher for negative high-arousal than for positive high-arousal items in both age groups (t(51) = 2.90, p < 0.01). For the low-arousal items, there was an interaction between valence and age (F(1,44) = 4.84, p< 0.05, hp2 = 0.10), with young adults’ ratings higher for negative low-arousal items (t < 1.00, p > 0.10), and older adults’ ratings higher for positive low-arousal items (t(22) = 2.48, p < 0.05). Thus, “feelings,” “thoughts,” and “order” revealed similar patterns, with an age-related “positivity effect” occurring only for the low-arousal items.
For “other associations,” the ANOVA for high-arousal items revealed only a main effect of valence (F(1,44) = 8.05, p< 0.01, hp2 = 0.16), with ratings higher for negative high-arousal than for positive high-arousal items in both age groups (t(51) = 2.87, p < 0.01). The ANOVA conducted on the low-arousal items revealed no main effects or interactions (all p> 0.25)
Analysis of Hits and False Alarms for Ratings
Though the distinction between the retrieval cue and the target item in Experiment 2 reduced the concern that participants could not distinguish their retrieval experience from their memory of the encoding episode, we nevertheless conducted a item (hit, false alarm) × detail × age ANOVA to compare the ratings given to hits and false alarms for the emotional items in both age groups. This analysis revealed a significant effect of item type (F(1,38) = 30.39, p<.001, Hp2 = 0.44), reflecting the fact that ratings were higher for hits than for false alarms (t(39) = 5.35, p < .001). The ANOVA also revealed a main effect of detail (F(5,34) = 29.27, p<.001, Hp2 = 0.81), qualified by an interaction between detail type and age (F(5,34) = 3.19, p<.05, Hp2 = .32). This interaction reflected the fact that although young and older adults gave comparable ratings for most details, younger adults’ ratings for visual details were higher than were older adults’ ratings on that dimension (mean (SE) rating in younger adults = 3.92 (0.15) and in older adults = 3.51 (0.15)). As in Experiment 1, the fact that participants’ ratings were systematically higher for hits than for false alarms provides evidence that they were rating the qualities of their memories, rather than the qualities of their current experience.
Discussion
The results of Experiment 2 replicated those of Experiment 1 in a few important ways. First, once again there were no age differences in the valence-based effects on phenomenological memory for high-arousal items. Second, when there were effects of valence on phenomenological memory for high-arousal items, they were such that negative high-arousal items were given higher ratings than positive high-arousal items. Third, the effects of valence on high-arousal items existed for all of the internal details ratings (thoughts, feelings, other associations) but not for all contextual event details ratings. Thus, negative valence seems to enhance the phenomenological qualities of some, but not all, aspects of a phenomenological memory, and those aspects that it boosts most readily may be more tied to a person’s subjective experience of the event than to the event’s contextual details. These effects of negative valence on memory for high-arousal items seem to be preserved over the lifespan.
The results of Experiment 2 went beyond those of Experiment 1, however, in suggesting that age differences in the effects of valence on phenomenological memory may be greater when the information is not high in arousal. Across three dimensions (thoughts, feelings, order), young adults’ ratings were higher for negative low-arousal information whereas older adults’ ratings were higher for positive low-arousal information. Interestingly, for each category that displayed an interaction between age and valence, the interaction arose because of a “positivity effect” in older adults’ memories, with positive low-arousal events being associated with more phenomenological detail. However, these effects were evidenced even though there was no “positivity effect” in terms of the overall quantity of information remembered (e.g. no positivity effect for corrected recognition scores). In fact, the corrected recognition scores revealed that older adults showed better memory for negative low-arousal items than for positive low-arousal ones. These results emphasize that quantitative measures of emotional memory may miss important distinctions between young and older adults’ memories, a point which we expand upon in the general discussion.
General Discussion
These experiments revealed the importance of considering a stimulus’ arousal level as well as the phenomenological characteristics remembered about that stimulus when examining how aging influences valence-based effects on phenomenological memory. Age differences appear to occur only for a subset of phenomenological characteristics and only when stimuli are of low arousal.
Neither experiment revealed evidence of age differences in the effects of valence on the phenomenological qualities associated with high-arousal information: For both young and older adults, negative high-arousal stimuli were subjectively retained with richer internal details than were positive high-arousal stimuli. Although the null effect of age, like any null effect, must be interpreted with caution, the result fits well with prior research on arousal-based processing across the lifespan. Prior studies examining how age influences emotion’s ability to capture attention (e.g., Hahn, Carlson, Singer & Gronlund, 2006; Leclerc & Kensinger, 2008b; Mather & Knight, 2006), to yield quantitative benefits to memory (e.g., Kensinger, 2008; Tomaszczyk et al., 2008), or to elicit changes in the neural activity within regions of the affective processing network (Leclerc & Kensinger, submitted), also have failed to find differences when high-arousal stimuli have been used. Though future research will be required to elucidate the basis for the age-related similarities in memories for high-arousal items, a likely explanation is that high-arousal stimuli are processed in a fairly automatic fashion, benefiting from rapid alerting systems during encoding (e.g., Anderson, et al., 2003; Ohman & Mineka, 2001) and from prioritized consolidation in memory due to interactions between the amygdala and the hippocampus (e.g., Kensinger, 2004; McGaugh, 2004). Given the relative preservation of automatic processing with aging (Fleischman, et al., 2004; Jennings & Jacoby, 1993), and of arousal-related neural responses across the adult lifespan (Leclerc & Kensinger, 2008a), it makes sense that aging would have relatively little impact on the phenomenological quality of memories for high-arousal stimuli.
The fact that both age groups retained negative high-arousal information with greater subjective detail than positive high-arousal information is consistent with a range of evidence suggesting that automatic processes may be particularly likely to be engaged for threat-related stimuli (see LeDoux, 1996) and that arousal-related modulation of the amygdala may be stronger when negative stimuli are processed than when positive stimuli are processed (Anders, Eippert, Weiskopf, & Veit, 2008; Straube, Pohlack, Mentzel, & Miltner, 2008). These findings also are consistent with a number of studies that have assessed memory for stimuli presented within a laboratory setting: Many studies have revealed subjectively more vivid memories for negative information than for positive information (e.g., Ochsner, 2000; Dewhurst & Parry, 2000) as well as an enhanced ability to remember details intrinsically related to the negative stimuli (e.g., Reisberg & Heuer, 2004). Thus, the present results may suggest that these effects of negative high-arousal emotion on phenomenological memory remain relatively constant across the lifespan, boosting the vividness with which intrinsic, internal details about an experience are remembered. An important caveat to this conclusion, however, is that when autobiographical memories are assessed, there often is not the advantage for negative information; rather, positive autobiographical experiences often are reported as being more vivid, and as including more internal detail, than negative experiences (D’Argembeau et al., 2003; Destun & Kuiper, 1999; Schaefer & Phillipot, 2005). A recent study (D’Argembeau & Van der Linden, 2008) suggested that positive experiences may be retrieved more vividly than negative ones when they are highly self-relevant; by contrast, negative information may be retrieved more vividly when the information is less applicable to one’s sense of self. This conclusion would be consistent with the evidence that negative stimuli presented in a laboratory setting (and therefore probably less tied to one’s sense of self) are more likely to be vividly remembered, whereas positive autobiographical experiences are more likely to be subjectively rich. Nevertheless, future research will be needed to test the validity of D’Argembeau & van der Linden’s hypothesis and to examine whether the dissociation they describe is stable across the lifespan.
It is interesting to note that the effects of negative high-arousal emotion on ratings existed despite no effect of emotion on the overall recognition rates. The lack of a recognition memory enhancement is consistent with a number of studies: It often is the case that emotion does not enhance recognition memory performance, particularly when memory is assessed after fairly short delays (e.g., Dolan et al., 2000; Leiphart, Rosenfeld, & Gabrieli, 1993; Sharot et al., 2004). Moreover, there have been prior reports of qualitative effects of emotion (either on subjective vividness or on objective detail) even in the absence of quantitative effects on recognition memory (e.g., Kensinger, Garoff-Eaton, & Schacter, 2006; Somerville, Wig, Whalen, & Kelley, 2006). One message to take away from this literature is that many of emotion’s effects on memory may be most notable when memory characteristics, rather than simply memory discrimination, are considered. The present results support that sentiment.
In contrast to the age similarities in memory for high-arousal stimuli, significant age differences were noted in terms of the qualitative characteristics of memory for low-arousal stimuli. In Experiment 2, young adults often reported remembering more internal details about negative low-arousal stimuli, whereas older adults’ ratings were higher for positive low-arousal stimuli. These age differences were apparent specifically in Experiment 2. Though it is not clear why the valence-based age differences were only revealed in Experiment 2 and not in Experiment 1, we suspect that the methodological improvements to Experiment 2 allowed us to detect these differences. In particular, the design of Experiment 2 led to enhanced variability in participant responses, and it also eliminated the overlap between the retrieval cue (the sentence description) and the studied picture. These improvements may have allowed us to detect age differences that could not be revealed using the design of Experiment 1. Another potentially important difference is that the encoding task used in Experiment 2 required participants to make a decision that was incidental to the emotionality of the stimulus (participants were asked to judge the photographic quality of the images); age-related changes in emotional memory may be most apparent when task instructions do not in any way focus participants on the emotionality of a scene (and see Mather, 2006 for further discussion). Although the “approach or move away” decision used in Experiment 1 did not require participants to explicitly judge the emotionality of the scenes, it did likely focus their attention on emotional elements of the scene and may therefore have reduced age differences in how the scenes were encoded.
Regardless of the exact reasons why the age differences were apparent in Experiment 2 and not in Experiment 1, the critical finding is that when there were age differences in memory, they were in the direction of a “positivity effect.” This result is consistent with extensive evidence suggesting that older adults’ may show a preferential retention of positive information as compared to young adults (e.g., Mather & Carstensen, 2005), and it further suggests that the effect can be reflected not only in the quantity of information retained but also in the subjective quality of the remembered experiences.
As noted in the discussion following Experiment 2, the fact that the “positivity effect” only occurred for low-arousal stimuli and not for high-arousal stimuli is also consistent with a growing body of literature suggesting that the effect may be most robust when memory is not supported by automatic processes (such as those that were delineated above for high-arousal information) but instead is supported by elaborative and controlled processes (see Mather, 2006; Kensinger & Leclerc, in press, for further discussion). Though the present study was not designed to elucidate the mechanisms supporting memory for low-arousal stimuli versus high-arousal stimuli, prior research has suggested that memory for low-arousal stimuli may benefit from engagement of self-referential processes, semantic elaboration, and autobiographical association rather than from automatic orienting or prioritized consolidation (discussed by Anderson et al., 2006; Buchanan et al., 2006; Kensinger & Corkin, 2004; Koenig & Mecklinger, 2008; Talmi & Moscovitch, 2004). Hence, the present results suggest that age differences in the controlled processing of emotional information can affect not only the quantity of information retained (as described by Leclerc & Kensinger, in press; Mather, 2006) but also the qualitative characteristics of those retained events. In fact, in the present study, low-arousal stimuli were only associated with a qualitative “positivity effect” and not with a quantitative one: it was not the case that older adults recognized proportionally more positive stimuli than negative stimuli.
In summary, the results of the present study suggest that aging does not uniformly affect the relation between emotion and phenomenological memory characteristics. For high-arousal stimuli, young and older adults show remarkably similar effects of emotion on memory phenomenology, with negative emotion enhancing memory ratings for internal details more than for external event details. Age differences were more pronounced when phenomenological characteristics of memories for low-arousal stimuli were examined, with older adults showing a “positivity effect.” These effects arose despite no “positivity effect” in the overall proportion of positive versus negative stimuli that were recognized. These findings emphasize that the phenomenological characteristics of a memory, and the arousal level of the retrieved experience, need to be considered in order to allow for a more complete appreciation of age differences in emotional memory.
Figure 1.
The effect of valence and arousal on young and older adults’ ratings for feelings (panel a) and thoughts (panel b). Plotted scores have subtracted out the participants’ ratings to neutral items, and therefore represent difference scores; positive values indicate higher ratings for the emotional pictures than the neutral baseline. All ratings were acquired using a five-point Likert Scale, so values could range from +4 (i.e., a rating of 5 for the emotional picture and a rating of 1 for the neutral picture) to −4 (i.e., a rating of 5 for the neutral picture and a rating of 1 for the emotional picture). Error bars represent standard error of the mean.
Acknowledgments
This research was supported by NIH grant MH080833 (to E.A.K.) and by a National Defense Science and Engineering graduate fellowship (to K.R. M.). We thank Elizabeth Choi and Keely Muscatell for assistance with participant recruitment, testing, and data management. We also thank Scott Slotnick and Maya Tamir for helpful discussion and Jonathan Romiti for comments on an earlier version of the manuscript. Portions of this manuscript were included in a master’s thesis submitted by K.R.M.
Appendix
Memory Characteristics Questionnaire. For each picture judged to be “old,” participants were asked to rate their memories for the pictures on a series of dimensions, using a 5-point scale (1 = least/lowest, 5 = most/highest).
- Confidence: How certain you are that you have seen the picture before?
- Visual Detail: How many particulars do you remember about the picture such as color, clarity, complexity, etc.?
- Feelings and Reactions: How many personal feelings and reactions do you remember from when you viewed the picture?
- Remembered Thoughts: How many thoughts do you remember from when you viewed the picture?
- Temporal Order: How much do you remember about the location of the picture in the list? (e.g. surrounding photographs, if it appeared at the beginning, middle or end of the list)
- Other Associations: How many other associations do you remember about when you first viewed the picture (e.g., things that were happening in the room, sensations that you felt)
Footnotes
We had intended “other associations” to tap into non-affective sensations that participants may have had while viewing the images; we gave participants examples such as remembering that they’d had an itch or that they’d felt a draft in the room while viewing the image. However, we cannot rule out the possibility that some participants included affective sensations in this category.
The general pattern of results remained unchanged when uncorrected scores (raw ratings, without neutral subtracted out) were analyzed, although power to detect effects was slightly higher when using the corrected scores, possibly because of individual differences in how the scales were used.
References
- Anders S, Eippert F, Weiskopf N, Veit R. The human amygdala is sensitive to the valence of pictures and sounds irrespective of arousal: an fMRI study. Social, Cognitive, and Affective Neuroscience. 2008;3:233–243. doi: 10.1093/scan/nsn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK. Affective influences on the attentional dynamics supporting awareness. Journal of Experimental Psychology: General. 2005;134:258–261. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Greenwald MK, Petry MC, Lang PJ. Remembering pictures: pleasure and arousal in memory. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1992;18:379–390. doi: 10.1037//0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Etzel JA, Adolphs R, Tranel D. The influence of autonomic arousal and semantic relatedness on memory for emotional words. International Journal of Psychophysiology. 2006;61:26–33. doi: 10.1016/j.ijpsycho.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Amygdaloid complex lesions differentially affect retention of tasks using appetitive and aversive reinforcement. Behavioral Neuroscience. 1990;104:532–543. doi: 10.1037//0735-7044.104.4.532. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. A novel demonstration of enhanced memory associated with emotional arousal. Conscious Cognition. 1995;(4):410–421. doi: 10.1006/ccog.1995.1048. [DOI] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Carstensen LL. Social and emotional patterns in adulthood: Support for socioemotional selectivity theory. Psychology and Aging. 1992;7:331–338. doi: 10.1037//0882-7974.7.3.331. [DOI] [PubMed] [Google Scholar]
- Comblain C, D’Argembeau A, Van der Linden M. Phenomenal characteristics of autobiographical memories for emotional and neutral events in older and younger adults. Experimental Aging Research. 2005;31(2):173–189. doi: 10.1080/03610730590915010. [DOI] [PubMed] [Google Scholar]
- Comblain C, D’Argembeau A, Van der Linden M, Aldenhoff L. The effect of ageing on the recollection of emotional and neutral pictures. Memory. 2004;12:673–684. doi: 10.1080/09658210344000477. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Comblain C, Van der Linden M. Phenomenal characteristics of autobiographical memories for positive, negative, and neutral events. Applied Cognitive Psychology. 2003;17:281–294. [Google Scholar]
- D’Argembeau A, Van der Linden M. Remembering pride and shame: self-enhancement and the phenomenology of autobiographical memory. Memory. 2008;16:538–547. doi: 10.1080/09658210802010463. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Lane R, Chua P, Fletcher P. Dissociable temporal lobe activations during emotional episodic memory retrieval. Neuroimage. 2000;11:203–209. doi: 10.1006/nimg.2000.0538. [DOI] [PubMed] [Google Scholar]
- Dougal S, Rotello CM. “Remembering” emotional words is based on response bias, not recollection. Psychonomic Bulletin & Review. 2007;14:423–429. doi: 10.3758/bf03194083. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Buchanan TW, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal elderly persons. Emotion. 2003;3:239–253. doi: 10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- Destun LM, Kuiper NA. Phenomenal characteristics associated with real and imagined events: the effects of event valence and absorption. Applied Cognitive Psychology. 1999;13:175–86. [Google Scholar]
- Dewhurst SA, Parry LA. Emotionality, distinctiveness, and recollective experience. European Journal of Cognitive Psychology. 2000;12:541–551. [Google Scholar]
- Doerksen S, Shimamura AP. Source memory enhancement for emotional words. Emotion. 2001;1:5–11. doi: 10.1037/1528-3542.1.1.5. [DOI] [PubMed] [Google Scholar]
- Fleischman DA, Wilson RS, Gabrieli JDE, Bienias JL, Bennett DA. A Longitudinal Study of Implicit and Explicit Memory in Old Persons. Psychology and Aging. 2004;19:617–625. doi: 10.1037/0882-7974.19.4.617. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajck G. Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience. 2008;20:1–12. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL. Neural correlates of self-reflection. Philosophical Transactions: Biological Sciences. 2004;125:1808–1814. [Google Scholar]
- Gasper K. Do you see what I see? Affect and visual information processing. Cognition & Emotion. 2004;18:405–421. [Google Scholar]
- Gasper K, Clore GL. Attending to the big picture: Mood and global versus local processing of visual information. Psychological Science. 2002;13:34–40. doi: 10.1111/1467-9280.00406. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Carstensen LL, Pasupathi M, Tsai J, Skorpen CG, Hsu AYC. Emotion and aging: Experience, expression, and control. Psychology and Aging. 1997;12:590–599. doi: 10.1037//0882-7974.12.4.590. [DOI] [PubMed] [Google Scholar]
- Hahn S, Carlson C, Singer S, Gronlund SD. Aging and visual search: Automatic and controlled attentional bias to threat faces. Acta Psychologia. 2006;123:312–336. doi: 10.1016/j.actpsy.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Hamann S. Toward understanding emotion’s effects on memory. Emotion Review in press. [Google Scholar]
- Hashtroudi S, Johnson MK, Chrosniak LD. Aging and qualitative characteristics of memories for perceived and imagined complex events. Psychology and Aging. 1990;5:119–126. doi: 10.1037//0882-7974.5.1.119. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Is there an age-related positivity effect in visual attention? A comparison of two methodologies. Emotion. 2006a;6:511–516. doi: 10.1037/1528-3542.6.3.511. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye tracking study. Psychology and Aging. 2006b;21:40–48. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. Automatic versus intentional uses of memory: aging, attention, and control. Psychology and Aging. 1993;8:283–293. doi: 10.1037//0882-7974.8.2.283. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Foley MA, Suengas AG, Raye CL. Phenomenal characteristics of memories for perceived and imagined autobiographical events. Journal of Experimental Psychology: General. 1988;117:371–376. [PubMed] [Google Scholar]
- Kensinger EA. Remembering the details: Effects of emotion. Emotion Review. doi: 10.1177/1754073908100432. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA. Age differences in memory for arousing and nonarousing emotional words. Journal of Gerontology: Psychological Sciences. 2008a;63:13–18. doi: 10.1093/geronb/63.1.p13. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. How emotion affects older adults’ memories for event details. Memory. 2008b;17:1–12. doi: 10.1080/09658210802221425. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Remembering emotional experiences: The contribution of valence and arousal. Reviews in the neurosciences. 2004;15(4):241–251. doi: 10.1515/revneuro.2004.15.4.241. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Memory enhancement for emotional words: Are emotional words more vividly remembered than neutral words? Memory and Cognition. 2003;31:1169–1180. doi: 10.3758/bf03195800. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Memory for specific visual details can be enhanced by negative arousing content. Journal of Memory and Language. 2006;54:99–112. [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity in young and older adults. Journal of Gerontology: Psychological Sciences. 2007;62:208–215. doi: 10.1093/geronb/62.4.p208. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Leclerc CM. Age-related changes in the neural mechanisms supporting emotion processing and emotional memory. European Journal of Cognitive Psychology in press. [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(9):2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S, Mecklinger A. Electrophysiological correlates of encoding and retrieving emotional events. Emotion. 2008;8:162–173. doi: 10.1037/1528-3542.8.2.162. [DOI] [PubMed] [Google Scholar]
- LaBar KS. Beyond Fear: Emotional memory mechanisms in the human brain. Current Directions in Psychological Science. 2007;16:173–177. doi: 10.1111/j.1467-8721.2007.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. Gainesville, FL: The Center for Research in Psychophysiology; 1999. [Google Scholar]
- Larson C, Steuer E. Motivational relevance as a potential modulator of memory for affective stimuli: Can we compare snakes and cakes? Emotion Review in press. [Google Scholar]
- Lawton MP, Kleban MH, Rajagopal D, Dean J. The dimensions of affective experience in three age groups. Psychology and Aging. 1992;7:171–184. doi: 10.1037//0882-7974.7.2.171. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cognitive, Affective, and Behavioral Neuroscience. 2008a;8:153–164. doi: 10.3758/cabn.8.2.153. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Effects of age on detection of emotional information. Psychology and Aging. 2008b;23:209–215. doi: 10.1037/0882-7974.23.1.209. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Neural processing of emotional pictures and words: A comparison of young and older adults. doi: 10.1080/87565641.2010.549864. submitted. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The Emotional Brain. New York: New York Magazine; 1996. [Google Scholar]
- Leiphart J, Rosenfeld JP, Gabrieli JD. Event-related potential correlates of implicit priming and explicit memory tasks. International Journal Psychophysiology. 1993;15:197–206. doi: 10.1016/0167-8760(93)90003-8. [DOI] [PubMed] [Google Scholar]
- Leventhal H, Scherer K. The Relationship of Emotion to Cognition: A Functional Approach to a Semantic Controversy. Cognition & Emotion. 1987;1:3. [Google Scholar]
- Mather M. Disentangling the effects of arousal and valence on memory for intrinsic details. Emotion Review. doi: 10.1177/1754073908100435. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M. Why memories may become more positive as people age. In: Uttl B, Ohta N, Siegenthaler AL, editors. Memory and Emotion: Interdisciplinary Perspectives. Blackwell Publishing; 2006. pp. 135–159. [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:296–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Matlin MW, Stang DJ. The pollyanna principle: selectivity in language, memory, and thought. Cambridge, MA: Schenkman Pub. Co; 1978. [Google Scholar]
- May CP, Rahhal T, Berry EM, Leighton EA. Aging, source memory, and emotion. Psychology and aging. 2005;20:571–578. doi: 10.1037/0882-7974.20.4.571. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. Fleisch. [DOI] [PubMed] [Google Scholar]
- Murphy NA, Isaacowitz DM. Preferences for emotional information in older and younger adults: A meta-analysis of memory and attention tasks. Psychology & Aging. 2008;23:263–86. doi: 10.1037/0882-7974.23.2.263. [DOI] [PubMed] [Google Scholar]
- Neisser U, Harsch HN. Phantom flashbulbs: false recollections of hearing the news about the challenger. In: Winograd E, Neisser U, editors. Affect and Accuracy in Recall: Studies of “Flashbulb” Memories. Vol. 4. Cambridge University Press; New York: 1992. [Google Scholar]
- Ochsner KN. Are affective events richly recollected or simply familiar? The experience and process of recognizing feelings past. Journal of Experimental Psychology: General. 2000;129:242–261. doi: 10.1037//0096-3445.129.2.242. [DOI] [PubMed] [Google Scholar]
- Ohman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Peterson W, Birdsall T, Fox W. The theory of signal detectability. Information Theory, Transactions of the IRE Professional Group. 1954;4:171–212. [Google Scholar]
- Petrican R, Moscovitch M, Schimmack U. Cognitive resources, valence, and memory retrieval of emotional events. Psychology and Aging. 2008;23:585–594. doi: 10.1037/a0013176. [DOI] [PubMed] [Google Scholar]
- Rahhal TA, May CP, Hasher L. Truth and character: Sources that older adults remember. Psychological Science. 2002;13:101–105. doi: 10.1111/1467-9280.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg D, Hertel P, editors. Memory and emotion. New York: Oxford University Press; 2004. [Google Scholar]
- Rubin DC, Kozin M. Vivid memories. Cognition. 1984;16:63–80. doi: 10.1016/0010-0277(84)90037-4. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Philippot P. Selective effects of emotion on the phenomenal characteristics of autobiographical memories. Memory. 2005;13:148–160. doi: 10.1080/09658210344000648. [DOI] [PubMed] [Google Scholar]
- Schmolck H, Buffalo EA, Squire LR. Memory distortions develop over time: Recollections of the O.J. Simpson trial verdict after 15 and 32 months. Psychological Science. 2000;11:39–45. doi: 10.1111/1467-9280.00212. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Clinical Gerontology : A Guide to Assessment and Intervention. NY: The Haworth Press; 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version; pp. 165–173. [Google Scholar]
- Sharot T, Delgado MR, Phelps EA. How emotion enhances the feeling of remembering. Nature Neuroscience. 2004;7:1376–1380. doi: 10.1038/nn1353. [DOI] [PubMed] [Google Scholar]
- Shipley WC. Shipley Institute of Living Scale. Los Angeles: Western Psychological services; 1986. [Google Scholar]
- Somerville LH, Wig GS, Whalen PJ, Kelley WM. Dissociable medial temporal lobe contributions to social memory. Journal of Cognitive Neuroscience. 2006;18:1253–65. doi: 10.1162/jocn.2006.18.8.1253. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Morgan CA, III, Nicolaou AL, Charney DS. Consistency of memory for combat-related traumatic events in veterans of Operation Desert Storm. American Journal of Psychiatry. 1997;154:173–177. doi: 10.1176/ajp.154.2.173. [DOI] [PubMed] [Google Scholar]
- Spreen O, Benton AL. Neurosensory Center Comprehensive Examination for Aphasia: Manual of instructions (NCCEA) (rev. ed.) Victoria, BC: University of Victoria; 1977. [Google Scholar]
- Straube T, Pohlack S, Mentzel HJ, Miltner WH. Differential amygdala activation to negative and positive emotional pictures during an indirect task. Behavioral Brain Research. 2008;191:285–288. doi: 10.1016/j.bbr.2008.03.040. [DOI] [PubMed] [Google Scholar]
- Swets JA, Dawes RM, Monahan J. Psychological science can improve diagnostic decisions. Psychological science in the public interest. 2000;1:1–26. doi: 10.1111/1529-1006.001. [DOI] [PubMed] [Google Scholar]
- Talarico J, Rubin DC. Confidence, not consistency, characterizes flashbulb memories. Psychological Science. 2003;14:455–461. doi: 10.1111/1467-9280.02453. [DOI] [PubMed] [Google Scholar]
- Talmi D, Goshen-Gottstein Y. The long-term recency effect in recognition memory. Memory. 2006;14(4):424–436. doi: 10.1080/09658210500426623. [DOI] [PubMed] [Google Scholar]
- Talmi D, Moscovitch M. Can semantic relatedness explain the enhancement of memory for emotional words? Memory and Cognition. 2004;32:742–751. doi: 10.3758/bf03195864. [DOI] [PubMed] [Google Scholar]
- Tomaszczyk JC, Fernandes MA, MacLeod CM. Personal relevance modulates the positivity bias in recall of emotional pictures in older adults. Psychonomic Bulletin and Review. 2008;15:191–196. doi: 10.3758/pbr.15.1.191. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Technical manual for the Wechsler Adult Intelligence and Memory Scale. 3. New York: The Psychological Corporation; 1997. [Google Scholar]