Abstract
Background
Physical or psychological adversity in childhood is associated with a higher risk for depression in adulthood, and with persistent serotonergic abnormalities in humans and in animal models. We hypothesized that reported childhood abuse would be associated with lower brain serotonin transporter (5-HTT) binding potential (BPP, proportional to the number of available transporters) in adults. We examined healthy volunteers and subjects with major depressive disorder, a sample enriched for childhood abuse.
Methods
Regional brain 5-HTT BPP was measured using positron emission tomography with [11C]McN 5652 and a metabolite corrected arterial input function in 43 healthy volunteers and 23 subjects in a major depressive episode, ten of whom reported a history of sexual and/or physical abuse before age 15, and 13 of whom did not. As only two healthy volunteers reported childhood abuse, primary analyses were restricted to the depressed sample, with healthy controls presented as comparators.
Results
Depressed subjects reporting childhood abuse had lower 5-HTT BPP than non-abused depressed subjects across all brain regions examined (p=0.017). The groups did not differ in relevant demographic or clinical variables. Genotype frequencies of a functional polymorphism in the 5-HTT gene promoter (5-HTTLPR) did not differ between the groups.
Conclusions
Reported childhood abuse is associated with lower 5-HTT BPP in this sample of subjects with major depression, consistent with other reports that childhood adversity can lower serotonergic function permanently. Lower 5-HTT BPP may represent a biological pathway through which early life stress predisposes to the development of subsequent psychiatric illness, including major depressive disorder.
Keywords: serotonin transporter, abuse, positron emission tomography
Introduction
Adverse experiences early in life may alter brain function and increase the risk of developing subsequent psychiatric illness (Meaney, 2001; Nemeroff and Vale, 2005). In animal models and in humans, early stress or adversity appears to reduce brain 5-HT function. Deficiency in the serotonin (5-HT) system is associated with psychiatric conditions including major depressive disorder (MDD) (Mann and Arango, 1999), as well as suicidal and aggressive behaviors (Coccaro et al., 1997; Currier and Mann, 2008). Adult rhesus macaques that have previously experienced high rates of rejection from their mothers or repeated maternal separation (among females) have lower levels of the serotonin metabolite 5-HIAA in CSF (Higley et al., 1992; Maestripieri et al., 2006), and lower serotonin transporter (5-HTT) binding on PET scanning (Ichise et al., 2006). Such maternal separation in monkeys has been associated with subsequent depressive behaviors (Harlow and Suomi, 1974), as well as anxiety-like behavior, increased aggression, and increased alcohol consumption (Higley et al., 1991; Suomi et al., 1992).
Similarly, humans raised in low socioeconomic circumstances or who report high rates of neglect early in life exhibit impaired 5-HT function, including blunted prolactin response to fenfluramine challenge (Manuck et al., 2005), and lower cerebrospinal fluid (CSF) 5-hydroxyindoleacetic acid (5-HIAA) (Roy, 2002). Childhood abuse and neglect in humans have been associated with increased risk for MDD in adulthood (Widom et al., 2007), suggesting a consistent association between childhood adversity, abnormal serotonergic markers, and psychopathology in both humans and in animal models.
Alterations in markers of 5-HT signaling as a result of early-life stress may be attributable to changed receptor availability and/or function. The serotonin transporter (5-HTT) is responsible for 5-HT reuptake from the synaptic cleft, and availability of this protein is associated with neural 5-HT function and depression-related behavior (Ansorge et al., 2004; Fuller et al., 1991; Parsey et al., 2006b). 5-HTT availability is correlated with the density of serotonergic axons, suggesting that 5-HTT binding is reflective at least of axonal density, if not of serotonergic neuronal density (Soucy et al., 1994). Rats experiencing nutritional deprivation or repeated maternal separation as infants exhibit reduced neural 5-HTT protein in the raphe nucleus as adults (Jahng et al., 2007; Lee et al., 2007). This could be indicative of a reduction in serotonergic neuron density in the raphe nucleus. As all cortical and subcortical serotonergic fibers arise from the raphe, a reduction in serotonergic fibers from the raphe would likely be accompanied by reduced 5-HTT in these regions. Indeed, maternally-deprived rhesus macaques exhibit lower 5-HTT availability across a broad array of cortical and subcortical structures (Ichise et al., 2006), suggesting a generalized effect of childhood adversity on 5-HTT across the post-synaptic terminal field of serotonergic neurons. While there are clearly pleiotropic neurochemical and morphological effects of early life stress on brain development (see discussion as well as (De Bellis, 2005; Nemeroff, 2004; Teicher et al., 2003)), these data suggest one possible pathway of childhood adversity leading to impaired serotonergic neurotransmission, reflected in reduced 5-HTT, predisposing to the development of subsequent psychopathology including MDD.
To our knowledge, the impact of reported childhood adversity on adult brain 5-HTT binding in humans has never been assessed. Therefore, we set out to explore the relationship of reported childhood abuse on 5-HTT regional brain binding in adulthood. We have previously shown that 5-HTT BPP is significantly lower in major depressive disorder (MDD) than among healthy controls using PET with the [11C]McN 5652 radioligand (Parsey et al., 2006b). In the current study, we compared 5-HTT binding in the brain between subjects from that sample with and without a reported history of childhood abuse. While we intended to examine the effects of reported childhood abuse on 5-HTT among subjects with MDD as well as among healthy controls, only two of 43 healthy controls in that sample endorsed a history of childhood abuse. We therefore restricted our primary hypothesis and analysis to subjects with MDD, a sample with a higher prevalence of childhood abuse. We included healthy controls as an additional comparison group, but did not include them in statistical analyses to avoid confounding the effect of diagnosis with that of childhood abuse. We hypothesized that depressed subjects reporting childhood physical or sexual abuse would have lower 5-HTT binding in brain regions previously associated with serotonergic abnormalities in depression compared with depressed subjects denying childhood abuse. In an exploratory fashion in this small group, we acquired data regarding clinical course following naturalistic treatment for depression after PET scanning, to assess whether reported childhood abuse was associated with differential clinical outcomes, given previous reports of diminished response to the antidepressant nefazodone among subjects with a history of childhood trauma (Nemeroff et al., 2003). While the current study was not powered to assess gene-environment interactions on 5-HTT binding, we also characterized the genotype of a functional polymorphism in the 5-HTT gene (5-HTTLPR) in this sample (Hu et al., 2005; Lesch et al., 1996), for which there is evidence of gene-environment interactions predisposing to the development of MDD (reviewed in (Caspi and Moffitt, 2006)).
Materials and Methods
Subjects
This is a further analysis of data from a previous study of 5-HTT binding in depression, which contains details regarding the clinical sample and imaging methods (Parsey et al., 2006b). All depressed subjects met the following inclusion criteria: (1) age 18 to 65 years; (2) DSM-IV criteria for a current major depressive episode (MDE); (3) ≥ two week medication-free period prior to PET scanning (four weeks for oral neuroleptics, six weeks for fluoxetine, and an exception of three days for short-acting benzodiazepines); (4) absence of current or lifetime history of alcohol or other drug abuse or dependence; (5) no lifetime exposure to 3,4-methylenedioxymethamphetamine (MDMA); (6) absence of significant current medical conditions; (7) absence of pregnancy; and (8) capacity to provide informed consent. In the previous study, we recruited 25 subjects currently in a major depressive episode. From that group, 23 subjects who supplied information regarding childhood history of abuse were included in the present study. Inclusion criteria for 43 healthy comparison subjects were similar; these subjects were required to have no psychiatric history and no history of a mood or psychotic disorder in their first-degree relatives. All subjects gave written informed consent for participation in this study. The study protocol was approved by the Institutional Review Board of the New York State Psychiatric Institute, and was carried out in accordance with the ethical guidelines enumerated in the Declaration of Helsinki.
Clinical Assessments
Diagnoses were determined using the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1995), conducted by experienced research masters and Ph.D. level psychologists. A team of experienced clinical research psychologists and psychiatrists reviewed all diagnoses. As part of a semi-structured interview, participants were asked whether they had a history of physical and/or sexual abuse over the course of their lifetime. If subjects endorsed a history of abuse, they were asked whether this abuse took place before age 15. Subjects who endorsed a history of abuse before age 15 were compared to those who did not. The Beck Depression Inventory (BDI) (Beck et al., 1961), Hamilton Depression Rating Scale (HAMD) (Hamilton, 1960), and Global Assessment Scale (GAS) (Endicott et al., 1976) were administered to assess depression severity and functional impairment. Lifetime history of aggression was measured using the Brown Goodwin Aggression History Scale (Brown et al., 1979). 10 of the 23 depressed subjects (43.5%) had made at least one prior suicide attempt. 11 depressed subjects (47.8%) had current co-morbid Axis I disorders, all of which were anxiety disorders (Table I).
Table I.
Clinical and Demographic Characteristics of the Sample
| Variable | MDD Reporting Childhood Abuse (N=10) | MDD Reporting No Childhood Abuse (N=13) | Healthy Controls (N=43) | T-test (depressed abused vs. unabused) | |||
|---|---|---|---|---|---|---|---|
| Continuous Variables | Mean | S.D. | Mean | S.D. | Mean | S.D. | p-value |
| Age (yrs) | 39.1 | 16.1 | 39.2 | 11.4 | 38.8 | 15.9 | 0.98 |
| 24-item Hamilton Depression Rating Scale | 24.2 | 7.2 | 25.8 | 7.6 | 0.68 | 0.91 | 0.62 |
| Beck Depression Index | 21.4 | 11.5 | 25.4 | 10.4 | 1.7 | 2.4 | 0.39 |
| Global Assessment Scale | 48.2 | 12.4 | 51.0 | 12.9 | 89.8 | 4.5 | 0.60 |
| # of prior major depressive episodes (MDEs) | 4.5 | 3.4 | 4.2 | 2.9 | 0 | 0 | 0.79 |
| Length of Current MDE (days)1 | 91.6 | 177.5 | 47.9 | 76.3 | N/A | N/A | 0.43 |
| # of 1st degree relatives with Major Depression | 1.0 | 1.2 | 1.0 | 1.0 | 0 | 0 | 1.00 |
| Years of Education | 14.8 | 1.8 | 15.0 | 3.6 | 16.4 | 2.9 | 0.79 |
| Lifetime Aggression Score | 16.7 | 4.9 | 15.3 | 3.6 | 14.1 | 3.9 | 0.48 |
| Categorical Variables | N | % | N | % | N | % | Fisher’s Exact p-value (depressed abused vs. unabused) |
| Gender | |||||||
| Male | 4 | 40.0 | 2 | 15.4 | 22 | 51 | 0.34 |
| Female | 6 | 60.0 | 11 | 84.6 | 21 | 49 | |
| Prior Suicide Attempts: | |||||||
| Yes | 6 | 60.0 | 4 | 30.8 | 0 | 0 | 0.22 |
| No | 4 | 40.0 | 9 | 69.2 | 43 | 100 | |
| Comorbid Anxiety Disorder | |||||||
| Yes | 4 | 40.0 | 7 | 53.9 | 0 | 0 | 0.68 |
| No | 6 | 60.0 | 6 | 46.2 | 43 | 100 | |
| Comorbid Post-Traumatic Stress Disorder | |||||||
| Yes | 1 | 10.0 | 3 | 23.1 | 0 | 0 | 0.60 |
| No | 9 | 90.0 | 10 | 76.9 | 43 | 100 | |
| Variable | MDD Reporting Childhood Abuse (N=10) | MDD Reporting No Childhood Abuse (N=13) | Healthy Controls (N=43) | Fisher’s Exact p-value (depressed abused vs. unabused) | |||
| N | % | N | % | N | % | ||
| Remission Status at 1 year2 | |||||||
| Remitter | 2 | 22.2 | 5 | 50.0 | N/A | N/A | 0.35 |
| Non-Remitter | 7 | 77.8 | 5 | 50.0 | N/A | N/A | |
| 5-HTTLPR Functional Genotype | |||||||
| L’L’ | 3 | 30.0 | 2 | 15.4 | 10 | 23.8 | 0.64 |
| L’S’ | 4 | 40.0 | 8 | 61.5 | 16 | 38.1 | |
| S’S’ | 3 | 30.0 | 3 | 23.1 | 16 | 38.1 | |
| Type of Reported Childhood Abuse | |||||||
| Sexual | 4 | 40.0 | N/A | 2 | 4.6 | ||
| Physical | 3 | 30.0 | N/A | 0 | 0 | ||
| Both | 3 | 30.0 | N/A | 0 | 0 | ||
one subject in the abused group is an outlier (584 days), accounting for observed non-statistically significant difference between groups.
clinical follow-up was not available for four subjects.
Following baseline assessment and PET scans, depressed patients received open, non-standardized antidepressant treatment. Remission, defined as ≥50% reduction of HAMD-24 score from baseline and final HAMD-24 score <10, was assessed among the 19 subjects who returned for clinical assessments one year following PET scanning to provide further clinical characterization of the sample; this clinical assessment was previously described in the absence of the characterization of abuse (Miller et al., 2008).
Genotyping
Genotyping of a functional polymorphism in the promoter region of the 5-HTT gene (5-HTTLPR) was performed as previously described (Parsey et al., 2006a). Triallelic genotypes were reclassified by level of in vitro expression as follows: S’S’ = (LGS=LGLG=SS); L’S’ = (LAS=LALG); L’L’ = LA LA (Hu et al., 2005).
Radiochemistry
[11C](+)-McN 5652, (+)-McN butyryl thioester tartrate, was produced as previously described (Frankle et al., 2004b). The injected dose of [11C]McN5652 did not differ between abused (mean=13.8mCi, SD=5.0) and non-abused (14.6mCi, SD=3.0) groups (t=0.47, df=21, p=0.65). Similarly, the injected mass of [11C]McN5652 did not differ between abused (mean=4.04μg, SD=1.53) and non-abused (mean=4.42μg, SD=1.07) groups (t=0.70, df=21, p=0.49).
Image Analysis and Modeling
PET and magnetic resonance imaging (MRI) data acquisition, analysis, and measurement of metabolite corrected arterial input functions were performed (for details, see (Parsey et al., 2006a; Parsey et al., 2006b; Parsey et al., 2000)). After a ten-minute transmission scan, [11C]McN5652 was injected intravenously and emission data acquired for 130 minutes. Regions of interest (ROIs) were traced on T1-weighted MRIs obtained for each individual subject using brain atlases (Duvernoy, 1991; Talairach and Tournoux, 1988) and published reports (Kates et al., 1997; Killiany et al., 1997). Six ROIs previously associated with serotonergic abnormalities in depression were included in this study: the anterior cingulate, amygdala, putamen, hippocampus, midbrain, and thalamus (Parsey et al., 2006b). Derivation of [11C]McN5652 regional distribution volumes (VT) was performed using likelihood estimation in graphical analysis (LEGA) (Ogden, 2003; Ogden et al., 2002; Parsey et al., 2003). VT is the sum of the specific (VS) and non-displaceable (free plus nonspecific binding = VND) distribution volumes. Binding potential (BPP) = VT − VND = fpBavail/KD where fp is the free fraction of radioligand in plasma, Bavail is the density of receptors available to bind radioligand in vivo, and KD is the dissociation constant, equal to koff/kon. This terminology is consistent with a recent consensus statement on outcome measure nomenclature in PET studies (Innis et al., 2007). We utilized a 12.1 ± 1.5 mL sample of the cerebellar gray matter as a measure of VND (Parsey et al., 2006b).
Statistics
Considering six ROIs at once, data from the two groups (depressed subjects with and without a reported history of childhood abuse) were analyzed using linear mixed effects models with brain region and group as fixed effects and subject as the random effect. To stabilize the variance and ensure modeling assumptions were met, analysis was performed on the natural log of the data, after first adding a quantity (2) to all measures to ensure positivity. The log transform was necessary primarily because of the unequal standard deviations (SD) of measurements across regions with differing binding levels (each SD is roughly proportional to its corresponding mean binding level). We have taken this approach to allow for valid statistical analysis of ROI-based PET data in mixed effects models (Miller et al., 2008; Oquendo et al., 2007; Parsey et al., 2006a; Parsey et al., 2006b; Parsey et al., 2006c; Parsey et al., 2006d; Sullivan et al., 2005). Others have used related statistical approaches, including linearizing transformation (Rabiner et al., 2002) and non-parametric testing (Meltzer et al., 2004) to address this issue in analyzing PET data. As the natural log is a monotone transformation, showing a difference in log(BPP) is equivalent to showing a difference (in the same direction) in BPP. Graphs of binding potential use actual (not log-transformed) BPP values. Reported p-values correspond to two-sided tests. Linear mixed effects models of binding were performed in R 2.1.0 (http://cran.r-project.org). Student’s t-tests were performed in Excel (Microsoft, 2003). Chi-square tests and Fisher’s exact tests were performed in SPSS for Macintosh OS X Version 11 (SPSS, Chicago, IL) to examine clinical and demographic variables. Statistics are presented as (test statistic, degrees of freedom, p-value).
Results
Clinical Characteristics
Table I presents demographic, clinical, and genetic information regarding the depressed subjects with or without a reported history of childhood abuse as well as healthy comparison subjects, who are not sub-divided by abuse history given the small number of healthy subjects reporting childhood abuse. Abused and unabused depressed subjects were comparable in terms of age, level of education obtained, severity of current depression, number of prior major depressive episodes, and frequency of comorbid anxiety disorders including post-traumatic stress disorder. While depressed subjects with a reported history of childhood abuse had a lower rate of remission following one year of naturalistic treatment than those who did not report abuse (22.2% vs. 50%), this difference was not statistically significant in this small sample (Fisher’s exact, p=0.35). Differences in sex ratio, history of prior suicide attempts, and length of current depressive episode were not statistically significant (Table I).
Serotonin Transporter Binding
5-HTT BPP was significantly lower in depressed subjects with a reported history of childhood abuse compared with depressed subjects without a reported history of childhood abuse with all regions of interest included in the model (Figure 1; F=6.76, df=1,21, p=0.017). Post-hoc analyses confirmed that this difference was significant in all regions examined: anterior cingulate (Figure 2; F=7.14, df=1,21, P=0.014), amygdala (F=4.60, df=1,21, p=0.044), dorsal putamen (F=4.48, df=1,21, p=0.046), hippocampus (F=4.63, df=1,21, p=0.043), midbrain (F=5.27, df=1,21, p=0.032), and thalamus (F=4.95, df=1,21, p=0.037). We did not find evidence for a differential effect of reported childhood abuse across the 6 brain regions examined (region by group interaction: F=0.91, df=5,105, p=0.48). There was no difference between the two groups in non-specific binding as quantified by log cerebellar VT (F=0.38, df=1,21, p=0.54).
Figure 1.
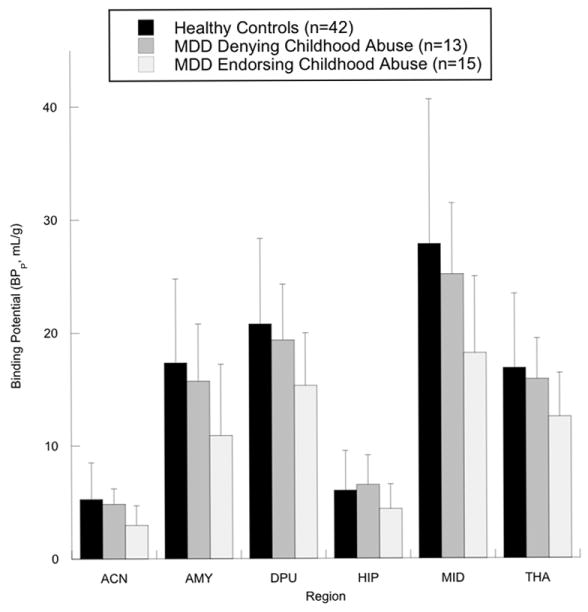
Depressed subjects with a reported history of childhood abuse have lower serotonin transporter binding potential than those who do not report childhood abuse (p<0.05 for all regions considered simultaneously in linear mixed effects model, and for each individual region considered separately). Healthy controls included as additional comparison group in figure. MDD = major depressive disorder, ACN = anterior cingulate, AMY = amygdala, DPU = dorsal putamen, HIP = hippocampus, MID = midbrain, THA = thalamus.
Genotype
There was no difference in the distribution of 5-HTTLPR functional genotypes between the depressed groups with and without a reported history of childhood abuse (Table I; Fisher’s exact, p=0.64).
Discussion
In this first pilot study, MDD subjects with a reported history of physical or sexual abuse before age 15 had lower 5-HTT BPP than depressed subjects without such an abuse history in all brain regions examined: midbrain, putamen, amygdala, thalamus, hippocampus, and anterior cingulate. The association of childhood abuse and lower 5-HTT binding is consistent with our hypothesis, and with a previous report of lower 5-HTT binding in maternally-deprived macaques as assessed by PET (Ichise et al., 2006). Lower platelet 5-HTT availability has also been demonstrated in subjects with bulimia who reported early sexual abuse (Steiger et al., 2004). While there are many factors leading to differential regulation of 5-HTT in platelets and brain, the direction of this finding is consistent with the data presented here.
We interpret lower 5-HTT BPP among subjects with a reported history of childhood abuse to reflect lower 5-HTT density. As BPP is equal to the product of the receptor density, receptor affinity (1/KD), and the free fraction of radioligand in plasma (fP), it is possible that observed differences in BPP reflect differences in 1/KD or fP. In a previous post-mortem study, however, lower 5-HTT binding among subjects with MDD was not associated with lower affinity (Perry et al., 1983). As fp is not measurable for the [11C]McN 5652 radioligand, we cannot exclude the possibility of differences in fp between groups. The results reported here cannot be explained by differences in intrasynaptic 5-HT concentrations, as the [11C]McN 5652 radioligand does not appear to be sensitive to endogenous neurotransmitter levels (Hummerich et al., 2006).
How early environment influences 5-HTT availability in adulthood is not known. Neuroanatomical, physiological or genomic alterations may contribute to low 5-HTT availability resulting from childhood abuse. The findings of lower CSF 5-HIAA in maternally-rejected or deprived monkeys (Higley et al., 1992; Maestripieri et al., 2006) suggest decreased serotonin release or neuron firing following these stressors. Lower 5-HTT BPP observed among depressed subjects with a reported history of abuse may therefore reflect a compensatory response to lower intra-synaptic 5-HT. Alternatively, it may reflect a deficit of serotonergic neurons in the raphe nuclei, of projections from these neurons to their terminal field, or of 5-HTT in terminal projections. The involvement of both cortical and sub-cortical regions is consistent with a previous finding in rhesus macaques (Ichise et al., 2006). As 5-HTT has been highly correlated with serotonergic neuron density (Soucy et al., 1994) and early footshock stress has been associated with fewer 5-HT immunoreactive cells in the medial raphe nucleus in rats (Konno et al., 2007), the diffuse reduction in 5-HTT BPP that we observed in abused subjects may reflect a reduction of serotonergic neurons in the raphe nuclei, which is not measurable non-invasively in vivo. While a greater number of serotonergic neurons in the dorsal raphe nucleus was previously reported among suicide victims compared to healthy controls assessed post-mortem (Underwood et al., 1999), that study did not examine the effects of childhood adversity, and focused on the phenomenon of suicide rather than the diagnosis of major depression. Methylation a CpG island downstream of the 5-HTT promoter region may be associated with less 5-HTT expression and onset of depression (Philibert et al., 2008), and could be a target for epigenetic effects of maternal deprivation or abuse. These effects could result in adult serotonin deficiencies associated with recurrent MDD in adulthood (Bhagwagar et al., 2002; Flory et al., 1998; Neumeister et al., 2004; Ruhe et al., 2007). Alternative possible mechanistic explanations for the observed association between 5-HTT BPP and reported abuse include reciprocal interactions between the serotonin system and the hypothalamic-pituitary-adrenal (HPA) axis (Tafet et al., 2001; Weidenfeld et al., 2002), which is dysregulated in individuals who experience childhood abuse (Nemeroff, 2004), or effects of brain-derived neurotrophic factor (BDNF) on serotonergic neuron differentiation and survival (Eaton and Whittemore, 1996; Mamounas et al., 2000; Mamounas et al., 1995), given a previous finding of decreased BDNF expression following acute stress (Smith et al., 1995).
If corroborated in subsequent prospective studies, this finding has potential clinical implications. Lower 5-HTT BPP in subjects with a history of childhood abuse may predispose to the development of depression. Several brain imaging studies have reported lower 5-HTT among subjects with major depressive disorder (Lehto et al., 2006; Malison et al., 1998; Newberg et al., 2005; Parsey et al., 2006b), although other studies have not replicated this finding (Cannon et al., 2007; Herold et al., 2006; Ichimiya et al., 2002; Meyer et al., 2004). While differences in radioligands, outcome measures used, and clinical characteristics may explain some of these discrepancies, the current study suggests that differential rates of childhood abuse among these samples may also contribute to variation in findings. Further, lower 5-HTT availability resulting from childhood abuse may limit the effectiveness of antidepressant medications. We have recently found that non-remission from MDD is associated with lower 5-HTT levels, consistent with this hypothesis (Miller et al., 2008). While not statistically significant in this modest sample, the group reporting childhood abuse had lower absolute rates of remission after one year of naturalistic antidepressant treatment than the non-abused group. If this were confirmed in a larger sample, it would raise the possibility that childhood abuse contributes to treatment resistance in depression due to a lack of availability of 5-HTT for pharmacological manipulation, or through lower serotonin release that would lessen the impact of serotonin reuptake inhibition. Consistent with this idea, a randomized controlled trial of the serotonin reuptake inhibitor and serotonin 2A receptor antagonist nefazodone versus structured psychotherapy for chronic depression found that subjects with a history of childhood trauma had lower remission rates with nefazodone than with structured psychotherapy (Nemeroff et al., 2003).
This study has limitations. Our analysis of the effects of abuse on SERT binding was confined to subjects with MDD, as our available sample of 43 healthy controls included only two subjects who reported childhood abuse. Of note, mean 5-HTT BPP among the two healthy volunteers reporting a history of childhood abuse was 16.4% lower across all regions compared with the 41 healthy volunteers denying childhood abuse, in the same direction as our finding among MDD subjects. This should be interpreted with caution given the very small sample size of abused controls. If childhood abuse is found to be associated with low 5-HTT among healthy controls in larger samples, this might suggest that low 5-HTT related to childhood adversity is not sufficient to produce a phenotype of adult depression, or that these healthy controls may be at higher risk for the development of subsequent psychiatric illness. Our sample was too small to evaluate gene-environment interactions with the 5-HTTLPR polymorphism. We and others have previously reported a lack of effect of 5-HTTLPR genotype on 5-HTT BPP (Murthy et al., 2008; Oquendo et al., 2007; Parsey et al., 2006a), although some studies have found such an effect (Praschak-Rieder et al., 2007; Reimold et al., 2007). Larger future studies could examine whether the ability to detect an effect of 5-HTTLPR genotype on in vivo 5-HTT binding is contingent on considering interactions between genotype and early life stress. The [11C]McN 5652 radioligand does not permit measurement of free-fraction in plasma (fP), which is required to estimate the more robust binding measure BPF (=Bavail/KD); in addition, [11C]McN 5652 has higher non-specific uptake than the radioligand [11C]DASB, for which fp is measurable (Frankle et al., 2004a). We did not examine the effects of physical or sexual abuse after age fifteen on 5-HTT BPP. Among the group of thirteen depressed subjects without a history of childhood abuse, only two endorsed a history of abuse from age fifteen or later, which limits any conclusions that could be drawn. Finally, childhood abuse history was assessed through the use of a semi-structured interview, and was not independently corroborated.
This study provides the first direct evidence of lower 5-HTT BPP in adulthood associated with a reported history of childhood abuse. Future studies with larger sample sizes may assess whether there is a critical period for the effects of abuse. The experience of adversity in childhood, a period of great neuroplasticity (Black et al., 1998), may be more salient for 5-HTT regulation than abuse experienced in adulthood. Use of a more detailed measure of childhood abuse, such as the childhood trauma questionnaire (Bernstein and Fink, 1998), will facilitate such studies. Targeted recruitment of psychiatrically healthy adults with and without a history of childhood abuse for 5-HTT quantification will allow us to assess the generalizability of this finding, and may speak to the neurobiological underpinnings of resiliency.
Acknowledgments
We would like to thank the staff of the Brain Imaging Division of The Department of Molecular Imaging and Neuropathology, as well as the Kreitchman PET Center. Funding for this study was provided by US PHS grants NIMH P30 MH46745, MH40695, MH40210, MH62185, MH015144, NARSAD, and the American Foundation for Suicide Prevention.
Footnotes
Disclosure/Conflict of Interest The authors of this manuscript have no conflicts of interest to report. They have the following disclosures to report:
Dr. Miller received financial compensation for psychiatric evaluations of subjects enrolled medication studies sponsored by Pfizer and Orexigen Therapeutics, unrelated to the current manuscript.
Dr. Kinnally has no disclosures to report.
Dr. Ogden has no disclosures to report.
Dr. Oquendo received financial compensation from Pfizer for the safety evaluation of a clinical facility, unrelated to the current manuscript, and was the recipient of a grant from Eli Lilly to support a year of salary for the Lilly Suicide Scholar, Enrique Baca-Garcia, M.D., Ph.D.
Dr. Parsey has received PET Imaging grants from Novartis Pharmaceuticals, Sepracor, Inc., Pfizer, and Eli Lilly Company, unrelated to the current manuscript.
Dr. Mann is principal investigator on PET Imaging grants from GSK and Novartis unrelated to the current manuscript.
References
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science (New York, NY) 2004;306(5697):879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbauh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood trauma questionnaire: A retrospective self-report. San Antonio: The Psychological Corporation; 1998. [Google Scholar]
- Bhagwagar Z, Whale R, Cowen PJ. State and trait abnormalities in serotonin function in major depression. Br J Psychiatry. 2002;180:24–28. doi: 10.1192/bjp.180.1.24. [DOI] [PubMed] [Google Scholar]
- Black J, Jones TA, Nelson CA, Greenough WT. Neuronal plasticity and the developing brain. In: Alessi NE, Coyle JT, Harrison SI, Eth S, editors. Handbook of child and adolescent psychiatry. New York: John Wiley; 1998. pp. 31–53. [Google Scholar]
- Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF. Aggression in human correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979;1:131–139. doi: 10.1016/0165-1781(79)90053-2. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, Manji HK, Drevets WC. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol Psychiatry. 2007;62(8):870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nature reviews. 2006;7(7):583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Trestman RL, Gabriel SM, Cooper TB, Siever LJ. Serotonin function in human subjects: intercorrelations among central 5-HT indices and aggressiveness. Psychiatry Res. 1997;73(1-2):1–14. doi: 10.1016/s0165-1781(97)00108-x. [DOI] [PubMed] [Google Scholar]
- Currier D, Mann JJ. Stress, genes and the biology of suicidal behavior. Psychiatr Clin North Am. 2008;31(2):247–269. doi: 10.1016/j.psc.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD. The psychobiology of neglect. Child Maltreat. 2005;10(2):150–172. doi: 10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- Duvernoy H. The human brain Surface, three-dimensional sectional anatomy and MRI. New York: Sringer-Verlag Wien; 1991. [Google Scholar]
- Eaton MJ, Whittemore SR. Autocrine BDNF secretion enhances the survival and serotonergic differentiation of raphe neuronal precursor cells grafted into the adult rat CNS. Experimental neurology. 1996;140(2):105–114. doi: 10.1006/exnr.1996.0121. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33(6):766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P, Version 2.0) New York: Biometrics Research Dept., New York State Psychiatric Institute; 1995. [Google Scholar]
- Flory JD, Mann JJ, Manuck SB, Muldoon MF. Recovery from major depression is not associated with normalization of serotonergic function. Biol Psychiatry. 1998;43(5):320–326. doi: 10.1016/s0006-3223(97)00480-0. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Huang Y, Hwang D-R, Talbot PS, Slifstein M, Van Heertum R, Abi-Dargham A, Laruelle M. Comparative Evaluation of Serotonin Transporter Radioligands 11C-DASB and 11C-McN 5652 in Healthy Humans. J Nucl Med. 2004a;45(4):682–694. [PubMed] [Google Scholar]
- Frankle WG, Huang Y, Hwang DR, Talbot PS, Slifstein M, Van Heertum R, Abi-Dargham A, Laruelle M. Comparative evaluation of serotonin transporter radioligands 11C-DASB and 11C-McN 5652 in healthy humans. J Nucl Med. 2004b;45(4):682–694. [PubMed] [Google Scholar]
- Fuller R, Wong D, Robertson D. Fluoxetine, a selective inhibitor of serotonin uptake. Medical Research Review. 1991;11(1):17–34. doi: 10.1002/med.2610110103. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psych. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF, Suomi SJ. Induced depression in monkeys. Behavioral Biology. 1974;12(3):273–296. doi: 10.1016/s0091-6773(74)91475-8. [DOI] [PubMed] [Google Scholar]
- Herold N, Uebelhack K, Franke L, Amthauer H, Luedemann L, Bruhn H, Felix R, Uebelhack R, Plotkin M. Imaging of serotonin transporters and its blockade by citalopram in patients with major depression using a novel SPECT ligand [123I]-ADAM. J Neural Transm. 2006;113(5):659–670. doi: 10.1007/s00702-005-0429-7. [DOI] [PubMed] [Google Scholar]
- Higley J, Suomi S, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biological Psychiatry. 1992;32(2):127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(16):7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Hummerich R, Schulze O, R‰dler T, Mikecz P, Reimold M, Brenner W, Clausen M, Schloss P, Buchert R. Inhibition of serotonin transport by (+)McN5652 is noncompetitive. Nuclear medicine and biology. 2006;33(3):317–323. doi: 10.1016/j.nucmedbio.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Ichimiya T, Suhara T, Sudo Y, Okubo Y, Nakayama K, Nankai M, Inoue M, Yasuno F, Takano A, Maeda J, Shibuya H. Serotonin transporter binding in patients with mood disorders: a PET study with [11C](+)McN5652. Biol Psychiatry. 2002;51(9):715–722. doi: 10.1016/s0006-3223(01)01351-8. [DOI] [PubMed] [Google Scholar]
- Ichise M, Vines D, Gura T, Anderson G, Suomi S, Higley J, Innis R. Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. Journal of Neuroscience. 2006;26(17):4638–4643. doi: 10.1523/JNEUROSCI.5199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jahng J, Kim J, Kim H, Kim B, Kang D, Lee J. Chronic food restriction in young rats results in depression- and anxiety-like behaviors with decreased expression of serotonin reuptake transporter. Brain Research. 2007;1150:100–107. doi: 10.1016/j.brainres.2007.02.080. [DOI] [PubMed] [Google Scholar]
- Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiat Res Neuroimag. 1997;75(1):31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Moss MB, Nicholson T, Jolesz F, Sandor T. An interactive procedure for extracting features of the brain from magnetic resonance images: The lobes. Human Brain Mapping. 1997;5(5):355–363. doi: 10.1002/(SICI)1097-0193(1997)5:5<355::AID-HBM4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Konno K, Matsumoto M, Togashi H, Yamaguchi T, Izumi T, Watanabe M, Iwanaga T, Yoshioka M. Early postnatal stress affects the serotonergic function in the median raphe nuclei of adult rats. Brain Research. 2007;1172:60–66. doi: 10.1016/j.brainres.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Lee J-H, Kim HJ, Kim JG, Ryu V, Kim B-T, Kang D-W, Jahng JW. Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neuroscience Research. 2007;58(1):32–39. doi: 10.1016/j.neures.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Lehto S, Tolmunen T, Joensuu M, Saarinen PI, Vanninen R, Ahola P, Tiihonen J, Kuikka J, Lehtonen J. Midbrain binding of [(123)I]nor-beta-CIT in atypical depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006 doi: 10.1016/j.pnpbp.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science (New York, NY) 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Higley JD, Lindell S, Newman T, McCormack K, Sanchez M. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques (Macaca mulatta) Behavioral Neuroscience. 2006;120(5):1017–1024. doi: 10.1037/0735-7044.120.5.1017. [DOI] [PubMed] [Google Scholar]
- Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB, Charney DS. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry. 1998;44(11):1090–1098. doi: 10.1016/s0006-3223(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20(2):771–782. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas LA, Blue ME, Siuciak JA, Altar CA. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci. 1995;15(12):7929–7939. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ, Arango V. Abnormalities of brain structure and function in mood disorders. In: Charney DS, Nestler EJ, Bunney BS, editors. Neurobiology of Mental Illness. New York: Oxford University Press; 1999. pp. 385–393. [Google Scholar]
- Manuck S, Bleil M, Petersen K, Flory J, Mann J, Ferrell R, Muldoon M. The socioeconomic status of communities predicts variation in brain serotonergic responsivity. Psychological Medicine. 2005;35(4):519–528. doi: 10.1017/s0033291704003757. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Reviews in Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, Mazumdar S, Mulsant BH, Houck PR, Lopresti BJ, Weissfeld LA, Reynolds CF. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29(12):2258–2265. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Houle S, Sagrati S, Carella A, Hussey DF, Ginovart N, Goulding V, Kennedy J, Wilson AA. Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry. 2004;61(12):1271–1279. doi: 10.1001/archpsyc.61.12.1271. [DOI] [PubMed] [Google Scholar]
- Miller JM, Oquendo MA, Ogden RT, Mann JJ, Parsey RV. Serotonin Transporter Binding as a Possible Predictor of One-Year Remission in Major Depressive Disorder. Journal of Psychiatric Research. 2008 doi: 10.1016/j.jpsychires.2008.01.012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy NV, Cowen PJ, Selvaraj S, Riedel WJ, Peers P, Kennedy J, Laruelle M, Rabiner E, Grasby PM. 5-HTTLPR Polymorphisms (SLC6A4 & rs25531) do not Affect 5-HT Transporter Expression in the Living Human Brain. Biological Psychiatry. 2008;63(1S):231S–232S. doi: 10.1016/j.neuroimage.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP, Jr, Weiss PM, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, Keller MB. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66(Suppl 7):5–13. [PubMed] [Google Scholar]
- Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, Bain EE, Luckenbaugh DA, Herscovitch P, Charney DS, Drevets WC. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry. 2004;61(8):765–773. doi: 10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Amsterdam JD, Wintering N, Ploessl K, Swanson RL, Shults J, Alavi A. 123I-ADAM Binding to Serotonin Transporters in Patients with Major Depression and Healthy Controls: A Preliminary Study. J Nucl Med. 2005;46(6):973–977. [PubMed] [Google Scholar]
- Ogden RT. On estimation of kinetic parameters in graphical analysis of PET imaging data. Statistics in Medicine. 2003;22:3557–3568. doi: 10.1002/sim.1562. [DOI] [PubMed] [Google Scholar]
- Ogden RT, Parsey RV, Mann JJ. Likelihood approach to parameter estimation in Logan graphical analaysis. Neuroimage. 2002;16(3):S73. [Google Scholar]
- Oquendo MA, Hastings RS, Huang YY, Simpson N, Ogden RT, Hu XZ, Goldman D, Arango V, Van Heertum RL, Mann JJ, Parsey RV. Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch Gen Psychiatry. 2007;64(2):201–208. doi: 10.1001/archpsyc.64.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Hu X, Goldman D, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, Mann JJ. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry. 2006a;163(1):48–51. doi: 10.1176/appi.ajp.163.1.48. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, Mann JJ. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry. 2006b;163(1):52–58. doi: 10.1176/appi.ajp.163.1.52. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Kegeles LS, Hwang DR, Simpson N, Abi-Dargham A, Mawlawi O, Slifstein M, Van Heertum RL, Mann JJ, Laruelle M. In vivo quantification of brain serotonin transporters in humans using [11C]McN 5652. J Nucl Med. 2000;41(9):1465–1477. [PubMed] [Google Scholar]
- Parsey RV, Ogden RT, Mann JJ. Determination of Volume of Distribution using Likelihood Estimation in Graphical Analysis: Elimination of Estimation Bias. Journal of Cerebral Blood Flow and Metabolism. 2003;23(12):1471–1478. doi: 10.1097/01.WCB.0000099460.85708.E1. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ. Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006c;31(8):1745–1749. doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, Van Heertum RL, Arango V, Mann JJ. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006d;59(2):106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Perry EK, Marshall EF, Blessed G, Tomlinson BE, Perry RH. Decreased imipramine binding in the brains of patients with depressive illness. Br J Psychiatry. 1983;142:188–192. doi: 10.1192/bjp.142.2.188. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Sandhu H, Hollenbeck N, Gunter T, Adams W, Madan A. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(5):543–549. doi: 10.1002/ajmg.b.30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praschak-Rieder N, Kennedy J, Wilson AA, Hussey D, Boovariwala A, Willeit M, Ginovart N, Tharmalingam S, Masellis M, Houle S, Meyer JH. Novel 5-HTTLPR Allele Associates with Higher Serotonin Transporter Binding in Putamen: A [11C] DASB Positron Emission Tomography Study. Biological Psychiatry. 2007;62(4):327–331. doi: 10.1016/j.biopsych.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Wilkins MR, Turkheimer F, Gunn RN, Udo de Haes J, de Vries M, Grasby PM. 5-Hydroxytryptamine1A receptor occupancy by novel full antagonist 2-[4-[4-(7-chloro-2,3-dihydro-1,4-benzdioxyn-5-yl)-1-piperazinyl]butyl]-1,2-benzi sothiazol-3-(2H)-one-1,1-dioxide: a[11C][O-methyl-3H]-N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl )cyclohexanecarboxamide trihydrochloride (WAY-100635) positron emission tomography study in humans. J Pharmacol Exp Ther. 2002;301(3):1144–1150. doi: 10.1124/jpet.301.3.1144. [DOI] [PubMed] [Google Scholar]
- Reimold M, Smolka MN, Schumann G, Zimmer A, Wrase J, Mann K, Hu XZ, Goldman D, Reischl G, Solbach C, Machulla HJ, Bares R, Heinz A. Midbrain serotonin transporter binding potential measured with [11C]DASB is affected by serotonin transporter genotype. J Neural Transm. 2007;114(5):635–639. doi: 10.1007/s00702-006-0609-0. [DOI] [PubMed] [Google Scholar]
- Roy A. Self-rated childhood emotional neglect and CSF monoamine indices in abstinent cocaine-abusing adults: possible implications for suicidal behavior. Psychiatry Research. 2002;112(1):69–75. doi: 10.1016/s0165-1781(02)00176-2. [DOI] [PubMed] [Google Scholar]
- Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12(4):331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15(3 Pt 1):1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy JP, Lafaille F, Lemoine P, Mrini A, Descarries L. Validation of the transporter ligand cyanoimipramine as a marker of serotonin innervation density in brain. J Nucl Med. 1994;35(11):1822–1830. [PubMed] [Google Scholar]
- Steiger H, Gauvin L, Israel M, Kin NM, Young SN, Roussin J. Serotonin function, personality-trait variations, and childhood abuse in women with bulimia-spectrum eating disorders. J Clin Psychiatry. 2004;65(6):830–837. doi: 10.4088/jcp.v65n0615. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Oquendo MA, Simpson N, Van Heertum RL, Mann JJ, Parsey RV. Brain serotonin1A receptor binding in major depression is related to psychic and somatic anxiety. Biol Psychiatry. 2005;58(12):947–954. doi: 10.1016/j.biopsych.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Rasmussen KLR, JD H. Primate models of behavioral and physiological change in adolescence. In: McAnarney K, Kreipe RE, Orr DP, Gormerci GD, editors. Textbook of adolescent medicine. Philadelphia: Saunders; 1992. pp. 135–140. [Google Scholar]
- Tafet GE, Toister-Achituv M, Shinitzky M. Enhancement of serotonin uptake by cortisol: a possible link between stress and depression. Cognitive, affective & behavioral neuroscience. 2001;1(1):96–104. doi: 10.3758/cabn.1.1.96. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. Three-dimensional proportional system: an approach of cerebral imaging. New York: Theime Medical Publisher; 1988. [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27(1-2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, Arango V. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. 1999;46(4):473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Weidenfeld J, Newman ME, Itzik A, Gur E, Feldman S. The amygdala regulates the pituitary-adrenocortical response and release of hypothalamic serotonin following electrical stimulation of the dorsal raphe nucleus in the rat. Neuroendocrinology. 2002;76(2):63–69. doi: 10.1159/000064430. [DOI] [PubMed] [Google Scholar]
- Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007;64(1):49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]


