Abstract
OBJECTIVE
The goal was to assess the impact of universal bilirubin screening on severe hyperbilirubinemia and phototherapy use.
METHODS
In this retrospective cohort study of 358 086 infants of ≥35 weeks and ≥2000 g born between January 1, 1995, and June 30, 2007, we obtained demographic data, bilirubin levels, and codes for inpatient phototherapy from existing databases. We compared the incidence of high total serum bilirubin (TSB) levels and phototherapy before and after implementation of universal screening and examined risk factors for high TSB levels.
RESULTS
A total of 38 182 infants (10.6%) were born at facilities that had implemented universal bilirubin screening. Compared with infants born at facilities that were not screening, these infants had a 62% lower incidence of TSB levels exceeding the American Academy of Pediatrics exchange guideline (0.17% vs 0.45%; P < .001), received twice the inpatient phototherapy (9.1% vs 4.2%; P < .001), and had slightly longer birth hospitalization lengths of stay (50.9 vs 48.7 hours; P < .001). Of those receiving phototherapy, 56% after initiation of universal screening had TSB levels at which phototherapy was recommended by the guideline, compared with 70% before screening. The adjusted odds ratio for developing TSB levels exceeding the guideline value was 0.28 (95% confidence interval: 0.20–0.40) for those born at a facility using TSB screening and 0.28 (95% confidence interval: 0.19–0.42) for those born at a facility using transcutaneous bilirubin screening.
CONCLUSIONS
Universal bilirubin screening was associated with a significantly lower incidence of severe hyperbilirubinemia but also with increased phototherapy use.
Keywords: bilirubin, jaundice, newborn, phototherapy, screening
The 2004 American Academy of Pediatrics (AAP) guideline on the management of hyperbilirubinemia recommends that every newborn be assessed for the risk of developing severe hyperbilirubinemia, by using predischarge total serum bilirubin (TSB) or transcutaneous bilirubin (TcB) measurements and/or assessment of clinical risk factors before discharge. 1 However, the effects of pre-discharge bilirubin screening on hyperbilirubinemia and phototherapy have not been studied adequately.2,3 In an 18-hospital system, Eggert et al4 showed that, after the initiation of a universal bilirubin screening program, the incidence of TSB levels of ≥25 mg/dL decreased from 1 in 1522 infants to 1 in 4037 infants. However, the authors did not provide information on how this result was achieved, such as increased use of phototherapy during the birth hospitalization, identification of more infants with hyperbilirubinemia, better follow-up monitoring, or improved lactation support.
In September 2005, the Northern California Kaiser Permanente Medical Care Program (NC-KPMCP) implemented the 2004 AAP guidelines by recommending that all newborns undergo TSB or TcB measurements before discharge. In addition, follow-up monitoring of these newborns, according to the risk group determined by plotting of their bilirubin measurements on the nomogram presented by Bhutani and colleagues2,5–7 and the use of a clinical risk index,8 was suggested.
The objective of this study was to determine the impact of universal bilirubin screening on the incidence of TSB levels above the 2004 AAP exchange level. In addition, we evaluated the mechanism for any differences in the incidence of severe hyperbilirubinemia by examining temporal trends in bilirubin testing and phototherapy use and comparing management with the AAP guidelines.
METHODS
Study Design
We used a historical cohort design to compare the incidence of severe hyperbilirubinemia and phototherapy use before and after implementation of universal bilirubin screening.
Subjects and Settings
We included all live-born infants born in 11 NC-KPMCP hospitals between January 1, 1995, and June 30, 2007, if their gestational age was ≥35 weeks and their birth weight was ≥2000 g. The NC-KPMCP serves a population of 3.3 million members, which constitutes ~30% of the insured population in Northern California. All NC-KPMCP facilities share the same common medical record numbers and database systems, which permits linkage of maternal and neonatal records to each other and to multiple information systems (eg, laboratory and hospitalization data). The NC-KPMCP institutional review board and the University of California, San Francisco, Committee on Human Research approved this project.
The NC-KPMCP implementation of the AAP guideline includes bilirubin screening before discharge, with either TcB or TSB measurements. TcB measurements must be confirmed with TSB measurements if the TcB level is ≥ 15 mg/dL or if the TcB level plus 3 mg/dL is above the 2004 AAP phototherapy treatment line. Since 1998, as a result of reviews of internal analyses, 2 randomized trials,9,10 and a case-control study,11 all infants born at NC-KPMCP hospitals undergo amandatory follow-up visit within 48 hours after discharge, with a pediatrician or nurse practitioner. In addition, a small number of infants receive home visits, at the discretion of the discharging physician. With the implementation of universal bilirubin screening, the suggested timing of follow-up visits was based on amodified clinical risk index8 and the bilirubin values plotted on the nomogram described by Bhutani et al6 (Tables 1 and 2).
TABLE 1.
Modified Clinical Risk Index for Hyperbilirubinemia
| Subtotal A | Subtotal B | Subtotal C | |||
|---|---|---|---|---|---|
| Gestational Age |
Score | Feeding | Score | Other | Score |
| 35 wk | 12 | Breast milk only | 6 | Jaundice requiring phototherapy in parent or sibling |
6 |
| 36 wk | 10 | Formula only | −6 | Cephalhematoma or bruising | 4 |
| 37 wk | 7 | Breast milk + formula | 0 | Asian | 4 |
| 38 wk | 4 | Maternal age of ≥25 y | 3 | ||
| 39 wk | 2 | Male | 1 | ||
| 40 wk | 0 | ||||
| 41 wk | −2 | ||||
| 42 wk | −4 | ||||
Total score = subtotal A + subtotal B + subtotal C.
TABLE 2.
Recommended Follow-up Period (days) on the Basis of Clinical Risk Index Score TSB and TcB Risk Group Measurements
| Clinical Risk Index Score |
TSB or TcB Risk Group | |||
|---|---|---|---|---|
| Low | Low-Intermediate | High-Intermediate | High | |
| ≥15 | 1–2 | 1 | 1 | 1 |
| 10–14 | 1–3 | 1–2 | 1 | 1 |
| 5–9 | a | 1–3 | 1–2 | 1 |
| <5 | a | a | 1–3 | 1 |
TSB levels were recommended if TcB values were ≥15 mg/dL or within 3 mg/dL of the AAP phototherapy. Bilirubin values were plotted on the nomogram described by Bhutani et al6 to determine risk group.
Very low risk of significant jaundice, but follow-up evaluation for other reasons may be required.
Of the 11 hospitals in the NC-KPMCP, 4 adopted universal TcB screening policies, 5 adopted universal TSB screening policies, and 2 had not adopted either as of June 2007. For facilities reporting TcB screening, nursery directors reported initiation of universal screening in October 2005. For TSB screening, we considered the implementation date to be the month in which the facility achieved screening of ≥ 95% of all newborns, which ranged from September 2004 to February 2007.
Outcome Variables
From existing KPMCP databases, we obtained all TSB values from an infant’s first month of life, by using previously described methods.11–16 We excluded any TSB measurements for which a corresponding conjugated or direct bilirubin measurement constituted ≥20% of the TSB level. We also obtained direct antiglobulin test results, if the test were performed. For all hospitalizations in the first month of life, we ascertained the use of phototherapy from International Classification of Diseases, Ninth Revision procedure codes. For each TSB value, we compared the value with the 2004 AAP treatment curves for phototherapy and exchange transfusions. Each infant’s risk group was assigned on the basis of gestational age and direct antiglobulin test results (low risk: ≥38 weeks and negative or missing direct antiglobulin test results; medium risk: ≥38 weeks and positive direct antiglobulin test results or <38 weeks and negative or missing direct antiglobulin test results; high risk: <38 weeks and positive direct antiglobulin test results). We defined missed phototherapy as having a TSB level above the level at which the AAP recommends phototherapy, for a given time point and risk group, but not receiving phototherapy. In contrast, we defined subthreshold phototherapy as receiving phototherapy but never having a TSB value over the threshold for phototherapy according to the AAP guideline. We defined appropriate phototherapy as receiving phototherapy and having ≥1 TSB value over the threshold for phototherapy according to the AAP guideline.
Statistical Methods
Characteristics of infants born before and after implementation of universal bilirubin screening were compared by using χ2 and Student’s t tests, as appropriate. We used χ2 and Fisher’s exact tests to compare the incidence of severe hyperbilirubinemia and phototherapy in these groups. To assess the odds of developing a TSB level of ≥25 mg/dL or above the 2004 AAP exchange transfusion threshold or receiving phototherapy, we used multivariate logistic regression models, including an indicator variable for being born at a facility after implementation of TSB or TcB screening. Covariables included were gender, gestational age, small-for-gestational age status,17 large-for-gestational age status,17 race, facility, and AAP risk group. We performed all analyses with Stata 9.0 (Stata Corp, College Station, TX).
RESULTS
A total of 319 904 infants were born at a facility before universal bilirubin screening and 38 182 infants after implementation of screening. The mean number of TSB tests per infant in the first month of life was 0.8 ± 1.7 tests per infant (mean ± SD) before implementation, compared with 1.9 ± 2.0 tests per infant after implementation (P <.001). The characteristics of the 2 groups were otherwise similar except that, after implementation of universal screening, the proportion of Asian infants was greater (22% vs 18%; P < .001) and more infants were in the AAP medium-risk group (14.2% vs 12.4%; P <.001) (Table 3). The mean length of birth hospitalization was 48.7 hours before universal screening and 50.9 hours after implementation, an increase of 2.2 hours (95% confidence interval [CI]: 1.4 –2.9 hours). We also compared lengths of birth hospitalization with stratification according to phototherapy during the birth hospitalization. In the phototherapy group, the mean length of stay was 125.7 hours before universal screening and 97.2 hours after implementation of screening, a decrease of 28.5 hours (95% CI: 23.5–33.4 hours). In the group not receiving phototherapy, the mean length of stay was 46.6 hours before universal screening and 47.6 hours after implementation of screening, an increase of 1.0 hour (95% CI: 0.3–1.7 hours).
TABLE 3.
Characteristics of Infants Born at Facilities With and Without Universal Bilirubin Screening
| No Universal Screening (N = 319 904) |
Universal Screening (N = 38 182) |
P | |
|---|---|---|---|
| Gestational age, mean ± SD, wk | 39.2 ± 1.4 | 39.0 ± 1.4 | <.001 |
| Birth weight, mean ± SD, g | 3459 ± 505 | 3418 ± 499 | <.001 |
| Male, n (%) | 163 406 (51) | 19 706 (52) | .05 |
| SGA, n (%) | 3702 (1.2) | 408 (1.1) | .1 |
| LGA, n (%) | 8571 (2.7) | 975 (2.2) | .2 |
| Black, n (%) | 26 259 (8.2) | 3048 (8.0) | .1 |
| Asian, n (%) | 56 248 (18) | 9052 (22) | <.001 |
| AAP risk group, n (%) | |||
| Low risk | 276 386 (87.3) | 35 643 (85.5) | <.001 |
| Medium risk | 39 821 (12.5) | 5437 (14.2) | |
| High risk | 697 (0.2) | 102 (0.3) |
SGA indicates small for gestational age; LGA, large for gestational age.
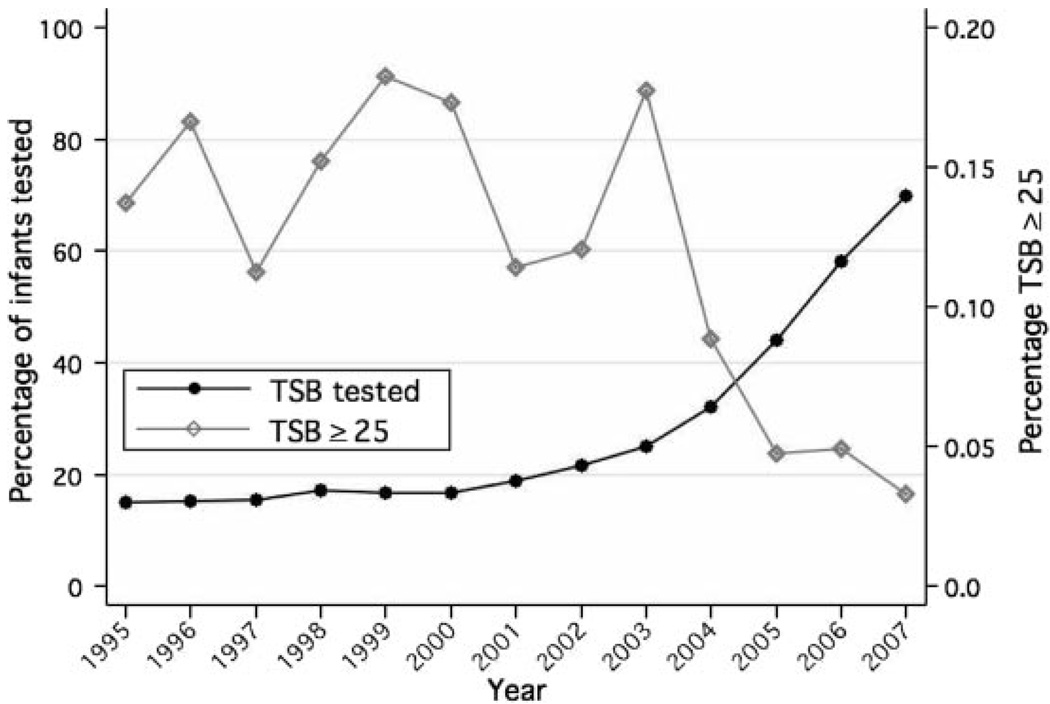
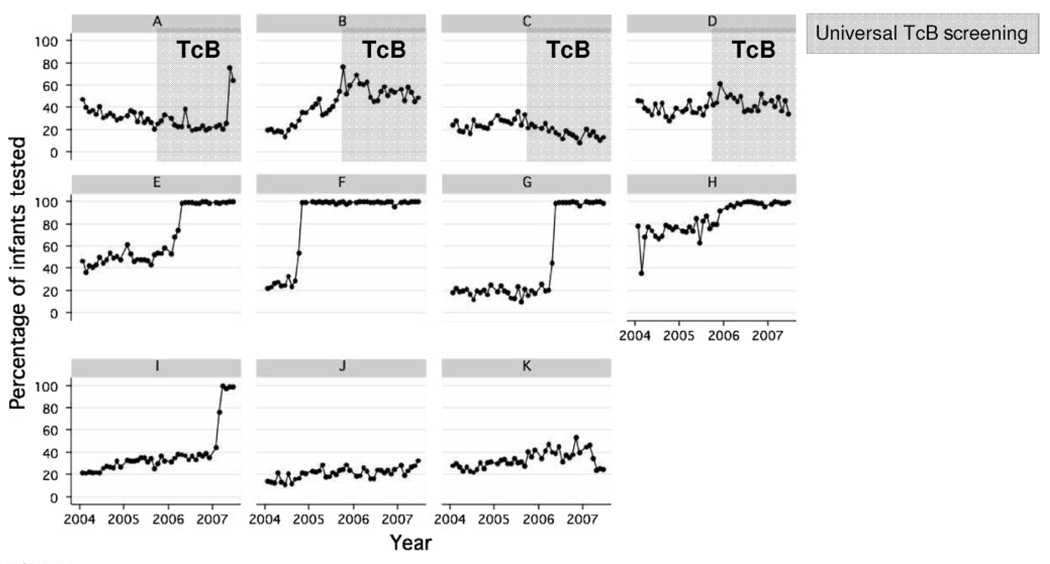
Over the 12-year period between 1995 and 2007, as the proportion of infants who underwent TSB measurements during their birth hospitalization increased, the proportion of infants who had maximal TSB levels of ≥25 mg/dL decreased (Fig 1). The proportion of infants tested remained relatively constant until 2001, when it began to increase, with more-significant increases beginning in 2003. In Fig 2, the monthly proportions of infants who underwent TSB screening at each facility between 2004 and 2007 are shown. Facilities A, B, C, and D initiated universal TcB screening in October 2005. Facilities J and K did not institute a screening program. The facilities that implemented universal TSB screening achieved compliance rates near 100%. In facilities that implemented universal TcB screening, TSB levels were measured only if TcB levels were high.
FIGURE 1.
Temporal trends (1995–2007) in TSB testing and TSB levels of ≥25 mg/dL. TcB testing not included in figure.
FIGURE 2.
Temporal trends (2004 –2007) in TSB testing according to facility.
There was a 62% reduction in the incidence of maximum TSB levels above the exchange guideline, from 0.45% before universal screening to 0.17% after screening (P<.001) (Table 4). Similarly, there was a 74% reduction in the incidence of maximum TSB levels of 25 to 29.9 mg/dL, from 0.12% before universal screening to 0.031% after screening (P<.001). In contrast, there was a 56% increase in the incidence of maximum TSB levels of 15 to 19.9 mg/dL, from 9.59% before universal screening to 14.94% after screening (P <.001).
TABLE 4.
Incidence of Hyperbilirubinemia
| Incidence, % | Change, % |
P | ||
|---|---|---|---|---|
| No Universal Screening |
Universal Screening |
|||
| TSB level at or above AAP exchange levela | 0.45 | 0.17 | −62 | <.001 |
| TSB level of 15–19.9 mg/dL | 9.59 | 14.94 | +56 | <.001 |
| TSB level of 20–24.9 mg/dL | 2.02 | 1.37 | −32 | <.001 |
| TSB level of 25–29.9 mg/dL | 0.12 | 0.031 | −74 | <.001 |
| TSB level of ≥ 30 mg/dL | 0.012 | 0.0052 | −57 | .3 |
From the 2004 AAP exchange transfusion guideline.1
Our multivariate logistic regression analysis showed that being born at a facility using universal bilirubin screening was protective. The adjusted odds ratio (OR) for developing a TSB level of ≥25 mg/dL was 0.22 (95% CI: 0.11– 0.48) for those born at a facility using TSB screening and 0.25 (95% CI: 0.12– 0.53) for those born at a facility using TcB screening, compared with facilities and years when universal screening was not being performed. Similarly, the adjusted OR for developing a TSB level over the 2004 AAP exchange threshold was 0.28 (95% CI: 0.20–0.40) for those born at a facility using TSB screening and 0.28 (95% CI: 0.19–0.42) for those born at a facility using TcB screening. The adjusted OR for phototherapy during the birth hospitalization was 3.03 (95% CI: 2.84 –3.24) for those born at a facility using TSB screening and 2.43 (95% CI: 2.24 –2.64) for those born at a facility using TcB screening. The adjusted OR for readmission for phototherapy was 1.30 (95% CI: 1.17–1.45) for those born at a facility using TSB screening and 2.12 (95% CI: 1.93–2.34) for those born at a facility using TcB screening.
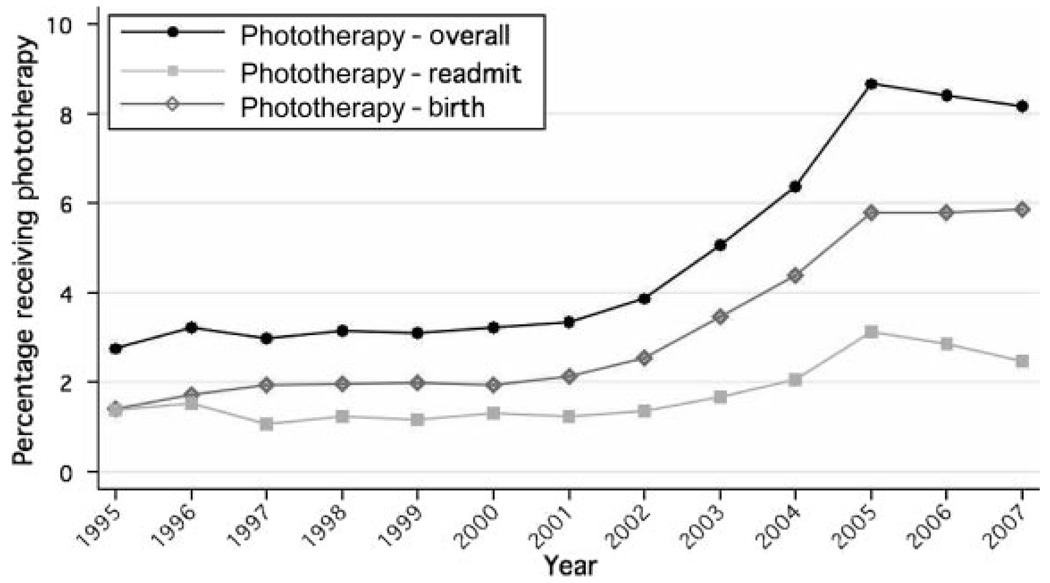
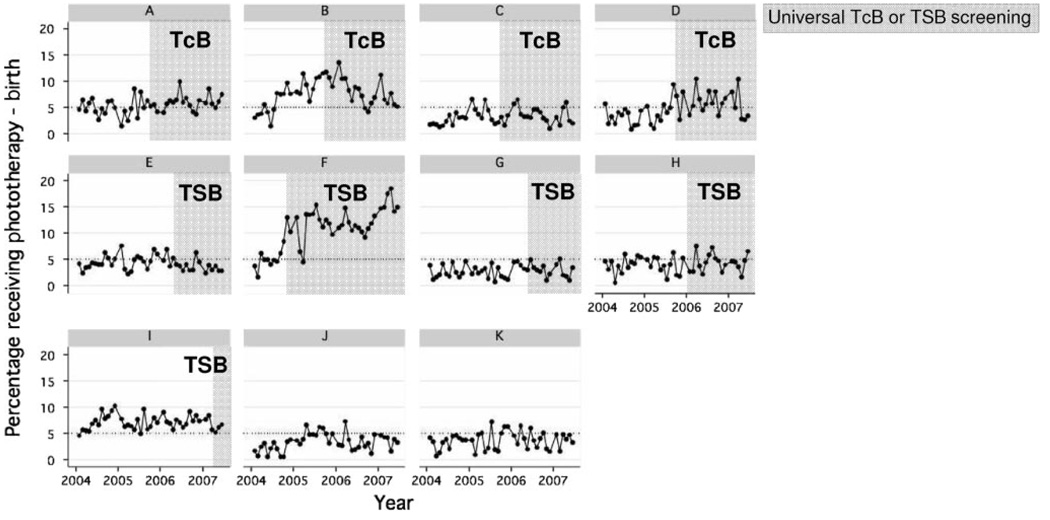
Phototherapy use began to increase after 2001 and increased dramatically between 2003 and 2005, before leveling off (Fig 3). Although there was an increase in readmissions for phototherapy after screening (2.7% vs 1.6%; P <.001), the overall increase in phototherapy use was attributable mainly to a large increase in phototherapy during the birth hospitalization (2.7% vs 6.7%; P < .001). There was marked interfacility variation in phototherapy use and in changes over time (Fig 4). Facilities B, D, and F had months after the implementation of universal screening when the proportion of infants receiving phototherapy exceeded 10%.
FIGURE 3.
Temporal trends (1995–2007) in phototherapy (PT) use.
FIGURE 4.
Temporal trends (2004 –2007) in phototherapy use during the birth hospitalization according to facility.
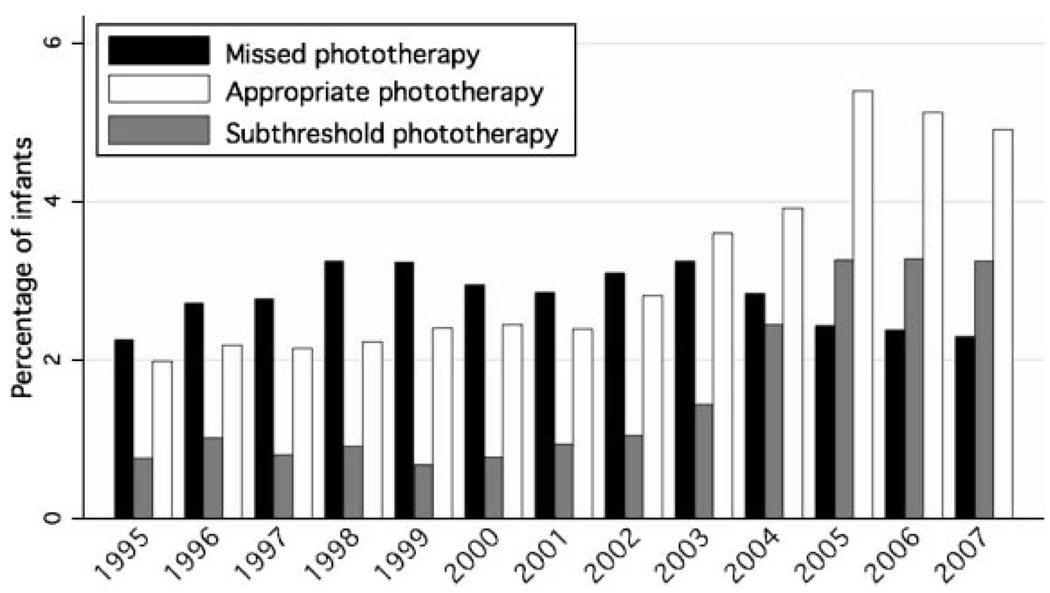
To assess the appropriateness of the phototherapy use, we compared all TSB values with the 2004 AAP guideline on the management of hyperbilirubinemia. 1 Among infants who received phototherapy during their birth hospitalization, the proportion of infants who had TSB measurements indicating the need for phototherapy decreased from 60% before screening to 43% after screening. Among infants who were readmitted for phototherapy, corresponding proportions were larger and less affected by screening (89% vs 84%). Figure 5 depicts the proportions of infants with subthreshold phototherapy, missed phototherapy, and appropriate photo-therapy according to year. The proportion with subthreshold phototherapy began to increase in 2003 and plateaued in 2005. In contrast, the proportion with missed phototherapy was relatively constant and then decreased slightly in 2005–2007. The proportion of infants receiving appropriate phototherapy began to increase in 2003, with a more-significant increase beginning in 2005.
FIGURE 5.
Comparison of phototherapy use with the 2004 AAP guideline.
Examination of discharge diagnoses identified 5 infants with International Classification of Diseases, Ninth Revision codes for kernicterus. All of the infants were born before September 2005. For 2 of the infants, the diagnosis was clearly an error, because neither the clinical course nor the maximal TSB levels (5.6 and 16.0 mg/dL) were consistent with the diagnosis. A third infant had a maximal TSB level of 27.5 mg/dL, with equivocal MRI results; however, outpatient records at 4 years of age indicated that the condition of the child was completely normal, which suggests that the diagnosis was in error. Of the remaining cases, 1 infant was critically ill from birth with multiple-organ failure, had a maximal TSB level of 27.3 mg/dL, and died at 4 days of age, with kernicterus confirmed at autopsy; the second infant, who is still alive with significant sequelae, had a first TSB level of 48.0 mg/dL at 81 hours of age and positive blood culture results for Enterococcus fecalis and coagulase-negative Staphylococcus.
DISCUSSION
We found a dramatic decrease in the incidence of TSB levels of ≥25 mg/dL as the proportion of infants who underwent bilirubin screening during their birth hospitalization increased. We saw no difference in the impact of screening according to whether the facility performed TSB or TcB testing. These results are reassuring that screening with a noninvasive method worked as well as screening with a blood test. It is important to remember that TSB confirmation was recommended in conjunction with TcB screening if the values were within 3 mg/dL of the AAP phototherapy threshold or ≥15 mg/dL which might have avoided any inaccuracy of TcB results at higher bilirubin levels. Our results are consistent with the findings of Eggert et al,4 who also reported a decreased incidence of severe hyperbilirubinemia with universal bilirubin screening. With universal bilirubin screening, we seem to be identifying more infants who require phototherapy who might otherwise have been missed or identifying infants at earlier points in their courses. After implementation of universal screening, a larger proportion of infants received appropriate phototherapy. Furthermore, the incidence of TSB levels of 15 to 19.9 mg/dL increased while the incidence of more-severe hyperbilirubinemia decreased, which suggests that one mechanism for the decrease was earlier identification and treatment of hyperbilirubinemia.
Although the results of our study are encouraging with respect to the potential benefits of universal screening, we caution that other factors might have contributed to our findings. The reduction in the incidence of TSB levels of ≥25 mg/dL began before the implementation of universal screening and seemed to plateau with universal screening. This might be related to the increases in TSB testing that were observed in the years before universal screening or a general increase in the level of physician concern regarding hyperbilirubinemia.
The mechanism through which universal bilirubin screening reduces severe hyperbilirubinemia is likely multifactorial. The proportion of infants who received appropriate phototherapy increased with universal screening, and there was a small decrease in the proportion with missed phototherapy. The universal screening program was linked to follow-up recommendations. Other factors, such as better lactation support or earlier formula supplementation, also might have contributed to the reduction in hyperbilirubinemia. Because the trend toward fewer cases of severe hyperbilirubinemia began before universal screening was implemented, increased clinician concern about jaundice and kernicterus that predated screening might have led to management changes that contributed to the decrease.
Perhaps the more-important question is whether universal screening will reduce the incidence of kernicterus. This question is difficult to answer, given the infrequency of kernicterus. In our study, only 2 cases were identified among 358 086 infants, which made it difficult to draw any statistical conclusions. If TSB levels of ≥25 mg/dL are considered a proxy or intermediate outcome for kernicterus, then universal bilirubin screening may be a potentially important intervention to reduce the incidence of kernicterus. Kernicterus may occur despite universal screening, however, because TSB levels of ≥25 mg/dL occurred despite universal screening (0.036% in our population). This might be attributable to missed follow-up care, sepsis, glucose-6-phosphate dehydrogenase deficiency, 18 failure of physicians to treat high bilirubin levels,19 feeding difficulties, or other factors.
We also found that, with increased bilirubin testing, there were significant increases in phototherapy during the birth hospitalization and readmissions for phototherapy, often at TSB levels below those recommended by the AAP. This was in contrast to studies by Petersen et al3 and Eggert et al4 that found reductions in readmissions. In the study by Petersen et al,3 however, there was a high baseline readmission rate (4.5%, compared with 1.6% in our study). In the study by Eggert et al,4 the readmission rate was low at baseline (0.55%) and improved only slightly (to 0.43%). There was significant heterogeneity among the facilities in this study regarding the use of phototherapy, which indicates that universal screening by itself does not lead to significantly higher rates of phototherapy. We speculate that physician concern (anxiety) about hyperbilirubinemia, coupled with increased testing, could led to subthreshold use of phototherapy.20,21
Neonatologists at some facilities reported starting phototherapy for infants with TSB levels lower than those recommended by the AAP in hopes of preventing readmissions for phototherapy. Some additional reasons for starting phototherapy at subthreshold TSB levels might include a high rate of increase in TSB levels, concern about the reliability of follow-up monitoring, or additional risk factors. Physician practices to initiate phototherapy also might have been influenced by payer factors such as the potential for parental co-payments for hospital readmissions.
The aim of the 2004 AAP guideline was to “reduce the frequency of severe neonatal hyperbilirubinemia and bilirubin encephalopathy and minimize the risk of unintended harm such as increased anxiety, decreased breastfeeding, or unnecessary treatment for the general population and excessive cost and waste.”1 Our data suggest that, although there has been some success regarding the first goal, it might have come at the cost of sometimes-excessive treatment. Somewhat reassuring were the findings that the mean length of birth hospitalization increased by only 2.2 hours with universal bilirubin screening and the length of stay for infants receiving phototherapy actually decreased by >1 day. The decreased length of stay for infants receiving phototherapy might have been attributable to more-prompt initiation of phototherapy, which resulted in shorter stays, or to more infants receiving subthreshold phototherapy, which reduced the mean length of stay for all infants receiving phototherapy.
Our study has some limitations. Phototherapy use was derived from procedure codes and not chart review, which probably led to a slight (<10%) underestimation of phototherapy use.22 We did not have information on how other interventions, including breastfeeding, formula supplementation, and lactation support, might have changed over the time period. We also did not have complete information on all risk factors (eg, sepsis and ill appearance) that might have influenced the decision to start phototherapy and altered our classification of subthreshold phototherapy. However, these factors are unlikely to have changed over time and would not explain the temporal changes in subthreshold phototherapy.
CONCLUSIONS
Universal bilirubin screening, with either TcB or TSB measurements, was associated with increased identification of newborns needing phototherapy and a significantly lower incidence of severe hyperbilirubinemia. There also was a substantial increase in the use of phototherapy, often at bilirubin levels lower than those recommended by the AAP. These results show the importance of monitoring the use of phototherapy as well as the frequency of bilirubin testing and hyperbilirubinemia after guideline implementation. With additional education, the reduction in serious hyperbilirubinemia with universal bilirubin screening may be achieved without excessive phototherapy use.
WHAT’S KNOWN ON THIS SUBJECT
The 2004 AAP guideline on the management of hyperbilirubinemia recommends that every newborn be assessed for the risk of developing severe hyperbilirubinemia, through predischarge TSB or TcB measurements and/or assessments of clinical risk factors.
WHAT THIS STUDY ADDS
Universal bilirubin screening can increase the identification of newborns needing phototherapy and significantly decrease the incidence of severe hyperbilirubinemia. There also may be an increase in the use of phototherapy at bilirubin levels lower than recommended by the AAP.
ACKNOWLEDGMENT
This work was supported by grant RO1 HD047557 from the National Institute of Child Health and Human Development.
Abbreviations
- TSB
total serum bilirubin
- TcB
transcutaneous bilirubin
- AAP
American Academy of Pediatrics
- NC-KPMCP
Northern California Kaiser Permanente Medical Care Program
- OR
odds ratio
- CI
confidence interval
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114(1):297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- 2.Bhutani VK, Johnson LH, Schwoebel A, Gennaro S. A systems approach for neonatal hyperbilirubinemia in term and near-term newborns. J Obstet Gynecol Neonatal Nurs. 2006;35(4):444–455. doi: 10.1111/j.1552-6909.2006.00044.x. [DOI] [PubMed] [Google Scholar]
- 3.Petersen JR, Okorodudu AO, Mohammad AA, Fernando A, Shattuck KE. Association of transcutaneous bilirubin testing in hospital with decreased readmission rate for hyperbilirubinemia. Clin Chem. 2005;51(3):540–544. doi: 10.1373/clinchem.2004.037804. [DOI] [PubMed] [Google Scholar]
- 4.Eggert LD, Wiedmeier SE, Wilson J, Christensen RD. The effect of instituting a prehospital-discharge newborn bilirubin screening program in an 18-hospital health system. Pediatrics. 2006;117(5) doi: 10.1542/peds.2005-1338. Available at: www.pediatrics.org/cgi/content/full/117/5/e855. [DOI] [PubMed] [Google Scholar]
- 5.Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, Johnson LH. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106(2) doi: 10.1542/peds.106.2.e17. Available at: www.pediatrics.org/cgi/content/full/106/2/e17. [DOI] [PubMed] [Google Scholar]
- 6.Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103(1):6–14. doi: 10.1542/peds.103.1.6. [DOI] [PubMed] [Google Scholar]
- 7.Keren R, Luan X, Friedman S, Saddlemire S, Cnaan A, Bhutani VK. A comparison of alternative risk-assessment strategies for predicting significant neonatal hyperbilirubinemia in term and near-term infants. Pediatrics. 2008;121(1) doi: 10.1542/peds.2006-3499. Available at: www.pediatrics.org/cgi/content/full/121/1/e170. [DOI] [PubMed] [Google Scholar]
- 8.Newman TB, Liljestrand P, Escobar GJ. Combining clinical risk factors with serum bilirubin levels to predict hyperbilirubinemia in newborns. Arch Pediatr Adolesc Med. 2005;159(2):113–119. doi: 10.1001/archpedi.159.2.113. [DOI] [PubMed] [Google Scholar]
- 9.Lieu TA, Braveman PA, Escobar GJ, Fischer AF, Jensvold NG, Capra AM. A randomized comparison of home and clinic follow-up visits after early postpartum hospital discharge. Pediatrics. 2000;105(5):1058–1065. doi: 10.1542/peds.105.5.1058. [DOI] [PubMed] [Google Scholar]
- 10.Escobar GJ, Braveman PA, Ackerson L, et al. A randomized comparison of home visits and hospital-based group follow-up visits after early postpartum discharge. Pediatrics. 2001;108(3):719–727. doi: 10.1542/peds.108.3.719. [DOI] [PubMed] [Google Scholar]
- 11.Escobar GJ, Gonzales VM, Armstrong MA, Folck BF, Xiong B, Newman TB. Rehospitalization for neonatal dehydration: a nested case-control study. Arch Pediatr Adolesc Med. 2002;156(2):155–161. doi: 10.1001/archpedi.156.2.155. [DOI] [PubMed] [Google Scholar]
- 12.Escobar GJ, Fischer A, Kremers R, Usatin MS, Macedo AM, Gardner MN. Rapid retrieval of neonatal outcomes data: the Kaiser Permanente Neonatal Minimum Data Set. Qual Manag Health Care. 1997;5(4):19–33. [PubMed] [Google Scholar]
- 13.Kuzniewicz MW, Escobar GJ, Wi S, Liljestrand P, McCulloch C, Newman TB. Risk factors for severe hyperbilirubinemia among infants with borderline bilirubin levels: a nested case-control study. J Pediatr. 2008;153(2):234–240. doi: 10.1016/j.jpeds.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman TB, Escobar GJ, Gonzales VM, Armstrong MA, Gardner MN, Folck BF. Frequency of neonatal bilirubin testing and hyperbilirubinemia in a large health maintenance organization. Pediatrics. 1999;104(5):1198–1203. [PubMed] [Google Scholar]
- 15.Escobar GJ. The neonatal “sepsis work-up”: personal reflections on the development of an evidence-based approach toward newborn infections in a managed care organization. Pediatrics. 1999;103 1 suppl E:360–373. [PubMed] [Google Scholar]
- 16.Selby JV. Linking automated databases for research in managed care settings. Ann Intern Med. 1997;127(8):719–724. doi: 10.7326/0003-4819-127-8_part_2-199710151-00056. [DOI] [PubMed] [Google Scholar]
- 17.Fenton TR. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371(9606):64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson LR, Escobar GJ, Takayama JI, Newman TB. Phototherapy use in jaundiced newborns in a large managed care organization: do clinicians adhere to the guideline? Pediatrics. 2003;111(5) doi: 10.1542/peds.111.5.e555. Available at: www.pediatrics.org/cgi/content/full/111/5/e555. [DOI] [PubMed] [Google Scholar]
- 20.Watchko JF. Vigintiphobia revisited. Pediatrics. 2005;115(6):1747–1753. doi: 10.1542/peds.2004-1748. [DOI] [PubMed] [Google Scholar]
- 21.Watchko JF, Oski FA. Bilirubin 20 mg/dL = vigintiphobia. Pediatrics. 1983;71(4):660–663. [PubMed] [Google Scholar]
- 22.Newman TB, Kuzniewicz MW, Liljestrand P, Wi S, McCulloch C, Escobar GJ. Numbers needed to treat with phototherapy according to American Academy of Pediatrics guidelines. Pediatrics. 2009;123(5):1352–1359. doi: 10.1542/peds.2008-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]