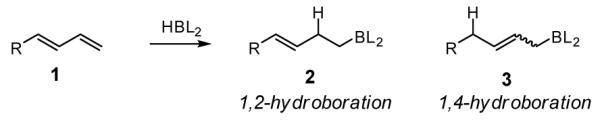
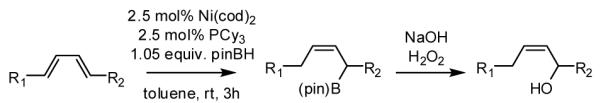
Since its first demonstration by Manning and Noth,1 catalytic hydroboration has become a powerful tool for organic synthesis.2 While this reaction is often applied to the transformation of alkenes and alkynes, the hydroboration of other hydrocarbon substrates such as dienes,3 allenes,4 and enynes5 remains relatively unexplored. Selective reaction of diene substrates, in particular, is remarkably useful; these reactions convert simple hydrocarbon building blocks into alkene-containing organoboranes, compounds which are versatile intermediates in organic synthesis.6 In this regard, only a few examples of catalytic diene hydroboration have been described with none able to effectively convert terminally-substituted dienes such as 1 to allylboronates 3. Both Ni(II)/dppe3a and Rh(I)/dppe3c were found to catalyze the hydroboration of dienes and, similar to the non-catalyzed reaction with 9-BBN,7 furnish the 1,2-addition product (2, Scheme 1) selectively. Suzuki and Miyaura found that 1,4-hydroboration product can be accomplished with Pd and Rh catalysis. This is an excellent reaction with 2-substituted and 2,3-disubstituted butadienes, but for terminally substituted dienes is limited to cyclic substrates (i.e. cyclohexadiene).3b Recently, Ritter described an iron catalyst that exhibits remarkable selectivity in the 1,4-hydroboration of 2-substituted dienes.3d This is a powerful method for the regio- and stereoselective synthesis of many useful allyl boronates, but it is less general for terminally-substituted diene substrates that lack substitution at the 2-position.8 In this report, we describe a catalytic 1,4-hydroboration of 1-substituted dienes that is highly regio- and stereoselective and is an effective complement to the above-described methods. Significantly, this reaction provides synthetically useful allylboronates in an operationally convenient fashion and, upon oxidation, it delivers stereodefined substituted allylic alcohols which are often difficult to access through single-step synthesis routes.9
Scheme 1.
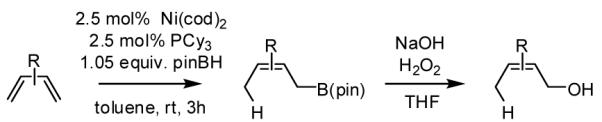
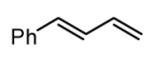
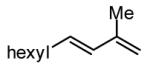
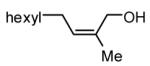
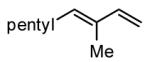
During our studies on the Pt-catalyzed enantioselective 1,4-diboration of dienes,10 we considered that a borane, used in place of B2(pin)2, might deliver substituted allylboronates as the reaction product. An initial survey examined the reaction of 1,3-decadiene and pinacolborane at room temperature (Scheme 2). While the Pt-based catalysts were ineffective (as were Pd-based catalysts), 2.5 mol% Ni(cod)2 combined with PCy3 provides a particularly effective catalyst that efficiently converts 1,3-decadiene to the primary (Z)-allylic boronate ester in just 20 minutes.11 Subsequent to this finding, a survey of the substrate scope was undertaken to address parameters that might influence the reaction. Several conclusions can be drawn from these experiments (Table 1). First, substrates with aromatic substituents were found to react equally as well as substrates containing simple alkyl groups (entries 1 and 2). Second, multiply-substituted dienes react with high levels of stereocontrol (entries 3-6) and can provide products with difficult-to-access substitution patterns. Third, as suggested by the product alkene configuration, the reactive diene conformer is likely the S-cis arrangement and, accordingly, substrates less able to adopt this conformation are less reactive. For example, the S-cis conformer for entry 5 suffers an A(1,3) interaction and this substrate requires higher temperature (60 °C) for reaction; the more hindered example in entry 6 possesses off-setting A(1,3) interactions in the S-cis and S-trans rotamers and this reaction proceeds efficiently at room temperature. Lastly, a variety of synthetically-common functional groups are tolerated. For instance, the reaction tolerates the presence of silyl ethers, benzyl ethers, pthalimides, esters and unprotected alcohols (entries 7-11).
Scheme 2.
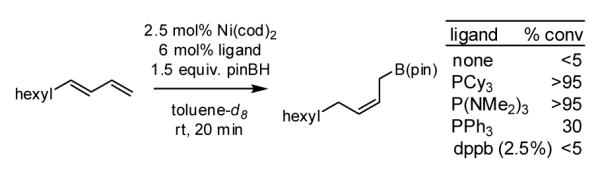
Table 1.
Ni-Catalyzed 1,4-Hydroboration of Simple 1,3-Dienesa
 | |||
|---|---|---|---|
| entry | substrate | product | yield (%)b |
| 1 |
|
|
85 |
| 2 |
|
|
91c |
| 3 |
|
|
93 |
| 4 |
|
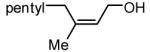
|
81 |
| 5 |
|
|
29d |
| 6 |
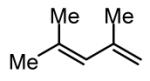
|
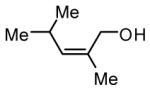
|
63e |
| 7 |
|
|
56 |
| 8 |
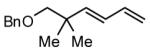
|
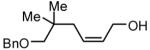
|
89 |
| 9 |
|
|
81f |
| 10 |
|
|
61f |
| 11 |
|
|
72g |
| 12 |
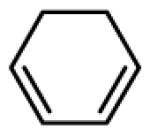
|
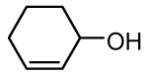
|
60 |
Reactions conducted at [substrate]=0.25M and oxidized with 30% H2O2 and 3M NaOH.
Isolated yield of purified material. Values are an average of two experiments.
1 mol% catalyst Ni(cod)2 and 1 mol% PCy3 employed for this experiment.
Reaction 12 h at 60 °C. Product isolated with an equimolar quantity of 4,8-dimethyl-3,7-nonadien-2-ol.
Reaction for 12 h at 25 °C.
Oxidation with buffered (pH=7) H2O2.
2.1 equiv. pinBH employed.
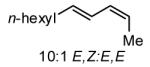
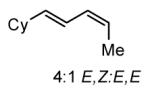
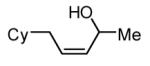
The Ni-catalyzed diene hydroboration is sufficiently sensitive to diene substituents that internal dienes react in a highly regioselective fashion. As revealed by the oxidation products in Table 2, borylation occurs exclusively at the less hindered carbon and, notably, the regiocontrol is high even when the required discrimination is between a methyl and an n-alkyl group (entry 4). Also of note, high stereo- and regioselection are obtained even when mixtures of diene stereoisomers are employed in the reaction (entries 5 and 6).
Table 2.
Ni-Catalyzed 1,4-Hydroboration of Functionalized Dienesa
 | ||||
|---|---|---|---|---|
| entry | substrate | product | regioselection | yield (%)b |
| 1 |
|
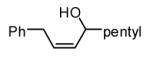
|
10:1 | 84c |
| 2 |
|
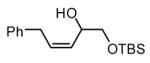
|
>20:1 | 91d |
| 3 |
|
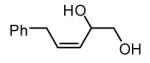
|
>20:1 | 54de |
| 4 |
|
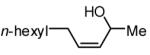
|
5:1 | 61c |
| 5 |
|
|
>20:1 | 82c |
| 6 |
|
|
>20:1 | 83c |
See footnote a, Table 1.
Isolated yield. Values are an average of two experiments.
Reaction for 12 h.
1 mol% Ni(cod)2 and 1 mol% PCy3 employed for this experiment.
2.1 equivalents pinBH employed.
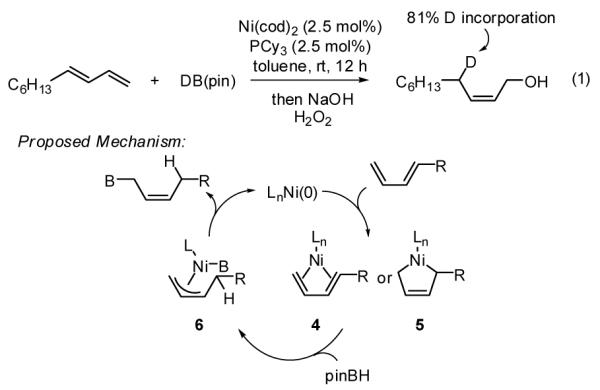
To probe mechanistic features, the hydroboration of 1,3-decadiene was conducted with DB(pin) and the oxidation product analyzed spectroscopically (Scheme 3, eq. 1). The deuterium atom was observed only at the C4 site in the product. This outcome, combined with the observations that the hydroboration reaction is ineffective with styrene and that an effective reaction appears to require access to the S-cis diene conformation, suggests that this process may proceed through a mechanism such as that depicted in Scheme 3. Initial association of Ni(0) and the diene is anticipated to furnish either η4-diene complex 4 or nickelacycle 5. While coordination of an L2Ni(0) fragment to butadiene has been shown to result in the former structure,12 the later bonding mode is observed in the reaction of heterobutadienes with Ni(0)13 and was proposed to account for the Ni-catalyzed coupling of 1,3-dienes and organoboronates.14 Subsequent reaction with HB(pin) furnishes the least hindered π-allyl complex 6 which provides the product.
Scheme 3.
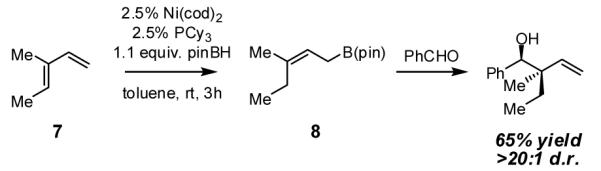
In addition to its utility as a method for allylic alcohol synthesis, the diene hydroboration reaction provides convenient access to allylboron reagents which would be difficult to prepare otherwise. For example, as depicted in Scheme 4, hydroboration of commercially available 3-methyl-1,3-pentadiene (7) provides allylboronate 8. As might be expected, addition of benzaldehyde to the unquenched hydroboration mixture furnishes the quaternary center-containing allylation product in a highly stereoselective fashion and in good yield. Further development of this reaction and study of its mechanism are in progress.
Scheme 4.
Supplementary Material
Acknowledgement
Support provided by the NIGMS (R01 GM-64451) and the NSF (DBI-0619576; BC Mass Spec Center).
Footnotes
Supporting Information Available: Characterization and procedures. This information is available free of charge through the internet at http://pubs.acs.org.
References
- (1).Männig D, Nöth H. Angew. Chem. Int. Ed. Engl. 1985;24:878. [Google Scholar]
- (2)(a).Reviews: Crudden CM, Edwards D. Eur. J. Org. Chem. 2003:4695. Beletskaya I, Pelter A. Tetrahedron. 1997;53:4957. Burgess K, Ohlmeyer MJ. Chem. Rev. 1991;91:1179.
- (3)(a).Zaidlewicz M, Meller J. Tetrahedron Lett. 1997;38:7279. [Google Scholar]; (b) Satoh M, Nomoto Y, Miyaura N, Suzuki A. Tetrahedron Lett. 1989;30:3789. [Google Scholar]; (c) Matsumoto Y, Hayashi T. Tetrahedron Lett. 1991;32:3387. [Google Scholar]; (d) Wu JY, Moreau B, Ritter T. J. Am. Chem. Soc. 2009;131:12915. doi: 10.1021/ja9048493. [DOI] [PubMed] [Google Scholar]
- (4).Yamamoto Y, Fujikawa R, Yamada A, Miyaura N. Chem. Lett. 1999:1069. [Google Scholar]
- (5).Matsumoto Y, Naito M, Hayashi T. Organometallics. 1992;11:2732. [Google Scholar]
- (6).For an excellent review of the allylboration reaction, see: Lachance H, Hall DG. In: Organic Reactions. Denmark SE, editor. Vol. 73. Wiley; New York: 2009.
- (7).Brown HC, Liotta R, Kramer GW. J. Org. Chem. 1978;43:1058. [Google Scholar]
- (8).Cyclohexadiene and nopadiene react well whereas both 1,2- and 1,4 hydroboration occur with 1,3-decadiene. See supporting info, ref. 3d.
- (9).For a recent synthesis of (Z) allylic alcohols along with a comprehensive survey of other methods, see: Kerrigan MH, Jeon S-J, Chen YK, Salvi L, Carroll PJ, Walsh PJ. J. Am. Chem. Soc. 2009;131:8434. doi: 10.1021/ja809821x.
- (10).Burks HE, Kliman LT, Morken JP. J. Am. Chem. Soc. 2009;131:9134. doi: 10.1021/ja809610h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11)(a).For Ni-catalyzed hydroboration of alkynes, see: Gridnev ID, Miyaura N, Suzuki A. Organometallics. 1993;12:589. For a review of Ni-catalyzed reactions of dienes, see: Kimura M, Tamaru Y. In: Modern Organonickel Chemistry. Tamaru Y, editor. Wiley-VCH; Weinheim, Germany: 2005. pp. 137–170.
- (12).Benn R, Betz P, Goddard R, Jolly PW, Kokel N, Kruger C, Topalovic I. Z. Naturforsch. 1991;46:1395. [Google Scholar]
- (13).Karsch HH, Leithe AW, Reisky M, Witt E. Organometallics. 1999;18:90. [Google Scholar]
- (14).Shirakawa E, Takahashi G, Tsuchimoto T, Kawakami Y. Chem Commun. 2002:2210. doi: 10.1039/b207185a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


