Abstract
IL-13 and eotaxin play important, inter-related roles in asthma models. In the lungs, CysLT, produced by the 5-LO-LTC4S pathway, mediate some local responses to IL-13 and eotaxin; in bone marrow, CysLT enhance IL-5-dependent eosinophil differentiation. We examined the effects of IL-13 and eotaxin on eosinophil differentiation. Semi-solid or liquid cultures were established from murine bone marrow with GM-CSF or IL-5, respectively, and the effects of IL-13, eotaxin, or CysLT on eosinophil colony formation and on eosinophil differentiation in liquid culture were evaluated, in the absence or presence of: a) the 5-LO inhibitor zileuton, the FLAP inhibitor MK886, or the CysLT1R antagonists, montelukast and MK571; b) mutations that inactivate 5-LO, LTC4S, or CysLT1R; and c) neutralizing mAb against eotaxin and its CCR3 receptor. Both cytokines enhanced GM-CSF-dependent eosinophil colony formation and IL-5-stimulated eosinophil differentiation. Although IL-13 did not induce eotaxin production, its effects were abolished by anti-eotaxin and anti-CCR3 antibodies, suggesting up-regulation by IL-13 of responses to endogenous eotaxin. Anti-CCR3 blocked eotaxin completely. The effects of both cytokines were prevented by zileuton, MK886, montelukast, and MK571, as well as by inactivation of the genes coding for 5-LO, LTC4S, and CysLT1R. In the absence of either cytokine, these treatments or mutations had no effect. These findings provide evidence for: a) a novel role of eotaxin and IL-13 in regulating eosinophilopoiesis; and b) a role for CysLTRs in bone marrow cells in transducing cytokine regulatory signals.
Keywords: secreted regulatory products, lipid mediators, signaling cascade
Introduction
Allergic pulmonary inflammation and hemopoiesis are often regarded as unrelated processes. However, they share a number of chemical mediators endowed with systemic and local activities, which may provide means of communication between inflammatory sites and the bone marrow. Such communication might be important in the case of eosinophils, characteristically associated with chronic infiltrates in asthma, as eosinophils are produced in bone marrow and usually enter inflammatory sites as mature, postmitotic effector cells, which are programmed to undergo apoptosis in a relatively short time. As a consequence, long-term eosinophil infiltration is highly dependent on chronically increased eosinophilopoiesis [1, 2]. The search for chemical mediators capable of ensuring the long-distance, functional coupling between eosinophil infiltrates in the lungs, on the one hand, and eosinophil production in the bone marrow, on the other hand, is therefore of interest.
IL-5 is produced by Th2 lymphocytes in many sites, including the lungs and bone marrow [3]. IL-5 is indispensable to long-term eosinophilia, as it is the major factor inducing eosinophil differentiation from lineage-committed precursors [1,2,3,4,5]. In addition, IL-5 extends the lifespan of infiltrating eosinophils and further activates or enhances their effector capabilities [6, 7]. However, IL-5 also promotes eosinophilia through mechanisms requiring its interaction with eotaxin and IL-13, two powerful chemical mediators of allergic inflammation. IL-5 interacts with eotaxin to initiate mobilization of bone marrow eosinophils to peripheral blood [8, 9] and with eotaxin and IL-13 to promote accumulation of eosinophils in the airways [10,11,12]. In murine models of asthma, IL-5, eotaxin, and IL-13 are all considered essential for the maintenance of eosinophilic infiltrates [10, 11]. Promotion of eosinophilia is part of the wide-ranging role of IL-13 in asthma [13, 14]. Some studies have reported that IL-13 induces eotaxin production [15, 16], thus raising the issue of whether its effects are mediated by eotaxin rather than synergic with those of eotaxin.
IL-13 has strong regulatory effects on various hemopoietic cell subtypes [17, 18], and eotaxin acts on the bone marrow to mobilize eosinophil progenitors [8, 9]. Nevertheless, the effects of either cytokine on eosinophil production from bone marrow cells (eosinophilopoiesis) remain unexplored. We have addressed here the possibility that eotaxin and/or IL-13 modulate eosinophilopoiesis. In addition, we have evaluated the relationship of their modulatory effects to the production and action of CysLT, which are powerful mediators of allergic inflammation. Such a relationship is suggested by a number of previous studies as follows: a) some of the effects of IL-13 [19] and eotaxin [20] in inflammatory sites are blocked by interference with CysLT production and signaling; b) CysLT feed back positively on IL-13 production [21, 22]; c) CysLT1Rs are required for production of eotaxin by IL-13-stimulated lung fibroblasts [23]; d) CysLT stimulate eosinophil colony formation in humans [24]; and e) CysLT strongly potentiate the effects of IL-5 in murine bone marrow culture [25]. As CysLT might provide an effective means to transduce the modulatory effects of eotaxin and IL-13 on eosinophilopoiesis, we examined here whether IL-13 or eotaxin requires CysLT production or signaling to modulate eosinophilopoiesis.
MATERIALS AND METHODS
Animals and animal procedures
Male and female BALB/c mice, bred at CECAL-FIOCRUZ (Rio de Janeiro, Brazil), were used at 6–8 weeks of age. Animal housing and handling followed procedures approved by the Institutional Committee on Ethical Handling of Laboratory Animals (Protocol CEUA #P0107-02). Where indicated, mice lacking: a) the CysLT1R (Cys-LT1R KO), generated on the BALB/c and C57BL/6 background [26]; b) the CysLT2R KO, generated on the C57BL/6 background [27]; or c) LTC4S, generated in the BALB/c background [28], bred at Brigham and Women’s Hospital (Boston, MA, USA), were used, along with their wild-type littermate controls, as approved by the Animal Care and Use Committee of the Dana-Farber Cancer Institute (Brookline, MA, USA; Protocol Number 02-122). Where indicated, 5LO–/– mice, generated in the S129 background [29] and bred at the Department of Pharmacology, Faculdade de Medicina de Ribeirão Preto-Universidade de São Paulo (Ribeirão Preto, Brazil), were used as approved by the Institutional Ethics Committee.
Reagents
Heat-inactivated FCS and culture media were from Hyclone (Logan, UT, USA); agar Noble, L-glutamine, penicillin, streptomycin, and NBT/BCIP from Sigma Chemical Co. (St. Louis, MO, USA); MK886 and MK571 from Calbiochem (Merck KgaA-affiliated, Darmstadt, Germany); LTD4 and montelukast from Cayman Chemical Co. (Ann Arbor, MI, USA); recombinant murine cytokines (GM-CSF, IL-5, IL-13, eotaxin) and antibodies specific for murine CCR3 or eotaxin, along with the appropriate control antibodies of the IgG2a isotype from R&D Systems (Minneapolis, MN, USA); SA-ALP from MabTech (Cincinnati, OH, USA); and liquid diaminobenzidin solution from Dako Cytomation (Dako Denmark A/S, Glostrup, Denmark).
Bone marrow cell studies
Bone marrow cells were obtained by flushing the two femurs of naive mice with RPMI-1640 medium containing 1% FCS. Initial studies were carried out in semi-solid cultures to define whether either cytokine had an impact on lineage-committed progenitors (colony-forming cells) [4, 30, 31]. Subsequently, to assess the effects of blockers (drugs and antibodies), liquid culture assays were used to facilitate sequential addition and proper mixing of these reagents to previously plated cells [32]. Blockers (inhibitors and antibodies) were added before positive stimuli (cytokines), all being present from the beginning of the culture without replenishment. Semi-solid (clonal) cultures were established by seeding 2 × 105 cells in 1 mL in 35 mm triplicate culture dishes in a mixture of IMDM with 20% FCS and agar Noble (0.3% final concentration) in the presence of GM-CSF (2 ng/mL), alone or in association with IL-13 (0.01–1 ng/mL) or eotaxin (0.01–1 ng/mL). Colonies (defined as the progeny of a single progenitor, totaling >50 cells) were scored at Day 7. The frequency of eosinophil-containing colonies was determined on agar layers dried, mounted on microscope slides, stained for EPO [33], and scored under high magnification (400×) [4]. For representative images of EPO+ and EPO– colonies, see Figure 1. We have confirmed previously that these conditions were adequate for counting total myeloid colonies and for accurate differential counts of myeloid colony types on dried agar layers [4, 30, 31]. Liquid bone marrow cultures were established by seeding 106 bone marrow cells in 1 mL RPMI-1640 medium, with 10% FCS in the presence of IL-5 (1 ng/mL), alone or in association with IL-13 (0.01–1 ng/mL) or eotaxin (0.01–1 ng/mL) and incubated at 37°C, 5% CO2/95% air, for 7 days before counting total cells in a hemocytometer and determining the frequency of cells positive for EPO after staining of cytocentrifuge smears for EPO [33]. There is excellent agreement among eosinophil counts by EPO cytochemical staining, by CCR3 immunostaining, and by Giemsa hematological staining [32]. Eosinophil numbers were calculated from total and differential counts. These conditions were shown previously to support eosinophil proliferation and terminal differentiation and to allow detection of enhancing and suppressive effects [4, 30,31,32]. In the absence of IL-5, eosinophil differentiation does not occur, and cultures present virtually no EPO+ cells by Day 7 [4], containing only macrophages and endothelial/stromal cells, which survive from the bone marrow inoculum, regardless of IL-5. Accordingly, no eosinophils were produced in the presence of eotaxin or IL-13 alone.
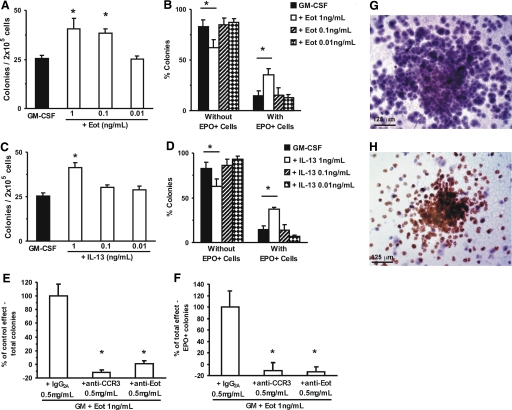
Figure 1.
Effect of eotaxin and IL-13 on eosinophil colony formation. (A–D) Data are numbers of colonies formed by 2 × 105 bone marrow cells (mean±sem) by Day 7 of semisolid culture with GM-CSF (2 ng/ml), alone or in association with the indicated concentrations of eotaxin (Eot; A) or IL-13 (C) or the frequency of colonies with (right) or without (left) EPO+ cells (i.e., eosinophil-containing and eosinophil-free, respectively) formed with GM-CSF alone or in association with eotaxin (B) or IL-13 (D). (E and F) Data are the percent of the enhancing effect of eotaxin on total (E) or EPO+ (F) colony counts, observed after exposure to irrelevant control (IgG2a) or to neutralizing (CCR3- or eotaxin-specific) antibodies, relative to controls incubated without antibodies. Photomicrographs (G and H) illustrate the morphology of colonies with (H, brown, coarse cytoplasmic granulation) and without (G) EPO+ cells after counterstaining. *, P < 0.05, for the differences between the indicated points and the respective GM-CSF (negative) controls (A–D) or between the indicated points and respective isotype control antibodies (E and F). (A–D) n = 6; (E and F) n = 3; (G and H) images representative of three experiments.
Eotaxin quantitation
Endogenous eotaxin production was quantitated (pg/mL) in 10× concentrated culture supernatants (100 μl) conditioned by 106 bone marrow cells for 4 days by ELISA (Quantikine™) using MAB420 and BAF420 anti-eotaxin antibodies, according to the manufacturer’s instructions. ELISPOT assays were done in 96-well nitrocellulose plates (MAHA S4510, Millipore, Billerica, MA, USA), sensitized with 5 μg/mL MAB420 (50 μL/well, 16 h, 4°C), washed (3×), and blocked (1 h, 37°C) in medium/10% FCS. Cultures were established for 5 days with bone marrow cells (200 μL/well, 1–0×105 cells) in the same medium plus cytokines (1 ng/mL IL-5 and IL-3, separately or in combination). Plates were washed 3× in PBS/1% FCS, incubated with biotinylated BAF420 antibody (1 μg/mL, 4 h, 37°C), washed further 3× in PBS-Tween 20, incubated with SA-ALP (1:1000, 50 μL/well, 1 h, 37°C), and developed with NBT/BCIP (50 μL/well, 40 min). ELISPOTs were photographed and enumerated in an Immunospot analyzer (CTL Analyzers, Cleveland, OH, USA).
Statistical analysis
Data (mean±sem) were analyzed with Systat for Windows, Version 4, from Systat Inc. (Evanston, IL, USA), using factorial ANOVA with the Tukey (Honestly Significant Difference) correction for multiple comparisons of means observed with different treatments. For the entire study, the variation in baseline responses to IL-5 in liquid culture [(10.22±0.74)×104 cells/ml, mean±sem, n=28], as well as in the enhanced responses in the presence of IL-5 and eotaxin [(19.28±1.96)×104 cells/ml, n=17] or IL-13 [(18.71±1.55)×104 cells/ml, n=18], was consistent with studies published previously using this methodology [25, 30, 31, 33]. To facilitate comparisons between the effects of the two cytokines in the two different culture systems, the magnitude of the average increase in response to IL-5 or GM-CSF induced by IL-13, and eotaxin was expressed as percent enhancement relative to the respective (IL-5 or GM-CSF) controls, calculated as: [(response in the presence of IL-13 or eotaxin)–(response in the controls)/(response in the controls)] × 100.
RESULTS
Effects of IL-13 and eotaxin on eosinophilopoiesis
To determine whether IL-13 and eotaxin have an impact on eosinophilopoiesis, we initially evaluated their effects on formation of myeloid colonies by bone marrow progenitors cultured in the presence of the multilineage hemopoietic factor, GM-CSF. As shown in Figure 1A, eotaxin increased the total numbers of myeloid colonies significantly (which include eosinophil colonies) [4, 30,31,32], developing in the presence of GM-CSF from BALB/c bone marrow progenitors at 1 and 0.1 (but not 0.01) ng/ml (59.6±21.7% and 51.6±8.5% enhancement, P=0.031 and P=0.014, respectively). As shown in Figure 1B, in the presence of 1 ng/mL eotaxin (but not at lower concentrations), this increase in total colony numbers was accompanied by a significantly increased frequency of eosinophil colonies (142.7±41.1% increase, P=0.028), which was mirrored by a nonselective decrease in the frequency of the other colony types (G, GM, and M). Eosinophil-containing colonies were “pure” (Eos) or “mixed” (GMEos), as described previously [4], and types were increased similarly by eotaxin. As shown in Figure 1C, IL-13 significantly increased the total numbers of GM-CSF-stimulated myeloid colonies at 1 ng/ml (62.8±10.7% enhancement, P=0.008) but not at lower concentrations. As reported by others [23], IL-13 by itself had no colony-stimulating activity (not shown). As further shown in Figure 1D, at this concentration, IL-13 increased the frequency of eosinophil colonies significantly (154.6±37.3% increase, P=0.043)—pure and mixed—and G, GM, and M colony numbers were decreased to a comparable extent.
EPO+ and EPO– colony numbers contributed to the increase in total colony counts observed with either cytokine. By far, EPO+ colony counts made the largest contribution (two-thirds) to this increase in both cases, EPO–colony counts accounting for the remaining one-third.
We have further examined the possibility that these effects reflected, to some extent, the presence of a contaminant able to increase colony counts nonspecifically. By definition, this hypothetical contaminant would not lose its activity when treatments that specifically neutralize the cytokine are used. We took advantage of the availability of anti-CCR3 and anti-eotaxin neutralizing antibodies to block the effects of eotaxin on colony counts as a control for the presence of a nonspecific stimulus in the cytokine preparation. (i.e., a stimulus able to increase colony counts independently of eotaxin). Both neutralizing antibodies abolished the enhancing effect of eotaxin on total colony counts (Fig. 1E) as well as eosinophil-containing colonies (Fig. 1F), thus ruling out the possibility of colony-count increases being induced by a contaminant rather than by an eotaxin itself. EPO– (Fig. 1G) and EPO+ (Fig. 1H) colonies, in these conditions, were identified unambiguously and conformed to morphological types described previously [4].
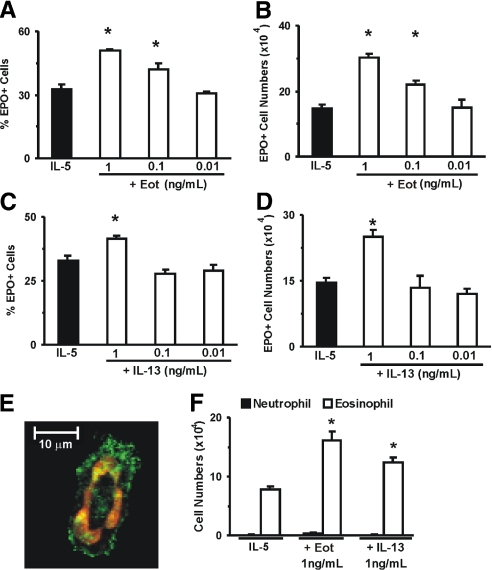
We have further evaluated whether terminal differentiation of eosinophils from committed precursors in IL-5-stimulated liquid cultures would be affected by either cytokine. As shown in Figure 2A, the frequency of EPO+ cells, comprising all stages of murine eosinophil differentiation, from the earliest precursors to fully mature eosinophils [4], was increased significantly by eotaxin at 1 and 0.1 (but not 0.01) ng/mL. This increased frequency was reflected in a dose-dependent increase in eosinophil numbers recovered from the culture at Day 7, as shown in Figure 2B. Eotaxin did not induce eosinophil differentiation in the absence of IL-5 (not shown). As shown further in Figure 2C, the frequency of EPO+ cells was also increased by IL-13 at 1 (but not 0.1–0.01) ng/ml. As shown in Figure 2D, this increased frequency was reflected in increased absolute numbers of EPO+ cells. IL-13 by itself did not induce eosinophil differentiation (not shown).
Figure 2.
Effect of eotaxin and IL-13 on IL-5-induced eosinophil differentiation. Data (mean±sem, n=3) are the percent EPO+ cells (A and C), the numbers of EPO+ cells (B and D), or the numbers of Giemsa-stained neutrophils and eosinophils (F) present by Day 7 in 1 mL liquid cultures, established with IL-5 (1 ng/ml), alone or in association with the indicated concentrations of eotaxin (A and B) or IL-13 (C and D). *, P < 0.05, for the differences between the indicated points and the respective IL-5 (negative) controls. Photomicrograph under confocal microscopy (E, ×630 original magnification, representative of three experiments) illustrates typical morphology of eosinophils in liquid culture following immunostaining for CCR3 (green) and nuclear staining with ToPro3 (red) [32].
Together, observations in semisolid and liquid culture conditions indicate that eotaxin and IL-13 enhance eosinophilopoiesis in the presence (but not in the absence) of GM-CSF and IL-5. As 1 ng/mL of either cytokine consistently had an enhancing effect, this was the concentration used in subsequent experiments designed to evaluate the contribution of CysLT in the observed cytokine effects (see below).
We controlled further for the accuracy of EPO staining in identifying eosinophils. Liquid cultures containing EPO+ cells contained cells that expressed CCR3 and presenting the donut-shaped nuclei typical of mature murine eosinophils (Fig. 2E); EPO and CCR3 staining agreed with detection of eosinophils by Giemsa (Fig. 2F). By contrast, neutrophils, identifiable by Giemsa staining, were not detectable by Day 7 in liquid cultures established with IL-5, alone or associated with eotaxin or IL-13 (Fig. 2F). These different identification methods consistently support the idea that production of eosinophils, not of other bone marrow cell types, is enhanced by eotaxin and IL-13 in semisolid and liquid cultures.
Relationship between the effects of IL-13 and eotaxin
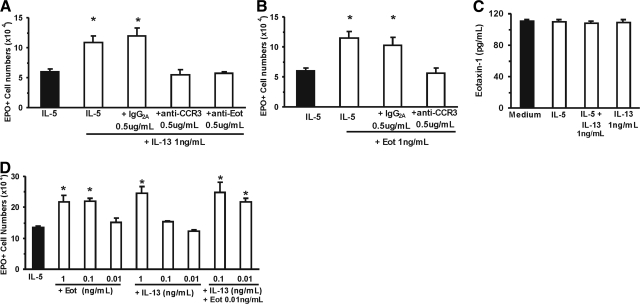
The similarity of effects for both cytokines, along with the published evidence that eotaxin is induced by IL-13 in some models, prompted us to examine whether the effectiveness of the latter would be affected by targeting production or function of the former. As shown in Figure 3A, IL-13 enhanced responses to IL-5 significantly (80.1±17.4% enhancement, P=0.049) in the absence but not in the presence of specific neutralizing antibodies to eotaxin or to its receptor, CCR3. Unlike the specific neutralizing antibodies, an irrelevant antibody of the same isotype (IgG2a) at the same concentration had no effect. As shown further in Figure 3B, eotaxin enhanced responses to IL-5 significantly (91.1±21.1% enhancement, P=0.004) in the absence but not in the presence of anti-CCR3. These observations confirm that eotaxin is acting, as expected, through CCR3 and further raise the possibility that the effects of IL-13 depend on the endogenous production of eotaxin in the culture.
Figure 3.
Evidence that eotaxin is required for the effects of IL-13. (A, B, and D) Data (mean±sem, n=3) are the numbers of EPO+ cells present by Day 7 in 1 mL liquid cultures established with IL-5 (1 ng/ml), alone or in association with the indicated concentrations of IL-13 (A and D) or eotaxin (B and D) or antibodies (neutralizing antibodies to eotaxin or to CCR3 or control antibody of IgG2a isotype). (C) The amounts of eotaxin detected in 10× concentrated liquid culture supernatants conditioned in the presence of medium alone, IL-5 alone, IL-13 alone, or an association of IL-5 and IL-13. *, P < 0.05, for the differences between the indicated points and the respective IL-5 (negative) controls.
We evaluated initially whether endogenous production of eotaxin was detectable in liquid bone marrow culture and whether an enhancing effect of IL-5, IL-13, or both on endogenous eotaxin production would be detectable. As shown in Figure 3C, small amounts of eotaxin were detected by ELISA in culture supernatants (0.1 ng/mL in 10× concentrated supernatants, corresponding to 0.01 ng/mL in the culture), but no difference was observed between cultures maintained in medium alone and those stimulated by IL-5 or IL-13. In addition, numbers of ELISPOTs formed by bone marrow cells in the presence of IL-5 (19.3±4.8, mean±sem) and IL-5 + IL-13 (14±1.5) were not significantly different (P=0.868).
In view of our inability to show induction of eotaxin production in response to IL-13, we examined the alternative possibility, namely that IL-13 does not induce eotaxin but still requires eotaxin to be present, as it acts synergically with endogenous eotaxin to stimulate eosinophilopoiesis. If so, enhancement of eosinophilopoiesis should be detectable in the presence of an association of IL-13 and eotaxin concentrations, which would have no effect if present separately. As shown in Figure 3D, IL-13, at concentrations that do not enhance eosinophilopoiesis significantly (0.1–0.01 ng/mL), strongly synergized with exogenous eotaxin at a very low concentration (0.01 ng/mL), which had no effect when added alone to the culture. These observations show that IL-13 and eotaxin, at low concentrations, enhance eosinophilopoiesis synergically. Furthermore, as the levels of eotaxin in the synergic combination approximate those produced spontaneously in the culture (see above), these results are consistent with a synergic, eotaxin-requiring mechanism for the effects of IL-13.
Role of CysLT in the modulation of eosinophilopoiesis by eotaxin and IL-13
As CysLT transduce the enhancing effects of nonsteroidal anti-inflammatory drugs on eosinophilopoiesis [25] and mediate some effects of IL-13 and eotaxin in sites of allergic inflammation [19, 20], we next evaluated whether targeting CysLT production or function would also have an impact on the ability of either cytokine to up-regulate eosinophilopoiesis. We took a comprehensive approach, targeting several critical steps in CysLT biosynthesis (involving 5-LO, FLAP, or LTC4S) and signal transduction (involving CysLT1R and CysLT2R). Where available, pharmacological and gene inactivation strategies were used to achieve comparable outcomes.
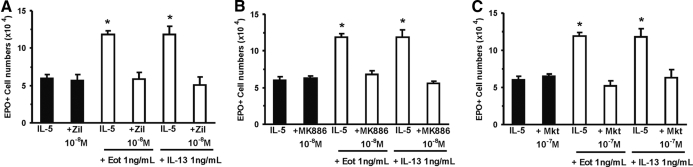
As shown in Figure 4A, eotaxin and IL-13 enhanced eosinophil production relative to IL-5 controls, in the absence but not in the presence of the 5-LO inhibitor, zileuton (98.6±8.5% and 98.0±17.9% enhancement, P=0.001 and P=0.013, respectively). By contrast, zileuton, in the absence of either cytokine, had no effect. As shown further in Figure 4B, the effects of both cytokines were blocked by the FLAP inhibitor MK886, which had no effect of its own, in the absence of either cytokine. Finally, as shown in Figure 4C, both were blocked completely by the CysLT1R antagonist, montelukast. Again, montelukast had no effect in the absence of eotaxin or IL-13. The response to IL-13 was also abolished by another CysLT1R antagonist, MK571 (not shown).
Figure 4.
Pharmacological inhibition of CysLT generation and function blocks the effects of eotaxin and IL-13. Data (mean±sem, n=3) are the numbers of EPO+ cells present by Day 7 in 1 mL liquid cultures established with IL-5 (1 ng/ml), alone or in association with the indicated concentrations of IL-13, eotaxin, and drugs targeting 5-LO (Zil, zileuton, A), FLAP (MK886, B), or CysLT1R (Mkt, montelukast, C). *, P < 0.05, for the differences between the indicated points and the respective IL-5 (negative) controls.
To confirm these pharmacological inhibition profiles, we examined the response to both cytokines in bone marrow cells from different strains of mice presenting distinct mutations related to the production (5-LO, LTC4S) or function (CysLT1R, CysLT2R) of CysLT.
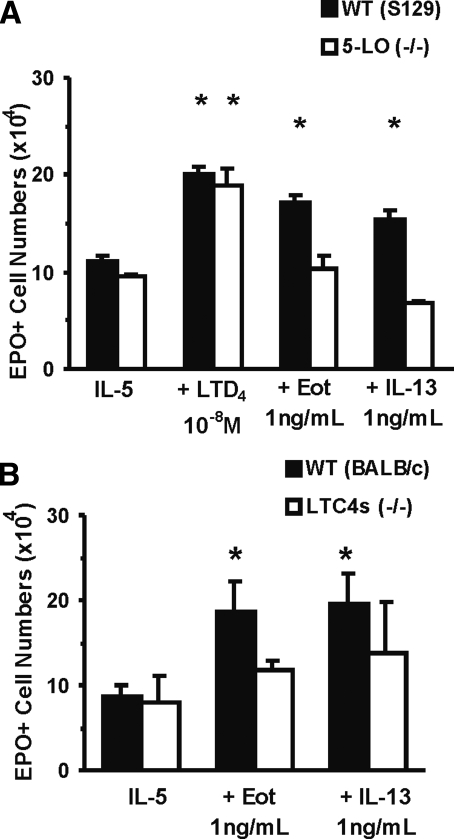
As shown in Figure 5A, eotaxin and IL-13 enhanced (54.2±4.6% and 38.7±6.0%, P<0.001 and P=0.002, respectively) IL-5-stimulated eosinophil production in cultured bone marrow from wild-type controls (S129) but not from 5LO–/– mice of the same genetic background. By contrast, as expected, the end-product, LTD4, was equally effective in wild-type and mutant bone marrow, showing that the defect in mutant bone marrow is a result of a lack of production rather than of responsiveness to CysLT. As shown further in Figure 5B, eotaxin and IL-13 were effective (114.9±39.7% and 126.0±39.8% enhancement, P=0.033 and P=0.033, respectively) on bone marrow from wild-type controls (BALB/c) but not from LTC4S-deficient mice of the same background.
Figure 5.
Effect of mutations preventing CysLT generation on the responses to eotaxin and IL-13. Data (mean±sem, n=3) are the numbers of EPO+ cells/ml present by Day 7 in liquid culture established with IL-5 (1 ng/ml), alone or in association with the indicated concentrations of IL-13, eotaxin, or LTD4 from bone marrow of wild-type (WT) control mice (A, S129; B, BALB/c) or mutant mice [A, 5-LO (−/−); B, LTC4s (−/−)]. *, P < 0.05, for the differences between the indicated points and the respective IL-5 (negative) controls.
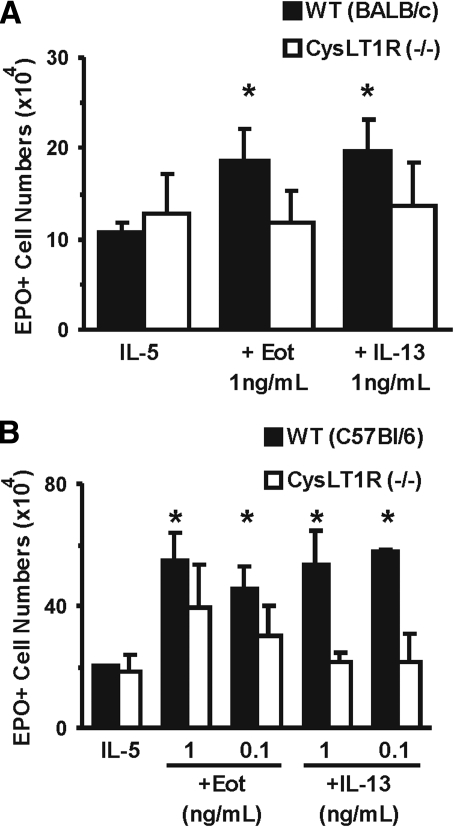
Further evidence for the involvement of CysLT in mediating responses to both cytokines was provided by experiments with receptor-deficient mouse strains. As shown in Figure 6A, eotaxin and IL-13 enhanced eosinophil production in wild-type (BALB/c) bone marrow (73.3±32.0% and 82.2±32.1% enhancement, P=0.022 and P=0.022, respectively) but not CysLT1R-deficient bone marrow of the same background. As shown further in Figure 6B, eotaxin and IL-13 were effective (for the highest concentration of each, 170.0±43.1% and 164.4±54.5% enhancement, P=0.018 and P=0.002, respectively) in bone marrow from wild-type controls of a different background (C57BL/6) but were totally ineffective in the corresponding CysLT1R-deficient mice, thereby confirming that lack of effect is determined by inactivation of CysLT1R and apparently independent of other background genes.
Figure 6.
Effect of mutations preventing CysLT signaling on the responses to eotaxin and IL-13. Data (mean±sem, n=3) are the numbers of EPO+ cells/ml present by Day 7 in liquid culture established with IL-5 (1 ng/ml), alone or in association with the indicated concentrations of IL-13 or eotaxin from bone marrow of wild-type controls (A, BALB/c; B, C57Bl/6) or CysLT1R-deficient mice [CysLT1R (–/–) A and B] of the corresponding strains. *, P < 0.05, for the differences between the indicated points and the respective IL-5 (negative) controls.
By contrast, there was no impact of gene inactivation of CysLT2R on the ability of eotaxin to enhance IL-5-stimulated eosinophilopoiesis in bone marrow culture. CysLT2R-deficient mice and wild-type controls of the same background responded to eotaxin, and this response was abolished by montelukast, indicating the involvement of CysLT1R (not shown).
DISCUSSION
The findings reported here provide evidence of a novel role for eotaxin and IL-13. Both cytokines up-regulate eosinophilopoiesis in the presence of the more conventional hemopoietic cytokines, IL-5 and GM-CSF. In GM-CSF-stimulated semisolid cultures, both cytokines increased total colony counts, as expected from the multilineage activity of this hemopoietic factor. This increase was accounted for by a strong stimulation of eosinophil (EPO+) colony formation and by a weaker effect on other colony types. In IL-5-stimulated liquid cultures, both increased eosinophil differentiation exclusively, in a way consistent with the selectivity of this cytokine for eosinophils. The effects of eotaxin in semisolid and liquid culture were abolished by neutralizing antibodies to its receptor, CCR3. This indicates that these effects are a result of eotaxin itself, rather than of any contaminant. The evidence from semisolid and liquid cultures, taken together, indicates that eotaxin and IL-13 do not induce eosinophilopoiesis by themselves. Both modify, instead, the effects of GM-CSF and IL-5 in a way that increases eosinophil production. These observations expand the list of regulatory activities associated with IL-13 and eotaxin and document further an important effect of the latter during early stages of eosinophil development, as opposed to its well-recognized chemoattractant activity for terminally differentiated eosinophils.
This study documents further that the modulatory signals from both cytokines are strictly dependent on the generation of 5-LO products and on signaling through CysLT1Rs. Stimulation of eosinophilopoiesis by CysLT has been documented previously in humans [24] as well as in mice [25]. This mechanism was shown previously to be required for transducing the regulatory signals from nonsteroidal anti-inflammatory drugs in bone marrow culture [25]. We now show that it provides a mechanism for transducing the effects of cytokines on eosinophilopoiesis as well.
Together, our observations suggest that the primary cellular target of cytokine action in our system should be able to: a) express CCR3 constitutively and b) respond to eotaxin binding of CCR3 by producing CysLT. This profile likely corresponds to a cell of the eosinophil lineage in the early stage of a colony-forming cell (progenitor) or at later maturation stages (precursor). However, our observations suggest that noneosinophil (EPO–) colony counts may also increase to some extent as a result of eotaxin stimulation. As CCR3 is not necessarily expressed by progenitors of other hemopoietic lineages, it is possible that these primary targets release, following contact with eotaxin, secondary mediators capable of stimulating progenitors from other lineages in the presence of GM-CSF.
Although CysLT are important mediators of allergic inflammation [34] and have been shown to affect hemopoiesis in various ways [24, 25], their possible contribution to the physiological regulation of hemopoiesis remains uncertain for a number of reasons. Local regulation of hemopoiesis by CysLT would require evidence of their production by bone marrow cells. To our knowledge, however, endogenous CysLT production from bone marrow cells in response to standard stimuli, such as Ca2+ ionophore, has not yet been demonstrated. Alternatively, systemic regulation could, in principle, take place if CysLT were released in sufficiently large amounts in the circulation [34]. However, in these conditions, CysLT are known to have untoward cardiovascular, digestive, and respiratory effects, which would make this an unlikely mechanism for physiological regulation. Our observations, by providing a link between the effects of exogenously added eotaxin and local production of CysLT, may modify this picture substantially, as eotaxin is released from a number of sites and acts systemically in very low concentrations [35], which are not associated with any untoward effects. Systemically administered eotaxin is active on bone marrow cells in vivo [9]. We have shown that: a) eotaxin, in effective concentrations in the culture, acts by inducing or facilitating activation of CysLT generation by bone marrow cells; and b) IL-13 greatly enhances the ability of eotaxin to do so. Hence, physiological regulation of eosinophilopoiesis by CysLT might be easier to understand if eotaxin, released from allergic inflammatory sites, acted systemically in bone marrow (especially if aided by IL-13) through local generation of CysLT.
Our observations show that the involvement of CysLT is separate from but dependent on induction of eosinophilopoiesis by IL-5. On the one hand, neither cytokine induced eosinophil production in the absence of IL-5. On the other hand, none of the inhibitors of CysLT synthesis and activity tested here and none of the gene inactivation strategies used had an effect on responses to IL-5 alone. Every one of them had a significant impact only in the presence of IL-13 or eotaxin. Overall, this supports our view that the 5-LO pathway does not contribute to signaling by IL-5 [25], but endogenous CysLT production is important for the up-regulation of eosinophilopoiesis by eotaxin and IL-13.
The bone marrow may not be the only site where eosinophilopoiesis is susceptible to modulation by eotaxin and IL-13. An alternative possibility is that CysLT mediates regulatory effects of eotaxin and IL-13 on eosinophil progenitors and precursors recruited to the lungs. This possibility is supported by evidence that allergen-challenged lungs: a) are important sources of CysLT [17,18,19,20, 27]; b) produce IL-5, IL-13, and eotaxin [11]; and c) accumulate eosinophil progenitors and precursors, including CD34+ IL-5Rα+ cells [36], in response to systemically active signals originating in lung tissue [37]. Although it is of interest, this possibility was not examined here because of the necessary focus on bone marrow in this study.
One critical issue in this study concerns the mechanism underlying the dependence of IL-13 on eotaxin and CCR3. This has been examined with every tool available to us. Two distinct possibilities exist: a) an inductive mechanism, in which IL-13 depends on eotaxin (and on CCR3) to act, as it induces eotaxin, and that it is eotaxin acting as a secondary mediator, which enhances eosinophilopoiesis, by acting on CCR3; and b) a synergic mechanism, in exogenously added IL-13, acts synergically with endogenously produced eotaxin. These two possibilities can be distinguished by measuring the endogenous production of eotaxin by bone marrow cells and evaluating the effect, if any, of exogenous IL-13 on this production. Our results did not support an inductive mechanism: Although we detected eotaxin-producing cells in bone marrow culture and measured the eotaxin content of culture supernatants, neither was increased significantly following IL-13 exposure. Instead, our results showed that a synergic mechanism is possible, as we demonstrated effective enhancement of eosinophilopoiesis in cultures exposed to an association of IL-13 and eotaxin in concentrations that would have no effect if present separately in the culture. Our observations were also consistent with the possibility of a synergic interaction between IL-13 exogenously added at relatively high concentrations (1 ng/mL) and eotaxin at the concentration endogenously produced in the cultures (0.01 ng/mL).
A related and important issue is whether the CCR3 pathway would mediate CysLT production by bone marrow cells in sufficient amount. Our interpretation of the data is that it points unequivocally to CysLT being produced in the culture. Two different inhibitors of LT synthesis and a gene inactivation approach provided exactly the same result: When LT synthesis is inhibited, neither IL-13 nor eotaxin enhances eosinophil production. Furthermore, it is CysLT that are involved, as pharmacological and gene inactivation approaches are consistent in showing a role for LTC4S and for a specific subtype of the CysLTR. We consider our approach through a combination of pharmacological and genetic tools more rigorous than an attempt to quantify CysLT production over a 7-day period in culture, as it demonstrates unequivocally that CysLT production and CysLT signaling are necessary. Although an absolute requirement can always be demonstrated clearly, the measurement of the amounts produced is subject to numerous sources of uncertainty: a) CysLT production could be taking place anytime during the 7-day period of culture; b) CysLT are likely degraded during this period, and total production is not necessarily reflected in amounts measured at any given time-point; and c) CysLT production is associated with cells and likely to reach higher effective concentrations in the immediate vicinity of the producer cells than in the total volume of the culture, which is what is accessible for immunoassay. For these reasons, we take the view that synthesis of CysLT and signaling by CysLT1R are absolutely required for the effects we observed; the logical conclusion is that they are produced and act in this system.
AUTHORSHIP
Tulio Queto carried out most experiments in bone marrow culture, including ELISPOT and neutralization assays; Maria I. Gaspar-Elsas conducted and supervised studies on colony formation; Daniela Masid-de-Brito and Zilton F. M. Vasconcelos participated in confocal microscopy and liquid culture experiments; Fausto K. Ferraris and Carmen Penido performed eotaxin quantitation; Fernando Q. Cunha, Yoshihide Kanaoka, and Bing K. Lam supervised studies with genetically modified mice generated and/or maintained in their laboratories; and Pedro Xavier-Elsas performed the initial observations, supervised and designed all subsequent studies, and wrote the original manuscript as well as its revisions.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (grant number HL082695 to B. K. L.), CNPq (grants 474979/2004-0, 471176/2007-9, and 478253/2008-7 to P. X-E. and M. I. G-E. and Research Productivity Fellowships to P. X-E., M. I. G-E., and F. Q. C.), and FAPERJ (APQ1 grant number E-26/110.188/2008 to P. X-E.), as well as by FIOCRUZ. Expert, user-friendly support in critical experiments was provided by the FIOCRUZ core facilities for ELISPOT (J. C. Lima Jr.) and confocal microscopy (B. Pascarelli and P. P. Manso).
Footnotes
Abbreviations: 5-LO=5-lipoxygenase, 5LO–/–=lacking 5-LO, BCIP=5-bromo-4-chloro-3-indolyl-phosphate, CysLT=cysteinyl leukotrienes, CysLT1R=type 1 CysLT receptor, EPO=eosinophil peroxidase, EPO+/EPO–=EPO-positive/EPO-negative, FLAP=5-LO-activating protein, KO=knockout, LTC4S=leukotriene C4 synthase, SA-ALP=streptavidin-conjugated alkaline phosphatase
References
- Elsas P X, Elsas M I. Eosinophilopoiesis at the cross-roads of research on development, immunity and drug discovery. Curr Med Chem. 2007;14:1925–1939. doi: 10.2174/092986707781368487. [DOI] [PubMed] [Google Scholar]
- Rosenberg H F, Phipps S, Foster P S. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–1310. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol. 2008;20:288–294. doi: 10.1016/j.coi.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Gaspar-Elsas M I, Joseph D, Elsas P X, Vargaftig B B. Rapid increase in bone-marrow eosinophil production and responses to eosinopoietic interleukins triggered by intranasal allergen challenge. Am J Respir Cell Mol Biol. 1997;17:404–413. doi: 10.1165/ajrcmb.17.4.2691. [DOI] [PubMed] [Google Scholar]
- Sehmi R, Wood L J, Watson R, Foley R, Hamid Q, O'Byrne P M, Denburg J A. Allergen-induced increases in IL-5 receptor α-subunit expression on bone-marrow-derived CD34+ cells from asthmatic subjects. A novel marker of progenitor cell commitment towards eosinophilic diferentiation. J Clin Invest. 1997;100:1–10. doi: 10.1172/JCI119789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Hayashi Y, Sugama Y, Miura Y, Kasahara T, Kitamura S, Torisu M, Mita S, Tominaga A, Takatsu K. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor. J Exp Med. 1988;167:1737–1742. doi: 10.1084/jem.167.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Suda T, Ohta S, Tominaga K, Miura Y, Kasahara T. Analysis of the survival of mature human eosinophils: interleukin-5 prevents apoptosis in mature human eosinophils. Blood. 1991;78:2542–2547. [PubMed] [Google Scholar]
- Mould A W, Matthaei K I, Young I G, Foster P S. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Invest. 1997;99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palframan R T, Collins P D, Williams T J, Rankin S M. Eotaxin induces a rapid release of eosinophils and their progenitors from the bone-marrow. Blood. 1998;91:2240–2248. [PubMed] [Google Scholar]
- Pope S M, Brandt E B, Mishra A, Hogan S P, Zimmermann N, Matthaei K I, Foster P S, Rothenberg M E. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001;108:594–601. doi: 10.1067/mai.2001.118600. [DOI] [PubMed] [Google Scholar]
- Mattes J, Mahalingam S, Kuehr J, Webb D C, Simson L, Hogan S P, Koskinen A, McKenzie A N J, Dent L A, Rothenberg M E, Matthaei K I, Young I G, Foster P S. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med. 2002;195:1433–1444. doi: 10.1084/jem.20020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould A W, Ramsay A J, Matthaei K I, Young I G, Rothenberg M E, Foster P S. The effect of IL-5 and eotaxin expression in the lung on eosinophil trafficking and degranulation and the induction of bronchial hyperreactivity. J Immunol. 2000;164:2142–2150. doi: 10.4049/jimmunol.164.4.2142. [DOI] [PubMed] [Google Scholar]
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben T Y, Karp C L, Donaldson D D. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- Grünig G, Warnock M, Wakil A E, Venkayya R, Brombacher F, Rennick D M, Sheppard D, Mohrs M, Donaldson D D, Locksley R M, Corry D B. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xia Y, Nguyen A, Lai Y H, Feng L, Mosmann T R, Lo D. Effects of Th2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells. J Immunol. 1999;162:2477–2487. [PubMed] [Google Scholar]
- Terada N, Hamano N, Nomura T, Numata T, Hirai K, Nakajima T, Yamada H, Yoshie O, Ikeda-Ito T, Konno A. Interleukin-13 and tumor necrosis factor-α synergistically induce eotaxin production in human nasal fibroblast. Clin Exp Allergy. 2000;30:348–355. doi: 10.1046/j.1365-2222.2000.00750.x. [DOI] [PubMed] [Google Scholar]
- Lai Y H, Heslan J M, Poppema S, Elliott J F, Mosmann T R. Continuous administration of IL-13 to mice induces extramedullary hemopoiesis and monocytosis. J Immunol. 1996;156:3166–3173. [PubMed] [Google Scholar]
- Sugawara T, Katayama N, Nishii K, Mahmud N, Mitani H, Ohishi K, Masuya M, Minami N, Shiku H. Distinctive actions of interleukin-13 and interleukin-4 on growth of hematopoietic progenitors. Int J Oncol. 1999;14:471–477. doi: 10.3892/ijo.14.3.471. [DOI] [PubMed] [Google Scholar]
- Vargaftig B B, Singer M. Leukotrienes mediate murine bronchopulmonary hyperreactivity, inflammation, and part of mucosal metaplasia and tissue injury induced by recombinant murine interleukin-13. Am J Respir Cell Mol Biol. 2003;28:410–419. doi: 10.1165/rcmb.2002-0032OC. [DOI] [PubMed] [Google Scholar]
- Hisada T, Salmon M, Nasuhara Y, Chung K F. Cysteinyl-leukotrienes partly mediate eotaxin-induced bronchial hyperresponsiveness and eosinophilia in IL-5 transgenic mice. Am J Respir Crit Care Med. 1999;160:571–575. doi: 10.1164/ajrccm.160.2.9810101. [DOI] [PubMed] [Google Scholar]
- Vargaftig B B, Singer M. Leukotrienes, IL-13, and chemokines cooperate to induce BHR and mucus in allergic mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2003;284:L260–L269. doi: 10.1152/ajplung.00226.2002. [DOI] [PubMed] [Google Scholar]
- Wu A Y, Chik S C, Chan A W, Li Z, Tsang K W, Li W. Anti-inflammatory effects of high-dose montelukast in an animal model of acute asthma. Clin Exp Allergy. 2003;33:359–366. doi: 10.1046/j.1365-2222.2003.01615.x. [DOI] [PubMed] [Google Scholar]
- Chibana K, Ishii Y, Asakura T, Fukuda T. Up-regulation of cysteinyl leukotriene 1 receptor by IL-13 enables human lung fibroblasts to respond to leukotriene C4 and produce eotaxin. J Immunol. 2003;170:4290–4295. doi: 10.4049/jimmunol.170.8.4290. [DOI] [PubMed] [Google Scholar]
- Braccioni F, Dorman S C, O'byrne P M, Inman M D, Denburg J A, Parameswaran K, Baatjes A J, Foley R, Gauvreau G M. The effect of cysteinyl leukotrienes on growth of eosinophil progenitors from peripheral blood and bone marrow of atopic subjects. J Allergy Clin Immunol. 2002;110:96–101. doi: 10.1067/mai.2002.125000. [DOI] [PubMed] [Google Scholar]
- Elsas P X, Queto T, Mendonça-Sales S C, Elsas M I, Kanaoka Y, Lam B K. Cysteinyl leukotrienes mediate the enhancing effects of indomethacin and aspirin on eosinophil production in murine bone marrow cultures. Br J Pharmacol. 2008;153:528–535. doi: 10.1038/sj.bjp.0707586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa A, Austen K F, Kanaoka Y. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J Biol Chem. 2002;277:20820–20824. doi: 10.1074/jbc.M203163200. [DOI] [PubMed] [Google Scholar]
- Beller T C, Maekawa A, Friend D S, Austen K F, Kanaoka Y. Targeted gene disruption reveals the role of the cysteinyl leukotriene 2 receptor in increased vascular permeability and in bleomycin-induced pulmonary fibrosis in mice. J Biol Chem. 2004;279:46129–46134. doi: 10.1074/jbc.M407057200. [DOI] [PubMed] [Google Scholar]
- Kanaoka Y, Maekawa A, Penrose J F, Austen K F, Lam B K. Attenuated zymosan- induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J Biol Chem. 2001;276:22608–22613. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- Guerrero A T G, Verri W A, Jr, Cunha T M, Silva T A, Schivo I R S, Dal-Secco D, Canetti C, Rocha F A C, Parada C A, Cunha F Q, Ferreira S H. Involvement of LTB4 in zymosan-induced joint nociception in mice: participation of neutrophils and PGE2. J Leukoc Biol. 2008;83:122–130. doi: 10.1189/jlb.0207123. [DOI] [PubMed] [Google Scholar]
- Gaspar-Elsas M I, Maximiano E S, Joseph D, Alves L, Topilko A, Vargaftig B B, Xavier-Elsas P. Upregulation by glucocorticoids of responses to eosinopoietic cytokines in bone-marrow from normal and allergic mice. Br J Pharmacol. 2000;129:1543–1552. doi: 10.1038/sj.bjp.0703145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar-Elsas M I, Joseph D, Lintomen L, Maximiano E S, Bodstein M, Xavier-Elsas P, Vargaftig B B. Murine myeloid progenitor responses to GM-CSF and eosinophil precursor responses to IL-5 represent distinct targets for downmodulation by prostaglandin E(2) Br J Pharmacol. 2000;130:1362–1368. doi: 10.1038/sj.bjp.0703403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar-Elsas M I, Queto T, Vasconcelos Z, Jones C P, Lannes-Vieira J, Xavier-Elsas P. Evidence for a regulatory role of α4-integrins in the maturation of eosinophils generated from the bone-marrow in the presence of dexamethasone. Clin Exp Allergy. 2009;39:1187–1198. doi: 10.1111/j.1365-2222.2009.03289.x. [DOI] [PubMed] [Google Scholar]
- Ten R M, Pease R L, McKean D J, Bell M P, Gleich G J. Molecular cloning of the human eosinophil peroxidase. Evidence for the existence of a peroxidase multigene family. J Exp Med. 1989;169:1757–1769. doi: 10.1084/jem.169.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaoka Y, Boyce J A. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol. 2004;173:1503–1510. doi: 10.4049/jimmunol.173.3.1503. [DOI] [PubMed] [Google Scholar]
- Williams T J, Jose P J. Role of eotaxin and related CC chemokines in allergy and asthma. Chem Immunol. 2000;78:166–177. doi: 10.1159/000058825. [DOI] [PubMed] [Google Scholar]
- Southam D S, Widmer N, Ellis R, Hirota J A, Inman M D, Sehmi R. Increased eosinophil-lineage committed progenitors in the lung of allergen-challenged mice. J Allergy Clin Immunol. 2005;115:95–102. doi: 10.1016/j.jaci.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Xavier-Elsas P, Santos-Maximiano E, Queto T, Mendonça-Sales S, Joseph D, Gaspar-Elsas M I C, Vargaftig B B. Ectopic lung transplantation induces the accumulation of eosinophil progenitors through an allergen- and interleukin-5-dependent mechanism. Clin Exp Allergy. 2007;37:29–38. doi: 10.1111/j.1365-2222.2006.02623.x. [DOI] [PubMed] [Google Scholar]