Abstract
Chloride serves as a critical component of innate host defense against infection, providing the substrate for MPO-catalyzed production of HOCl in the phagosome of human neutrophils. Here, we used halide-specific fluorescent sensors covalently coupled to zymosan particles to investigate the kinetics of chloride and iodide transport in phagosomes of human neutrophils. Using the self-ratioable fluorescent probe specific for chloride anion, we measured chloride dynamics within phagosomes in response to extracellular chloride changes by quantitative fluorescence microscopy. Under the experimental conditions used, normal neutrophils showed rapid phagosomal chloride uptake with an initial influx rate of 0.31 ± 0.04 mM/s (n=5). GlyH-101, a CFTRinh, decreased the rate of uptake in a dose-dependent manner. Neutrophils isolated from CF patients showed a significantly slower rate of chloride uptake by phagosomes, having an initial influx rate of 0.043 ± 0.012 mM/s (n=5). Interestingly, the steady-state level of chloride in CF phagosomes was ∼26 mM, significantly lower than that of the control (∼68 mM). As CFTR transports chloride as well as other halides, we conjugated an iodide-sensitive probe as an independent approach to confirm the results. The dynamics of iodide uptake by neutrophil phagosomes were monitored by flow cytometry. CFTRinh172 blocked 40–50% of the overall iodide uptake by phagosomes in normal neutrophils. In a parallel manner, the level of iodide uptake by CF phagosomes was only 20–30% of that of the control. Taken together, these results implicate CFTR in transporting halides into the phagosomal lumen.
Keywords: fluorescent halide probes, chloride sensor, iodide sensor, ion transport, hypochlorous acid
Introduction
Neutrophils constitute 50–60% of the circulating leukocyte pool in humans and represent the first line of cellular defense in innate immune response to infection. Efficient antimicrobial action occurs in the phagosome of neutrophils, where secreted granule contents and reactive O2 species, generated by the NADPH oxidase, combine to create an environment noxious to the ingested microbe. Critical to optimal antimicrobial action in the neutrophil phagosome is the generation of HOCl, a process requiring H2O2 produced by the phagocyte NADPH oxidase, the azurophilic granule protein MPO, and chloride anion [1], as indicated in the following reaction 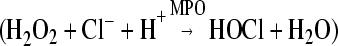 . In addition to the direct involvement in oxidant production, chloride has been reported to act as a counter ion to neutralize membrane potential changes induced by activation of the NADPH oxidase [2] and to modulate phagosomal pH and ionic strength [3]. We reported recently that chloride availability to phagosomes is required not only for oxidant-mediated but also for nonoxidant-dependent bacterial killing [4]. Although these data suggest that chloride anion plays a pivotal role in neutrophil function, the mechanisms of chloride anion transport into the phagosomal lumen have not been defined fully. We have developed two zymosan-conjugated halide probes that can be phagocytosed by neutrophils. Using a quantitative fluorescence microscopy technique to record the dynamic changes of chloride in the phagosomal lumen of live neutrophils, we found that chloride transport into neutrophil phagosomes was compromised when the CFTR, a cAMP-activated ClC, was blocked by specific inhibitors or impaired by inherited defects present in patients with CF. Similar results were obtained by using an iodide-specific probe monitored by flow cytometry. Taken together, the data suggest that CFTR contributes to halide transport into neutrophil phagosomes.
. In addition to the direct involvement in oxidant production, chloride has been reported to act as a counter ion to neutralize membrane potential changes induced by activation of the NADPH oxidase [2] and to modulate phagosomal pH and ionic strength [3]. We reported recently that chloride availability to phagosomes is required not only for oxidant-mediated but also for nonoxidant-dependent bacterial killing [4]. Although these data suggest that chloride anion plays a pivotal role in neutrophil function, the mechanisms of chloride anion transport into the phagosomal lumen have not been defined fully. We have developed two zymosan-conjugated halide probes that can be phagocytosed by neutrophils. Using a quantitative fluorescence microscopy technique to record the dynamic changes of chloride in the phagosomal lumen of live neutrophils, we found that chloride transport into neutrophil phagosomes was compromised when the CFTR, a cAMP-activated ClC, was blocked by specific inhibitors or impaired by inherited defects present in patients with CF. Similar results were obtained by using an iodide-specific probe monitored by flow cytometry. Taken together, the data suggest that CFTR contributes to halide transport into neutrophil phagosomes.
MATERIALS AND METHODS
Chemicals and reagents
Zymosan-A, 6-bromohexanoic acid, 6-methoxyquinoline, NaGlu, fresh human male type AB serum, NA, CHC, DIDS, SITS, Ficoll-Hypaque, and other common chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA). 6-Carboxytetramethyl-rhodamine hydroxysuccinimide ester and RG were obtained from Molecular Probes/Invitrogen (Eugene, OR, USA). Percoll and Na36Cl were obtained from GE Healthcare (Piscataway, NJ, USA). GlyH-101 and CFTRinh172 were obtained from Calbiochem (La Jolla, CA, USA).
Derivatization of zymosan chloride probe and iodide probe
MQHA was synthesized from 6-bromohexanoic acid and 6-methoxyquinoline. The covalent conjugation of MQHA and TMR to zymosan particles was achieved as described [5,6,7]. The MQHA-TMR-conjugated fluorescence probe responded to changes of chloride levels, indicative of collisional quenching. After ingestion in the presence of 5 mM azide, the two types of probe fluorescence were stable and did not diminish when the cells were extracted with detergent, indicating that both fluorchromes were covalently associated with the zymosan particles. When plotted according to the Stern-Volmer equation: F0blue/Fblue = Ksv [Cl−] + 1, where F0blue and Fblue indicate the fluorescent intensities at 0 mM chloride and tested chloride concentrations, respectively, and Ksv = the Stern-Volmer constant. As reported, Ksv = 30–35 M–1 for the probe particles in a physiological buffer environment [5]. The iodide-sensitive probe was prepared similarly by substitution of MQHA with the N-hydroxysuccinimide ester of the iodide-specific sensor RG. This probe is iodide-specific and does not respond to chloride at physiological, relevant concentrations [8].
Modified Ringer’s solutions
The physiological NaCl R was composed of 135 mM NaCl, 1.2 mM MgCl2, 1.2 mM CaCl2, 2.4 mM K2HPO4, 0.6 mM KH2PO4, 20 mM HEPES (pH 7.3), and 10 mM dextrose. The NaGlu R was made by substitution of the above chloride salts with equal molar concentration of gluconate salts, except 4 mM calcium gluconate was used to compensate for the mild calcium-chelating effect of gluconate. Sodium azide (5 mM) was added routinely to all of the buffers to prevent MPO-mediated bleaching of the fluorchromes, as described by Jankowski and colleagues [7]. For iodide-uptake studies, NaI Ringer’s buffer was made by substituting 135 mM NaI for 135 mM NaCl in the Ringer’s buffer recipe above.
Opsonization of dual-labeled zymosan
The derivatized zymosan probes (10 mg/ml) were opsonized by mixing with unheated human AB-pooled serum for 30 min at 37°C. The probe particles were washed three times with the NaGlu R and held on ice until use. Opsonization had no effect on the fluorescence properties of the particles or on their responses to halide anions [5].
Isolation of neutrophils from normal subjects and CF patients
Human peripheral blood neutrophils were isolated using the Percoll gradient method described previously [9]. In all cases, endotoxin-free reagents and plasticware were used to prevent activation of the cells. The Institutional Review Boards of Louisiana State University Health Sciences Center at New Orleans (LA, USA), Ochsner Medical Center at New Orleans (LA, USA), and University of Texas Medical Branch at Galveston (TX, USA) approved the human subject protocol. A total of nine CF patients were recruited. The genotypes of the CF population are as follows: Four were ΔF508 homozygotes, two were ΔF508 compound heterozygotes—ΔF508/1717-1(G→A*) and ΔF508/3272-26(A→G*)—and three had unknown mutations with severe CF disease, positive sweat tests, and pancreatic enzyme supplement. For the cytosolic chloride-uptake studies, large quantities of neutrophils (∼109 cells) were needed and prepared from buffy coats obtained from the Ochsner Blood Bank in New Orleans (LA, USA). Excess erythrocytes were sedimented at 1 g for 30 min at room temperature after adding an equal volume of 3% (w/v) dextran (500,000 MW; Sigma-Aldrich) in PBS. The leukocyte-rich supernatant was centrifuged at 300 g for 20 min, and the cell pellets were resuspended in 100 ml PBS. Cell suspension (16 ml) was then underlayered with 13 ml Ficoll-Hypaque (density, 1.077 g/ml) and centrifuged at 800 g for 30 min at room temperature. The cell pellet was resuspended in 10 ml PBS, and the residual RBCs were removed by brief hypotonic lysis as described previously [4].
Measurement of chloride dynamics in phagosomes by quantitative fluorescence microscopy
Neutrophils, pre-equilibrated in the NaGlu R for 1 h on ice, were incubated at 4°C with opsonized MQHA-TMR-zymosan particles for 30 min to synchronize their phagocytosis. The cell-zymosan mixtures were pipetted into Delta T Bioptech chambers and allowed to settle and adhere for 15 min at 37°C. The preparations were then rinsed several times with starting NaGlu R, as indicated in the text, to remove free particles, mounted on a thermostatic stage at 37°C, and imaged using a Leica DM-RXA upright epifluorescence microscope, equipped with a Lambda LS 75 W Xenon lamp, fitted with a Lambda 10-2 microprocessor-controlled, high-speed filter wheel to allow rapid, real-time ratioing of two differing filter cassettes. Fluorescence imaging data were collected every 30 s using a 63× water-immersion lens (numerical aperture, 0.9), the appropriate filter sets for MQHA (350 nm Ex; 400 nm Em; 600 ms exposure) and TMR (540 nm Ex; 565 nm Em; 2 ms exposure), and a Sensicam QE camera (Intellegent Imaging Innovations, Denver, CO, USA). Image analysis for computation of a fluorescent ratio of TMR/MQAE was performed using commercial software to calculate each fluorescent intensity over an integrated area minus the background-subtracted pixel intensities. Medium, as indicated, was pumped over the specimen with a peristaltic pump at a rate of 1 ml/ml. The data from seven to 11 single cells were imaged in each recording, and the background- and photobleach-corrected red and blue fluorescence (F-red and F-blue, respectively) was calculated. F-red/F-blue, which is independent of probe concentration, was calculated for each time-point and compared with a standard in situ calibration curve generated at the end of the experiment. Calibration was performed by using potassium chloride/potassium gluconate Ringer’s buffers in combination with 7 μM nigericin and 10 μM tributyl tin acetate, which in concert, act as a specific ionophore for chloride [10]. Alternatively, calibration was performed using the Ringer’s buffer containing 0.1% Triton X-100 and known chloride concentrations. Ksv were calculated for each particle cell set by plotting background fluorescence-corrected F0blue/Fblue as a function of chloride concentration. Background or autofluorescence was measured by bathing the cell preparations in 135 mM potassium thiocyanate, a potent quencher of MQHA, at the end of each set of experimental measurements. A plot of F0blue/Fblue yielded a straight line from which the Ksv could be estimated from the slope of a linear-regression statistical curve fit, made with a standard statistical software package. Extracellular probe particles in the physiological buffer typically had Ksv of 30–35 M–1, which can be distinguished from intraphagosomal particles with Ksv of 7–25 M–1.
Measurement of iodide dynamics in phagosomes by flow cytometry
Iodide influx into the phagosomes was measured by two-color flow cytometry. Neutrophils, resuspended in the NaGlu R and iodide-free gluconate Ringer’s buffer (1×107/ml), were mixed with a serum-opsonized RG-TMR-zymosan probe (20 μg/ml) and incubated for 15 min at 37°C for phagocytosis. CFTRinh172, when applied, was added prior to phagocytosis and was present throughout the experiment at the indicated concentrations. After phagocytosis, the cells in 0.05 ml were diluted directly in 0.45 ml 135 mM NaI Ringer’s solution containing 0.004% (v/v) trypan blue to quench the fluorescence of extracellular, nonphagocytosed probe particles. Calibration of the flow cytometric data was performed by diluting the cells into calibration solutions of various volumetric ratios of the iodide-free gluconate Ringer’s buffer and the iodide-rich KI Ringer’s buffer ranging from 0 mM KI to 135 mM KI. Tributyl tin acetate (10 μM) and nigericin (7 μM) were present in all calibration solutions prior to the addition of the cells. The green and red fluorescent signals were monitored using the standard FL1 and FL2 filter sets, respectively, at 10 s intervals for the first 1 min and at 60 s intervals thereafter with a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). At least 3000-gated events were collected in a 3- to 4-s period. The fluorescence ratio of the green RG to the red TMR of the internalized zymosan probe particles was calculated by dividing the MCF units of the red fluorescence by that of the green fluorescence after correcting both values for background obtained by adding potassium thiocyanate, a strong fluorescent quencher, to a final concentration of 135 mM at the end of each experiment. Inspection of these data indicated that the red fluorophore that was used to serve as a reference decreased unexpectedly with increasing iodide concentration. As the green RG sensor was covalently attached to the particles, it was decided to analyze the data using the green probe alone by calculation of F0/F for the MCF. The overall change in F0/F (green) was approximately twofold from 0 to 135 mM iodide concentrations. In contrast, F-red/F-green changed by only 15%. Hence, monitoring RG alone provided a considerably more robust assay with a larger dynamic range and less susceptibility to noise in the measurements. Flow cytometry data were analyzed using the WinMDI data processing program developed by Joseph Trotter at The Scripps Research Institute (La Jolla, CA, USA).
Measurement of cytosolic chloride uptake
Neutrophils were pre-equilibrated for 1 h on ice in the NaGlu R to deplete intracellular, exchangeable chloride. Preliminary isotope-dilution experiments using 36Cl–established that chloride uptake by zymosan-activated cells reached equilibrium within 10–20 min at 37°C and contained ∼16 mM intracellular chloride that was not exchangeable over the course of the experiment. To measure the rate of uptake of total chloride, we used the technique described by Simchowitz and De Weer [11]. Briefly, the gluconate-equilibrated neutrophils exposed to opsonized zymosan for 15 min at 37°C were centrifuged, and the pellet was resuspended in the prewarmed 127 mM NaCl R at 1 × 107/ml. Aliquots (1 mL) were taken at selected times (2, 5, and 10 min) in triplicate and layered over Nyosil M25 silicon oil and centrifuged at 10,000 g for 1 min. The cells pelleted through the oil phase within 5 s. The aqueous top layer was removed by aspiration, followed by repeated rinses with water to remove any residual radioactivity. After careful aspiration of the oil phase, the cell pellet was dissolved in 0.1% Triton X-100 in water and freeze-thawed once. After centrifugation, the supernatants were assayed for chloride using a colorimetric assay [12]. The total free ionic chloride concentration inside the cell was calculated by subtracting the nonexchangable chloride (defined as that present in the chloride-depleted cells at the start of the experiment) from the total chloride level measured at times after resuspension in 127 mM NaCl R. Sodium azide (5 mM) was present in all buffers to minimize the MPO-mediated conversion of free cytosolic chloride ions to HOCl, which would not be detected in the spectrophotometric assay. The radioactive measurement of cytosolic chloride uptake was performed similarly by adding 1.5 μCi/ml Na36Cl to 127 mM NaCl R. At various time-points (2, 5, and 10 min), the cells were sampled and layered onto the heavy silicon oil for quick separation of the cells as described above. Radioactivity was examined by dissolving the cell pellet in 0.1% Triton X-100 in water and counted in 10 ml Scintiverse II liquid scintillation fluid in a Packard TriCarb liquid scintillation counter. The specific activity of total cell-bound chloride was obtained by determination of radioactivity in dpm and assay of total cell-bound chloride by colorimetry as described above.
Statistical analysis
Wilcoxon-Mann-Whitney nonparametric analysis was performed after the preliminary inspection of the data revealed a non-normal distribution. Otherwise, Student’s t-test was used. Intergroup comparisons were done using ANOVA test. P values <0.05 were considered statistically significant.
RESULTS
Zymosan-conjugated self-ratioable fluorescent probe for sensing chloride levels in neutrophil phagosomes
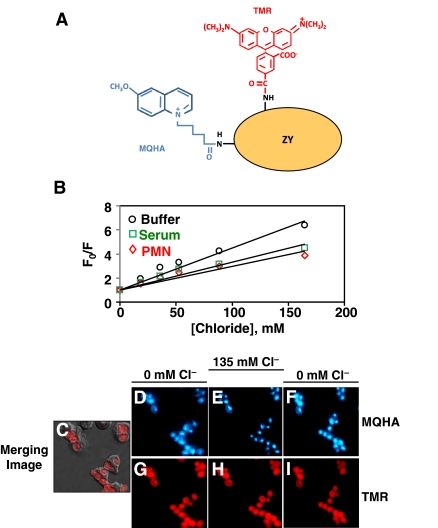
A dual-labeled fluorescent chloride probe (Fig. 1A) was prepared by chemical conjugation of MQHA and TMR to zymosan-A particles [5,6,7]. The probe has been proven to indicate authentically chloride levels extracellularly and intracellularly and is insensitive to other physiological ions including hydronium ion [5, 6]. The blue fluorescence responded to changes in chloride concentration, obeying the Stern-Volmer-quenching relationship in a physiological buffer, as well as in the presence of neat serum or the proteins (17 mg/ml) released from neutrophils by phorbol ester stimulation (Fig. 1B). The Ksv did decrease from 32 M–1 to ∼22 M–1, but linearity was maintained. This result indicates the need for in situ calibration of the probe’s response to chloride. To record the dynamics of chloride in phagosomes of live neutrophils in response to extracellular chloride changes, freshly isolated peripheral blood neutrophils were resuspended in NaGlu R for 1 h on ice to deplete the exchangeable intracellular chloride. Then, the serum-opsonized zymosan-probe particles were incubated with the neutrophils in the chloride-free buffer at 4°C to synchronize their phagocytosis and then pipetted into a Delta T Bioptech chamber and allowed to settle and adhere for 15 min at 37°C. After several rinses with NaGlu R to remove free zymosan-probe particles, the specimens were mounted on a temperature-controlled microscope stage. The chloride depletion procedure lowers total cellular chloride, providing a more favorable chloride gradient for measuring ion influx, and does not affect phagocytosis or superoxide responses [4, 13]. Fluorescent signals from cells in typical views were acquired by quantitative fluorescence microscopy. Displayed (Fig. 1, C-I) is a representative field of phagocytosed zymosan-probe particles within normal neutrophils bathed in isotonic media with varied chloride concentrations. The blue MQHA fluorescence in phagosomes responded to changes of chloride concentration in the bath medium from 0 mM to 135 mM and back to 0 mM (Fig. 1, D–F). The MQHA blue fluorescence was quenched after the medium was changed to 135 mM NaCl R, and fluorescence intensity was restored to its initial level after the medium was returned to 0 mM chloride. As expected, the corresponding red TMR fluorescence in the same cells (Fig. 1, G–I) did not respond to changes in chloride concentration.
Figure 1.
Zymosan-conjugated self-ratioable fluorescent probe for sensing chloride levels. (A) A schematic drawing of the zymosan (ZY)-ratioable fluorescent probe used in chloride dynamic measurement. MQHA is a blue fluorofluor (λEx=340 nm; λEm=430 nm), which is quenched specifically by free halide anions. TMR is a red-emitting fluorofluor (λEx=520 nm; λEm=590 nm), which is insensitive to most physiological ions (including H+) and serves as a reference fluorescence. (B) Fluorescence responses of the MQHA-TMR-zymosan probe to chloride changes in the cell-free system. The MQHA-TMR-labeled zymosan probe (20 μg/ml) was incubated in NaGlu R (Buffer, ○), neat human serum (Serum, □), or neutrophil-releasate proteins [polymorphonuclear neutrophil (PMN)], ⋄; 17 mg/ml]. The solutions were titrated by adding small volumes of 1 M NaCl, and the fluorescence intensities were determined by a fluorescent plate reader. The data are plotted according to the Stern-Volmer collisional-quenching equation: F0/F = 1 + Ksv[Cl−]. The Ksv in the buffer, serum, or releasate was obtained from the linear regression lines and was 34, 20, and 20 M–1, respectively. (C–I) Microscopic images of a representative field of normal neutrophils 15 min after ingestion of serum-opsonized MQHA-TMR-zymosan particles. Merged image of phase-contrast micrograph with the corresponding MQHA and TMR fluorescence micrographs (C). Blue MQHA fluorescence associated with the zymosan particles within the phagosomes initially in 0 mM chloride buffer NaGlu R (D). Ten minutes after the extracellular medium was changed to 135 mM chloride buffer NaCl R (E). After another 10 min, the bath medium was changed back to 0 mM chloride medium (F). Displayed (G–I) are the corresponding images of the red TMR fluorescence.
CFTR-mediated chloride transport in phagosomes of normal and CF neutrophils
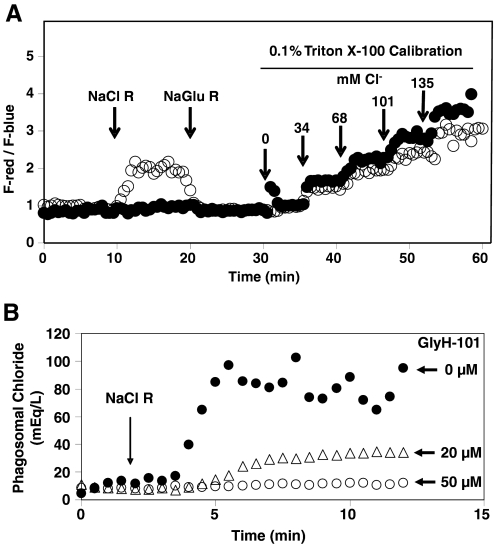
We compared the dynamics of chloride concentration change in phagosomes of normal and CF neutrophils. The fluorescence intensities of TMR and MQHA were similarly recorded by quantitative fluorescence microscopy in real time. F-red/F-blue, which is independent of probe concentration, was calculated at each time-point and compared with a standard calibration curve generated at the end of each record. Calibration was performed by sequentially changing to media containing varied concentrations of NaCl R, supplemented with 0.1% Triton X-100. Isotonicity and ionic strength were held constant by substitution of sodium gluconate for sodium chloride to maintain the total Na+ level of 135 mEq/L. Under these conditions, intracellular chloride exchanges rapidly with extracellular chloride, allowing the system to be calibrated internally and the chloride concentrations calculated for each time-point from the observed ratios of the TMR and MQHA fluorescent intensities. As demonstrated (Fig. 2A), the fluorescence ratio (F-red/F-blue) of ingested particles was low initially in normal and CF phagosomes when the cells were bathed in 0 mM chloride buffer (NaGlu R), indicating a relatively low chloride concentration. However, after the medium was changed to 135 mM NaCl R at the 10-min mark, the F-red/F-blue ratio increased in normal cells (Fig. 2A, ○), indicating an increase in phagosomal chloride concentration. Remarkably, little change in the ratio in CF neutrophils was detected over a 10-min period after the buffer change to 135 mM chloride (Fig. 2A, •). At the 20-min mark, the medium was changed back to 0 mM NaCl R (NaGluc R). This resulted in a rapid decrease in the fluorescence ratio in the normal neutrophils, indicating chloride efflux out of the phagosomes. CF neutrophils were essentially unresponsive again to the buffer change over this 10-min period. At the 30-min mark, the cells were bathed sequentially in a series of calibration buffers over a range of chloride concentrations at 6 min intervals. The F-red/F-blue ratio increased similarly in both cell types (Fig. 2A). These results indicate that the influx and efflux of chloride in the phagosomal compartment were completely reversible in normal neutrophils but that the phagosome of CF neutrophils, in contrast, seemed to be remarkably impermeable to chloride anion.
Figure 2.
F-red/F-blue ratios of intraphagosomal probe or phagosomal chloride levels measured by quantitative fluorescence microscopy. (A) F-red/F-blue ratios of a representative neutrophil from a normal or CF subject in response to extracellular chloride changes. After phagocytosis of the probe particles by normal neutrophils (○) in the NaGlu R, the initial ratio F-red/F-blue was ∼1 and rose rapidly after the medium was changed to 135 mM NaCl buffer (NaCl R) at the 10-min mark. Changing the medium back to the NaGlu R restored the ratio to ∼1 rapidly. CF neutrophils (•) showed little response to the buffer changes in the first 30 min of the experiment. At 30 min, a series of isoosmotic 0.1% Triton X-100 solutions containing varied chloride concentrations was introduced sequentially to calibrate the system at 6 min intervals. As expected, the fluorescent ratio rose in response to chloride, as the detergent allowed rapid equilibration of the extracellular medium with the intraphagosomal solution. (B) Effect of GlyH-101 on the rates of chloride uptake and the steady-state chloride levels in phagosomes of normal neutrophils. In the untreated cells (•), the level of chloride rose at a rapid rate and achieved a steady-state level of 70–80 mM for this sample. GlyH-101 was present in the medium at 20 μM (▵) or 50 μM (○) as indicated. The rate and extent of chloride steady-state level were reduced in a dose-dependent manner.
The above data suggested that under the experimental conditions, CF neutrophils were relatively resistant to transport of chloride anion in their phagosomes. We reasoned that if this defect were a result of CFTR dysfunction, use of a CFTRinh would recapitulate the phenomenon in normal neutrophils. To test this prediction, we determined whether GlyH-101, a highly specific CFTRinh, impeded the transport of chloride into the phagosomes of normal neutrophils after CFTR blockade. GlyH-101 blocks CFTR-mediated chloride transport by occluding the extracellular-facing pore of the channel in a rapid and reversible manner and does not inhibit other known ClCs at concentrations below 25 μM [14]. The drug was added prior to phagocytosis and present throughout the experiment to insure contact with the luminal surface of the phagosome Normal neutrophils treated with 0, 20, or 50 μM GlyH-101 exhibited a dose-dependent inhibition of chloride influx into phagosomes (Fig. 2B).
Using this experimental approach, chloride transport in neutrophils from five normal donors and five CF patients was evaluated (Table 1). When the extracellular chloride level was changed from 0 mM to 135 mM, the steady-state level of chloride in the phagosomes of CF neutrophils was significantly lower (∼26 mM) than that of the control (∼68 mM). Furthermore, the initial influx rate for the CF phagosomes (0.043±0.012 mM/s, n=5) was ∼7.2-fold lower than that of the control (0.31±0.04 mM/s, n=5). Similarly, when the extracellular chloride was changed from 135 mM to 0 mM, the initial efflux rate for the CF phagosomes (0.063±0.013 mM/s, n=5) was significantly lower than that of the normal cells (0.26±0.05 mM/s, n=5). Normal neutrophils treated with the CFTRinh GlyH-101 mimicked the behavior of CF cells, strongly implicating involvement of this ClC in the process.
TABLE 1.
Summary of Kinetic Parameters of Transport of Chloride in Neutrophil Phagosomes of Normal and CF Neutrophilsa
| Extracellular medium change from 0 mM to 135 mM CI−
|
Extracellular medium change from 135 mM to 0 mM Cl−
|
|||
|---|---|---|---|---|
| Initial influx rate mM/s | Steady-state [Cl−]PL (mM) | Initial efflux rate mM/s | Steady-state [Cl−]PL (mM) | |
| Normal controls (n = 5) | 0.31 ± 0.04 | 67.7 ± 7.3 | −0.26 ± 0.05 | 15.9 ± 4.3 |
| Normals GlyH-101 (n = 5) | 0.013 ± 0.067b | 27.7 ± 4.4b | −0.095 ± 0.022c | 12.9 ± 4.2 |
| CF (n = 5) | 0.043 ± 0.012b | 25.5 ± 3.3b | −0.063 ± 0.13c | 17.0 ± 3.3 |
The influx rate represents the maximal initial slope of the chloride change in mM/s after changing the medium from NaGlu R (0 mM chloride) to NaCl R (135 mM chloride) at the 10-min mark. The efflux rate represents the opposite rate (hence, indicated by negative signs) when the buffer was changed from NaCl R (135 mM chloride) to NaGlu R (0 mM chloride) at the 20-min mark. The steady-state [Cl−]PL represents the phagosomal chloride level 10 min after each respective buffer change.
P < 0.001;
P < 0.05 by Wilcoxon-Mann-Whitney nonparametric analysis (each was compared with the corresponding normal controls). [Cl−]PL, phagosomal chloride concentration.
Cytosolic chloride uptake is independent of CFTR
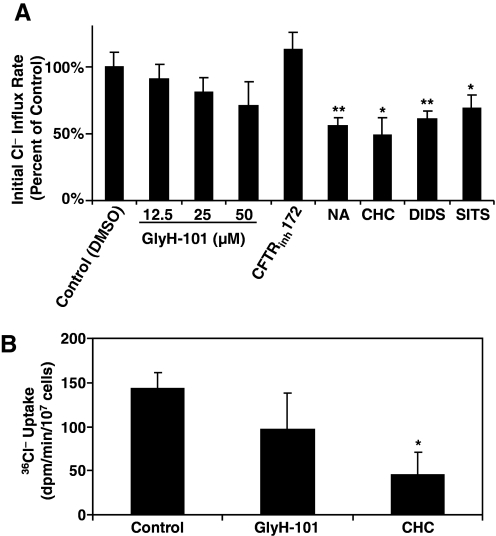
For extracellular chloride anion to reach the lumen of intact phagosomes, it must first cross the plasma membrane to reach the cytoplasm, from which it in turn is transported across the phagosomal membrane and into the phagosome. Consequently, failure of chloride uptake into phagosomes may reflect defective chloride transport from the extracellular medium to the cytoplasm, from the cytosol to the phagosomes, or from a combination of both. To define if CFTR contributes to chloride transport from extracellular medium to the cytosol, we measured the change in cytosolic chloride concentration in response to a range of extracellular chloride concentrations (0–135 mM). As done previously, normal neutrophils were resuspended in the NaGlu R for 1 h on ice to deplete the intracellular chloride. After opsonized zymosan particles were fed to neutrophils in the chloride-free buffer for 15 min at 37°C, cells were resuspended in 135 mM chloride buffer for varied times (2, 5, and 10 min) in the presence or absence of ion channel inhibitors. After rapid centrifugation through silicon oil, total measurable cellular chloride in the cell pellet was assessed colorimetrically [12], and the initial rates of chloride uptake were calculated. The relative initial rate of chloride influx in percent as compared with the control for each condition was plotted (Fig. 3A). Neither GlyH-101 (12.5, 25, and 50 μM) nor CFTRinh172 (10 μM), specific CFTRinh, significantly affected the rate of chloride influx to the cytosol. GlyH-101-treated cells trended toward a lower rate of uptake, especially at the high dose (50 μM), which could be a result of the nonspecific, off-target effect of the inhibitor. In contrast, other anion channel-blockers, such as NA (100 μM), CHC (10 mM), DIDS (100 μM), and SITS (100 μM), decreased significantly the rate of chloride uptake by the cells. Furthermore, combinations of GlyH-101 with CHC did not appear to have any significantly synergistic effect (data not shown).
Figure 3.
Effects of chloride or anion channel inhibitors on the initial rate of chloride uptake to the cytosol of zymosan-activated normal neutrophils. (A) Effects of chloride or anion channel inhibitors on the initial rate of chloride uptake to the cytosol of zymosan-activated neutrophils by colorimetric assay. Neutrophils, depleted of chloride by incubation in the NaGlu R for 1 h at 4°C, were allowed to phagocytose opsonized zymosan particles for 15 min at 37°C. The cells were then incubated with the stated concentrations of channel inhibitors in 135 mM NaCl R for 2, 5, and 10 min. Samples in triplicate were collected at each time-point and assayed for the total chloride content with a colorimetric assay as described in Materials and Methods. The initial rate of chloride uptake for each condition was calculated and compared with that of the DMSO control. The final concentration(s) for each inhibitor were GlyH-101 (CFTRinh, 12.5 μM, 25 μM, and 50 μM), CFTRinh172 (CFTRinh, 20 μM), NA (100 μM), CHC (10 mM), DIDS (100 μM), and SITS (100 μM). *, P < 0.05; **, P < 0.01. (B) Effects of chloride or anion channel inhibitors on the initial rate of radioactive 36Cl uptake to the cytosol of zymosan-activated neutrophils, which from five donors, similarly depleted of chloride, were allowed to phagocytose opsonized zymosan particles for 15 min at 37°C. The cells were then incubated with the stated concentrations of channel inhibitors in 135 mM chloride buffer containing radioactive 36Cl− (1.5 μCi/ml) for 2, 5, and 10 min. Then, the cells were assayed for 36Cl− uptake. The initial rates were determined from kinetic plots over a 10-min period. *, P < 0.05, compared with the control group (n=5).
To assess chloride uptake using a different method, we measured the rate of influx of radioactive 36Cl− into neutrophils in the presence or absence of CFTRinhGlyH-101 and the general anion inhibitor CHC. Cell-associated 36Cl− radioactivity increased over time when neutrophils, preactivated with opsonized zymosan for 15 min in the NaGlu R, were transferred to the chloride-rich medium (135 mM chloride) containing 36Cl− for various times (2, 5, and 10 min). GlyH-101 (25 μM) did not significantly block the influx of 36Cl− across the cytoplasmic membrane. In contrast, CHC significantly inhibited the chloride uptake at the cytoplasmic membrane (Fig. 3B), consistent with published results [11]. These data suggest that in contrast to its activity at the phagosomal membrane, CFTR likely did not play a major role in chloride transport across the cytoplasmic membrane. This conclusion concurs with our previous finding that the rates of iodide uptake into the cytoplasm by normal and CF neutrophils are comparable [13].
Zymosan-conjugated fluorescent probe for sensing iodide levels in neutrophil phagosomes
Despite their varied anion selectivity, channels generally recognized as ClCs are capable of transporting iodide [15, 16]. As our data demonstrated that CFTR supported chloride transport across phagosomal but not plasma membrane, we sought to assess more rigorously anion flux selectively into the phagosomes. As channels that support chloride transport likewise mediate transport of iodide, we quantitated phagosomal idodide uptake as an independent approach to extend our chloride transport observations. To this end, we used an iodide-specific zymosan probe prepared in a way similar to that used for the chloride zymosan probe shown in Figure 1A, except that RG, a long-wavelength, iodide-specific sensor, was substituted for MQHA. We selected this probe to assess iodide transport in phagosomes, as there is little or no endogenous iodide to interfere with the interpretation of results; RG is exquisitely photostable, and the cellular autofluorescence background is lower as a result of the long excitation wavelength; the emission wavelength is suitable for detection by flow cytometry; and a bulk population of cells can be analyzed rapidly through flow cytometry.
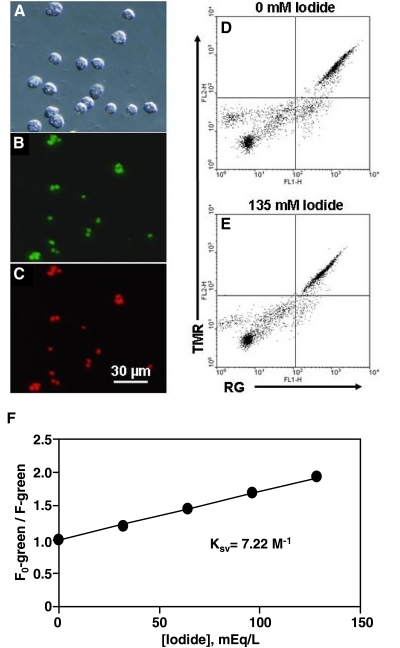
Micrographs of the RG-TMR-zymosan particles 15 min after phagocytosis by normal neutrophils are shown in Figure 4, A–C. For kinetic study of iodide uptake by phagosomes, neutrophils resuspended in the NaGlu R and iodide-free gluconate buffer were fed the opsonized RG-TMR-zymosan probe for 15 min at 37°C. The cells were subjected subsequently to analysis by flow cytometry. Figure 4, D and E, shows a two-color dot plot of the ingested dual-labeled probe in response to 0 mM and 135 mM iodide in the presence of nigericin and tributyl tin acetate. When the extracellular iodide was changed from 0 mM to 135 mM, MCF for RG decreased significantly in response to such a change. Unexpectedly, the MCF for TMR, the reference fluorophore, was also reduced for reasons unknown, thus undermining the rationale for monitoring the TMR fluorophore as a reference. Consequently, as the iodide sensor was covalently attached to the zymosan particles, and the cells were diluted accurately and gated for single-cell analysis, we elected to measure RG, the green iodide probe only. Shown in Figure 4F, the F0/F ratio of the green fluorescence remained linear and indicated iodide concentrations faithfully. The linear relationship was fitted into the Stern-Volmer collisional quenching equation and yielded a Ksv of 7.2 M–1, lower than the Ksv for the probe in a cell-free medium (14 M–1, data not shown). Presumably, the reduced Ksv for the probe within neutrophils reflects the increased viscosity in the protein-rich phagosomal lumen created by release of granules during phagocytosis.
Figure 4.
Zymosan-conjugated fluorescent probe for sensing iodide levels. (A–C) Microscopic images of the iodide-specific probe in human neutrophils. Phase-contrast image of RG-TMR-zymosan particles in human neutrophils 15 min after ingestion (A). The corresponding RG fluorescence and TMR fluorescence images (B and C). (D–F) Two-color flow cytometric analysis of the RG-TMR-zymosan probe in neutrophils in response to extracellular iodide changes. The green RG fluorescence [FL1-height (FL1-H)] and the red TMR fluorescence (FL2-H) were gated electronically on the neutrophil population, as defined by forward- and 90° light-scatter. A flow cytometric dot plot shows the MCF change of the probe in the isoosmotic high potassium Ringer’s calibration buffer containing 0 mM (D) or 135 mM (E) iodide and also containing 10 μM tributyl tin acetate and 7 μM nigericin as ionophores. (F) The calibration curve was generated using a series of defined calibration buffers with varied iodide concentrations. The data were plotted as the ratio of the MCF of RG in iodide-free Ringer’s buffer (F0) to that of RG of the same cell population measured in the presence of a given iodide concentration. The Ksv was determined by linear regression analysis to be 7.22 M–1, according to the equation F0/F = 1 – Ksv[I−], where [I−] is the molar concentration of iodide.
Kinetics of iodide uptake into phagosomes assessed by flow cytometry
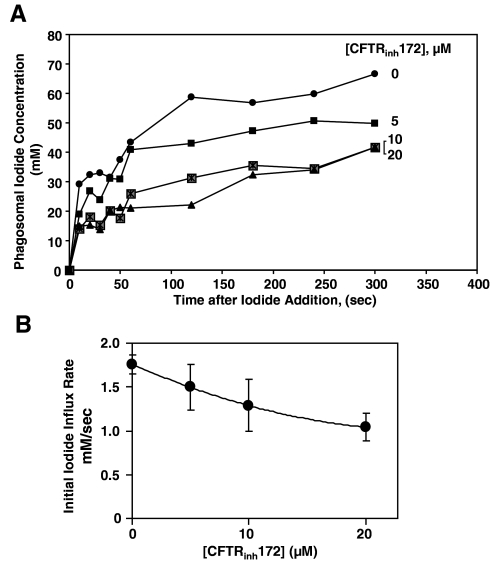
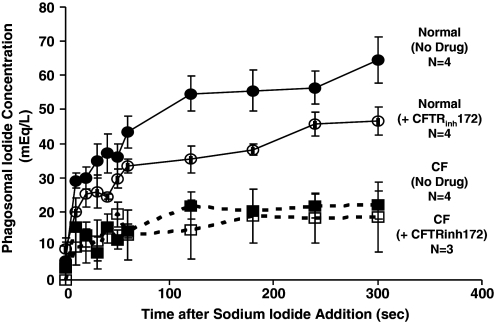
Neutrophils, resuspended in the NaGlu R and iodide-free gluconate Ringer’s buffer, were allowed to phagocytose the opsonized iodide-zymosan probe for 15 min. Aliquots were made and held on ice. For flow cytometric measurement, each aliquot was diluted into the room-temperature 135 mM NaI Ringer’s buffer in tenfold volume excess. CFTRinh172 (10 μM) was added prior to phagocytosis of zymosan probe and included in all of the buffers thereafter for the entire experiment. Preliminary experiments determined that the inhibitor did not alter phagocytosis (data not shown). Data were acquired at 10 s intervals for the 1st minute and at 60 s intervals thereafter, for a total of 5 min. Each data acquisition lasted 3–4 s, and at least 3000 particle-bearing cells were collected for each time-point. Calibration for each sample was performed in parallel as described above. Iodide uptake by normal phagosomes was quite rapid even at room temperature and was completed after 120 s (Fig. 5A). The initial influx rate of iodide attained in the phagosomes was slowed by the CFTRinh in a dose-dependent manner (Fig. 5B). The highest concentration of inhibitor (20 μM) reduced the initial rate of iodide uptake by 40–50% as compared with the inhibitor-free control. We also compared the dynamics of iodide uptake by CF phagosomes with that of the control. The level of iodide uptake by CF phagosomes was 20–30% of the control (Fig. 6).
Figure 5.
CFTRinh172 inhibits iodide uptake into phagosomes of normal neutrophils in a dose-dependent manner. (A) Iodide influx kinetics in phagosomes of normal neutrophils treated with varied doses of CFTRinh172. Neutrophils with ingested zymosan probe were diluted into 135 mM NaI Ringer’s buffer containing 0.004% trypan blue. The fluorescence parameters associated with the ingested RG-TMR-zymosan probe were acquired in the absence of any ionophores. CFTRinh172 was present during phagocytosis at the indicated concentrations and throughout the experiment. Maximum ratios (F0/F) of the green fluorescence, determined in the NaGlu R and iodide-free gluconate Ringer’s buffer in the presence and absence of drug, were used to establish the ratio for zero iodide concentration. Calibration was carried out using the protocol described in Materials and Methods. Data represent the average of duplicate determinations for a single neutrophil donor. (B) Dose-dependent effect of CFTRinh172 on iodide influx into phagosomes of normal neutrophils. The data were obtained for four different donors. Forty percent to 50% of iodide uptake was inhibited at 20 μM inhibitor with an apparent EC50 of 6 μM. The extent of iodide uptake into the phagosome was calculated by estimating the initial rate of iodide uptake for each donor within the linear region of the plot and averaging the results for all donors. Vertical bars represent sd of the mean.
Figure 6.
Iodide uptake into phagosomes of normal and CF neutrophils. By using the iodide-specific probe, iodide uptakes by phagosomes of normal neutrophils from four donors (•, solid line) or from four CF donors (▪, dashed line) were determined. A decrease rate and extent of iodide uptake were seen in CF neutrophils (▪, dashed line), which was slightly less than that seen in normal cells treated with 20 μM CFTRinh172 (○, solid line). CFTRinh172 did not affect the rate of uptake of iodide by CF neutrophils (□, dashed line).
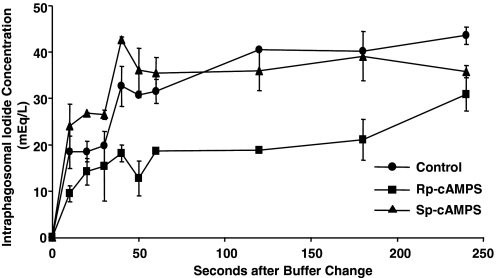
As CFTR is a cAMP-activated ClC, we reasoned that the modulation of cAMP should alter iodine transport into neutrophil phagosomes. To test this prediction, neutrophils were treated with 100 μM Rp-cAMPS, a potent and specific inhibitor of cAMP-mediated activation of cAMP-dependent protein kinases, or 100 μM Sp-cAMPS, a potent and specific activator of cAMP-dependent kinases, for 5 min. Next, the cells were allowed to phagocytose the zymosan-conjugated iodide probe for 15 min at 37°C. Then, the cell suspensions were placed on ice, and 50 μl aliquots were assayed for iodide uptake by flow cytometry, as described above. Treating neutrophils with the Rp-cAMPS inhibitor reduced phagosomal iodide uptake significantly by ∼50% (Fig. 7). In contrast, the cAMP analog Sp-cAMPS did not affect the uptake as compared with the no drug-treated cells. The Sp-cAMPS-treated cells had a rate of iodide transport and steady-state iodide level in phagosomes comparable with those of no drug-treated cells (Fig. 7), suggesting the cAMP-dependent halide transport was near-maximal capacity under the experimental conditions used. Consistent with our findings from studies of chloride flux, these data strongly implicate CFTR in halide transport into neutrophil phagosomes.
Figure 7.
cAMP-dependent iodide transport into neutrophil phagosomes. Neutrophils were incubated with the indicated drugs for 5 min prior to the addition of opsonized RG/TMR-zymosan particles in the gluconate buffer containing sodium azide. Fifteen minutes later, the cell suspensions were placed on ice, and 50 μl aliquots were assayed for iodide uptake by flow cytometry. When present, drugs were included in the iodide uptake solutions. Control cells (no drug) showed normal rates of uptake of iodide into the phagosomal lumen (•). In contrast, the cAMP antagonist Rp-cAMPS (100 μM; ▪) slowed the uptake by ∼50%, whereas the cAMP agonist isomer Sp-cAMPS (100 μM; ▴) had no significant effect over that seen in the controls.
DISCUSSION
In contrast to other phagocytic cells, such as macrophages and dendritic cells, neutrophils rely heavily on the generation of oxidants to mediate antimicrobial action in the phagosome. Prominent among the oxidant-dependent microbicidal species produced by neutrophils is the MPO-catalyzed generation of HOCl. Stimulated neutrophils produce HOCl at a rate of 134 mM/min, accounting for ∼89% of the O2 consumed by the NADPH-dependent oxidase [17]. To sustain generation of such high levels of HOCl, neutrophils require an adequate supply of chloride ion to the phagosome, as the amount of chloride incidentally acquired from the extracellular medium during phagocytosis would likely be consumed rapidly. However, the precise means by which chloride anion is delivered into the phagosome to support HOCl production is incompletely understood, prompting our interest in quantitating chloride and iodide transport into the phagosomes of human neutrophils. It is important to recognize that our experimental system required the use of sodium azide to inhibit MPO-mediated oxidation of the fluorescent probes, events that would have undermined their use for quantitative studies. Consequently, the phagosomal chloride in our system was not consumed continuously by MPO, as occurs in nature, and the values obtained should be recognized as likely higher than those in vivo during phagocytosis.
Multiple classes of anion channels preferentially transporting chloride have been characterized in mammalian cells: cAMP-activated ClCs, such as CFTR; calcium-activated ClCs; voltage-gated ClCs; ligand-gated ClCs; and volume-regulated ClCs [16, 18]. Two ClCs, CFTR and ClC-3, have been identified in human neutrophils and their phagosomes [13, 19]. Interestingly, loss of function of ClC-3 in mice is associated with an increased susceptibility to infections systemically, and the neutrophils of these mice have compromised NADPH oxidase activity, diminished phagocytosis, and impaired migration [19, 20]. In contrast, lack of functional CFTR in CF patients results in more frequent pulmonary but not systemic infections. The NADPH oxidase activity and phagocytosis rate of CF neutrophils appear to be normal or even higher when compared with their normal counterparts [4, 21,22,23]. A prominent clinical manifestation for CF disease is the presence of neutrophil-rich airway inflammation, suggesting the CF neutrophils exhibit normal transendothelial and transepithelial migration, a finding in stark contrast to the behavior of ClC-3−/− neutrophils. It is possible that these contrasting features reflect the different functional roles played by the two ClCs in phagocyte-dependent host defense.
As the data indicate (Figs. 2, 5, and 6), CF neutrophils were relatively deficient in transporting chloride and iodide ions across the phagosomal membrane. CFTRinh decreased the rates of chloride and iodide influx into phagosomes in a dose-dependent manner and also decreased their steady-state levels within the phagosomal compartment. These results imply that chloride uptake into the phagosomes of neutrophils of CF patients may be compromised by the underlying abnormal or absent CFTR. This functional defect would be predicted to cause a deficient bactericidal capacity against microbes that are especially susceptible to HOCl and also relatively resistant to non-HOCl oxidants such as H2O2, antimicrobial bactericidal peptides in neutrophil granules, and bactericidal proteases such as elastase, cathepsin G, and gelatinases.
The uptake of chloride was reduced significantly by general anion channel inhibitors, including NA, CHC, DIDS, and SITS. These agents are reported to block anion channel-mediated chloride uptake in neutrophils as well as to affect neutrophil cellular functions [24]. In contrast, neither CFTRinh172 nor GlyH-101 (Fig. 3), specific CFTRinh, had any significant effects on cytosolic chloride uptake by zymosan-activated cells at ≤25 μM, indicating a minor role of CFTR in transporting chloride across the plasma membrane when the cells were activated with the particulate zymosan stimulus. Using immunofluorescence staining and immunoblotting, we demonstrated previously that the majority of CFTR in human neutrophils is located in the intracellular secretory vesicles of resting neutrophils and the phagosomes of phagocytosing cells; there is little CFTR on the surface of unstimulated human neutrophils [13]. These localization results are in agreement with the whole cell patch-clamp studies of neutrophils, which show little CFTR-like chloride transport activity in resting neutrophils [25]. The selective localization of CFTR in secretory vesicles but not plasma membrane provides the neutrophil with a means by which to modulate anion transport rapidly into the nascent phagosome. The secretory vesicles of human neutrophils serve as an intracellular reservoir of functionally important membrane proteins, including formyl peptide receptors, complement receptors, and the membrane component of the phagocyte NADPH oxidase, which fuses rapidly with the forming phagosome during neutrophil activation [26]. Consequently, the initiation of phagocytosis triggers fusion of secretory vesicles with the developing phagosome, thereby recruiting CFTR into the phagosomal membrane and providing a mechanism to augment chloride transport into the phagosome for the support of MPO-mediated HOCl generation. It is noteworthy that despite their genetic defect, phagosomes of CF neutrophils exhibited limited chloride uptake (Table 1) or iodide uptake (Fig. 6). Similarly, CFTRinh did not block chloride or iodide influx to phagosomes completely under our experimental conditions. This residual halide transport in CF neutrophils and CFTR-inhibited normal neutrophils suggests that other ClCs, in addition to CFTR, contribute to overall halide transport into phagosomes. Future studies are needed to distinguish the individual roles of various ClCs in neutrophil phagosomal chloride transport and the extent to which they complement each other functionally.
Our data demonstrate that chloride and iodide enter into and equilibrate within the phagosomes very rapidly. The membrane potential of the phagosomal membrane in macrophages, a phagocyte closely related to neutrophils, is ∼+27 mV (lumen-positive) [27]. Assuming that phagosomes of neutrophils resemble those of macrophages, we speculate that after the ClCs are open, electrical potential and chemical potential will drive chloride anion into the phagosomal lumen. Furthermore, the rate of uptake of chloride must be fast, as O2 consumed per phagosome is mathematically estimated to be ∼2.5 mM/s when oxidase activity is maximal. As ∼89% of the O2 consumed is converted into HOCl by MPO, HOCl will be produced at a rate of ∼2.2 mM/s [17]. Consequently, chloride ion must enter the phagosomal lumen at a similar or faster rate, as one chloride ion is required to produce a molecule of HOCl. We observed a rate of chloride uptake of ∼0.31 mM/s in our studies under conditions at which the oxidase was operating at a submaximal state. Such a rapid chloride influx into phagosomes of neutrophils, as measured by our probes, concurs with the mathematical prediction of massive and rapid chloride attainment by phagosomes, which is essential to support a robust production of HOCl to complete their antimicrobial action.
AUTHORSHIP
R. G. P. and L. M. performed research and analyzed data; G. A. L. and V. G. V. identified and provided patient samples; and R. G. P., W. M. N., and G. W. designed the research and wrote the paper.
ACKNOWLEDGMENTS
The work was supported by grants to G. Wang from Louisiana Gene Therapy Consortium, Cystic Fibrosis Foundation (CFF WANG07I0), and National Institutes of Health (1R01AI72327). The authors are grateful to the volunteers who generously provided their blood samples for this study.
Footnotes
Abbreviations: CF=cystic fibrosis, CFTR=CF transmembrane conductance regulator, CFTRinh=CFTR inhibitor, CHC=α-cyano-4-hydroxycinnamate, Cl−=chloride ion, ClCs=chloride channels, DIDS=4,4′-diisothiocyanatostilbene-2,2′-disulfonate, F1/F2=fluorescence 1/2, HOCl=hypochlorous acid, KI=potassium iodide, Ksv=Stern-Volmer constant, MCF=mean channel fluorescence, MPO=myeloperoxidase, MQHA=6-methoxyquinoline-N-6-hexanoic acid, NA=niflumic acid, NaCl R=sodium chloride Ringer’s buffer, NaGlu R=sodium chloride-free gluconate Ringer’s buffer, NaI=sodium iodide, O2=oxygen, RG=rhodamine green, SITS=4-acetamido-4′-isothiocyanato-2,2′-stilbenedisulfonic acid, TMR=tetramethylrhodamine
References
- Klebanoff S J. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- Murphy R, DeCoursey T E. Charge compensation during the phagocyte respiratory burst. Biochim Biophys Acta. 2006;1757:996–1011. doi: 10.1016/j.bbabio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh R N, Maxfield F R. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Painter R G, Bonvillain R W, Valentine V G, Lombard G A, LaPlace S G, Nauseef W M, Wang G. The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J Leukoc Biol. 2008;83:1345–1353. doi: 10.1189/jlb.0907658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R G, Wang G. Direct measurement of free chloride concentrations in the phagosomes of human neutrophils. Anal Chem. 2006;78:3133–3137. doi: 10.1021/ac0521706. [DOI] [PubMed] [Google Scholar]
- Jayaraman S, Song Y, Vetrivel L, Shankar L, Verkman A S. Noninvasive in vivo fluorescence measurement of airway-surface liquid depth, salt concentration, and pH. J Clin Invest. 2001;107:317–324. doi: 10.1172/JCI11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski A, Scott C C, Grinstein S. Determinants of the phagosomal pH in neutrophils. J Biol Chem. 2002;277:6059–6066. doi: 10.1074/jbc.M110059200. [DOI] [PubMed] [Google Scholar]
- Jayaraman S, Teitler L, Skalski B, Verkman A S. Long-wavelength iodide-sensitive fluorescent indicators for measurement of functional CFTR expression in cells. Am J Physiol. 1999;277:C1008–C1018. doi: 10.1152/ajpcell.1999.277.5.C1008. [DOI] [PubMed] [Google Scholar]
- Kjeldsen L, Sengelov H, Borregaard N. Subcellular fractionation of human neutrophils on Percoll density gradients. J Immunol Methods. 1999;232:131–143. doi: 10.1016/s0022-1759(99)00171-4. [DOI] [PubMed] [Google Scholar]
- Chao A C, Widdicombe J H, Verkman A S. Chloride conductive and cotransport mechanisms in cultures of canine tracheal epithelial cells measured by an entrapped fluorescent indicator. J Membr Biol. 1990;113:193–202. doi: 10.1007/BF01870071. [DOI] [PubMed] [Google Scholar]
- Simchowitz L, De Weer P. Chloride movements in human neutrophils. Diffusion, exchange, and active transport. J Gen Physiol. 1986;88:167–194. doi: 10.1085/jgp.88.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorg G, Bertau M. Thiol-tolerant assay for quantitative colorimetric determination of chloride released from whole-cell biodehalogenations. Anal Biochem. 2004;328:22–28. doi: 10.1016/j.ab.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Painter R G, Valentine V G, Lanson N A, Jr, Leidal K, Zhang Q, Lombard G, Thompson C, Viswanathan A, Nauseef W M, Wang G. CFTR expression in human neutrophils and the phagosomal chlorination defect in cystic fibrosis. Biochemistry. 2006;45:10260–10269. doi: 10.1021/bi060490t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muanprasat C, Sonawane N D, Salinas D, Taddei A, Galietta L J, Verkman A S. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D N, Welsh M J. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- Jentsch T J, Stein V, Weinreich F, Zdebik A A. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- Winterbourn C C, Hampton M B, Livesey J H, Kettle A J. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J Biol Chem. 2006;281:39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- Verkman A S, Galietta L J. Chloride channels as drug targets. Nat Rev Drug Discov. 2009;8:153–171. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland J G, Davis A P, Bailey G, Nauseef W M, Lamb F S. Anion channels, including ClC-3, are required for normal neutrophil oxidative function, phagocytosis, and transendothelial migration. J Biol Chem. 2006;281:12277–12288. doi: 10.1074/jbc.M511030200. [DOI] [PubMed] [Google Scholar]
- Volk A P, Heise C K, Hougen J L, Artman C M, Volk K A, Wessels D, Soll D R, Nauseef W M, Lamb F S, Moreland J G. ClC-3 and IClswell are required for normal neutrophil chemotaxis and shape change. J Biol Chem. 2008;283:34315–34326. doi: 10.1074/jbc.M803141200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller D Y, Urbanek R, Gotz M. Increased degranulation of eosinophil and neutrophil granulocytes in cystic fibrosis. Am J Respir Crit Care Med. 1995;152:629–633. doi: 10.1164/ajrccm.152.2.7633718. [DOI] [PubMed] [Google Scholar]
- Van Der Vliet A, Nguyen M N, Shigenaga M K, Eiserich J P, Marelich G P, Cross C E. Myeloperoxidase and protein oxidation in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2000;279:L537–L546. doi: 10.1152/ajplung.2000.279.3.L537. [DOI] [PubMed] [Google Scholar]
- Brockbank S, Downey D, Elborn J S, Ennis M. Effect of cystic fibrosis exacerbations on neutrophil function. Int Immunopharmacol. 2005;5:601–608. doi: 10.1016/j.intimp.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Busetto S, Trevisan E, Decleva E, Dri P, Menegazzi R. Chloride movements in human neutrophils during phagocytosis: characterization and relationship to granule release. J Immunol. 2007;179:4110–4124. doi: 10.4049/jimmunol.179.6.4110. [DOI] [PubMed] [Google Scholar]
- Di A, Brown M E, Deriy L V, Li C, Szeto F L, Chen Y, Huang P, Tong J, Naren A P, Bindokas V, Palfrey H C, Nelson D J. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Steinberg B E, Touret N, Vargas-Caballero M, Grinstein S. In situ measurement of the electrical potential across the phagosomal membrane using FRET and its contribution to the proton-motive force. Proc Natl Acad Sci USA. 2007;104:9523–9528. doi: 10.1073/pnas.0700783104. [DOI] [PMC free article] [PubMed] [Google Scholar]