Abstract
Isolating elemental steps that comprise a protein reaction in solution is a difficult process. In this study, the use of sugar-derived glass matrices is evaluated as a biophysical tool to help dissect out elemental steps and isolate intermediates. Two features of the glass are utilized in this endeavor: i) the capacity of trehalose glass matrices to support thermal reduction over macroscopic distances; and ii) the ability of glass matrices to significantly damp large amplitude protein dynamics. The focus of the study is on the reaction of nitric oxide (NO) with a nitrite ion coordinated to the heme iron of hemoglobin (Hb). The thermal reduction property of the glass is used to generate NO from nitrite within the glass and the damping of protein dynamics is used to control entry of NO into the distal heme pocket of Hb where it can either interact with bound nitrite or bind to the heme iron. The results not only relate to earlier controversial studies addressing the reactions of Hb with NO and nitrite but also raise the prospect that these properties of sugar-derived glassy matrices can be exploited as a new biophysical tool to modulate and probe reactions of NO with hemeproteins as well as a wide range of other metalloproteins.
Many physiologically important protein-based reactions are exceedingly difficult to dissect in that contributing species and intermediates are often present at levels that make biophysical characterization problematic. Reaction kinetics are often used for an initial characterization of a protein reaction. Typically standard kinetics generated through rapid mix and other similar techniques are not effective in exposing the specific intermediates needed for piecing together a detailed molecular level mechanism. The use of sugar-derived matrices as a tool to facilitate such studies is considered in this work.
Sugar and polysaccharide-derived glasses can function as robust matrices for many biological molecules. The present study seeks to expand the potential use of such matrices as a biophysical tool to help systematically dissect complex protein reactions initiated by the binding or accessing of a small substrate molecules to an internal site within a protein. In solution, the many stochastic steps that are associated with such processes including the dynamics that allow entry of a substrate into the protein from the solvent, the subsequent diffusion of the substrate within the protein and the initial reactions which are typically too fast and too temporally intertwined to be easily separated and probed experimentally. The present study demonstrates how two properties of glassy matrices can be used to separate these intertwined process and thus allow for a more direct dissection of the mechanisms driving protein functionality. The two glass-associated properties are: i) selective damping of different protein dynamics; and ii) facilitating thermal reduction over macroscopic distances.
Glassy matrices derived from sugars such as trehalose have been used in numerous studies that focus on their capacity to stabilize and preserve therapeutic reagents in dry formulations1-4. Additionally, these glasses have been used as a biophysical tool to manipulate and probe protein dynamics5-10. These studies have revealed that within these matrices protein dynamics undergo significant damping. The pattern that is emerging from these studies is that incorporation of a protein into the extended hydrogen bonding network which contributes to the rigidity of the glass (i.e. exceptionally high viscosity), results in almost complete damping of certain categories of protein dynamics. The protein dynamics most vulnerable to this damping are those associated with large fluctuation of the surrounding volume. Such protein motions include partial unfolding, quaternary structure changes and the equilibrium fluctuations in hemoglobins and myoglobins that transiently open the so-called distal histidine gate that allows oxygen and water to enter and exit these proteins. On the other hand small amplitude motions that are slaved to local motions of hydration waters on the surface of the proteins but not requiring a large volume change can still be active for proteins within glassy matrices that still retain free waters not strongly integrated into the extended hydrogen bonding network. For example the side chain fluctuations that facilitate substrate diffusion within the interior of a protein are still active within the glass over an extensive temperature regime. As a result of the different degree of damping by the glass for the different protein motions, it is possible to use glassy matrices to selectively modify protein reactivity by greatly slowing some functionally important dynamics but not others5,8,11-13.
The other property of glassy matrices arises from the ability of glassy matrices to support electron transfer processes over macroscopic distances14,15. The basic concept is that an electron generated in the glass through either thermal ejection from an electron source such as glucose or photo ejection is capable of a sustained entropic search among the shallow potential minima associated with the energy landscape of the glass until captured by a suitable redox center. Whereas undoped trehalose and trehalose/sucrose glasses are not good electron sources for thermal reduction, glucose and tagatose doped versions of these glasses are. Thermal reduction of ferric hemeproteins has been observed 14,15 for glucose and tagatose doped glasses with tagatose being the more effective source of reducing equivalents. For a given glass, the temperature at which the reduction of glass-embedded molecules occurs, decreases with increasing redox potential. It was also demonstrated that the thermal electrons can traverse across the interface between two glassy films sandwiched together.
In the present work, a particular reaction of human adult hemoglobin (Hb) is used as the model reaction to test the general concept that thermal tuning of both protein dynamics and redox reactions within a glassy matrix can be used to expose mechanisms not readily accessed in solution. Hb's as well as myoglobins are ideal model proteins for such studies in that they manifest easily monitored changes in the UV/visible absorption spectrum that reflect redox status of the heme iron, degree of coordination (5 or 6 coordinate heme), spin state of the heme iron and identity of the sixth ligand for the heme iron (e.g. NO, CO, O2,H2O, OH-,NO2-). Additionally, for these proteins, functionally relevant dynamics impacted by the glassy matrices have been studied and identified 5,8,13,16 and thermal reduction properties established14,15. Significantly, these proteins retain their native structure within the glass even when heated to temperatures that reach the glass transition. Hb is used in the present study despite myoglobin being structurally simpler than Hb in that it has only a single heme site whereas Hb has one heme in each of the two types of subunits comprising the Hb tetramer. The reason for choosing Hb is that the reaction in question has been studied in much greater detail for Hb thus allowing for a direct comparison with earlier results. The issue of subunit heterogeneity in Hb is not a relevant issue for the present study but will addressed in future studies using iron/metal hybrid Hbs that allow for the isolation of subunit specific behavior.
In the present study, the efficacy of a glass based approach is evaluated with respect to the reaction of nitric oxide (NO) with nitrite that is coordinated to ferric (met) heme in hemoglobin (Hb). This reaction is currently of considerable interest but remains controversial. It has been proposed17 that this reaction represents a physiologically relevant pathway through which Hb can generate long lived bioactive forms of NO. Thus the reaction could provide a pathway that enables both cellular (red blood cells) and acellular Hbs to function as vasodilators under anoxic conditions. The short life of NO under many experimental conditions, the limiting solubility of NO, the rapidity with which NO binds to many active sites such as ferrous hemes, all limit the extent to which this reaction and other NO-mediated protein reactions can be probed mechanistically in solution. We verify that NO can be generated through the thermal reduction of nitrite within glassy matrices. This process creates conditions whereby access of NO to the active site (i.e. the heme within the distal heme pocket of the globin) within a protein is now under thermal control. The thermal control arises from the thermal reduction process that generates the NO and the thermal control of the significantly damped dynamics that allow access of the NO to the active site. The results from the present study are evaluated in relation to a recent related study in which externally applied gaseous NO is allowed to diffuse into the Hb-containing glass at ambient temperatures18.
In the present work, thermal reduction is used both to generate NO from nitrite and to reduce ferric (met) Hb to ferrous Hb. Differences in redox potentials for the two processes can allow both for a clear separation of time scales for these two redox reactions at a given temperature and for preferential activation at different temperatures. Thermal reduction of nitrite was previously demonstrated and utilized to generate NO releasing nanoparticles19.
In concert with the thermal reduction processes, temperature dependent modulation of glass damped protein dynamics is used to control entry of the thermally generated NO into the distal heme pocket of the Hb. The dynamics that control entry of ligands such as NO, O2 and H2O into the distal heme pocket of Hb (the active site) belong to a class of dynamics that are slaved8,11-13,20-22 to to large volume changing fluctuations of the surrounding solvent known as α fluctuations (or relaxations). At ambient temperatures these dynamics for Hb and Mb embedded in a glassy matrix are significantly damped so that they are not active over the time scales associated with conventional ligand photodissociation-recombination studies 6,9,10,23,24. With increasing temperatures, especially as the glass transition temperature is approached, the functional influence of these dynamics becomes apparent at progressively shorter time periods5,13.
The combination of the above properties are utilized in developing a strategy for protocols that allow for controlled access of NO into the distal heme pocket of Hb under conditions where there either is or is not a nitrite bound to the ferric heme iron. Our approach is first trap populations within the glass that are comprised of a substantial population of met Hb with either water or nitrite as the ligand and then use heating protocols both to thermally generate NO from free nitrite in the glass and to modulate the entry of the NO into the distal heme pocket.
Figure 1 shows the three different glass protocols employed in the present study. The single layer protocol consists of a single thin glassy film with all the potential reactants included in the single layer. Two types of glassy matrices are employed in making a given thin film. We use our earlier nomenclature14 to describe the specific types of glass matrices being used. G1 film refers to a glass derived from just trehalose and sucrose without any source of thermal electrons. The G3 glassy film differs from the G1 glass in that it has a thermal electron source in the form of tagatose included in the trehalose/sucrose glass14. Either glass can have additional additives such as nitrite or Hb (or both). The two layer sandwich protocol (middle scheme in Fig. 1) consists of making a sandwich comprised of two dry thin glassy films. The two films can be cleanly separated at the end of the heating cycle and probed separately. The third protocol consists of cut strip of glass (from a microscope slide) on which there are well-separated (∼ 2 cm) thin reactant containing glass films. These reactant containing films are linked to each other via a G1 “trehalose/sucrose glass wire”. Thus any two reactant containing glass films are linked to each other through the reactant free G1 glass wire as shown in the bottom panel of Fig 1. Silver paste is used to join the ends of the G1 wire to the reactant containing films. In cases where the experiment is designed to allow NO to diffuse from one film through the G1 wire to another film, the wire is directly attached to adjacent films as a sticky wet glass that is then allowed to dry. All of the mountings for the glass films are cut to a size that allows them to fit into the sample chamber of the UV/Vis absorption spectrophotometer that is used to record the optical spectra before and after heating cycles.
Figure 1.
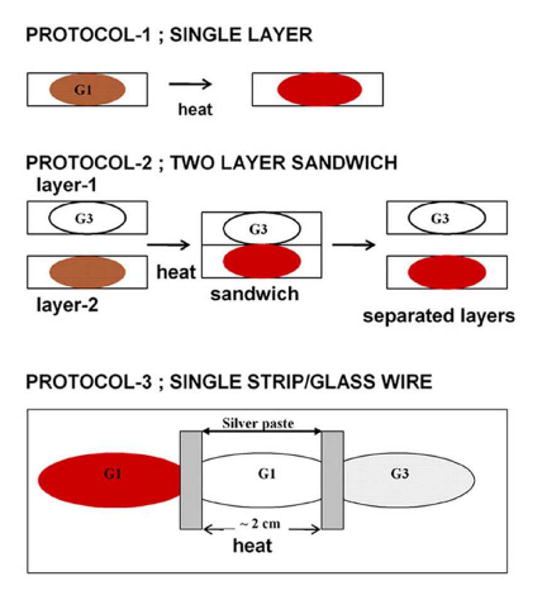
Glass protocols used in the heating experiments.
Results
Thermal reduction of aquomet HbA in a glassy film
The spectrum of aquomet HbA in a single G1 glass (trehalose/sucrose glass without any added source of thermal electrons such as tagatose) film as a function of 45 minute heating cycles each conducted at progressively higher temperatures is shown in Fig 2a. All absorption spectra in this and subsequent figures are taken at ambient temperature (∼ 25 C) after the sample is allowed to cool. The initial spectrum (labeled “before heating”) is derived from the sample after drying but prior to any of the heating protocols. The “before heating” spectrum is characteristic of aquomet HbA (ferric heme with water as the sixth ligand) with a small contribution from the hemichrome spectrum of HbA. The hemichrome is the met derivative in which the sixth ligand is the imidazole from the distal histidine (E7) rather than the water in the aquomet derivative25. The hemichrome population is observed to increase when samples are allowed to dry for more extended time periods. In Fig. 2a the progression of spectroscopic changes occurring with each 45 minute heating cycle is consistent with an increasing population of hemichrome HbA as is also observed with samples subjected to extended periods of drying without the heating.
Figure 2.
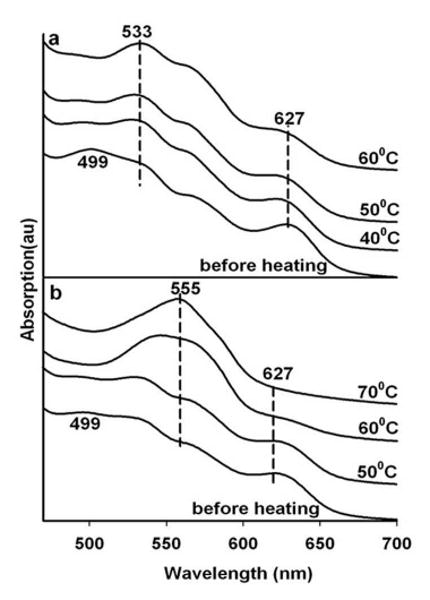
Heating induced changes in the visible absorption spectrum of aquomet Hb in a single thin glassy film derived from trehalose sucrose (referred to as a G1 glass) by itself (panel a) and sandwiched with a glassy film comprised of trehalose sucrose doped with tagatose (referred to as a G3 glass) (panel b).
The temperature dependent changes are shown in Fig. 2b for a 2 layer sandwich protocol in which a G1 aquomet HbA sample (i.e. a G1 glass film that also contains aquomet Hb) is sandwiched with a protein-free G3 thin film glass sample. In contrast to the pure G1 sample (Fig. 2a) which forms a hemichrome with increasing temperature, the sample in contact with the G3 layer undergoes reduction over the same heating cycle resulting in the appearance of a Q absorption band characteristic of a five coordinate high spin ferrous heme derivative of HbA (i.e. deoxy HbA). These changes do not occur with drying alone. Continued heating (hours) of the heat-generated ferrous five coordinate samples, results in the eventual appearance of a hemochrome spectrum 25 indicative of loss of the loosely associated water in the distal heme pocket and the binding of the E7 imidazole as sixth ligand to the ferrous heme. The sandwich experiment confirms the earlier report 14 that thermally generated electrons can flow between the two distinct layers of the sandwich.
Thermal reduction of met HbA in a glass under conditions of low concentration of added nitrite
Results of temperature cycling are shown in Fig. 3 for samples that now have nitrite added at a concentration of 1 mM. Under these conditions nitrite is not competitive with water for the sixth ligation site of the met heme. The starting spectrum is indicative of a population that is predominantly aquomet with very little or any met nitrite HbA (ferric heme with nitrite as the sixth ligand). The heating of the single layer G1 sample (Fig. 3a) mimics the pattern seen in the nitrite-free sample in Fig. 2a in that only the hemichrome spectrum appears with heating. A different result is obtained from a two layer sample made as a sandwich of a G1 layer containing both met Hb and 1 mM added nitrite and a blank G3 second layer that is a source of thermal electrons but free of both protein and nitrite (Fig. 3b). In this case there is a sequence of changes in the spectra consistent with appearance of a ferrous NO derivative of HbA (NOHb). The ferrous spectrum appears fully developed after the sample is subjected to heating at a temperature that is below that which is required for the thermal reduction of a G3 sample of nitrite-free met HbA (Fig. 2b). The results are consistent with thermal reduction of nitrite occurring at temperatures below those needed to reduce the heme directly and the reduction of the heme possibly occurring through an NO mediated reaction with met Hb.
Figure 3.
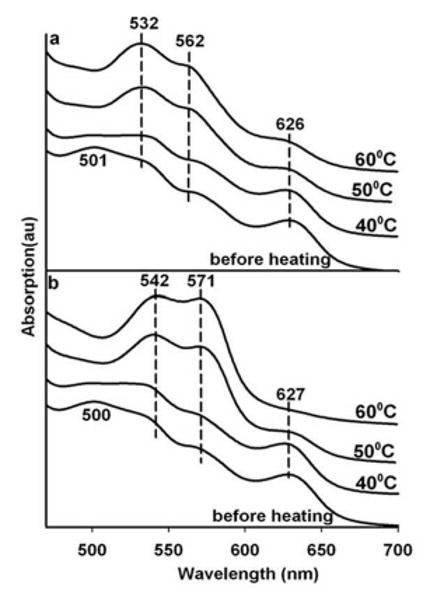
Heating induced changes in the visible absorption spectrum of aquomet Hb in a single thin glassy film derived from trehalose sucrose (referred to as a G1 glass) doped with 1 mM sodium nitrite. Panel a-the G1 glass by itself; and Panel b- the G1 glass containing Hb and nitrite sandwiched with a G3 glassy film.
Thermal reduction of met HbA mediated through NO formation from nitrite
Fig 4 shows the effect of heating a two layer sandwich sample containing a nitrite-free G1 layer with aquomet HbA and a G3 layer with 10 mM nitrite. In this case, in contrast to what was used for the sample giving rise to Fig 3, the nitrite is not in the protein containing glassy layer. It can be seen that with heating the starting aquomet spectrum evolves into a ferrous NO spectrum by 50 C indicating that the thermally generated NO can diffuse across the interface between the two layers. When the 10 mM nitrite is in the same G1 layer as the met Hb, the transition occurs at a slightly lower temperature. Higher concentrations of nitrite are required to produce a clearly discernable met nitrite spectrum as is seen for samples of HbA in the presence 100 mM nitrite (vide infra).
Figure 4.
Heating induced changes in the visible absorption spectrum of aquomet Hb in a thin G1 glassy film sandwiched with a G3 thin glassy film containing 10 mM sodium nitrite.
Thermal reduction of the met nitrite derivative of HbA
Met HbA in the presence of 100 mM nitrite both in solution and in the glass yields a spectrum indicative of a population that is largely if not entirely the met nitrite derivative of HbA. Fig. 5, depicts the evolution with sequential heating cycles of a two layer sandwich consisting of a G1 layer with met HbA in the presence of 100 mM nitrite and a blank G3 layer. Here the second layer (G3) supplies only electrons. It can be seen that the initial “before heating” spectrum which is characteristic of the met nitrite Hb derivative makes the transition to the ferrous NO spectrum by 50 C.
Figure 5.
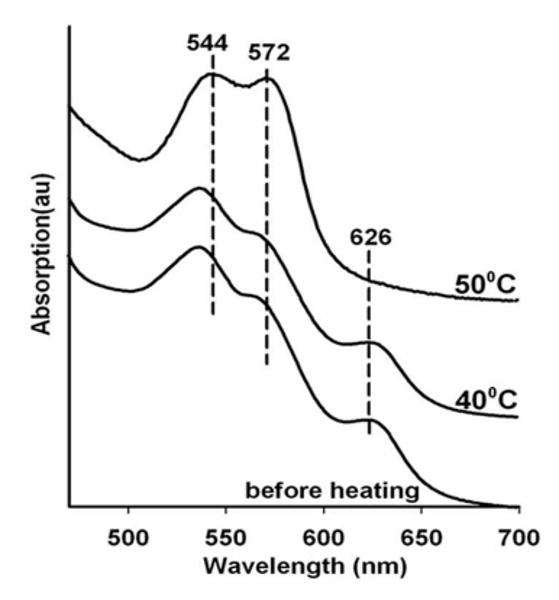
Heating induced changes in the visible absorption spectrum of nitrite met Hb in a thin G1 glassy film containing 0.1 M nitrite sandwiched with a G3 thin glassy film.
Thermal reduction under conditions where the rate of exposure of the heme-bound nitrite to NO is modulated by the glass protocol
The above spectra show no obvious intermediates. The likely limitation is that NO is being generated at sufficiently high levels to essentially flood the Hb environment with NO. Under such conditions it is improbable that one can isolate mechanistic steps for a process that requires more than one NO molecule. Instead protocols are required that limit the rate of delivery of NO to the distal heme pocket thus allowing for the potential temporal/thermal separation of sequential NO mediated steps.
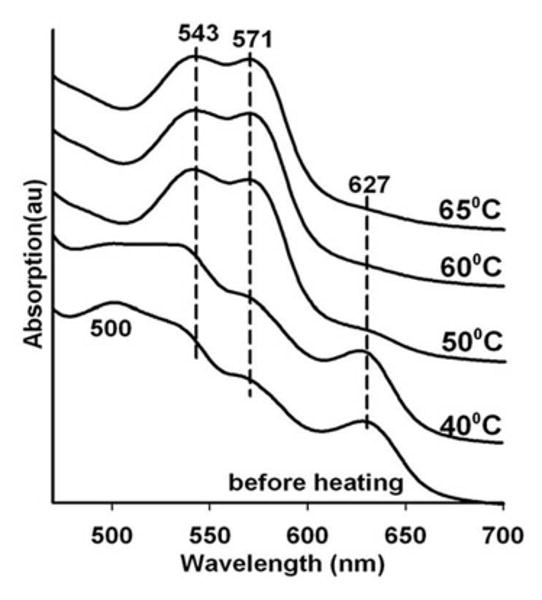
The results using the first variation on the standard two layer protocols are shown in Fig. 6 for a sandwich comprised of a G1 layer containing met Hb in the presence of 100 mM nitrite and a blank G3 layer. In this case the starting sample is allowed to dry for a much longer time period compared to the samples used to generate the previous figures. The increased drying of the glass further damps the large amplitude conformational fluctuations needed to transiently open access to the distal heme pocket. Thus, although the thermal reduction of the nitrite to NO is still generating a large “bolus” of NO, the enhanced drying results in a decreased access rate of the NO to the heme pocket. The temperature dependent progression of spectra shown in Fig 6 reveals spectra appearing between 45 and 65 C, that show a Q band absorption with α and β bands at ∼ 569 and 538 nm respectively and loss of the met Hb band at ∼ 627 nm. This intermediate spectrum evolves smoothly into the NOHb spectrum at 70 C. This same progression of spectra was reported previously for glass embedded18 and sol-gel encapsulated26 met nitrite Hb exposed to gaseous NO at ambient temperature.
Figure 6.
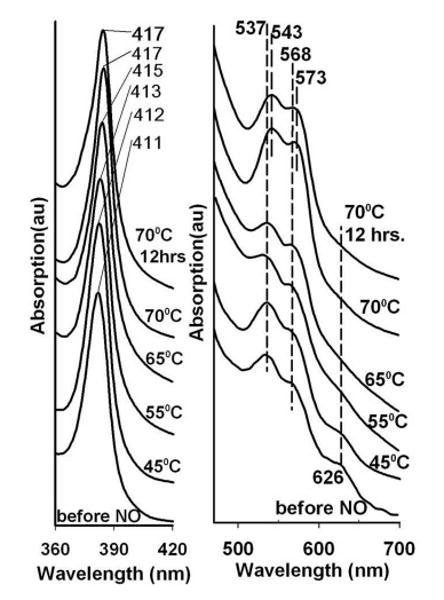
Heating induced changes in the visible absorption spectrum of nitrite met Hb in a very dry thin G3 glassy film containing 0.1 M nitrite. Also shown are the progressive heating induced changes to the Soret band in blue region of the spectrum.
The second variation on the standard protocol is designed to control the flux of thermally generated electrons. The issue being addressed is how to overcome the high level of NO resulting from the thermal reduction of the high concentration of nitrite. The high concentration of nitrite is needed to create a substantial starting population of met nitrite Hb. The high local concentration of nitrite is a potential source for a burst-like production of NO when subjected to a high flux of thermally generated electrons. This situation is hard to avoid when the protocol favors a large flux of thermally generated electrons. Here, a protocol is devised that allows retaining the high concentration of nitrite but limits NO production by limiting the rate and number of electrons being made available for reduction of nitrite. A protocol was developed in which the G1 layer containing the met nitrite Hb and high concentration of free nitrite is linked to a well-separated G3 layer though a 2 cm long blank G1 sugar wire on a single long glass slide. Silver glue is used to connect the 2 cm G1 wire to the protein/nitrite GI film and the G3 blank film (see bottom panel in Fig. 1). Fig. 7 shows the clear appearance of the intermediate as the strip is subjected to the heating protocol. The changes in the initial met nitrite Hb spectrum begin to appear at temperatures consistent with the thermal reduction profile of nitrite seen in the single and double layer film experiments described above.
Figure 7.
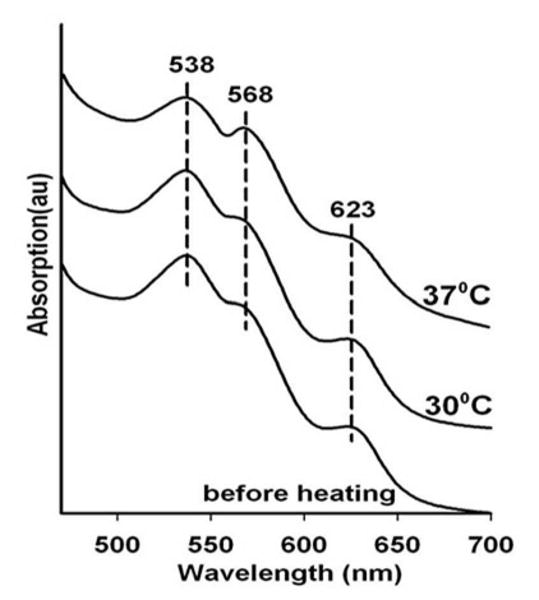
Heating induced changes in the visible spectrum of a nitrite met Hb sample in a thin G1 glass film containing 0.1 M nitrite that is linked to a spatially well-separated (2 cm) G3 film through a G1 (trehalose/sucrose) glassy wire.
These results are consistent with a model in which NO entering the distal hemepocket of the nitrite met derivative, does not displace the nitrite to form the met NO derivative. It is anticipated that under similar conditions, NO entering the distal heme of the aquomet derivative would initially generate the NO met derivative and not an intermediate. The challenge in verifying that anticipated behavior is to have controlled slow delivery of NO to the aquomet derivative in order to prevent a two step NO mediated reduction27-30 from dominating the observed spectral changes. This fine tuning for the NO delivery was achieved using a three layer strip variation of Protocol 3 shown in the bottom panel of Fig. 1 consisting of a central circular G1 film containing 0.1 M nitrite linked via “G1 wires” to two other separate circular films one on each side of the central located nitrite containing film. On one side is a circular G1 glass layer containing aquomet Hb and the other side of the nitrite layer is a circular G3 glass film. The rational for the set up is that heating will allow the G3 layer to supply electrons via a “G1 glass wire” to the central nitrite containing layer and thus generating NO. The NO then diffuses slowly through a G1 glass wire to the aquomet Hb containing G1 layer. Silver paste is used to join the edges of G1 glass wire to the G3 layer and the central nitrite-containing G1 layer; however, the central nitrite-containing G1 layer is directly linked to the Hb containing layer directly through the G1 wire without the use of the silver paste as a joiner linker. In this manner electrons but not NO can flow between the G3 and central layers; whereas, NO can diffuse between the central and Hb containing layers. The shown spectra in Fig. 8 shows the gradual appearance of the 535 and 565 nm peaks that are characteristic of the NO derivative of ferric hemoglobin.
Figure 8.
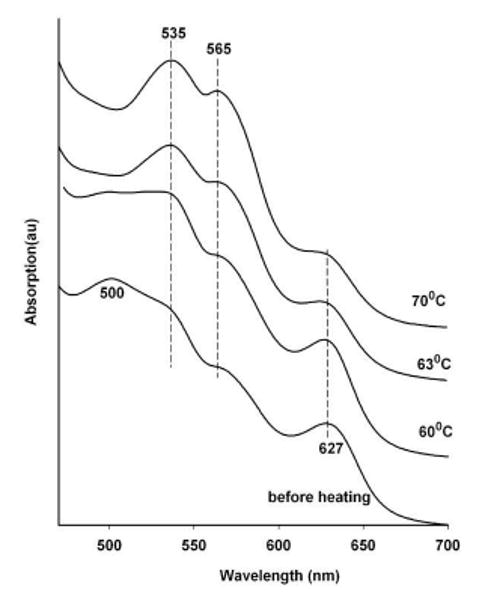
Heating induced changes in the visible absorption spectrum of aquo met Hb in a G1 glass film that is linked through a G1 glass wire to a spatially well separated source of thermally generated NO.
Discussion
The present study shows that thermal reduction in glassy matrices can be used to modulate both NO production and NO accessibility to the active site in Hb. Although the present work focused on NO reactivity with Hb in either the presence or the absence of bound nitrite, the implications are clearly general with respect to using this approach for many other NO reactions with proteins.
The key general findings from this study are: i) NO can be easily generated within glassy matrices through thermal reduction of nitrite; and ii) access of NO to the active site within a protein can be controlled by protocols that manipulate the properties (e.g. dryness) and configuration of the glassy samples. These glass associated properties were utilized within this study to isolate two important intermediates: i) the intermediate associated with the reaction of NO with nitrite bound to a ferric heme; and ii) the initial ferric NO intermediate that is associated with the autoreduction of ferric NO Hb to ferrous NO Hb. Given that the glassy samples are amenable to wide variety of probe techniques including virtually all optical spectroscopies as well as electron spin resonance based spectroscopies, the present results showing the trapping of intermediates clearly opens the door for more detailed spectroscopic studies that can be used to better dissect the nature of NO generated intermediates in a wide variety of proteins.
Methods
Materials
Hemoglobin, trehalose, sucrose, sodium nitrite, and proteins were all obtained from Sigma-Aldrich (Missouri, USA). Pure tagatose was obtained as a generous gift from Spherix Inc.
Methods
Stock solutions of (a) 80:20 mg/ml of trehalose:sucrose and (b) 60:20:20 mg/ml of trehalose: sucrose: tagatose, in deionized water were used to prepare G1 and G3 glassy films respectively. The G1 glasses have been shown to inert with respect to thermal reduction whereas the G3 glass is highly effective in supporting thermal reduction11. The nomenclature is derived from our earlier work11. Sodium nitrite is added to the stock solutions to create solutions with final concentrations of nitrite of 1mM, 10mM, and 100mM. Aliquots of stock aquomet Hb solutions in 50mM tris(HCl) (either pH 7.5 or 6.5) are added to the above solutions to achieve a final protein concentration of 0.25 mM.
Small aliquots of the resulting solutions are layered on glass plates and then dried in a dessicator for several days. The samples are stored in a sealed container with desiccant at room temperature. For the two-layer sandwich experiments, the protein-containing layer is sandwiched with a protein free layer, and then subjected to the heating protocols. For the single glass wire experiments (protocol 3 in the scheme figure), a circular protein-containing glass film is linked with a similar but well separated (∼ 2 cm) protein-free second glass circular glass film through a pure G1 glassy wire that is coupled to the two circular layers via silver paste. The single layer, sandwich and glass wire samples are subjected to 40 minute heating cycles in a sealed temperature controlled oven. After cooling, the visible absorption spectrum of each sample is generated on a PERKIN-ELMER (Lambda-2) UV-Vis absorption spectrometer. In several instances, the protein containing layer was re-dissolved and the UV/Vis spectrum taken. The two layers comprising the sandwich are easily separated without typically disrupting the integrity of the individual layers.
Acknowledgments
This work was supported through fundings from National Institutes of Health Grant P01HL071064 and FJC, A Foundation of Philanthropic Funds.
References
- 1.Colaco C, Sen S, Thangavelu M, Pinder S, Roser B. Biotechnology (N Y) 1992;10:1007. doi: 10.1038/nbt0992-1007. [DOI] [PubMed] [Google Scholar]
- 2.Crowe JH, Crowe LM, Chapman D. Science. 1984;223:701. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- 3.Liao YH, Brown MB, Nazir T, Quader A, Martin GP. Pharm Res. 2002;19:1847. doi: 10.1023/a:1021445608807. [DOI] [PubMed] [Google Scholar]
- 4.Newman YM, Ring SG, Colaco C. Biotechnol Genet Eng Rev. 1993;11:263. doi: 10.1080/02648725.1993.10647903. [DOI] [PubMed] [Google Scholar]
- 5.Dantsker D, Samuni U, Friedman JM, Agmon N. Biochim Biophys Acta. 2005;1749:234. doi: 10.1016/j.bbapap.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Cordone L, Cottone G, Giuffrida S, Palazzo G, Venturoli G, Viappiani C. Biochim Biophys Acta. 2005;1749:252. doi: 10.1016/j.bbapap.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Hagen SJ, Hofrichter HJ, Bunn HF, Eaton WA. Transfus Clin Biol. 1995;2:423. doi: 10.1016/s1246-7820(05)80066-7. [DOI] [PubMed] [Google Scholar]
- 8.Samuni U, Dantsker D, Roche CJ, Friedman JM. Gene. 2007;398:234. doi: 10.1016/j.gene.2007.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagen SJ, Hofrichter J, Eaton WA. Science. 1995;269:959. doi: 10.1126/science.7638618. [DOI] [PubMed] [Google Scholar]
- 10.Gottfried D, Peterson E, Sheikh A, Yang M, Wang J, Friedman J. J Phys Chem. 1996;100:12034. [Google Scholar]
- 11.Fenimore PW, Frauenfelder H, McMahon BH, Young RD. Proc Natl Acad Sci U S A. 2004;101:14408. doi: 10.1073/pnas.0405573101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frauenfelder H, Fenimore PW, McMahon BH. Biophys Chem. 2002;98:35. doi: 10.1016/s0301-4622(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 13.Samuni U, Roche CJ, Dantsker D, Friedman JM. J Am Chem Soc. 2007;129:12756. doi: 10.1021/ja072342b. [DOI] [PubMed] [Google Scholar]
- 14.Navati MS, Friedman JM. J Biol Chem. 2006;281:36021. doi: 10.1074/jbc.M606866200. [DOI] [PubMed] [Google Scholar]
- 15.Ray A, Friedman BA, Friedman JM. J Am Chem Soc. 2002;124:7270. doi: 10.1021/ja0201348. [DOI] [PubMed] [Google Scholar]
- 16.Dantsker D, Roche C, Samuni U, Blouin G, Olson JS, Friedman JM. J Biol Chem. 2005;280:38740. doi: 10.1074/jbc.M506333200. [DOI] [PubMed] [Google Scholar]
- 17.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Nat Chem Biol. 2007;3:785. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 18.Navati MS, Friedman JM. J Am Chem Soc. 2009;131:12273. doi: 10.1021/ja903364h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman AJ, Han G, Navati MS, Chacko M, Gunther L, Alfieri A, Friedman JM. Nitric Oxide. 2008;19:12. doi: 10.1016/j.niox.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Frauenfelder H, Fenimore PW, Young RD. IUBMB Life. 2007;59:506. doi: 10.1080/15216540701194113. [DOI] [PubMed] [Google Scholar]
- 21.Frauenfelder H, Fenimore PW, Chen G, McMahon BH. Proc Natl Acad Sci U S A. 2006;103:15469. doi: 10.1073/pnas.0607168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fenimore PW, Frauenfelder H, McMahon BH, Parak FG. Proc Natl Acad Sci U S A. 2002;99:16047. doi: 10.1073/pnas.212637899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Librizzi F, Viappiani C, Abbruzzetti S, Cordone L. J Chem Phys. 2002;116:1193. [Google Scholar]
- 24.Cordone L, Galajda P, Vitrano E, Gassmann A, Ostermann A, Parak F. European Biophysics Journal with Biophysics Letters. 1998;27:173. doi: 10.1007/s002490050123. [DOI] [PubMed] [Google Scholar]
- 25.Rachmilewitz EA, Peisach J, Blumberg WE. J Biol Chem. 1971;246:3356. [PubMed] [Google Scholar]
- 26.Roche CJ, Friedman JM. Nitric Oxide. 2009 doi: 10.1016/j.niox.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez BO, Lorkovic IM, Ford PC. Inorg Chem. 2004;43:5393. doi: 10.1021/ic049532x. [DOI] [PubMed] [Google Scholar]
- 28.Nagababu E, Ramasamy S, Rifkind JM. Nitric Oxide. 2006 doi: 10.1016/j.niox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Nagababu E, Ramasamy S, Rifkind JM. Biochemistry. 2007;46:11650. doi: 10.1021/bi700364e. [DOI] [PubMed] [Google Scholar]
- 30.Salgado MT, Nagababu E, Rifkind JM. J Biol Chem. 2009;284:12710. doi: 10.1074/jbc.M808647200. [DOI] [PMC free article] [PubMed] [Google Scholar]


