Abstract
Age is a major risk factor for cardiovascular diseases, not only because it prolongs exposure to several other cardiovascular risks, but also owing to intrinsic cardiac aging, which reduces cardiac functional reserve, predisposes the heart to stress and contributes to increased cardiovascular mortality in the elderly. Intrinsic cardiac aging in the murine model closely recapitulates age-related cardiac changes in humans, including left ventricular hypertrophy, fibrosis and diastolic dysfunction. Cardiac aging in mice is accompanied by accumulation of mitochondrial protein oxidation, increased mitochondrial DNA mutations, increased mitochondrial biogenesis, as well as decreased cardiac SERCA2 protein. All of these age-related changes are significantly attenuated in mice overexpressing catalase targeted to mitochondria (mCAT). These findings demonstrate the critical role of mitochondrial reactive oxygen species (ROS) in cardiac aging and support the potential application of mitochondrial antioxidants to cardiac aging and age-related cardiovascular diseases.
Introduction
Cardiovascular diseases are the leading causes of death, and the elderly (>65 y) account for greater than 80% of patients with ischemic heart disease, more than 75% of patients with congestive heart failure, and greater than 70% of patients with atrial fibrillation (AHA Statistics Committee report (Rosamond et al. 2007)). The exponential increase in mortality rate related to cardiovascular diseases in the geriatric population (NHLBI mortality and morbidity chart book 2007) implies that cardiac aging per se is a major risk factor for cardiovascular diseases. Intrinsic cardiac aging is defined as the slowly progressive age-dependent degeneration and decline in function which makes the heart more vulnerable to stress and contributes to increased cardiovascular mortality and morbidity in the elderly. However, intrinsic cardiac aging can be obscured by the cardiomyopathic changes seen in diabetes or hypertension, which are highly prevalent in the elderly population. Both diabetes and hypertension have been shown to accelerate cardiovascular senescence (Brodsky et al. 2004, Kosugi et al. 2006). However, intrinsic cardiac aging is also evident in rodents, even though diabetes, hypertension or elevated blood cholesterol is absent in many species, including mouse. Furthermore, the availability of genetically modified mice and the relatively short mouse lifespan have made mouse a premier model of mammalian aging for gerontologic studies, including those of intrinsic cardiac aging.
The pathophysiology of cardiac aging in humans and mice
Data from the Framingham Heart Study and the Baltimore Longitudinal Study on Aging (BLSA) demonstrate that the prevalence of left ventricular hypertrophy increases with age. Diastolic function, measured by the ratio of early to late ventricular filling (E/A) by Doppler echocardiography also declines with age. While systolic function (ejection fraction) is relatively preserved in subjects at rest, the maximal exercise capacity decreases with age and left ventricular (LV) wall thickness increases progressively with age in both sexes, indicating increasing LV hypertrophy (reviewed in Lakatta 2003, Lakatta and Levy 2003). Since the BLSA focused on subjects without hypertension or clinically apparent cardiovascular diseases, all of the above changes are likely manifestations of intrinsic cardiac aging. LV early diastolic filling (peak tissue E wave, Ea) is progressively compromised in age, which might be due to fibrosis and reduced ventricular compliance, coupled with slower reuptake of cytosolic calcium in myocardial cells, which further delays relaxation. The decline in early diastolic filling necessitates that atrial contraction during late diastolic phase (A wave; measured by tissue Doppler imaging as Aa) contribute to a larger fraction of LV filling. This likely increase atrial pressure and contribute to atrial hypertrophy, which can subsequently predispose to atrial fibrillation, the prevalence of which also increases with age. The decline in early diastolic filling is interpreted clinically as an evidence of diastolic dysfunction, defined as Ea/Aa <1 (Khouri et al. 2004). Diastolic dysfunction contributes to exercise intolerance in the elderly population and also predisposes to the development of diastolic heart failure. Diastolic heart failure, defined as symptoms of heart failure in the setting of preserved systolic function but impaired diastolic function, is prevalent in older individuals and markedly increases the risk of mortality (Bursi et al. 2006). It has been shown that diastolic heart failure accounts for more than 50% of patients over the age of 75 with the clinical diagnosis of congestive heart failure. In many individuals this was clinically unrecognized and untreated (Bursi et al. 2006).
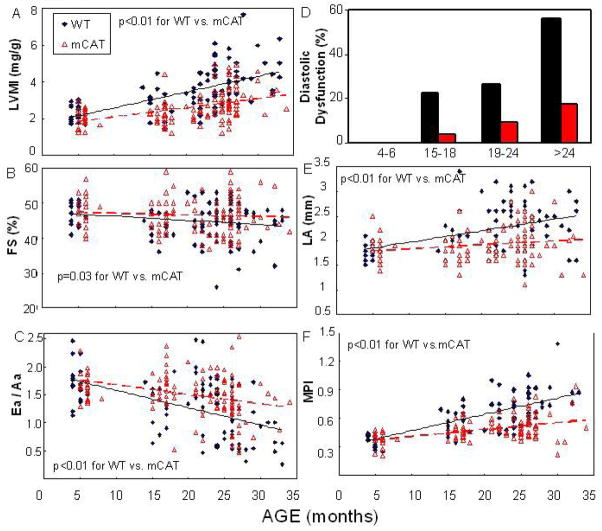
Cardiac aging in the murine model closely recapitulates the age-related cardiac changes in apparently healthy human population noted above. Echocardiography performed on a mouse longevity cohort in our lab demonstrated that there were significant age-dependent linear trends in increased left ventricular mass index (LVMI) and left atrial dimension, reduction in fractional shortening (FS) and diastolic function (Ea/Aa), as well as worsening of myocardial performance index (MPI) (Figure 1 black lines; linear trend P<0.05 for all)(Dai et al. 2009). LVMI increased by around 75% in the oldest group compared to a young adult group, indicating the increase prevalence of left ventricular hypertrophy with age (Figure. 1A). Systolic function measured by FS showed a modest decline of ~10% from the young adult to the oldest group(Figure. 1B). There is a substantial and significant decline of Ea/Aa with age (Figure. 1C), and the prevalence of diastolic dysfunction was dramatically increased to 55% in the oldest age group (Figure. 1D). As a consequence of the age-dependent decline of systolic and diastolic function, which increases left ventricular end-diastolic pressure, we observed enlargement of left atrium with age (Figure. 1E). The myocardial performance index was significantly increased (worsened) with age (Figure. 1F), and indicates that a greater fraction of systole is spent to cope with the pressure changes during isovolemic phases. This has been shown to reflect both LV systolic and diastolic dysfunction (Tei et al. 1997). In summary, echocardiographic findings indicate that the murine heart becomes hypertrophic with age and the decline in diastolic function is prominent. While systolic function only declines slightly with age, the maximal exercise capacity and O2 consumption also significantly decline with age (Schefer and Talan 1996). Furthermore, there is a significant decline in general myocardial performance, shown by worsening (increase) in myocardial performance index (MPI). The above changes have been also been observed in aging of C3H x C57Bl/6 F1 and C3H x BALB/c F1 mice and parallel those of C57Bl/6 mice (unpublished observations).
Figure 1.
Echocardiography of 170 WT and mCAT C57Bl/6 mice in a longevity cohort. (A) Left ventricular mass index (LVMI=LVM/body weight), (B) systolic function measured by percentage of fractional shortening (FS), (C) diastolic function measured by tissue Doppler imaging Ea/Aa, (D) the proportion of mice with diastolic dysfunction, defined as Ea/Aa<1, (E) left atrial dimension and (F) the myocardial performance index were analyzed. The linear trends across ages in WT mice were significant for all parameters (P<0.05 for all). The beneficial effect of mCAT vs. WT was analyzed by the interaction term between genotype and the age trend, and was significant in all cases (P <0.01 for all except fractional shortening, P =0.03). Data reanalyzed from (Dai et al. 2009)
Histopathologic changes in mouse hearts with age include subendocardial and interstitial fibrosis, hyaline cytoplasmic change, vacuolization of cytoplasm, variable and hypertrophic myocyte fiber size, collapse of sarcomeres, mineralization, and arteriolosclerosis. These can be designated as age-associated cardiomyopathy (Treuting et al. 2008). Morphometric analysis demonstrates cardiomyocytes hypertrophy (increased myocardial fiber size), increased cardiomyocytes apoptosis (Yan et al. 2007) and increased fibrosis and amyloid deposition with age. Interestingly, fibrosis in old mouse hearts was more commonly observed in the ventricular subendocardium (Dai et al. 2009), which might be due to exposure to higher wall stress in the endocardial layers.
Molecular mechanisms of cardiac aging: neurohormonal regulation
1. Renin Angiotensin Aldosterone System (RAAS)
RAAS activation has been implicated in a broad spectrum of cardiovascular diseases, including hypertension, coronary heart disease and congestive heart failure, as well as atrial fibrillation. The prevalence of all of the above diseases have been shown to increase with age in the Framingham Heart Study. Indeed, Angiotensin II (Ang II) directly induces cardiomyocyte hypertrophy and apoptosis, increases cardiac fibrosis and impairs cardiomyocyte relaxation (Domenighetti et al. 2005), consistent with the changes found in cardiac aging. We and others have shown that cardiac Ang II concentrations increase significantly in aged rodent hearts (Dai et al. 2009, Groban et al. 2006), probably related to increased tissue levels of angiotensin II converting enzyme (ACE)(Lakatta 2003). Though the mechanism of increased ACE in the aged heart is not well understood, long-term inhibition with Angiotensin receptor blockers or disruption of angiotensin receptor type I has been shown to reduce age-dependent cardiac pathology and prolong rat (Basso et al. 2007) and mouse (Benigni et al. 2009) survival. Thus, the activation of RAAS might play a central role in cardiac aging and age-associated cardiovascular diseases.
2. Adrenergic signaling
Chronic activation of β-adrenergic signaling is well known to be deleterious to the heart. This activation enhances cardiac metabolic demand secondary to increase in heart rate, contractility, afterload (blood pressure) and wall stress. Several clinical trials have shown that inhibition of β-adrenergic signaling by β-blockers provides survival benefit in patients with heart failure. Stimulation of β-adrenergic receptors (G-protein coupled receptors) activates adenylate cyclase, a key enzyme producing c-AMP as a secondary messenger. There are several isoforms of adenylate cyclase and type 5 (AC5) is the major form in the heart. Mice with disruption of AC5 were shown to have prolonged lifespan, likely mediated through upregulation of the Raf-1/pMEK/pERK pathway, which confers protection against stress, including oxidative stress (Yan et al. 2007). These mice were also shown to be protected from cardiac aging, including age-dependent cardiac hypertrophy, systolic dysfunction, apoptosis and fibrosis (Yan et al. 2007). Consistent with this, AC-5 disruption protected against chronic pressure overload-induced cardiac hypertrophy, apoptosis and failure by chronic catecholamine stimulation or aortic banding (Okumura et al. 2003, 2007).
3. Insulin/IGF1 signaling
Insulin/IGF-1 signaling has been implicated in the regulation of lifespan in vertebrate and invertebrate animal models. Both mice deficient in growth hormone (Bartke et al. 2001) and mice with mutation of the IGF-1 receptor (Holzenberger et al. 2003) have been shown to have prolonged lifespans. Furthermore, deficiency in IGF-1 signaling was shown to improve cardiac performance at advanced age in Drosophila as well as to attenuate age-associated cardiomyocyte dysfunction in mice (Li et al. 2008, Wessells et al. 2004). This contrasts with findings from the Framingham Heart Study, which showed that low serum IGF-1 levels were associated with increased risk of heart failure in elderly subjects without a history of myocardial infarction (Vasan et al. 2003). Moreover, low levels of serum GH and IGF-1 have been correlated with systolic dysfunction in heart failure patients. Consistent with this, GH replacement therapy improves heart failure symptoms and attenuates cardiac remodeling in human patients (Giustina et al. 1996) and in the rat model of experimental heart failure (Tajima et al. 1999). It also attenuates age-associated diastolic dysfunction and increases cardiac angiotensin II in senescent rats (Groban et al. 2006). Thus, further studies are required to address the controversial role of insulin/IGF1 signaling on cardiac aging.
Molecular mechanisms of cardiac aging: role of mitochondrial ROS in cardiac aging
Harman et al first proposed the free radical theory of aging more than five decades ago, postulating that the production of reactive oxygen species (ROS) is a major determinant of lifespan (Harman 1956). The deleterious effects of ROS on various cell and organ components might drive an age-dependent functional decline of cells and organ systems, leading to associated degenerative diseases. Within cells, ROS are produced in multiple compartments and by multiple enzymes including NADPH oxidase at the plasma membrane, oxidative phosphorylation within mitochondria, and by cyclooxygenases and xanthine oxidase in the cytoplasm. Although all of these sources contribute to the overall oxidative burden, mitochondria contribute the majority of ROS generation as a byproduct of electron transfer during oxidative phosphorylation. Most specifically, excess electrons from complex I and III can be transferred directly to O2 to generate superoxide anion (O2−), which is then converted to H2O2 by mitochondrial manganese superoxide dismutase. Mitochondrial H2O2 can diffuse into cytosol and nucleus, to activate redox-sensitive signaling. However, H2O2 is reduced by the Fenton reaction (Fe2+ chemistry) into a hydroxyl radical (OH•), the most reactive ROS species. Mitochondrial nucleic acids, lipids and proteins can be at highest risk from such damage. This has led to the mitochondrial variant of the free radical theory of aging (reviewed by Balaban et al. 2005), which proposes that mitochondrial ROS attack mitochondrial constituents, causing mitochondrial DNA damage and mitochondrial dysfunction, followed by a vicious cycle between increased mitochondrial damage and further production of ROS, which cause functional declines of cells and organ systems that eventually lead to death. Several studies have documented age-dependent impairment of mitochondrial function, mainly the decline in mitochondrial respiratory capacity (state 3) due to diminished activity of complexes I and IV, but relatively unaffected complexes II, III and V. This slower rate of mitochondrial electron transfer with age also favors mitochondrial superoxide production, leading to a positive feedback between complex I inhibition, mitochondrial ROS production (designated as ROS induced ROS release), mitochondrial DNA mutation and protein damages (reviewed by Navarro and Boveris 2007).
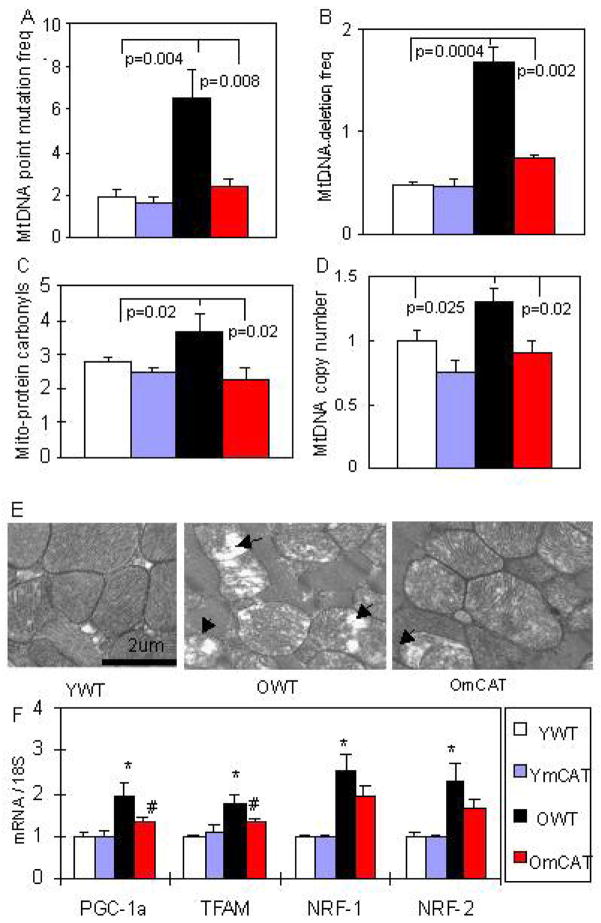
Because the heart is a vital organ with high metabolic demand and rich in mitochondria, it is especially vulnerable to mitochondrial oxidative damage. Indeed, mitochondrial energetics deficiency has been widely documented in heart failure in both human patients and mouse models (Ventura-Clapier et al. 2008). The mechanisms may include mitochondrial biogenesis that is inadequate to match the increasing demand/workload (reviewed in Goffart et al. 2004), increased mitochondrial uncoupling and decreased substrate availability (Murray et al. 2004), and increased mitochondrial DNA deletions (Dai et al, in press). In aging, we showed that mitochondrial DNA point mutation and deletion frequencies increase ~3 fold in the old mouse hearts, compared with young adult hearts (Figure. 2A, B). In addition, protein carbonyls in mitochondrial extracts, indicative of mitochondrial protein oxidative damage, significantly increase in the aged-heart (Figure. 2C). Oxidative damage to mitochondria is reflected in abnormal ultrastructure of mitochondria in old wild-type (WT) hearts, which showed disrupted cristae and vacuolation (loss of electron density) (Figure. 2E). This damage stimulates signaling for mitochondrial biogenesis, seen in the aged heart by an increase in mtDNA copy number (Figure. 2D) concomitant with significant upregulation of the master regulator PPAR- Coactivator-1-a (PGC-1a) and its downstream transcription factors mitochondrial transcription factor A (TFAM) and nuclear respiratory factors (NRFs) (Figure. 2F). Direct evidence for the critical role of mitochondrial ROS in cardiac aging was reinforced by our experiments using mice overexpressing catalase targeted to the mitochondria (mCAT). We have previously shown that mCAT mice had an 18% prolongation of lifespan, while mice overexpressing wild-type peroxisomal catalase (pCAT) did not (Schriner et al. 2005). We subsequently found that mCAT mice were significantly protected from the age-dependent increase in LVMI (Figure 1A, P <0.01), decline in systolic (FS%, Figure. 1B, P =0.03), diastolic function (Ea/Aa), (Figure. 1C, P <0.01) and increase in the prevalence of diastolic dysfunction (Figure. 1D), enlargement of left atrium (Figure. 1D, P <0.01), as well as worsening of myocardial performance (Figure. 1E, P <0.01)(Dai et al. 2009). Consistent with this, we found that mCAT attenuates age-dependent mitochondrial oxidative damage, as displayed by significant reductions of mtDNA mutation and deletion frequencies (Figure. 2AB), decreased mitochondrial protein carbonyls (Figure. 2C), better protection of ultrastructure of mitochondrial cristae (Figure. 2E) and attenuation of age-dependent activation of mitochondrial biogenesis (Figure. 2D,F).
Figure 2. Mitochondrial DNA mutations, oxidative damage and biogenesis in aged heart and the protective effect of mCAT.
(A, B) Mitochondrial DNA point mutation and deletion frequencies (both per million base pairs) increased significantly with age and were attenuated in old mCAT mouse hearts. (C) Protein carbonyls content (nmol/mg) from cardiac mitochondrial extracts increased significantly with age, while mCAT attenuated age-dependent oxidative damage to mitochondrial proteins. (D) Mitochondrial DNA copy number (normalized to young WT) increased significantly with age, and old mCAT mice had significantly less increase in mitochondrial DNA copy number. (E) Electron micrographs displayed disruption of cristae and vacuolation of mitochondria (loss of electron density) in old WT mouse hearts, which was better protected in old mCAT heart mitochondria. (F) Upregulation of genes involved in mitochondrial biogenesis in the aged heart, including PGC-1a, TFAM, NRF-1 and NRF-2, and changes in PGC-1a and TFAM were attenuated in old mCAT mice (*P <0.025 between young vs. old WT, # P <0.025 between old WT vs. mCAT; n=9–12 each group; old: 26–28 months, young:4–5 months old).
Another line of evidence indicating the role of mitochondria in aging was demonstrated by mice with homozygous mutation of mitochondrial polymerase gamma, which impairs the proofreading capacity of the enzyme and thus induces a substantial increase in mtDNA point mutations and deletions (Kujoth et al. 2005, Trifunovic et al. 2004). These mice had shortened lifespan and a phenotype of accelerated aging, including kyphosis, alopecia, anemia, osteoporosis and age-dependent cardiomyopathy (Trifunovic et al. 2004). The accumulation of mitochondrial DNA mutations have been shown to increase apoptotic rate (Kujoth et al. 2005), and observations that mitochondrial damage and cardiomyopathy in these mice can be partially rescued by mCAT suggests that it is at least partially mediated through mt-ROS (Dai et al, in press). Furthermore, it has been shown that accumulation of mtDNA deletions is better correlated with the premature aging phenotype in these mice than are mtDNA point mutations (Vermulst et al. 2008). Mouse models of mtDNA mutation were extensively reviewed in (Wallace and Fan 2009). In human, age-associated accumulation of mtDNA deletions have been documented in various tissues, including heart (Zhang et al. 1997, Corral-Debrinski et al. 1991).
Mechanism of age-dependent LV hypertrophy and diastolic dysfunction
Cardiac hypertrophy involves a complex network of molecular signaling (reviewed by Heineke and Molkentin 2006). We found that the calcineurin-NFAT pathway, which mediates pathological hypertrophy, is activated in age-dependent murine cardiac hypertrophy (Dai et al. 2009). Calcineurin is a phosphatase that dephosphorylates and activates the transcription factor NFAT, which then translocates into nucleus and interacts with several other transcription factors (e.g., GATA4) to initiate transcription of hypertrophic genes, such as atrial natriuretic peptides and brain natriuretic peptides.
Calcium handling proteins regulate the electro-mechanical coupling of cardiomyocytes. In old rodent hearts, we and others demonstrated the decline of sarcoplasmic reticulum ATPase (SERCA2) protein concentration (Xu and Narayanan 1998), concomitant with compensatory increase in the levels of Na+/Ca2+ exchanger (Dai et al. 2009, Koban et al. 1998). Oxidative damage to particular cysteine thiols could also impair SERCA2 activity (Adachi et al. 2004). Chronic reduction of SERCA protein level/function could lead to prolongation of Ca2+ decay rate, reduction in SR Ca2+-load and hence smaller amplitude of Ca2+ transients (Li et al. 2005). Indeed, our analysis of murine cardiac aging showed these Ca2+ transient changes and that decreased SERCA2 protein concentration is a predominant factor associated with age-dependent diastolic dysfunction. The aged heart might utilize the compensatory increase in the L-type Ca2+ currents(Josephson et al. 2002) and the significant prolongation of action potential duration to preserve SR loading and to keep the amplitude of intracellular Ca2+-transients and contractions in old cardiomyocytes. (Janczewski et al. 2002) The other factors contributing to age-dependent diastolic dysfunction include increased myocardial stiffness related to cardiac hypertrophy and fibrosis.
Increased susceptibility to stress in the aged heart
The aged myocardium is more susceptible to ischemia and hemodynamic stress than young myocardium (Isoyama and Nitta-Komatsubara 2002). Cells from aged hearts have a lower threshold for ROS induced ROS release and increased susceptibility to mitochondrial permeability transition pore (MPTP) induction (Juhaszova et al. 2005). MPTP is a voltage-dependent, high conductance “channel” located in the inner mitochondrial membrane. In the fully open state, it allows passive diffusion of solutes with molecular masses up to 1.5 kDa. MPTP opening may cause mitochondrial swelling, collapse of mitochondrial membrane potential (Δψm), ATP depletion, and eventually trigger apoptosis and/or cell death (Di Lisa and Bernardi 2006). Furthermore, ischemic preconditioning, the endogenous cardioprotective mechanism incited by repetitive ischemia that reduced the area of myocardial infarction, is impaired in the aged myocardium (reviewed by Juhaszova et al. 2005). The mechanism underlying this impairment might include decreased mitochondrial heat shock protein-70 (Nitta et al. 1994), reduced bioavailability of nitric oxide (Chou et al. 1998), damaged mitochondria that are vulnerable to stress, and diminished PKC translocation, all of which are believed to be required for the protective effect of ischemic preconditioning (Korzick et al. 2001, Tani et al. 2001).
Reduced regenerative capacity of the aged heart
Several studies have demonstrated that resident stem and progenitor cells in the adult heart are capable of regeneration (Beltrami et al. 2003, Hsieh et al. 2007). Transplantation of these cells has been shown to improve cardiac function in rodent models of experimental myocardial infarction (reviewed in Reinecke et al. 2008)). Although these cells are capable of continuously repopulating cardiomyocytes in adult hearts, they fail to prevent the progression of cardiovascular diseases. One possible explanation is the aging of cardiac stem cells, which might reduce their number and impair their regenerative capacity, either by senescence of the stem cell or as a consequence of a hostile niche in the aged heart. Anversa et al. (2005) reported that cardiac stem cells in older animals and patients with cardiovascular diseases had a higher rate of apoptosis, shorter telomeres and increased expression of the senescence marker p16INK4a. Furthermore, a recent study measuring 14C labeling (a retrospective birth dating method) in human hearts showed that the turnover or renewal rate of cardiomyocytes in young adults was approximately 1% annually, and this was significantly reduced to 0.45 % in the hearts of the elderly (Bergmann et al. 2009). Thus, the decline in number and regenerative capacity of cardiac stem cells might explain part of the increased susceptibility of the elderly to heart failure.
Cardiac aging in other model organisms
The emerging new techniques for analysis of Drosophila cardiac function in recent years has allowed the fruit fly genetic system to be used to study age-related functional changes in cardiac tissue. The Drosophila heart demonstrates an age-dependent decline in heart rate and contractility, increase in susceptibility to arrhythmia and pacing induced cardiac failure. Although the mouse has been a premier model for mammalian aging studies because of the availability of genetically modified mice and the relatively short mouse lifespan, research using nonhuman primates provides a valuable tool to investigate aging process which closely recapitulate human aging and allows the investigation of potential anti-aging interventions before human clinical trials. Longitudinal study of aging in rhesus monkeys (Macaca mulatta) conducted by the National Institute of Aging revealed that under normal diets rhesus monkeys develop several aging-related pathologies, including aortic and mitral valves degenerative calcifications, loss or degeneration of myocardial fibers with hypertrophy of remaining cardiomyocytes, lipofuscin accumulation and variable degrees of myocarditis, multifocal interstitial fibrosis, myocardial infarction and congestive heart failure (Lane et al. 1999, 2002, Mattison et al. 2003, Roth et al. 2004).
Conclusion
Cardiac aging in mice closely recapitulates human cardiac aging, which includes cardiac hypertrophy, fibrosis, diastolic dysfunction, reduced functional reserve and adaptive capacity to stress. These changes increase the risk of heart failure. Mitochondrial ROS appears to play a central role in cardiac aging. Future studies should consider the potential application of mitochondrial antioxidants in cardiac aging and age-related cardiovascular diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi T, Weisbrod RM, Pimentel DR, et al. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–7. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–95. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Bartke A, Coschigano K, Kopchick J, et al. Genes that prolong life: relationships of growth hormone and growth to aging and life span. J Gerontol A Biol Sci Med Sci. 2001;56:B340–9. doi: 10.1093/gerona/56.8.b340. [DOI] [PubMed] [Google Scholar]
- Basso N, Cini R, Pietrelli A, et al. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007;293:H1351–8. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- Benigni A, Corna D, Zoja C, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–30. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky SV, Gealekman O, Chen J, et al. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circ Res. 2004;94:377–84. doi: 10.1161/01.RES.0000111802.09964.EF. [DOI] [PubMed] [Google Scholar]
- Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. Jama. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- Chou TC, Yen MH, Li CY, et al. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension. 1998;31:643–8. doi: 10.1161/01.hyp.31.2.643. [DOI] [PubMed] [Google Scholar]
- Corral-Debrinski M, Stepien G, Shoffner JM, et al. Hypoxemia is associated with mitochondrial DNA damage and gene induction. Implications for cardiac disease. Jama. 1991;266:1812–6. [PubMed] [Google Scholar]
- Dai DF, Santana LF, Vermulst M, et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–97. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lisa F, Bernardi P. Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc Res. 2006;70:191–199. doi: 10.1016/j.cardiores.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Domenighetti AA, Wang Q, Egger M, et al. Angiotensin II-mediated phenotypic cardiomyocyte remodeling leads to age-dependent cardiac dysfunction and failure. Hypertension. 2005;46:426–32. doi: 10.1161/01.HYP.0000173069.53699.d9. [DOI] [PubMed] [Google Scholar]
- Giustina A, Lorusso R, Borghetti V, et al. Impaired spontaneous growth hormone secretion in severe dialated cardiomyopathy. Am Heart J. 1996;131:620–2. doi: 10.1016/s0002-8703(96)90552-9. [DOI] [PubMed] [Google Scholar]
- Goffart S, von Kleist-Retzow J-C, Wiesner RJ. Regulation of mitochondrial proliferation in the heart: power-plant failure contributes to cardiac failure in hypertrophy. Cardiovascular Research. 2004;64:198–207. doi: 10.1016/j.cardiores.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Groban L, Pailes NA, Bennett CD, et al. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–7. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Hsieh PC, Segers VF, Davis ME, et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–4. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoyama S, Nitta-Komatsubara Y. Acute and chronic adaptation to hemodynamic overload and ischemia in the aged heart. Heart Fail Rev. 2002;7:63–9. doi: 10.1023/a:1013701923065. [DOI] [PubMed] [Google Scholar]
- Janczewski AM, Spurgeon HA, Lakatta EG. Action potential prolongation in cardiac myocytes of old rats is an adaptation to sustain youthful intracellular Ca2+ regulation. J Mol Cell Cardiol. 2002;34:641–8. doi: 10.1006/jmcc.2002.2004. [DOI] [PubMed] [Google Scholar]
- Josephson IR, Guia A, Stern MD, et al. Alterations in properties of L-type Ca channels in aging rat heart. J Mol Cell Cardiol. 2002;34:297–308. doi: 10.1006/jmcc.2001.1512. [DOI] [PubMed] [Google Scholar]
- Juhaszova M, Rabuel C, Zorov DB, et al. Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res. 2005;66:233–44. doi: 10.1016/j.cardiores.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Khouri SJ, Maly GT, Suh DD, et al. A practical approach to the echocardiographic evaluation of diastolic function. J Am Soc Echocardiogr. 2004;17:290–7. doi: 10.1016/j.echo.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Koban MU, Moorman AF, Holtz J, et al. Expressional analysis of the cardiac Na-Ca exchanger in rat development and senescence. Cardiovasc Res. 1998;37:405–23. doi: 10.1016/s0008-6363(97)00276-9. [DOI] [PubMed] [Google Scholar]
- Korzick DH, Holiman DA, Boluyt MO, et al. Diminished alpha1-adrenergic-mediated contraction and translocation of PKC in senescent rat heart. Am J Physiol Heart Circ Physiol. 2001;281:H581–9. doi: 10.1152/ajpheart.2001.281.2.H581. [DOI] [PubMed] [Google Scholar]
- Kosugi R, Shioi T, Watanabe-Maeda K, et al. Angiotensin II receptor antagonist attenuates expression of aging markers in diabetic mouse heart. Circ J. 2006;70:482–8. doi: 10.1253/circj.70.482. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–4. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–7. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–54. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Roth GS. Calorie restriction in nonhuman primates: effects on diabetes and cardiovascular disease risk. Toxicol Sci. 1999;52:41–8. doi: 10.1093/toxsci/52.2.41. [DOI] [PubMed] [Google Scholar]
- Lane MA, Mattison J, Ingram DK, et al. Caloric restriction and aging in primates: Relevance to humans and possible CR mimetics. Microsc Res Tech. 2002;59:335–8. doi: 10.1002/jemt.10214. [DOI] [PubMed] [Google Scholar]
- Li Q, Ceylan-Isik AF, Li J, et al. Deficiency of insulin-like growth factor 1 reduces sensitivity to aging-associated cardiomyocyte dysfunction. Rejuvenation Res. 2008;11:725–33. doi: 10.1089/rej.2008.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SY, Du M, Dolence EK, et al. Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glycation endproducts and protein modification. Aging Cell. 2005;4:57–64. doi: 10.1111/j.1474-9728.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Lane MA, Roth GS, et al. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- Murray AJ, Anderson RE, Watson GC, et al. Uncoupling proteins in human heart. Lancet. 2004;364:1786–8. doi: 10.1016/S0140-6736(04)17402-3. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292:C670–86. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- Nitta Y, Abe K, Aoki M, et al. Diminished heat shock protein 70 mRNA induction in aged rat hearts after ischemia. Am J Physiol. 1994;267:H1795–803. doi: 10.1152/ajpheart.1994.267.5.H1795. [DOI] [PubMed] [Google Scholar]
- Okumura S, Takagi G, Kawabe J, et al. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci U S A. 2003;100:9986–90. doi: 10.1073/pnas.1733772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura S, Vatner DE, Kurotani R, et al. Disruption of type 5 adenylyl cyclase enhances desensitization of cyclic adenosine monophosphate signal and increases Akt signal with chronic catecholamine stress. Circulation. 2007;116:1776–83. doi: 10.1161/CIRCULATIONAHA.107.698662. [DOI] [PubMed] [Google Scholar]
- Reinecke H, Minami E, Zhu WZ, et al. Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ Res. 2008;103:1058–71. doi: 10.1161/CIRCRESAHA.108.180588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- Roth GS, Mattison JA, Ottinger MA, et al. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004;305:1423–6. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- Schefer V, Talan MI. Oxygen consumption in adult and AGED C57BL/6J mice during acute treadmill exercise of different intensity. Exp Gerontol. 1996;31:387–92. doi: 10.1016/0531-5565(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–11. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Tajima M, Weinberg EO, Bartunek J, et al. Treatment with growth hormone enhances contractile reserve and intracellular calcium transients in myocytes from rats with postinfarction heart failure. Circulation. 1999;99:127–34. doi: 10.1161/01.cir.99.1.127. [DOI] [PubMed] [Google Scholar]
- Tani M, Honma Y, Hasegawa H, et al. Direct activation of mitochondrial K(ATP) channels mimics preconditioning but protein kinase C activation is less effective in middle-aged rat hearts. Cardiovasc Res. 2001;49:56–68. doi: 10.1016/s0008-6363(00)00240-6. [DOI] [PubMed] [Google Scholar]
- Tei C, Nishimura RA, Seward JB, et al. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr. 1997;10:169–78. doi: 10.1016/s0894-7317(97)70090-7. [DOI] [PubMed] [Google Scholar]
- Treuting PM, Linford NJ, Knoblaugh SE, et al. Reduction of age- associated pathology in old mice by overexpression of catalase in mitochondria. J Gerontol A Biol Sci Med Sci. 2008;63:813–22. doi: 10.1093/gerona/63.8.813. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–23. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- Vasan RS, Sullivan LM, D’Agostino RB, et al. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann Intern Med. 2003;139:642–8. doi: 10.7326/0003-4819-139-8-200310210-00007. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–17. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- Vermulst M, Wanagat J, Kujoth GC, et al. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet. 2008;40:392–4. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Fan W. The pathophysiology of mitochondrial disease as modeled in the mouse. Genes Dev. 2009;23:1714–36. doi: 10.1101/gad.1784909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells RJ, Fitzgerald E, Cypser JR, et al. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–81. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- Xu A, Narayanan N. Effects of aging on sarcoplasmic reticulum Ca2+-cycling proteins and their phosphorylation in rat myocardium. Am J Physiol. 1998;275:H2087–94. doi: 10.1152/ajpheart.1998.275.6.H2087. [DOI] [PubMed] [Google Scholar]
- Yan L, Vatner DE, O’Connor JP, et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–58. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- Zhang C, Bills M, Quigley A, et al. Varied prevalence of age-associated mitochondrial DNA deletions in different species and tissues: a comparison between human and rat. Biochem Biophys Res Commun. 1997;230:630–5. doi: 10.1006/bbrc.1996.6020. [DOI] [PubMed] [Google Scholar]