Abstract
CD1d molecules are MHC class I-like molecules that present lipids to a unique subpopulation of T cells called natural killer T (NKT) cells. The cytoplasmic tail of human CD1d possesses a tyrosine-based endosomal targeting motif (YXXZ). As such, these molecules traffic through the endocytic pathway, where it is believed that they are loaded with the antigenic lipid that stimulates NKT cells. In the current study, it was found that the T322 residue in the human CD1d tail is a major signal controlling transport to the cell surface and thus its functional expression. Mimicking the phosphorylation of this residue or removal of the entire cytoplasmic tail negates its ability to regulate CD1d trafficking, resulting in lysosomal targeting and degradation. These results demonstrate an important role of a heretofore unknown signal in the cytoplasmic tail of CD1d that may have relevance to other type I integral membrane proteins that traverse through the endocytic pathway.
Keywords: CD1d, Antigen Presentation/Processing, Cell Trafficking
Introduction
CD1 molecules are non-polymorphic transmembrane glycoproteins encoded by genes located outside of the MHC locus (1). There are five members of the CD1 family: CD1a, CD1b, CD1c, CD1d and CD1e. Like MHC class I, all CD1 proteins consist of three extracellular domains (α1, α2 and α3), a transmembrane domain and cytoplasmic tail. The CD1 α3 domains are non-convalently associated with β2–microglobulin (β2m) forming a heterodimer soon after translation in the endoplasmic reticulum (ER) (2, 3). Unlike MHC class I and class II molecules that present peptide antigens, CD1 molecules present lipids to T cells. The CD1d molecule presents lipid antigens mostly to a unique subpopulation of T cells that express an invariant TCR α chain and surface markers also present on natural killer cells (4). These are thus named “invariant” natural killer T (NKT) cells. Activated NKT cells secrete both Th1 (e.g., IFN-γ, GM-CSF) and Th2 (e.g., IL-4) cytokines, playing important roles in both innate and adapt immunity (5), including anti-tumor, autoimmune and anti-microbial responses (6-8).
Microsomal triglyceride transfer protein (MTP), a protein involved in lipoprotein assembly (9), has been reported to be important for CD1d function in vivo. It is likely required for the loading of self-lipids into the hybrophobic groove formed by the α1 and α2 domains of CD1d when synthesized in the ER (10, 11). These self-lipids, including phosphatidylinositol (PI) and phosphatidylcholine (PC), generally cannot activate NKT cells. Instead, they likely play an analogous role to the invariant chain-derived CLIP peptide for MHC II (4). Either before or after lipid loading, the CD1d heavy chain binds to β2m and then is transported to the Golgi. In the Golgi, the glycans on CD1d are processed further before being transported to the plasma membrane (12, 13). From the surface, due to the YXXZ motif (Y is tyrosine, X stands for any amino acid and Z for bulky hydrophobic residue) in its cytoplasmic tail, surface CD1d is internalized and traffics through compartments of the endocytic pathway (5, 14). CD1d-mediated lipid antigen presentation requires lipid exchange in late endocytic compartments and re-expression on the cell surface to activate NKT cells (15, 16). Sphingolipid activator proteins (SAPs), especially saposin B, may facilitate lipid binding to CD1d (or other CD1 molecules) throughout the endocytic pathway (17-20). Among the lipid antigens that activate NKT cells, some are natural cellular ligands, such as isoglobotrihexosylceramide (iGb3), whereas others are microbial lipids such as α-glucuronosylceramide (GSL-1) from Sphingomonas (21-23). α-Galactosylceramide (α-GalCer), a glycolipid extracted from marine sponges, is recognized by invariant NKT cells in a CD1d-dependent manner (4, 24).
CD1d a type I transmembrane protein and its cytoplasmic tail contains at least one signal for endocytic trafficking (Supplementary Fig. 1). Both the human and mouse CD1d cytoplasmic tails contain an YXXZ motif, which is believed to be a binding motif for the AP2μ1 subunit and AP3, respectively (25). Destroying this motif causes the accumulation of both human and mouse CD1d on the cell surface (15, 26). Interestingly, the YXXZ motif is also required for CD1d downregulation caused by a microbial infection, such as HIV and Chlamydia (13, 27, 28). The lysine in the CD1d cytoplasmic tail is also believed to be important, as the mono-ubiquitination of lysines can also function as a signal for endocytic trafficking (29, 30), and infection with Kaposi’s Sarcoma Herpesvirus downregulates surface CD1d through ubiquitination of the K326 residue, a presumed means of immune evasion (31).
In the current study, we have identified contrasting signals in the cytoplasmic tail of human CD1d important for its intracellular distribution, endocytic trafficking and ability to present antigen. Our data strongly suggest that there are two major signals in the cytoplasmic tail that are important for lysosomal targeting. One directly targets the CD1d molecule to lysosomes and is Tyr (Y331)-based; the second is Thr (T322)-based and, under normal conditions, permits cell surface expression. When altered to mimic a phosphorylated form (T322D), CD1d is directed to lysosomes for degradation. The T322-based signal is dominant over that which is Y331-based and therefore controls the functional expression of CD1d. We speculate that this type of Thr-based signal for targeting lysosomes also exists in many other type I transmembrane proteins and probably has a broad application in their intracellular distribution and endocytic trafficking.
Material and Methods
CD1d and mutant constructs
The wildtype (WT) human CD1d (hCD1d) cDNA was excised from pBSKII-hCD1d (kindly provided by S. Balk) by XhoI/BamHI digestion (New England Biolabs, Ipswich, MA) and inserted into the pcDNA 3.1-neo vector to generate pcDNA3.1-neo-hCD1d WT (Invitrogen, Carlsbad, CA). The CD1d Y331A and TD-6 mutants were generated as described (13, 27) and also subcloned into pcDNA3.1-neo. The T322A and T322D mutants were generated by site-directed mutagenesis of pcDNA3.1 neo-hCD1dWT. The forward and reverse primers for T322A were: 5′-c att gtg ggc ttt gcc tcc cgg ttt aag-3′ and 5′-ctt aaa ccg gga ggc aaa gcc cac aat g-3′, respectively. The primers for T322D were: 5′-c att gtg ggc ttt gac tcc cgg ttt aag-3′ (forward) and 5′-ctt aaa ccg gga gtc aaa gcc cac aat g-3′ (reverse).
To generate hCD1d mutants in which the last 10 or all 14 cytoplasmic tail amino acids were deleted (TD-10 and TD-14, respectively), two reverse primers were designed either lacking the C-terminal 10 (5′-t ata gga tcc tca aaa ccg gga ggt aaa gcc-3′) or 14 amino acids (5′-t ata gga tcc tca aaa gcc cac aat gag gag g-3′). Both primers were flanked with BamHI sites as underlined. These two primers were paired with a T7 forward primer for PCR amplification of hCD1d. The PCR products were then digested with XhoI and BamHI, purified and subcloned into pcDNA 3.1-neo.
CD25/CD1d fusion proteins [containing the CD1d transmembrane domain with WT or mutant (T322D or TD-14) cytoplasmic tails] were generated by Ntsane Moleleki and Mark Peggie (MRC Protein Phosphorylation Unit, University of Dundee, Scotland, UK) and based in the pcDNA3.1-neo vector.
To generate CD1a/CD1d tail fusion proteins, CD1a cDNA was first amplified from C1R.CD1a cells (kindly provided by S. Balk) by PCR using two primers designed to flank the amplified PCR product with XhoI and BamHI sites. After XhoI/BamHI digestion, the amplified fragment was purified and inserted into the pcDNA3.1-neo vector to generate pcDNA3.1-neo-CD1a. There is a common XbaI site in the 3′ end of the α3 domain of both CD1d and CD1a molecules (32-34). Thus, the pcDNA3.1 neo-hCD1d vector was digested with XbaI to remove the CD1d extracellular domain and the extracellular domain of CD1a was excised from pcDNA3.1-neo-CD1a with XbaI and subcloned to generate a CD1a extracellular domain fused to the CD1d transmembrane and cytoplasmic tail. This construct was used as a template to generate T322D and TD-14 cytoplasmic tail mutants as described above.
Cell lines
HEK293 cells (kindly provided by Prof. Philip Cohen, MRC Protein Phosphorylation Unit, University of Dundee, Scotland, UK) were cultured in DMEM supplemented with 10% FBS, 2 mM L-glutamine. These cells were transfected with the pcDNA3.1-neo-based vectors containing hCD1d with the WT or indicated cytoplasmic tail mutants, or CD1a extracellular domain fused to the CD1d WT (or mutant) cytoplasmic domain, using polyethylenimine following standard techniques. C1R cells (a kind gift from S. Balk) were transfected by electroporation with pcDNA3.1-neo containing WT and cytoplasmic tail mutants of hCD1d (or CD1a/CD1d fusion proteins). To generate stable transfectants, the cells were selected and maintained in DMEM medium supplemented with 10% FBS, 2 mM L-glutamine and 500 μg/ml of G418.
Antibodies and related reagents
Purified, unlabeled stocks of the CD1d-specific mAbs 51.1.3 (for immunoprecipitation) and 42.1 (for flow cytometry and confocal microscopy) were generous gifts from M. Exley (Brigham and Women’s Hospital, Harvard University). Purified 6B11 (anti-human invariant NKT cells), FITC-conjugates of mAb against human LAMP-1, CD1a, and CD25, as well as phycoerythrin (PE)-conjugated 42.1 were from BD Biosciences (San Diego, CA). A PE-labeled goat anti-mouse immunoglobulin antiserum was purchased from Dako (Carpenteria, CA). A Texas Red-conjugated donkey anti-rabbit immunoglobulin antiserum was from Jackson ImmunoResearch Laboratories (West Grove, PA). A Texas Red-conjugated goat anti-mouse immunoglobulin antiserum and Hoechst stain were purchased from Molecular Probes (Portland, OR). The HB95 hybridoma (pan-HLA class I-specific mAb) was a kind gift from J. Yewdell and J. Bennink (Laboratory of Viral Diseases, NIAID, NIH). Antibodies against the endoplasmic reticulum (ER) marker Sec61β were from Millipore (Billerica, MA), whereas the anti-CD1d free HC-specific mAb (C3D5, for immunoprecipitation and Western analysis) was from Santa Cruz Biotechnology (Santa Cruz, CA). A FITC-conjugated rabbit anti-PE antibody was obtained from Rockland Immunochemicals, Inc. (Gilbertsville, PA). The CD1d-binding lipid α-galactosylceramide (α-GalCer) was generated as described (35) or purchased from Alexis Biochemicals (San Diego, CA). Recombinant human IL-2, IL-4 and GM-CSF were from PeproTech, Inc. (Rocky Hill, NJ), whereas antibody pairs for the human IL-4 and GM-CSF ELISA assays (described below) were obtained from BD-Biosciences and Biolegend (San Diego, CA), respectively. Peptide:N-Glycosidase F (PNGase F) and Endoglycosidase H (Endo H) were purchased from New England Biolabs. Monensin (GolgiStop) was from BD Bioscienses.
Culture of human NKT cells
Human NKT cells were generated following the protocol described by Balk, et al. (36). Briefly, human PBMCs were isolated from de-identified donated whole human blood (Indiana Blood Center, Indianapolis, IN) by density gradient centrifugation on Ficoll-Hypaque (GE Healthcare, Piscataway, NJ). Vα24-Jα18+ NKT cells were purified by positive selection using the 6B11 mAb followed by goat anti-mouse immunoglobulin coupled to magnetic beads (Miltenyi Biotec, Auburn, CA). After magnetic bead separation, the NKT cells were expanded by stimulation with irradiated allogeneic human PBMC in the presence of 100 ng/ml α-GalCer and 10 ng/ml recombinant human IL-2 (rhIL-2).
T cell stimulation assays
HEK293-hCD1d (WT and mutants; 1×105 cells/well) were incubated with human NKT cells at an effector to target (E:T) ratio of 1:1 in the presence of 1 ng/ml of rhIL-2 in the presence or absence of α-GalCer (100 ng/ml) for 48 h. Secreted IL-4 and GM-CSF levels were measured by ELISA. The anti-CD1d-β2m mAb (42.1; 500 ng/ml) was used to block CD1d-mediated antigen presentation in some experiments.
Confocal microscopy
Staining for confocal microscopy was performed as described previously (15). Briefly, HEK293 cells were plated in sterile glass-bottom 35-mm dishes coated with collagen (MatTek, Ashland, MA). After the cells became 50-80% confluent, they were washed, fixed and then permeabilized by 0.1% saponin in Hank’s balanced salt solution (HBSS) with 0.1% bovine serum albumin (HBSS/BSA). To stain the CD1d-β2m complex, cells were incubated with the CD1d-β2m complex-specific 42.1 mAb followed by a Texas Red-conjugated donkey anti-mouse immunoglobulin antiserum. After blocking the free reactive sites with normal mouse serum (Sigma-Aldrich, St. Louis, MO), the cells were incubated with a FITC-conjugated anti-human LAMP-1 mAb. To stain the free CD1d heavy chain (HC), the cells were incubated with the anti-CD1d free HC mAb followed by a FITC-conjugated anti-mouse immunoglobulin antiserum. ER-specific staining was performed by incubating the cells with a rabbit anti-Sec61β polyclonal antiserum followed by a Texas Red-conjugated anti-rabbit immunoglobulin antiserum. For staining the nucleus, cells were immersed in HBSS/BSA with 0.1% saponin-containing Hoechst (1:2000) for 5 min. Just prior to confocal analysis, the cells were placed in mounting medium [10 mM Tris (pH 8.5), 2% DABCO]. The cells were viewed on a Zeiss LSM-510 laser scanning confocal microscope modified for one-photon microscopy using an oil immersion lens at 100×. Analysis of the relative level of CD1d colocalization with LAMP-1 was performed using MetaMorph software (version 5; Molecular Devices, Sunnyvale, CA).
Flow cytometry
Aliquots of cells were washed three times in PBS and then fixed in 1% paraformaldehyde. For surface staining, the cells were washed three times in HBSS/BSA and incubated with the appropriate mAb followed by a PE-conjugated rabbit anti-mouse immunoglobulin antiserum or FITC-labeled anti-CD1a (or anti-CD25) mAb. For intracellular staining, the cells were permeabilized in HBSS/BSA with 0.1% saponin and then incubated with the appropriate mAb followed by a PE-conjugated rabbit anti-mouse immunoglobulin antiserum in the presence of saponin. Analysis was performed by flow cytometry as previously described (15).
Co-immunoprecipitations
Cells were lysed in lysing buffer [10 mM Tris (pH 7.4), 150 mM NaCl, 0.5 mM EDTA, 2% CHAPS], and the lysates were incubated with the CD1d-β2m complex-specific (42.1; IgG2b) or anti-CD1d HC [C3D5 (IgG2a)] mAb coupled to protein G-conjugated Sepharose beads (Pierce, Rockford, IL) overnight at 4 °C. The lysates were then washed four times in PBS containing 0.02% azide, resuspended in SDS-loading buffer [4% SDS, 100 mM Tris-HCl (pH 6.8), 20% glycerol, 2% 2-mercaptoethanol, 0.1% bromophenol blue], run on a 10% SDS-PAGE gel and then transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore). The blot was then processed using the anti-CD1d HC mAb and developed using chemiluminescence before exposure on film.
Statistical analysis
An unpaired two-tailed Student’s t-test was performed by Prism software (version 5.00 for Windows; GraphPad, San Diego, CA). A P value < 0.05 was considered significant. The error bars in the bar graphs show the standard deviation (SD) from the mean.
Results
Identification of two physical forms of CD1d in HEK293-CD1d cells
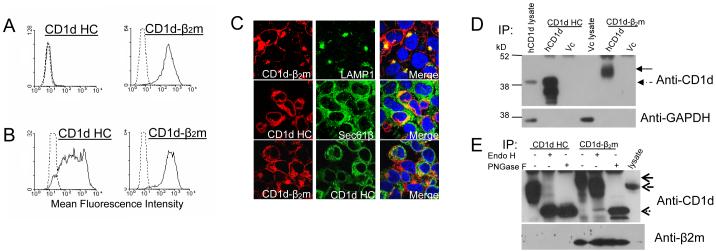
To study the subcellular distribution and intracellular trafficking of CD1d, a stable HEK293 cell line expressing WT human CD1d was generated. Three human CD1d-specific mAbs, C3D5, 42.1 and 51.1, were used to characterize this stable CD1d-expressing cell line. It has been reported that the C3D5 mAb binds only to the CD1d heavy chain (HC) whereas the 42.1 and 51.1 mAbs bind to the CD1d-β2m heterodimer (12). In the current study, an analysis by flow cytometry indicated that the HEK293-CD1d cells express no detectable free CD1d HC on the surface, but a high level of the CD1d-β2m heterodimer (Fig. 1A). Intracellular staining of the same cells revealed substantial CD1d HC (Fig. 1B). By confocal microscopy, it was apparent that the free HC (as detected by the C3D5 mAb) was restricted to the perinuclear area and co-localized with the Sec61β translocon, an ER marker (Fig. 1C). In contrast, the CD1d-β2m complex-specific mAb mostly stained molecules on the cell surface and areas distal from the cell nucleus. There was some degree of co-localization with the lysosomal/late endosomal marker LAMP-1 (Fig. 1C), as has been previously described (37). The overlap between the anti-CD1d HC and anti-CD1d-β2m mAb staining was quite limited (Fig. 1C), suggesting the existence of two different forms of CD1d inside the cell. Thus, the free CD1d HC appears to reside mainly in the ER, whereas the CD1d-β2m heterodimer is found on the cell surface and in endocytic subcellular organelles.
FIGURE 1.
Identification of two physical forms of CD1d in HEK293 cells. HEK293 cells stably transfected with CD1d were fixed and stained with the CD1d HC- or CD1d-β2m-specific mAb (solid line) or isotype control mAb (dotted line) followed by a PE-conjugated second antibody in the absence (A) or presence (B) of saponin. C, HEK293-CD1d cells were fixed and stained with anti-CD1d-β2m (red), anti-LAMP-1 (green) and Hoechst (blue) (upper panel), anti-CD1d HC (red), anti-Sec61β (green) and Hoechst (blue) (middle panel), or anti- CD1d-β2m (red), anti-CD1d HC (green) and Hoechst (blue) (lower panel). D, HEK293-CD1d and vector control (Vc) lysates were immunoprecipitated with the CD1d HC- or CD1d-β2m-specific mAb and run on a 10% SDS-PAGE gel under reducing conditions. The proteins were then transferred onto a PVDF membrane and probed with anti-CD1d (C3D5) for Western blot analysis. The same membrane was then stripped and reprobed with an anti-GAPDH antibody. The solid arrow points to glycosylated CD1d, whereas the dashed arrow points to the unglycosylated form. E, HEK293-CD1d lysates were immunoprecipitated with the anti-CD1d HC or anti-CD1d-β2m mAb and the immunoprecipitants were digested with Endo H or PNGase F. The proteins were resolved on 10% SDS-PAGE gel followed by Western blot analysis with the anti-CD1d HC mAb as described above. Total lysates of HEK293-CD1d were loaded on the gel as a control. The top arrow points to glycosylated CD1d, the middle arrow points to partially glycosylated CD1d and the bottom arrow points to unglycosylated CD1d. The blot below shows that only the CD1d-β2m-specific (but not CD1d free HC-specific) mAb recognizes the CD1d-β2m heterodimer.
In addition to the flow cytometry and confocal microscopic analyses, human CD1d expressed by HEK293 was also characterized by co-immunoprecipitation with the anti-CD1d HC and anti-CD1d-β2m heterodimer mAb. CD1d that was pulled down by the anti-CD1d-β2m heterodimer mAb showed slower mobility (~42 kD) than that immunoprecipitated with the CD1d HC-specific mAb (~38 kD). The band corresponding to the CD1d/β2m complex was almost undetectable in the total cell lysate (Fig. 1D). Our results suggest that the CD1d-β2m heterodimer is more glycosylated than the free CD1d HC, as has been previously reported (12, 13). Thus, the free CD1d HC is non- (or poorly) glycosylated and the CD1d-β2m heterodimer is at least partially (if not fully) glycosylated (Fig. 1C). Further, the HC is much more abundant than the heterodimer. CD1d has four potential N-linked glycosylation sites and one of the glycans (glycan 2 at Asn42) is particularly important for CD1d-β2m association (38). Human epithelial cells and some cell lines express non-glycosylated CD1d (12, 39, 40), whereas other cell lines express a glycosylated form (12). To determine whether the CD1d molecules expressed in HEK293 cells are glycosylated, immunoprecipitants of CD1d using the free HC- and CD1d-β2m-specific mAb were digested with Endo H or PNGase F. The free CD1d HC was partially-glycosylated and Endo H-sensitive (Fig. 1E), suggesting it is mainly located in the ER. On the other hand, the CD1d-β2m heterodimer was Endo H-resistant (Fig. 1E), indicating that this complex had been processed in the Golgi, whereas the high mannose glycans on CD1d were modified [reviewed in (41)]. We also found that β2m was co-immunoprecitated with CD1d molecules pulled down using the anti-CD1d-β2m (but not CD1d HC-specific mAb; Fig. 1E). These data further confirmed that there are two different forms of CD1d: the free HC is located mainly in the ER, whereas the glycosylated CD1d-β2m heterodimer is processed in the Golgi before its expression on the cell surface.
Destroying the YXXZ motif results in increased surface expression of the CD1d-β2m heterodimer, whereas substitution of Thr 322 with Asp or deletion of the entire CD1d tail reduces its surface expression
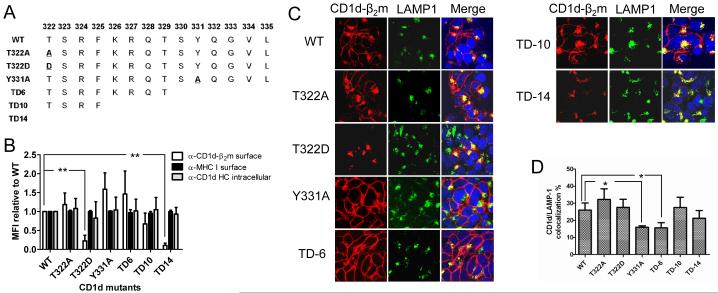
The cytoplasmic tail of CD1d contains a YXXZ motif, which is believed to be utilized for binding to adaptor proteins involved in clathrin-dependent endocytosis, such as AP2 (25). It has been shown previously that an Y331A mutation in the CD1d tail reduces its rate of endocytosis (26). For other glycoproteins, it has been reported that the phosphorylation of threonine, serine and/or tyrosine residues in the cytoplasmic tail affects their intracellular trafficking (42, 43). To determine whether the phosphorylation of the most N-terminal amino acids (T and S) in the CD1d cytoplasmic tail and/or destroying the YXXZ motif would affect its trafficking, a broad series of mutants were generated by site-directed mutagenesis (Supplementary Fig. 1). We then compared the expression of CD1d from mutants to that of the WT by running FACS and confocal microscopy. We found there was almost no difference of CD1d HC expression but great difference of CD1d-β2m heterodimer expression among the mutant cell lines (Supplementary Fig. 2A). We also found some difference in the co-localization of CD1d-β2m to a late-endosomal/lysosomal marker LAMP-1 among different mutant cell lines (Supplementary Fig. 2B). For simplicity, the data from six representative mutants: T322A, T322D, Y331A, and those with a deletion of the last six (TD-6), 10 (TD-10) or all 14 (TD-14) C-terminal amino acids are presented here (Fig. 2), with the results from the other mutants also presented in the Supplementary Data. The replacement of a Thr with Asp is commonly used to mimic its phosphorylated form (44). None of the six mutants, similar to WT, were detected by the anti-CD1d HC mAb on the surface (data not shown), but expressed the same level of CD1d HC as the WT intracellularly as determined by flow cytometry (Fig. 2B, Supplementary Fig. 3). Interestingly, when the cells were stained with the anti-CD1d-β2m complex for flow cytometry analysis, the T322D mutant was expressed at a very low level on the cell surface (Fig. 2A), as was the mutant with the entire cytoplasmic tail deleted (TD-14; Fig. 2B). In contrast, the T322A mutant was expressed at a comparable level as WT CD1d (Fig. 2B). Notably, the T322D and TD-14 mutants could be detected as a CD1d-β2m heterodimer, but likely only in lysosomes, as determined by co-localization with LAMP-1 (Fig. 2C). The Y331A mutant was expressed at a significantly higher level on the surface (Fig. 2B) and showed less co-localization with LAMP-1 as compared to WT (Fig. 2C, 2D). As expected (15, 26), the TD-6 mutant, lacking the YXXZ motif (Fig. 2A), was also expressed at a higher level on the surface (Fig. 2B) and showed less co-localization with LAMP-1 than WT (Fig. 2C, 2D). Although the TD-10 mutant also lacks the YXXZ motif, it actually showed more co-localization with LAMP-1, with a surface level below that of WT (albeit not statistically significant; Fig. 2B, 2C and 2D). This suggests that besides the YXXZ motif, there are other lysosomal targeting signals in the cytoplasmic tail of CD1d. These signals would only become dominant when the last 10 amino acids are absent.
FIGURE 2.
CD1d cytoplasmic tail mutants show different surface and intracellular expression patterns. A, Amino acid sequence of the cytoplasmic tail mutants used in the experiments shown in this figure. WT: wild type. B, WT- and mutant CD1d-expressing HEK293 cells were fixed and stained with the anti-CD1d-β2m mAb (white bars) or the pan-anti-HLA class I-specific anti-MHC I mAb (black bars) followed by a PE-conjugated anti-mouse Ig antiserum. For staining with the anti-CD1d HC mAb (gray bars), the cells were permeabilized with 0.1% saponin. Analysis was by flow cytometry. The data are displayed as the mean fluorescence intensity (MFI) relative to WT (WT = 1). Each bar represents the mean of the MFI in six experiments ± SD. **, p<0.01. C, HEK293-CD1d WT and the indicated mutants were fixed, permeabilized and stained with the anti-CD1d-β2m (red) and anti-LAMP-1 (green) mAb, and Hoechst (blue). The stained cells were then analyzed by confocal microscopy. D, The level of CD1d (anti-CD1d-β2m-reactive) and LAMP-1 co-localization in the graph below was determined by MetaMorph analysis. *, p<0.05.
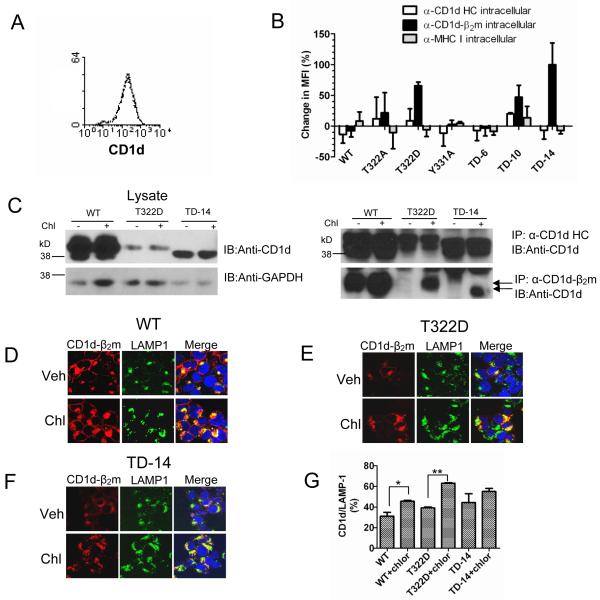
Chloroquine rescues CD1d-β2m heterodimer expression in the T322D and TD-14 mutants
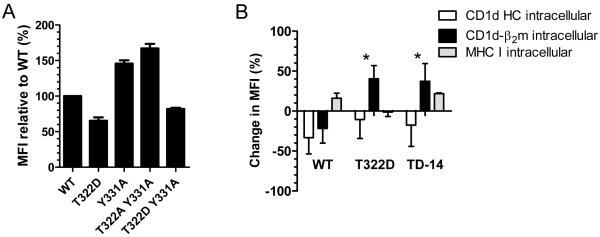
Due to the fact that the CD1d-β2m heterodimer was mostly observed in LAMP-1+ compartments with the T322D and TD-14 mutants, it was hypothesized that changes in lysosomal targeting signals in these mutants would result in their degradation in lysosomes. To test this hypothesis, WT CD1d and the six mutants described above were incubated with the lysosomotropic inhibitor chloroquine to prevent acidification of this compartment. Chloroquine treatment increased the intracellular level of the T322D, TD-10 and TD-14 mutants as CD1d-β2m heterodimers (Fig. 3B, Supplementary Fig. 4), but had no effect on the HC overall. Chloroquine did not alter the intracellular level of WT CD1d (Fig. 3A). These results were supported by co-immunoprecipitation (Fig. 3C) and confocal microscopy analyses (Figs. 3D-G). For the latter experiments, chloroquine treatment generally increased the co-localization of CD1d and LAMP-1 in cells expressing WT CD1d as well as the T322D and TD-14 mutants (Fig. 3D-G). Therefore, these data suggest that the substitution of T322 with D or a total deletion of the cytoplasmic tail (TD-14) causes lysosomal targeting and, consequently, likely degradation of the CD1d-β2m heterodimer in that compartment.
FIGURE 3.
Chloroquine prevents the intracellular loss of the T322D and TD-14 CD1d mutants. A, HEK293-CD1d WT cells were treated with vehicle (solid line) or 20 μM of chloroquine (dotted line) overnight. The cells were then fixed, permeablized and stained with the anti-CD1d-β2m mAb for flow cytometry analysis. B, HEK293 cells expressing CD1d WT or the CD1d mutants were treated with or without 20 μM of chloroquine overnight. The cells were then fixed, permeablized and stained with anti-CD1d HC (white bars), anti-CD1d-β2m (black bars), or anti-MHC I (gray bars) mAbs for flow cytometry analysis. The increase in MFI after overnight treatment of chloroquine was calculated using this formula: change of MFI = 100% * (MFIchl − MFIveh)/ MFIveh. The data shown in the figure are the mean change of the MFI ± SD of two independent experiments. MFIchl : MFI from chloroquine treated cells; MFIveh: MFI from vehicle treated cells. C, CD1d WT-, T322D- and TD-14-expressing HEK293 cells were treated with or without 20 μM of chloroquine overnight, lysed and then the lysates were immunoprecipitated with the anti-CD1d HC or anti-CD1d-β2m mAb. Total lysates of each treatment were loaded onto the gel as a control. The membrane was probed with the CD1d HC-specific mAb for Western blot analysis. The same membrane was stripped and reprobed with anti-GAPDH. The arrows point to an anti-CD1d-β2m-reactive band in the T322D and TD-14 mutants after chloroquine treatment. HEK293-CD1d WT (D), T322D (E) and TD-14 (F) cells were treated overnight with 20 μM of chloroquine (or vehicle). The cells were then fixed, permeabilized and stained with the anti-CD1d-β2m mAb (red), anti-LAMP-1 (green) and Hoechst (blue). The stained cells were then analyzed by confocal microscopy. G, The level of CD1d (CD1d-β2m complex) and LAMP-1 co-localization was determined by MetaMorph analysis. *, p<0.05; **, p<0.01.
Threonine-based signals in the CD1d cytoplasmic tail control the intracellular trafficking of fusion proteins
We have shown that the T322D or TD-14 mutations cause less expression of CD1d on the surface in HEK293 cells. To test whether this phenomenon is only limited to fibroblast cells, we also generated stable C1R (human B lymphoblastoid) cell lines expressing CD1d WT, T322D and TD-14. C1R cells also expressed less CD1d on the surface with the T322D and TD-14 mutants as compared to WT (Supplementary Fig. 5). This result is further support for the existence of a lysosomal targeting signal besides the YXXZ motif.
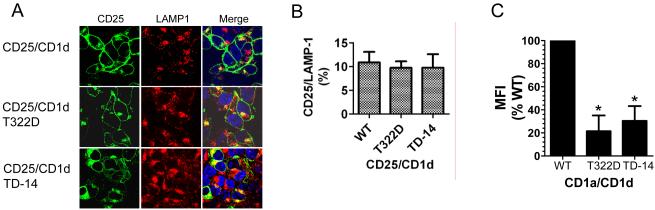
To determine if only the CD1d cytoplasmic tail contains signals that control trafficking to lysosomes, chimeric constructs of the CD25 extracellular domain fused to the transmembrane domain of CD1d and WT (or mutated) cytoplasmic tail of CD1d were generated. The chimeric constructs were used to transfect HEK293 cells and stable transfectants were characterized by confocal microscopy. The CD25/CD1d fusion protein could be observed on the plasma membrane and in the cytosol, with some level of co-localization with LAMP-1 (Fig. 4A and 4B). When the cytoplasmic tail was deleted (CD25/CD1d TD-14), CD25 was only detected in the perinuclear area and in lysosomes (Fig. 4A), suggesting that the CD1d cytoplasmic tail is required for its targeting to the plasma membrane (26, 45, 46). Thus, as was observed with the CD1d TD-14 construct (Fig. 3), deletion of the entire cytoplasmic tail of CD1d caused lysosomal targeting of the CD25 /CD1d (TD-14) fusion protein. Surprisingly, the T322D mutation in the CD25 /CD1d fusion protein showed a similar intracellular distribution and co-localization with LAMP-1 as the WT chimeric protein in HEK293 cells (Fig. 4A, 4B). However, one explanation could be that the T322D residue is a signal for lysosomal targeting, whereas the other 13 amino acids of the cytoplasmic tail contains recycling signals for trafficking back to the plasma membrane. As a single chain protein, CD25 is likely much more easily recycled back to the plasma membrane than the CD1d-β2m heterodimer. Thus, to test this hypothesis, chimeric proteins with the CD1a extracellular domain fused to the transmembrane and cytoplasmic domains of WT (or mutated) CD1d were generated. Like CD1d, CD1a is a β2m-associated molecule (47). As observed with the intact CD1d molecule, the T322D and TD-14 mutations in the CD1a chimeric construct resulted in reduced cell surface expression of CD1a, whereas when the WT CD1d tail was used, much higher levels of CD1a could be detected (Fig. 4C), consistent with our data above with intact WT CD1d.
FIGURE 4.
The cytoplasmic tail from the T322D and TD-14 CD1d mutants alter the surface expression of CD25 or CD1a fusion proteins. A, HEK293 cells were transfected with the CD25 extracellular domain fused to WT, T322D or TD-14 CD1d tail. The cells were stained with an anti-CD25 (green) and anti-LAMP-1 (red) mAb, and Hoechst (blue), and analyzed by confocal microscopy. B, The level of CD25 and LAMP-1 co-localization in the graph below was determined by MetaMorph analysis. C, Constructs encoding the CD1a extracellular domain fused to the WT, T322D or TD-14 CD1d tail mutants were transfected to HEK293 cells. The cells were stained with anti-CD1a for flow cytometry analysis. The data are shown as the MFI relative to the WT (WT as 100%). Each bar corresponds to the MFI mean ± SD. *, p<0.05.
The cytoplasmic tail of CD1d is required for antigen presentation to human NKT cells
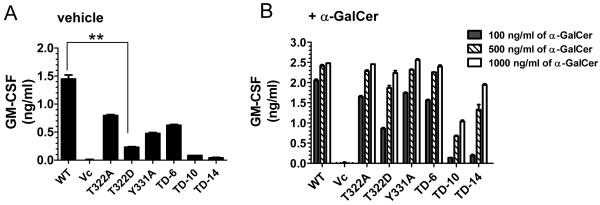
CD1d molecules present lipid antigens to NKT cells for their activation and these antigens are likely acquired in a late endocytic compartment(s) (4). As it was shown above that the CD1d cytoplasmic tail contains two different signals for its intracellular trafficking, it is reasonable to postulate that mutations in those signals would affect antigen presentation to NKT cells. To test this hypothesis, HEK293 cells expressing WT CD1d or those with mutations in their cytoplasmic tail were treated with vehicle or α-GalCer [a synthetic glycolipid that strongly stimulates NKT cells in a CD1d-restricted manner (4)] and co-cultured with human NKT cells generated from normal PBMC. HEK293 cells expressing CD1d WT were able to activate human NKT cells to secrete cytokines that can be blocked by an anti-CD1d antibody (Supplementary Fig. 6), suggesting that the NKT cell activation is CD1d-specific. In the absence of α-GalCer, the T322A mutant activated NKT cells to the same level as WT CD1d (Fig. 5A). In contrast, the mutants lacking the lysosomal-targeting motif YXXZ (i.e., Y331A, TD-6, TD-10 and TD-14) were less effective at activating NKT cells. Antigen presentation by the T322D mutant, which was expressed at a lower surface level than WT was also reduced (Fig. 5A). However, the decrease in NKT cell stimulation was not simply due to less CD1d surface expression or a defect in the antigen presenting capacity of mutant CD1d molecules; the addition of α-GalCer to these cultures resulted in NKT activation by the mutants at comparable levels to that induced by WT CD1d (Fig. 5B), suggesting that the CD1d mutants are indeed functional.
FIGURE 5.
WT CD1d and the cytoplasmic tail mutants differ in their antigen presentation abilities. CD1d WT- and the indicated CD1d tail mutant-expressing cells were co-cultured with PBMC-derived human NKT cells (E:T = 1:1) for 48 h in the presence of vehicle (PBS+0.02% Tween 20; A) or the indicated concentrations of α-GalCer (B). Supernatants were harvested and the production of GM-CSF was measured by ELISA. Each bar is the mean of triplicate samples ± SD. **, p<0.01. The data shown are representative of three independent experiments.
Threonine-based (T322) signals in the CD1d cytoplasmic tail regulate direct trafficking from the TGN to lysosomes
Our data suggest there are two lysosomal targeting signals in the cytoplasmic tail of CD1d: threonine- (T322) and tyrosine-based (YXXZ motif). To determine which signal is dominant, we generated T322A/Y331A and T322D/Y331A double mutants and analyzed their surface expression by flow cytometry. The level of the T322A/Y331A double mutant on the cell surface was comparable to the Y331A single mutant. In contrast, the T322D/Y331A double mutant was expressed at a level below that of the WT (Fig. 6A, Supplementary Fig. 7), but not as low as the T322D single mutant, suggesting that the T322-based signal acts upstream of the YXXZ motif, and is dominant. Most likely, the T322D and TD-14 mutants are directly targeted from the TGN to lysosomes. To further address this hypothesis, WT CD1d and the T322D and TD-14 mutants were treated with monensin overnight to block protein transport in the Golgi. This treatment caused a reduction in intracellular WT CD1d, but raised the levels of both the T322D and TD-14 mutants (Fig. 6B). Therefore, these results strongly suggest that two signals in the cytoplasmic tail of CD1d regulate its trafficking to lysosomes; the T322D mutation or the deletion of the entire cytoplasmic tail of CD1d causes direct targeting of CD1d to lysosomes for degradation.
FIGURE 6.
The T322 signal in the CD1d cytoplasmic tail is dominant. A, HEK293 cells were transfected with WT CD1d or the T322D and Y331A single mutants, or T322A/Y331A and T322D/Y331A double mutants to generate stable transfectants. The cells were stained with an anti-CD1d-β2m mAb for flow cytometry analysis. The data are shown as the percent of the MFI relative to WT. Each bar represents the mean of the MFI ± SD. B, CD1d WT-, T322D- and TD-14-expressing HEK293 cells were treated with or without monensin (GolgiStop, 1:1500) overnight. The cells were then fixed, permeablized and stained with anti-CD1d HC (white bars), anti-CD1d-β2m (black bars), or anti-MHC I (gray bars) mAb for flow cytometry analysis. The increase in MFI after monensin treatment was calculated using this formula: increase of MFI = 100% * (MFImon − MFIveh)/ MFIveh. MFImon : MFI from monensin treated cells; MFIveh: MFI from vehicle treated cells. The data shown in the graph represent the mean increase of MFI ± SD of two independent experiments combined. *, p<0.05.
Discussion
Of the CD1 isoforms that are antigen-presenting molecules, only CD1a lacks the adaptor protein-binding motif YXXZ in its cytoplasmic tail (5). As a likely consequence, CD1a is mostly expressed on the cell surface (48). In contrast, the other CD1 family members CD1b, CD1c and CD1d all possess the YXXZ motif, resulting in their localization both on the surface and in early and late endocytic compartments (37). It has been suggested that the cytoplasmic tails of these CD1 molecules contain signals for their intracellular distribution and endosomal trafficking [reviewed in (5)]. The data presented in the current study suggest that the cytoplasmic tail is also important for the stability of the CD1d-β2m heterodimer. We speculate that the cytoplasmic tail of CD1d contains signals for directing the molecule to other compartments. When these signals are missing or altered, the molecule traffics to lysosomes for its degradation (by a default pathway). Thus, our data show that the T322D mutant causes a substantial decrease in the cell surface level of CD1d. Rather than an alteration of the Thr-based signal, it is possible that the T322D mutation simply caused conformational changes in the extracellular domain of CD1d which reduced its ability to be detected by the CD1d-β2m complex-specific mAb. However, were that to be the case, it is unlikely that we would have been able to detect an increase in the T322D and TD-14 mutants in lysosomes in chloroquine- and monensin-treated cells.
Based on our results, we propose a model to explain how the two signals in the cytoplasmic tail influence the intracellular distribution and the trafficking of the CD1d-β2m heterodimer and its surface expression (Fig. 7). After its synthesis in the ER, CD1d molecules quickly associate with β2m and are transported to the TGN. There, they are further glycosylated and transported to the plasma membrane with the help of the T322 residue in the cytoplasmic tail. The T322D mutant [equivalent to its phosphorylation (49)] or deletion of the entire cytoplasmic tail, would result in the loss of signals that target the CD1d-β2m complex to the plasma membrane and thereby diverts this complex to lysosomes. Here, β2m dissociates from the CD1d HC and the molecule is degraded. From the cell surface, CD1d is endocytosed via clathrin-dependent vesicles due to its YXXZ motif. In late endosomes and/or lysosomes, CD1d meets and binds the lipid antigens that are required for NKT cell activation (4). CD1d is then returned to the cell surface for recognition by NKT cells. When the Y331 residue is replaced with an Ala or the YXXZ motif is deleted (e.g. TD-6), the CD1d-β2m complex remains on the cell surface, rather than traversing through endocytic compartments. As a result, this molecule is expressed at a higher level than WT and has a reduced ability to activate NKT cells. In TD-10, CD1d shows reduced surface expression and more co-localization with LAMP-1+ compartments, suggesting that the KRQT sequence present in TD-6 (but not in TD-10; Supplementary Fig. 1) may also play a role in efficient plasma membrane trafficking. In fact, the K326R mutant of CD1d is expressed on the surface at reduced levels (Supplementary Fig. 2A). It is well-known that lysines are targets for different types of ubiquitylation, and each type can have distinct effects on such proteins, including directing their degradation by proteasomes (50), and monoubiquination has also been shown to be an endocytic trafficking signal (29). Of immunological importance, the CD1d K326 residue also seems to be an important target for immune evasion by KSHV (31).
FIGURE 7.
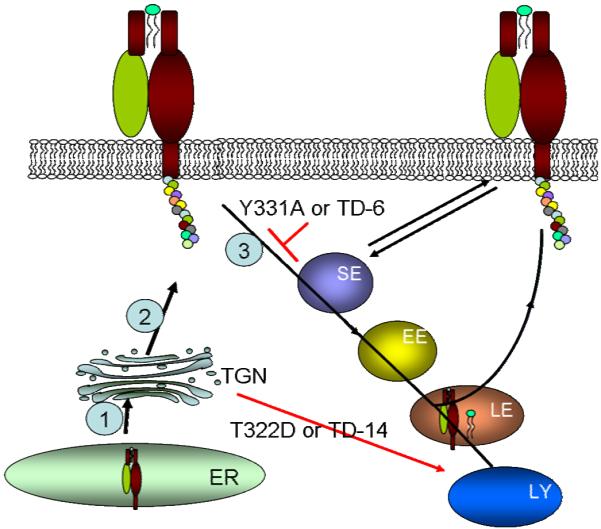
Model illustrating how Thr- and Tyr-based signals in the cytoplasmic tail of CD1d control its functional expression. The black lines and arrows show the typical pathway for newly-synthesized CD1d, whereas the red lines and arrow show the effect of specific mutations of these signals on CD1d intracellular trafficking. Details are provided in the Discussion. 1: CD1d is synthesized in the ER. 2: After association with β2m, CD1d is transported to the TGN, where it undergoes glycosylation modification. 3: CD1d is endocytosed from the cell surface through clathrin-dependent vesicles. SE: sorting endosome; EE: early endosome; LE: late endosome; ER: endoplasmic reticulum; TGN: trans-Golgi network; TD-14: CD1d mutant in which the entire 14 residue cytoplasmic tail was deleted; TD-6: CD1d mutant in which the C-terminal six residues in the cytoplasmic tail were deleted.
We and other investigators have clearly shown that the YXXZ motif in mouse CD1d1 is required for mouse CD1d-mediated antigen presentation to invariant NKT cells (15, 51). In this report, our data also suggest that the YXXZ motif is required for human CD1d-mediated antigen presentation. However, this conclusion appears to be in conflict with some prior publications suggesting that this motif is less important with human CD1d (32, 52). The difference in our study could be because we used endothelial cells (HEK293) rather than B lymphoblastoid cells such as C1R cells (32) or cells derived from peripheral blood lymphocytes (52). Of further relevance to our study, Colgan et al found that in epithelial cells, the YXXZ motif is required for CD1d autocrine signaling (53). We also observed that CD1d molecules lacking this motif activate NKT cells the same as WT does in C1R cells (data not shown), suggesting that distinct antigen presenting cells differentially activate NKT cells. This could be due to cell (or tissue)-specific lipid antigens presented by CD1d. This is not unprecedented (54). Nevertheless, we can most certainly draw the conclusion that the YXXZ motif is important for human CD1d-mediated antigen presentation in epithelial cells.
Two forms of CD1d have been shown to exist in both primary human intestinal epithelium and a number of cell lines (12, 13, 53, 55). They are either associated with β2m or exist as free heavy chains. Usually, the β2m-associated form is glycosylated, whereas the β2m-independent form can be glycosylated in some cell lines such as C1R, and some epithelial cell lines such as FO-1 (12, 13), but appears to be non-glycosylated in most epithelial cell lines, including HEC, HeLa, HT-29 and T84 cells (39, 53, 55, 56). The relationship between these two forms of CD1d has remained unclear, although it is known that the group 1 CD1b molecule is trapped in the ER when β2m is absent (3). Our confocal microscopy data indicate that the CD1d HC is also retained in the ER in HEK293 cells. Thus, consistent with a prior report (57), the results of the current study suggest that the CD1d HC accumulates in the ER possibly due to a shortage of β2m, and association of the HC with β2m likely serves as a potential “release” signal to permit CD1d to begin its exit from the ER. Small amounts of free CD1d HC may be targeted to the plasma membrane as has been suggested (12, 13, 57). Another remaining question is whether the β2m-independent form of CD1d is functional in NKT cell activation. One study reported that spleen cells from β2m −/− mice are able to activate CD1d-specific T cells, suggesting that the free CD1d HC alone can activate NKT cells (58). However, in our study, cells expressing the T322D and TD-14 human CD1d mutants, which appear to be mostly free HC, were considerably less able to activate NKT cells, even when α-GalCer was added exogenously. Therefore, our data suggest that only the CD1d-β2m heterodimer is functional in antigen presentation to NKT cells in the human system.
The current study suggests there are also signals in the cytoplasmic tail of CD1d that prevent lysosomal targeting. Such signals are likely used for trafficking to endosomes or recycling back to the surface. It has been shown that a “lysosomal avoiding” motif in the cytoplasmic tail of P-selectin acts at a later stage of trafficking through the endocytic pathway [e.g., from late endosomes to lysosomes (59)]. The mannose-6-phosphate receptor possesses a pair of aromatic acids in its cytoplasmic tail that prevents it from being targeted to lysosomes for degradation (60). For CD1d, the “lysosomal avoiding” signals are likely located in the first eight N-terminal amino acids in the cytoplasmic tail (TSRFKRQT), with the T322 residue being most critical. Evidence consistent with this idea includes: 1. the TD-6 mutant expresses a higher level of CD1d, TD-10 expresses a lower level of CD1d on the surface than WT, and TD-14 expresses the lowest level of CD1d; 2. T322D is expressed at a nearly comparably low level of expression on the surface as TD-14.
Although we have shown that substituting an Asp for a Thr [a regularly used method to mimic phosphorylation of Thr (49)] at position 322 causes lysosomal targeting of CD1d, one important question is whether T322 is phosphorylated under normal conditions. Due to the fact that human primary APCs express a very low level of endogenous CD1d (61), it would be extremely difficult technically to detect CD1d phosphorylation on the T322 residue in fresh CD1d+ cells. Despite the fact that we obviated the low expression of CD1d in fresh CD1d+ APCs by using transfected cell lines, because we are proposing that the phosphorylation of T322 would cause degradation of CD1d based on results with the T322D mutant, we do not expect phosphorylation of T322 to be the norm. In fact, we have been unable to detect phosphorylation of Thr residues in CD1d molecules, suggesting that phosphorylated T322 may be below the level of detection by Western blot analyses under the conditions we used (data not shown). However, we have found that an HSV infection causes less degradation of the T322A/S323A double mutant as compared to CD1d WT (data not shown). This suggests that the decrease in CD1d surface expression observed following an HSV-1 infection (56; J.L. and R.R.B, unpublished observation) may be due to phosphorylation of the T322 residue and consequent faster degradation of CD1d.
In summary, our results show that a Thr-based signal in the cytoplasmic tail controls the functional expression of CD1d. It is highly likely that phosphorylation of this residue (or complete loss of the cytoplasmic domain) results in a default program to direct the CD1d molecule to lysosomes for degradation. Such regulation of the intracellular trafficking of a glycoprotein which is thus normally in a non-phosphorylated state has many applications to a number of other molecules that also traverse through the endocytic pathway, with critical relevance to the function of that cell.
Supplementary Material
Acknowledgements
We would like to thank the Indiana Center for Biological Microscopy for important help in these studies, as well as Ntsane Moleleki and Mark Peggie for generating the CD25/CD1d fusion constructs. We also would like to thank the Flow Cytometry Resource Facility, Indiana University School of Medicine for their assistance. Philip Cohen and Mark Exley generously provided the HEK293 and, 51.1 and 42.1 mAbs, respectively. We also would like to thank Renukaradhya Gourapura for helpful discussion and Claire Willard for expert technical assistance.
Abbreviations used in this paper
- AP
adaptor protein
- β2m
β2–microglobulin
- HC
heavy chain
- NKT
natural killer T
- LAMP-1
lysosome-associated membrane protein 1
- α-GalCer
α-Galactosylceramide
- MFI
mean fluorescence intensity
- YXXZ
Tyrosine-based motif, X stands for any amino acid and Z for bulky hydrophobic residue
Footnotes
This work was supported by National Institutes of Health grants RO1 AI46455 and PO1 AI056097 (to R.R.B.), and NSF CHE-0194682 from the National Science Foundation (to J.G.H.).
Disclosures
There are no financial conflicts of interest by any of the authors.
References
- 1.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 2.Huttinger R, Staffler G, Majdic O, Stockinger H. Analysis of the early biogenesis of CD1b: involvement of the chaperones calnexin and calreticulin, the proteasome and β2-microglobulin. Int Immunol. 1999;11:1615–1623. doi: 10.1093/intimm/11.10.1615. [DOI] [PubMed] [Google Scholar]
- 3.Sugita M, Porcelli SA, Brenner MB. Assembly and retention of CD1b heavy chains in the endoplasmic reticulum. J Immunol. 1997;159:2358–2365. [PubMed] [Google Scholar]
- 4.Brutkiewicz RR. CD1d ligands: the good, the bad, and the ugly. J Immunol. 2006;177:769–775. doi: 10.4049/jimmunol.177.2.769. [DOI] [PubMed] [Google Scholar]
- 5.Moody DB, Porcelli SA. Intracellular pathways of CD1 antigen presentation. Nat Rev Immunol. 2003;3:11–22. doi: 10.1038/nri979. [DOI] [PubMed] [Google Scholar]
- 6.Renukaradhya GJ, Khan MA, Vieira M, Du W, Gervay-Hague J, Brutkiewicz RR. Type I NKT cells protect (and type II NKT cells suppress) the host’s innate antitumor immune response to a B-cell lymphoma. Blood. 2008;111:5637–5645. doi: 10.1182/blood-2007-05-092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berzofsky JA, Terabe M. NKT cells in tumor immunity: opposing subsets define a new immunoregulatory axis. J Immunol. 2008;180:3627–3635. doi: 10.4049/jimmunol.180.6.3627. [DOI] [PubMed] [Google Scholar]
- 8.Arrenberg P, Halder R, Kumar V. Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens. J Cell Physiol. 2009;218:246–250. doi: 10.1002/jcp.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon DA, Jamil H, Sharp D, Mullaney D, Yao Z, Gregg RE, Wetterau J. Secretion of apolipoprotein B-containing lipoproteins from HeLa cells is dependent on expression of the microsomal triglyceride transfer protein and is regulated by lipid availability. Proc Natl Acad Sci U S A. 1994;91:7628–7632. doi: 10.1073/pnas.91.16.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brozovic S, Nagaishi T, Yoshida M, Betz S, Salas A, Chen D, Kaser A, Glickman J, Kuo T, Little A, Morrison J, Corazza N, Kim JY, Colgan SP, Young SG, Exley M, Blumberg RS. CD1d function is regulated by microsomal triglyceride transfer protein. Nat Med. 2004;10:535–539. doi: 10.1038/nm1043. [DOI] [PubMed] [Google Scholar]
- 11.Dougan SK, Salas A, Rava P, Agyemang A, Kaser A, Morrison J, Khurana A, Kronenberg M, Johnson C, Exley M, Hussain MM, Blumberg RS. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J Exp Med. 2005;202:529–539. doi: 10.1084/jem.20050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HS, Garcia J, Exley M, Johnson KW, Balk SP, Blumberg RS. Biochemical characterization of CD1d expression in the absence of beta2-microglobulin. J Biol Chem. 1999;274:9289–9295. doi: 10.1074/jbc.274.14.9289. [DOI] [PubMed] [Google Scholar]
- 13.Kawana K, Quayle AJ, Ficarra M, Ibana JA, Shen L, Kawana Y, Yang H, Marrero L, Yavagal S, Greene SJ, Zhang YX, Pyles RB, Blumberg RS, Schust DJ. CD1d degradation in Chlamydia trachomatis-infected epithelial cells is the result of both cellular and chlamydial proteasomal activity. J Biol Chem. 2007;282:7368–7375. doi: 10.1074/jbc.M610754200. [DOI] [PubMed] [Google Scholar]
- 14.Brutkiewicz RR, Lin Y, Cho S, Hwang YK, Sriram V, Roberts TJ. CD1d-mediated antigen presentation to natural killer T (NKT) cells. Crit Rev Immunol. 2003;23:403–419. doi: 10.1615/critrevimmunol.v23.i56.30. [DOI] [PubMed] [Google Scholar]
- 15.Roberts TJ, Sriram V, Spence PM, Gui M, Hayakawa K, Bacik I, Bennink JR, Yewdell JW, Brutkiewicz RR. Recycling CD1d1 molecules present endogenous antigens processed in an endocytic compartment to NKT cells. J Immunol. 2002;168:5409–5414. doi: 10.4049/jimmunol.168.11.5409. [DOI] [PubMed] [Google Scholar]
- 16.Jayawardena-Wolf J, Benlagha K, Chiu YH, Mehr R, Bendelac A. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity. 2001;15:897–908. doi: 10.1016/s1074-7613(01)00240-0. [DOI] [PubMed] [Google Scholar]
- 17.Zhou D, Cantu C, 3rd, Sagiv Y, Schrantz N, Kulkarni AB, Qi X, Mahuran DJ, Morales CR, Grabowski GA, Benlagha K, Savage P, Bendelac A, Teyton L. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 2004;303:523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang SJ, Cresswell P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat Immunol. 2004;5:175–181. doi: 10.1038/ni1034. [DOI] [PubMed] [Google Scholar]
- 19.Yuan W, Qi X, Tsang P, Kang SJ, Illarionov PA, Besra GS, Gumperz J, Cresswell P. Saposin B is the dominant saposin that facilitates lipid binding to human CD1d molecules. Proc Natl Acad Sci U S A. 2007;104:5551–5556. doi: 10.1073/pnas.0700617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winau F, Schwierzeck V, Hurwitz R, Remmel N, Sieling PA, Modlin RL, Porcelli SA, Brinkmann V, Sugita M, Sandhoff K, Kaufmann SH, Schaible UE. Saposin C is required for lipid presentation by human CD1b. Nat Immunol. 2004;5:169–174. doi: 10.1038/ni1035. [DOI] [PubMed] [Google Scholar]
- 21.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 22.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 23.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 24.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 25.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 26.Rodionov DG, Nordeng TW, Pedersen K, Balk SP, Bakke O. A critical tyrosine residue in the cytoplasmic tail is important for CD1d internalization but not for its basolateral sorting in MDCK cells. J Immunol. 1999;162:1488–1495. [PubMed] [Google Scholar]
- 27.Cho S, Knox KS, Kohli LM, He JJ, Exley MA, Wilson SB, Brutkiewicz RR. Impaired cell surface expression of human CD1d by the formation of an HIV-1 Nef/CD1d complex. Virology. 2005;337:242–252. doi: 10.1016/j.virol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Chen N, McCarthy C, Drakesmith H, Li D, Cerundolo V, McMichael AJ, Screaton GR, Xu XN. HIV-1 down-regulates the expression of CD1d via Nef. Eur J Immunol. 2006;36:278–286. doi: 10.1002/eji.200535487. [DOI] [PubMed] [Google Scholar]
- 29.Shin JS, Ebersold M, Pypaert M, Delamarre L, Hartley A, Mellman I. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature. 2006;444:115–118. doi: 10.1038/nature05261. [DOI] [PubMed] [Google Scholar]
- 30.Acconcia F, Sigismund S, Polo S. Ubiquitin in trafficking: The network at work. Exp Cell Res. 2009;315:1610–1618. doi: 10.1016/j.yexcr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez DJ, Gumperz JE, Ganem D. Regulation of CD1d expression and function by a herpesvirus infection. J Clin Invest. 2005;115:1369–1378. doi: 10.1172/JCI24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Vα24+ CD4−CD8− T cells. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin LH, Calabi F, Milstein C. Isolation of CD1 genes: a family of major histocompatibility complex-related differentiation antigens. Proc Natl Acad Sci U S A. 1986;83:9154–9158. doi: 10.1073/pnas.83.23.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balk SP, Bleicher PA, Terhorst C. Isolation and characterization of a cDNA and gene coding for a fourth CD1 molecule. Proc Natl Acad Sci U S A. 1989;86:252–256. doi: 10.1073/pnas.86.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du W, Gervay-Hague J. Efficient synthesis of alpha-galactosyl ceramide analogues using glycosyl iodide donors. Org Lett. 2005;7:2063–2065. doi: 10.1021/ol050659f. [DOI] [PubMed] [Google Scholar]
- 36.Exley MA, Bigley NJ, Cheng O, Shaulov A, Tahir SM, Carter QL, Garcia J, Wang C, Patten K, Stills HF, Alt FW, Snapper SB, Balk SP. Innate immune response to encephalomyocarditis virus infection mediated by CD1d. Immunology. 2003;110:519–526. doi: 10.1111/j.1365-2567.2003.01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugita M, Cao X, Watts GF, Rogers RA, Bonifacino JS, Brenner MB. Failure of trafficking and antigen presentation by CD1 in AP-3-deficient cells. Immunity. 2002;16:697–706. doi: 10.1016/s1074-7613(02)00311-4. [DOI] [PubMed] [Google Scholar]
- 38.Paduraru C, Spiridon L, Yuan W, Bricard G, Valencia X, Porcelli SA, Illarionov PA, Besra GS, Petrescu SM, Petrescu AJ, Cresswell P. An N-linked glycan modulates the interaction between the CD1d heavy chain and β2-microglobulin. J Biol Chem. 2006;281:40369–40378. doi: 10.1074/jbc.M608518200. [DOI] [PubMed] [Google Scholar]
- 39.Balk SP, Burke S, Polischuk JE, Frantz ME, Yang L, Porcelli S, Colgan SP, Blumberg RS. β2-microglobulin-independent MHC class Ib molecule expressed by human intestinal epithelium. Science. 1994;265:259–262. doi: 10.1126/science.7517575. [DOI] [PubMed] [Google Scholar]
- 40.Somnay-Wadgaonkar K, Nusrat A, Kim HS, Canchis WP, Balk SP, Colgan SP, Blumberg RS. Immunolocalization of CD1d in human intestinal epithelial cells and identification of a β2-microglobulin-associated form. Int Immunol. 1999;11:383–392. doi: 10.1093/intimm/11.3.383. [DOI] [PubMed] [Google Scholar]
- 41.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 42.Jones BG, Thomas L, Molloy SS, Thulin CD, Fry MD, Walsh KA, Thomas G. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCulloch CV, Morrow V, Milasta S, Comerford I, Milligan G, Graham GJ, Isaacs NW, Nibbs RJ. Multiple roles for the C-terminal tail of the chemokine scavenger D6. J Biol Chem. 2008;283:7972–7982. doi: 10.1074/jbc.M710128200. [DOI] [PubMed] [Google Scholar]
- 44.Lee SY, Wenk MR, Kim Y, Nairn AC, De Camilli P. Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc Natl Acad Sci U S A. 2004;101:546–551. doi: 10.1073/pnas.0307813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jayawardena-Wolf J, Benlagha K, Chiu YH, Mehr R, Bendelac A. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity. 2001;15:897–908. doi: 10.1016/s1074-7613(01)00240-0. [DOI] [PubMed] [Google Scholar]
- 46.Lawton AP, Prigozy TI, Brossay L, Pei B, Khurana A, Martin D, Zhu T, Spate K, Ozga M, Honing S, Bakke O, Kronenberg M. The mouse CD1d cytoplasmic tail mediates CD1d trafficking and antigen presentation by adaptor protein 3-dependent and -independent mechanisms. J Immunol. 2005;174:3179–3186. doi: 10.4049/jimmunol.174.6.3179. [DOI] [PubMed] [Google Scholar]
- 47.Martin LH, Calabi F, Lefebvre FA, Bilsland CA, Milstein C. Structure and expression of the human thymocyte antigens CD1a, CD1b, and CD1c. Proc Natl Acad Sci U S A. 1987;84:9189–9193. doi: 10.1073/pnas.84.24.9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugita M, Grant EP, van Donselaar E, Hsu VW, Rogers RA, Peters PJ, Brenner MB. Separate pathways for antigen presentation by CD1 molecules. Immunity. 1999;11:743–752. doi: 10.1016/s1074-7613(00)80148-x. [DOI] [PubMed] [Google Scholar]
- 49.Sakaguchi M, Miyazaki M, Takaishi M, Sakaguchi Y, Makino E, Kataoka N, Yamada H, Namba M, Huh NH. S100C/A11 is a key mediator of Ca2+-induced growth inhibition of human epidermal keratinocytes. J Cell Biol. 2003;163:825–835. doi: 10.1083/jcb.200304017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilkinson KD. Ubiquitination and deubiquitination: targeting of proteins for degradation by the proteasome. Semin Cell Dev Biol. 2000;11:141–148. doi: 10.1006/scdb.2000.0164. [DOI] [PubMed] [Google Scholar]
- 51.Chiu YH, Park SH, Benlagha K, Forestier C, Jayawardena-Wolf J, Savage PB, Teyton L, Bendelac A. Multiple defects in antigen presentation and T cell development by mice expressing cytoplasmic tail-truncated CD1d. Nat Immunol. 2002;3:55–60. doi: 10.1038/ni740. [DOI] [PubMed] [Google Scholar]
- 52.Chen X, Wang X, Keaton JM, Reddington F, Illarionov PA, Besra GS, Gumperz JE. Distinct endosomal trafficking requirements for presentation of autoantigens and exogenous lipids by human CD1d molecules. J Immunol. 2007;178:6181–6190. doi: 10.4049/jimmunol.178.10.6181. [DOI] [PubMed] [Google Scholar]
- 53.Colgan SP, Hershberg RM, Furuta GT, Blumberg RS. Ligation of intestinal epithelial CD1d induces bioactive IL-10: critical role of the cytoplasmic tail in autocrine signaling. Proc Natl Acad Sci U S A. 1999;96:13938–13943. doi: 10.1073/pnas.96.24.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park SH, Roark JH, Bendelac A. Tissue-specific recognition of mouse CD1 molecules. J Immunol. 1998;160:3128–3134. [PubMed] [Google Scholar]
- 55.Somnay-Wadgaonkar K, Nusrat A, Kim HS, Canchis WP, Balk SP, Colgan SP, Blumberg RS. Immunolocalization of CD1d in human intestinal epithelial cells and identification of a β2-microglobulin-associated form. Int Immunol. 1999;11:383–392. doi: 10.1093/intimm/11.3.383. [DOI] [PubMed] [Google Scholar]
- 56.Yuan W, Dasgupta A, Cresswell P. Herpes simplex virus evades natural killer T cell recognition by suppressing CD1d recycling. Nat Immunol. 2006;7:835–842. doi: 10.1038/ni1364. [DOI] [PubMed] [Google Scholar]
- 57.Kang SJ, Cresswell P. Calnexin, calreticulin, and ERp57 cooperate in disulfide bond formation in human CD1d heavy chain. J Biol Chem. 2002;277:44838–44844. doi: 10.1074/jbc.M207831200. [DOI] [PubMed] [Google Scholar]
- 58.Amano M, Baumgarth N, Dick MD, Brossay L, Kronenberg M, Herzenberg LA, Strober S. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: β2-microglobulin-dependent and -independent forms. J. Immunol. 1998;161:1710–1717. [PubMed] [Google Scholar]
- 59.Blagoveshchenskaya AD, Hewitt EW, Cutler DF. A balance of opposing signals within the cytoplasmic tail controls the lysosomal targeting of P-selectin. J Biol Chem. 1998;273:27896–27903. doi: 10.1074/jbc.273.43.27896. [DOI] [PubMed] [Google Scholar]
- 60.Schweizer A, Kornfeld S, Rohrer J. Proper sorting of the cation-dependent mannose 6-phosphate receptor in endosomes depends on a pair of aromatic amino acids in its cytoplasmic tail. Proc Natl Acad Sci U S A. 1997;94:14471–14476. doi: 10.1073/pnas.94.26.14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spada FM, Borriello F, Sugita M, Watts GF, Koezuka Y, Porcelli SA. Low expression level but potent antigen presenting function of CD1d on monocyte lineage cells. Eur J Immunol. 2000;30:3468–3477. doi: 10.1002/1521-4141(2000012)30:12<3468::AID-IMMU3468>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.