SUMMARY
(Pro)renin receptor (PRR) binding to renin or prorenin mediates Ang II dependent and independent effects. PRR expression was increased in the kidneys of diabetic rats but its role in diabetic nephropathy is unknown. We investigated the contribution of PRR to the development of diabetic nephropathy through enhancement of renal production of tumor necrosis factor-α (TNF-α) and interleukine-1β (IL-1β).
Normoglicemic control and streptozotocin-induced diabetes Sprague-Dawley rats were studied. We evaluated urine albumin-to-creatinine ratio (UACR), renal interstitial fluid (RIF) levels of Ang II, TNF-α, and IL-1β, and the renal expression of TNF- α and IL-1β in control, non-treated diabetes, and diabetes treated with PRR blocker (PRRB), AT1 receptor blocker valsartan, or combined therapy, administered directly to the renal cortical interstitium for 14 days via osmotic minipump.
Compared to normoglycemic control, UACR and RIF Ang II, TNF-α, and IL-1β were significantly higher in diabetic rats. PRRB or valsartan individually and combined significantly reduced UACR, RIF TNF-α and IL-1β levels. Renal expressions of TNF-α and IL-1β were higher in non-treated diabetic rats and significantly reduced by PRRB or valsartan individually and combined. Renal PRR expression was increased in non-treated and PRRB treated diabetic rats, and reduced in rats receiving valsartan alone or combined therapy. RIF Ang II was not influenced by PRRB, while valsartan alone and combined with PRRB significantly increased its levels.
We conclude that PRR is involved in the development and progression of kidney disease in diabetes by enhancing renal production of inflammatory cytokines TNF-α and IL-1β, independently of renal Ang II effects.
Keywords: cytokines, diabetic nephropathy, prorenin receptor, renin-angiotensin system
INTRODUCTION
Diabetic nephropathy is the leading cause for the development of end-stage renal disease (1). Previous studies suggested that the renin-angiotensin system (RAS) contributes to the development and progression of kidney disease in diabetes. The efficacy of the RAS blockade was demonstrated in large multicenter randomized trials (2–3) involving patients at various stages of diabetic nephropathy with slowing of the progression of renal injury. However, although the RAS inhibitors are widely used, the prevalence of diabetic nephropathy continues to be high (1). In diabetes, the RAS was shown to activate renal inflammation (4). The enhanced production of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 were demonstrated to play important role in the development and progression of diabetic nephropathy (5).
The discovery of the (pro)renin receptor (PRR) as a new member of the RAS (6) brought new concepts to possible mechanisms contributing to organ damage in diabetes and hypertension. PRR consists of 350 amino acids protein with a single transmenbrane domain that binds both prorenin and renin. This receptor has high affinity for prorenin allowing the renin precursor to become catalytically active without proteolytic cleavage of its prosegment (7). The binding of PRR by renin or prorenin can determine the ultimate generation of angiotensin II (Ang II) (7–8). In addition, independent of Ang II production, PRR was shown to activate intracellular protein phosphorylation and matrix formation (9–10). Recently, a putative peptidic PRR blocker (PRRB) knows as ‘handle region peptide’ (HRP) was developed (11–12). This drug contains the amino acids sequence that mimics the handle region segment of PRR and thus binds competitively to this receptor, thereby preventing its activation by prorenin (13). Treatment of diabetic rodents with PRRB led to prevention or reversal of diabetic nephropathy, including decrease in albuminuria (12,14–15). However, the pathophysiological mechanisms related to PRR activation in diabetes remain unclear.
In the present study, we hypothesized that in presence of diabetes, PRR contributes to the development of diabetic nephropathy, independent of Ang II generation, through enhancement of renal production of inflammatory factors TNF-α and IL-1β.
METHODS
Animal preparation
Experiments were approved by the University of Virginia Animal Care and Use Committee and conducted in male Sprague-Dawley rats (Charles River Laboratories; Wilmington, MA) weighing 230 to 260g. Animals were given food and tap water ad libitum and a minimum of one week was allowed to adjust to our animal care facility. Rats were divided randomly into five groups: normoglycemic control group (n = 8), diabetes group (DM; n = 10), DM treated with PRRB group (DM + PRRB; n = 10), DM treated with valsartan group (DM + VAL; n = 10), and DM treated with both PRRB and valsartan group (DM + PRRB + VAL; n = 9). Diabetes was induced by intraperitoneal injection of 65 mg/kg of streptozotocin (STZ; Sigma-Aldrich, Saint Louis, MO). Normoglycemic control rats were injected with an equal volume of vehicle (0.9% NaCl). The decapeptide NH3-RILLKKMPSV-COOH (HRP) was dissolved in saline and an infused at 0.2 mg/kg/2 wks. The angiotensin AT1 receptor blocker valsartan (Novartis, East Hanover, NJ, USA) was used at 2 mg/kg/day, a dose which does not affect blood pressure in rats (16). Both drugs were administered directly to the left renal cortical interstitium for 14 days via osmotic minipump (model 2002; Alzet, Cupertino, CA, USA). Controls and non-treated diabetic rats were implanted with a sham osmotic minipump containing 0.9% NaCl.
For minipump implantation and kidney infusion, one day after STZ or vehicle (0.9% NaCl) injection, rats were anesthetized with ketamine (80 mg/kg; i.p.) and xylazine (8 mg/kg; i.p.) and placed on a heating pad throughout the surgery period. The osmotic minipump was implanted subcutaneously in the subscapular region of all rats. Thereafter, a midline laparotomy was performed and the left kidney was properly isolated. A PE-10 catheter connected to each minipump was tunneled subcutaneously through a bevel-tipped stainless-steel tube to emerge into the abdominal cavity and the distal end of the catheter was placed under the left renal capsule and glued on the surface of the kidney using Vetbond (3M Animal Care Products, St. Paul, MN, USA) (17). The abdominal wall was then sutured and rats were allowed one week to recover.
Body weight, blood glucose, and 24-h urine measurements
Body weight, blood glucose, and 24-h urine collections were obtained before and at the end of the second week post-diabetes induction. Blood glucose from tail vein was also monitored 72-h after STZ administration and at the end of the study using a glucometer (Bayer HealthCare, Mishawaka, IN, USA). For urine collections, rats were placed in individual metabolic cages for a period of 24-h and urine samples were kept at −80°C until assayed. Urinary albumin was determined by ELISA using Nephrat (Exocell, Philadelphia, PA, USA), and urine creatinine by a creatinine assay kit (Cayman Chemical, Ann Arbor, MI, USA). Changes in urinary albumin to creatinine ratio (UACR) were used as a marker for diabetic nephropathy.
In vivo renal interstitial fluid (RIF) collections
To determine the RIF Ang II, TNF-α, and IL-1β we constructed a microdialysis probe as previously described (4,18–20). In this technique, substances with a molecular mass > 40,000 Da cannot cross the dialysis membrane but allowing the free passage of Ang II, TNF-α, and IL-1β. Two weeks after development of diabetes and drugs treatment, acute RIF collections were performed under sodium pentobarbital anesthesia (50 mg/kg i.p.; Sigma-Aldrich, St. Louis, MO, USA). A midline laparotomy was performed and a dialysis probe was placed in the left kidney cortex. In brief, a 30-gauge needle was tunneled approximately 1–2 mm from the outer renal surface for about 0.5 cm before it exited by penetrating the capsule again. The tip of the needle was then inserted into one end of the dialysis probe, and the needle was pulled together with the dialysis tube until the dialysis fiber was situated into the renal cortex. To prevent dislodging, dialysis probe was glued to the surface of the kidney using Vetbond. Thereafter, the inflow tube of the dialysis probe was connected to a gas-tight syringe filled with saline and perfused at a rate of 3 µl/min using an infusion pump. After a 60-min period for stabilization following completion of surgical procedures, the effluent was collected from the outflow tube in nonheparinized plastic tubes over ice over five periods of 60-min each. At the end of each experiment, animals were euthanized and kidneys were harvested and weighed. Kidney mass was calculated as kidney weight in miligrams divided by body weight in grams. Kidney tissue was immediately frozen in liquid nitrogen and stored at −80°C for mRNA analysis.
RIF storage and assays
The RIF collections were stored at −80°C until assayed. Both RIF TNF-α and IL-1β were measured using an enzyme immunoassay kit (R&D Systems, Minneapolis, MN, USA). The RIF Ang II samples were measured using enzyme immunoassay kit (SPI-BIO, France). RIF recovery rate of each substance is presented as picograms per minute.
Quantitative real time reverse transcriptase-polymerase chain reaction (RT-PCR)
The procedures for mRNA measurements were performed as previously described (21). The frozen kidneys were thawed and homogenized on ice. The total renal RNA was extracted using RNeasy Kit (Qiagen, Valencia, CA, USA). The quality of RNA was confirmed by ethidium bromide staining in 1% formaldehyde agarose gel. Single-stranded cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Gene-specific primers were as follows: for β-actin, forward sequence 5'-AGCCATGTACGTAGCCATCC-3' and reverse sequence 5'-ACCCTCATAGATGGGCACAG-3'; for TNF-α, forward sequence 5’-ACTCCCAGAAAAGCAAGCAA-3’ and reverse sequence 5’-CGAGCAGGAATGAGAAGAGG-3’; and for IL-1β, forward sequence 5’-AGGCTTCCTTGTGCAAGTGT-3’ and reverse sequence 5’-TGAGTGACACTGCCTTCCTG-3’. Quantitative RT-PCR was performed using iCycler (Bio-Rad), and threshold cycle number was determined using iCycler software version 3.0 (Bio-Rad). Reactions were performed in triplicate, and threshold cycle numbers were averaged. The mRNA results for specific target genes were normalized to β-actin mRNA.
Western blot analysis
Preparation of kidney tissue lysate and protein quantitation was performed as previously described (21). Antibody to PRR (Abcam, Cambridge, MA, USA) was used in the Western blot. Signal detection was carried out by using Super Signal West Pico Chemiluminescent Subtract (Pierce Biotechnology, Rockford, IL, USA). The blot was treated with Restore Western Blot Stripping Buffer (Pierce Biotechnology) according to the manufacturer's recommendation, followed by reprobing with a monoclonal antibody against β-actin (Sigma, St Louis, MO, USA). Densitometry of the bands was done using Image Master™ TotalLab version 2.0 (Amersham, Piscataway, NJ, USA). The band density of PRR was normalized to the corresponding density of β-actin. The arbitrary unit of band densities was represented as the expression level of PRR.
Statistical analysis
All data are expressed as mean ± SEM. Statistical analysis was performed using SPSS 17.0 (SPSS, Inc). Data were compared among groups using one-way analysis of variance (ANOVA) followed by a Tukey test for post-hoc comparisons. Pearson's correlation coefficient was used to correlate UACR with RIF TNF-α and IL-1β. A value of P < 0.05 was considered to be significant.
RESULTS
Body weight, blood glucose, urine output, and left kidney mass index data of all rats at the end of two weeks post-induction of diabetes and with different treatments are presented in Table 1. At baseline there were no significant differences in body weight, blood glucose, or urine volume between all studied groups. Fasting blood glucose before STZ administration was 98 ± 1 mg/dl and increased to 379 ± 9 mg/dl (P < 0.001) three days after STZ administration. Blood glucose remained elevated during the whole period of study in diabetic animals. Compared to normoglycemic control group, two weeks after induction of diabetes there was a significant decrease in body weight and increase in blood glucose, urine volume, and left kidney mass index in all diabetic animals (P < 0.001). There was no significant changes in these parameters during PRRB or valsartan treatment individually and combined.
Table 1.
Body weight, blood glucose, 24-h urine output, and left kidney mass index at the end of two weeks study in normoglycemic control rats, diabetic non-treated rats (DM), and diabetic rats treated with (pro)renin receptor blocker (DM + PRRB), valsartan (DM + VAL), or PRRB plus valsartan (DM + PRRB + VAL).
| Control (n=8) |
DM (n=10) |
DM+PRRB (n=10) |
DM+VAL (n=10) |
DM+PRRB+VAL (n=9) |
|
|---|---|---|---|---|---|
| Body Weight g | 331±5 | 264±14* | 274±9* | 273±5* | 278±9* |
| Blood Glucose mg/dl | 85±4 | 461±23* | 452±15* | 421±9* | 426±20* |
| Urine Volume ml/d | 23±1 | 198±7* | 199±9* | 198±19* | 207±5* |
|
Left kidney mass index mg/g |
3.4±0.1 | 5.6±0.2* | 5.2±0.2* | 5.3±0.1* | 5.3±0.2* |
Data are mean ± SEM.
P < 0.05 versus control.
Expression of TNF-α, IL-1β, and PRR in the kidney of normoglycemic and diabetic rats
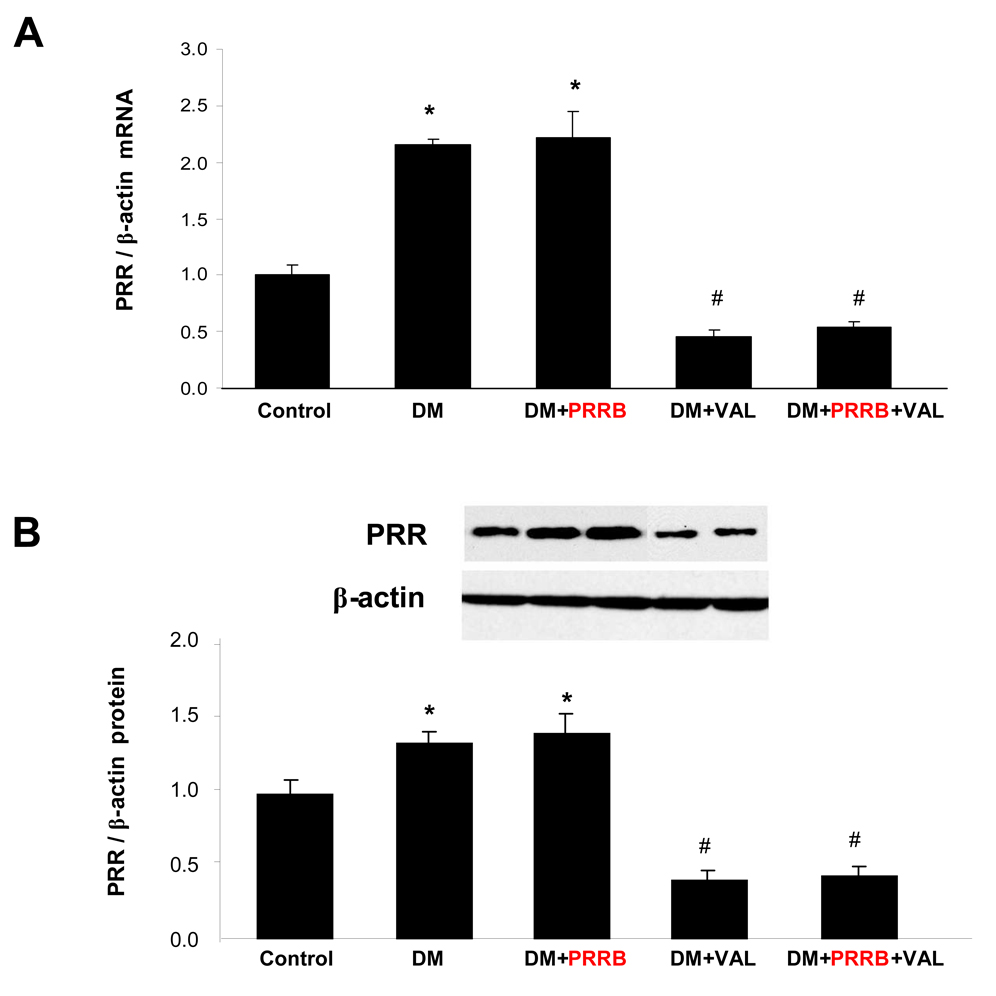
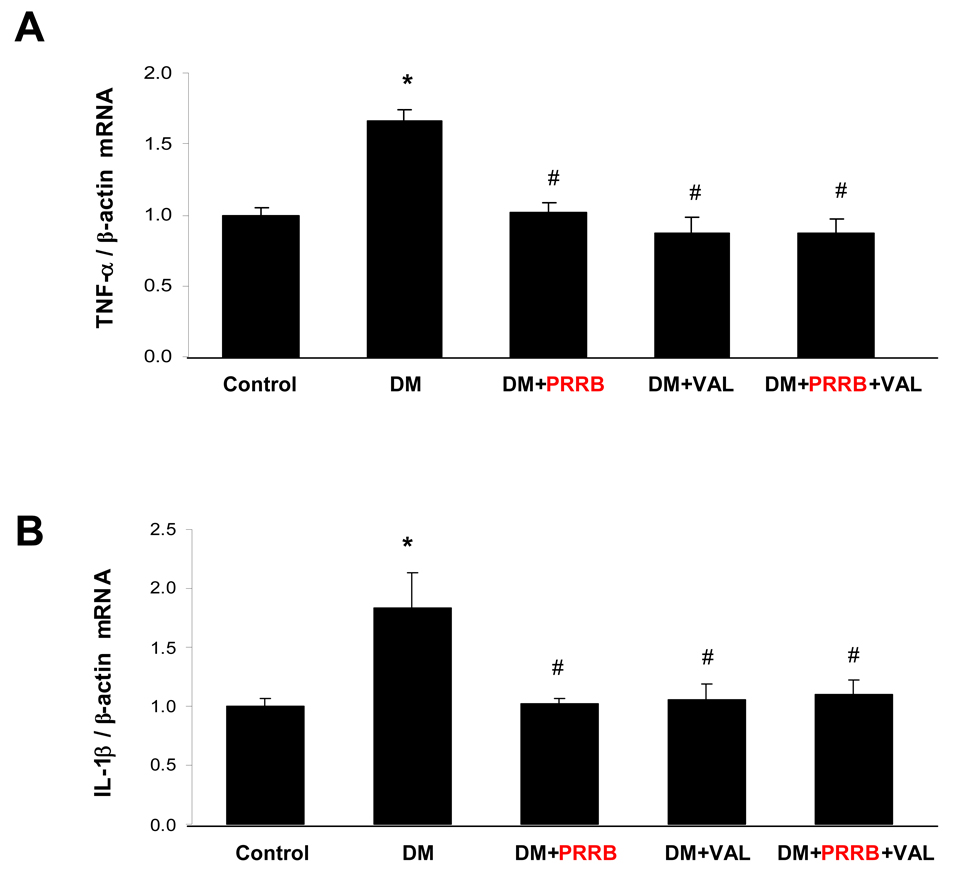
The PRR mRNA (Figure 1A) and protein (Figure 2B) expressions were significantly increased in non-treated and PRRB treated diabetic animals compared to normoglycemic control group (P < 0.01). In contrary, the receptor expression was significantly reduced in diabetic animals treated with valsartan alone or combined PRRB and valsartan compared to non-treated diabetes (P < 0.01). There were no significant differences in PRR mRNA and protein expressions between non-treated and PRRB treated diabetic animals or valsartan individually and combined. TNF-α (Figure 2A) and IL-1β mRNA (Figure 2B) expressions were significantly higher in non-treated DM group compared to normoglycemic control group (P < 0.01). Treatment with PRRB or valsartan individually or combined significantly reduced mRNA expression of both TNF-α and IL-1β compared to diabetes group (P < 0.01). There were no significant differences between TNF-α and IL-1β mRNA in diabetic groups treated with PRRB or valsartan individually and combined.
Figure 1.
Renal expressions of (pro)renin receptor (PRR) mRNA (A) and PRR protein (B) in normoglycemic control rats (n = 5), and streptozotocin (STZ)-induced diabetic rats (DM; n = 5) treated with PRR blocker (DM + PRRB; n = 5), valsartan (DM + VAL; n = 5), or PRRB plus valsartan (DM + PRRB +VAL; n = 5) after two weeks of STZ-induction of diabetes and drug treatment. Data are mean ± SEM. *P < 0.05 vs. control; #P < 0.05 vs. DM.
Figure 2.
Renal expressions of TNF-α mRNA (A) and IL-1β mRNA (B) in normoglycemic control rats (n = 5), and streptozotocin (STZ)-induced diabetic rats (DM; n = 5) treated with PRR blocker (DM + PRRB; n = 5), valsartan (DM + VAL; n = 5), or PRRB plus valsartan (DM + PRRB +VAL; n = 5) after two weeks of STZ-induction of diabetes and drug treatment. Data are mean ± SEM. *P < 0.05 vs. control; #P < 0.05 vs. DM.
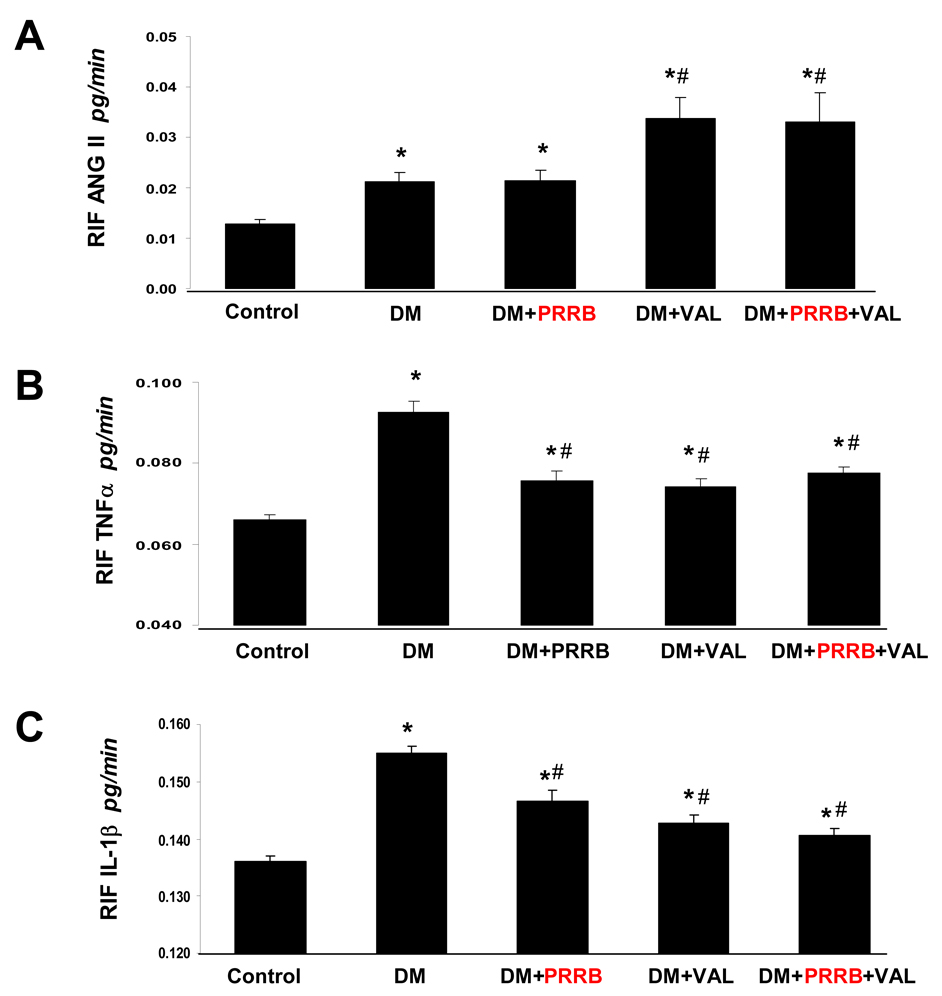
RIF Ang II in normoglycemic and diabetic rats
RIF Ang II recovery rates are showed in Figure 3A. Two weeks after STZ-induction of diabetes, RIF Ang II was significantly higher in all diabetic groups compared to the normoglycemic control group (P < 0.05). RIF Ang II did not change in response to PRRB treatment. In contrast, valsartan treatment caused 57% further increase in RIF Ang II compared to diabetes group or diabetes treated with PRRB group. RIF Ang II in the diabetes group treated with combined PRRB and valsartan was also significantly increased compared to the non-treated diabetes group or diabetes group treated with PRRB (P < 0.01), but not different from the DM group treated with valsartan alone.
Figure 3.
Renal interstitial fluid recovery rates of Ang II (A), TNF-α (B), and, IL-1β (C) in normoglycemic control rats (n = 8), and streptozotocin (STZ)-induced diabetic rats (DM; n = 10) treated with PRR blocker (DM + PRRB; n = 10), valsartan (DM + VAL; n = 10), or PRRB plus valsartan (DM + PRRB + VAL; n = 9) after two weeks of STZ-induction of diabetes and drug treatment. Data are mean ± SEM. *P < 0.05 vs. control; #P < 0.05 vs. DM.
RIF TNFα and IL-1β in normoglycemic and diabetic rats
Compared to normoglycemic control group, there were significant increases in RIF TNF-α (Figure 3B) and RIF IL-1β (Figure 3C) recovery rates in all diabetes groups (P < 0.04). Both PRRB and valsartan treatment attenuated the increases in RIF TNF-α and RIF IL-1β that were observed in the non-treated diabetes group (P < 0.02). There were no significant difference in RIF TNF-α and RIF IL-1β between rats treated with PRRB and valsartan. Compared to non-treated diabetes group, combined PRRB and valsartan treatment significantly reduced RIF TNF-α and RIF IL-1β (P < 0.05) but it was not different from the levels observed with individual PRRB or valsartan treatment.
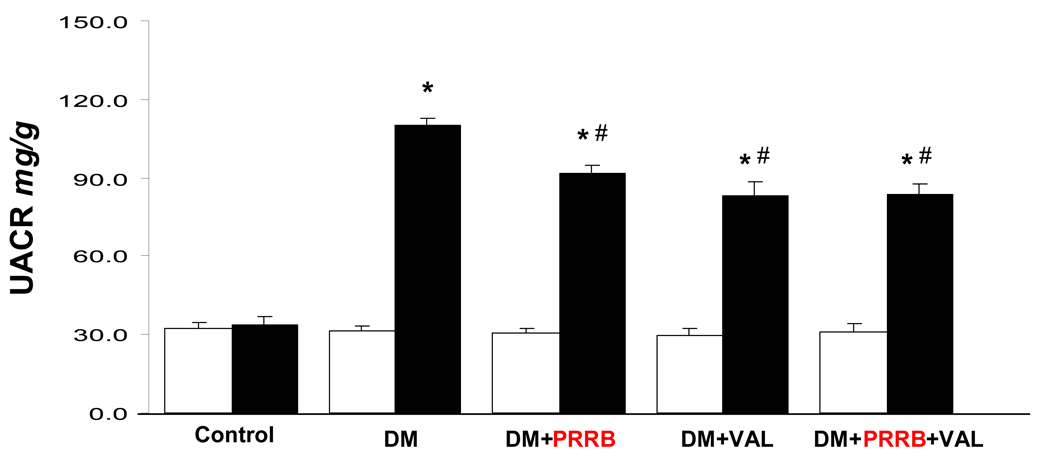
UACR in normoglycemic and diabetic rats
Two weeks after development of diabetes, there was significant increase (P < 0.001) in UACR in all diabetic animals compared to the normoglycemic control group (Figure 4). UACR decreased significantly in DM groups treated with PRRB or valsartan by 14% and 22%, respectively, compared to non-treated DM group (P < 0.01). Similarly, there was significant reduction in UACR in the DM group treated with combined PRRB and valsartan compared to non-treated DM group (P < 0.01). There were no significant differences between groups receiving individual or combined PRRB and valsartan treatment.
Figure 4.
Urinary albumin to creatinine ratio (UACR) of normoglycemic control rats (n = 8), and streptozotocin (STZ)-induced diabetic rats (DM; n = 10) treated with PRR blocker (DM + PRRB; n = 10), valsartan (DM + VAL; n = 10), or PRRB plus valsartan (DM + PRRB + VAL; n = 9) at baseline (open bars) and after two weeks of STZ-induction of diabetes and drug treatment (solid bars). Data are mean ± SEM. *P < 0.05 vs. control; #P < 0.05 vs. DM.
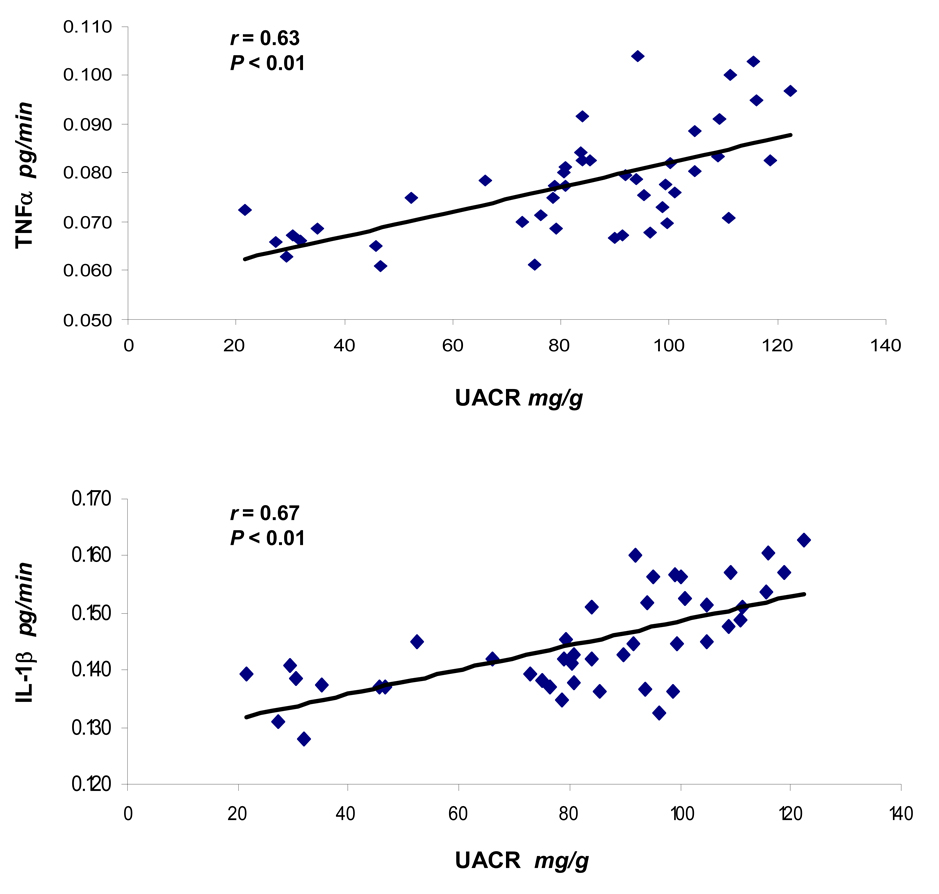
Relationship between UACR with RIF TNFα and IL-1β
At the end of two week study period, UACR of all animals (n = 47) were correlated with RIF TNFα (Figure 5A) and RIF IL-1β (Figure 5B). The analyses showed a direct correlation in both situations and significant Pearson’s coefficients (r = 0.63 and r = 0.67, respectively) were determined.
Figure 5.
Correlation between urinary albumin to creatinine ratio (UACR) of all rats (n = 47) with renal interstitial fluid (RIF) TNFα (A) and IL-1β (B) at the end of two week study period, investigated by Pearson’s correlation analysis. r = Pearson’s coefficient.
DISCUSSION
The present study was conducted to evaluate the role of PRR in development of renal inflammation in diabetic nephropathy. Our data demonstrated that in presence of diabetes, PRR blockade reduced albuminuria and in vivo renal production of the inflammatory cytokines TNF-α and IL-1β. The absence of changes in renal production of Ang II during PRR blockade suggests that the contribution of this receptor to development of renal inflammation in diabetes is additive to Ang II-mediated effects. Angiotensin AT1 receptor blockade reduced albuminuria as well as renal expressions of TNF-α and IL-1β, and increased renal Ang II levels in diabetic rats. These results point to different mechanisms associated with PRR and AT1 receptor in diabetic kidney disease and expand on our previous report demonstrating that stimulation of the AT1 receptor by Ang II mediates renal inflammation in diabetes (4). Collectively, our studies suggest both Ang II-dependent and independent mechanisms contributing to development of renal inflammation in diabetic nephropathy.
In diabetes, the expression and activity of all components of the RAS are increased (18,22), including prorenin (23). The relation between increased plasma prorenin and organ damage in diabetes remains unclear. Prorenin is a precursor of renin that was considered to be without any physiological significance until recent discovery of its receptor (6). PRR is expressed in the kidney mainly in the glomerular mesangial cells (6,9), vascular smooth muscle cells of renal vessels (6), distal renal tubule (7), and podocytes (10). Plasma prorenin levels were higher (23), while plasma renin levels were lower in diabetic patients than in normal healthy subjects (24). The presence of high prorenin levels together with increased renal PRR expression in diabetes (21) suggests contribution of this receptor to development of diabetic nephropathy. In support of this hypothesis, recent studies demonstrated that activation of PRR in diabetes plays a role in the development of microvascular complications such as retinopathy (25) and nephropathy (12). In addition, chronic administration of a PRRB to diabetic rodents prevented the development and progression of diabetic nephropathy (12,14–15). PRRB interferes with the binding of prorenin to its receptor, thereby preventing its nonproteolytic activation (11,13).
Our current data show increased expressions of PRR mRNA and protein in non-treated and in PRRB treated diabetic animals after two weeks of development of diabetes. PRR blockade did not influence PRR expression. In contrast, two weeks of AT1 receptor blockade with valsartan caused significant reductions in the renal expressions of PRR mRNA and protein. These results confirm our previous finding of reduction of PRR expression in diabetes by AT1 receptor blockade (21). The exact mechanism responsible for the downregulation of PRR expression with valsartan in diabetes needs further clarification. As demonstrated by in vitro studies where renin was associated with reduction of PRR expression (26), it is possible that increased production of renin during AT1 receptor blockade, as demonstrated by the increased levels of renal Ang II, contributes to downregulation of PRR expression. Another possibility is that blockade of AT1 receptor reduces the high oxidative stress and superoxide production (27) observed in the kidney of diabetic animals (28) leading to reduced renal PRR expression, as we have previously demonstrated (21).
In the current study, we confirmed that chronic treatment with PRRB reduced the development of diabetic nephropaty as demonstrated by decreased UACR. The PRRB dose used in the present study is high enough to work as a competitive inhibitor (29). Valsartan treatment also reduced UACR, as previously demonstrated (30). However, different mechanisms seem to be involved with PRRB or valsartan effects on albuminuria as judged by the differences of their influence on renal Ang II generation. Despite of this difference we did not observe further reductions of UACR when animals were concomitantly treated with both drugs. The reduced renal expression of PRR mRNA and protein in valsartan treated diabetic rats suggests that AT1 receptor blockade contributes to decreased PRR activity.
The pathophysiology of diabetic nephropathy involve multiple mechanisms including the RAS (22) and inflammation (5,31). Renal expressions of inflammatory cytokines such as TNF-α and IL-1β were demonstrated to increase in diabetes contributing to the development of diabetic nephropathy (30–32). In the current study, the association between the development of diabetic nephropathy and inflammatory cytokines was demonstrated by a positive correlation between UACR and the renal production of both TNF-α and IL-1β. The role of cytokines in development of diabetic nephropathy is complex, involving different mechanisms of cellular injury (5,32). Current study demonstrated that high levels of blood glucose stimulated renal production of both TNF-α and IL-1β. We also demonstrated reductions in the production of these renal inflammatory cytokines in diabetic rats with chronic PRRB or valsartan treatments that were not related to changes in blood glucose or body weight. The reductions in renal cytokines with PRRB treatment were independent of changes in renal Ang II. These findings are in agreement with a previous study showing reduction in the development of diabetic nephropathy with PRRB in the angiotensin II type 1a receptor-deficient mice (14), an animal model which no longer responds to Ang II. Based on these results, production of inflammatory molecules in diabetes involves both Ang II -dependent and -independent mechanisms.
(Pro)renin receptor activation by renin or prorenin mediates both Ang II dependent and independent pathways. The binding of renin and prorenin to the PRR, independently of Ang II, was demonstrated to initiate a cascade of intracellular signal transduction mechanisms, including increased phosphorylation of extracellular signal-related protein kinase 1 and 2 (ERK 1/2), increased mitogen-activated protein kinase activity (34–36), and increased production of transforming growth factor-β1 and matrix proteins with subsequent induction of mesangial cell proliferation (9). In contrast, PRR blockade with either small-interference RNA targeted to PRR mRNA or PRRB prevented the phosphorylation of ERK 1/2, independently of AT1 receptor blockade (9,37). This finding is supported by a recent study showed that PRR blockade inhibited the production of inflammatory molecules such as VEGF and ICAM-1 in diabetic AT1 receptor deficient mice (29). The increased expression of PRR and the production and action of cytokines in diabetic kidney are closely related. Our current findings of reduced renal interstitial levels and renal expressions of TNF-α and IL-1β after PRR blockade in diabetes with concomitant reduction of UACR suggest that this receptor is involved in the development of diabetic nephropathy.
In conclusion, PRR is involved in the development and progression of kidney disease in diabetes through enhancement of renal inflammatory mechanisms including TNF-α and IL-1β. The inflammatory mechanisms mediated by PRR activation in diabetes are mainly independent of the renal Ang II effects.
ACKNOWLEDGEMENTS
This study was supported by grant DK-078757 and HL091535 from the National Institutes of Health to Helmy M. Siragy, MD.
REFERENCES
- 1.KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am. J. Kidney Dis. 2007;49 Suppl 2:S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N. Engl. J. Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 4.Siragy HM, Awad A, Abadir P, Webb R. The angiotensin II type 1 receptor mediates renal interstitial content of tumor necrosis factor-α in diabetic rats. Endocrinology. 2003;144:2229–2233. doi: 10.1210/en.2003-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J. Am. Soc. Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen G, Danser AH. Prorenin and (pro)renin receptor: a review of available data from in vitro studies and experimental models in rodents. Exp. Physiol. 2008;93:557–563. doi: 10.1113/expphysiol.2007.040030. [DOI] [PubMed] [Google Scholar]
- 8.Batenburg WW, Krop M, Garrelds IM, et al. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J. Hypertens. 2007;25:2441–2453. doi: 10.1097/HJH.0b013e3282f05bae. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Wongamorntham S, Kasting J, et al. Renin increases mesangial cell transforming growth factor-b1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int. 2006;69:105–113. doi: 10.1038/sj.ki.5000011. [DOI] [PubMed] [Google Scholar]
- 10.Ichihara A, Sakoda M, Mito-Kurauchi A, Narita T, Kinouchi K, Itoh H. Drug discovery for overcoming chronic kidney disease (CKD): new therapy for CKD by a (pro)renin-receptor-blocking decoy peptide. J. Pharmacol. Sci. 2009;109:20–23. doi: 10.1254/jphs.08r07fm. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki F, Hayakawa M, Nakagawa T, et al. Human prorenin has "gate and handle" regions for its non-proteolytic activation. J. Biol. Chem. 2003;278:22217–22222. doi: 10.1074/jbc.M302579200. [DOI] [PubMed] [Google Scholar]
- 12.Ichihara A, Hayashi M, Kaneshiro Y, et al. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the "handle" region for nonproteolytic activation of prorenin. J. Clin. Invest. 2004;114:1128–1135. doi: 10.1172/JCI21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nabi AH, Biswas KB, Nakagawa T, Ichihara A, Inagami T, Suzuki F. 'Decoy peptide' region (RIFLKRMPSI) of prorenin prosegment plays a crucial role in prorenin binding to the (pro)renin receptor. Int. J .Mol. Med. 2009;24:83–89. doi: 10.3892/ijmm_00000210. [DOI] [PubMed] [Google Scholar]
- 14.Ichihara A, Suzuki F, Nakagawa T, et al. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J. Am. Soc. Nephrol. 2006;17:1950–1961. doi: 10.1681/ASN.2006010029. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi H, Ichihara A, Kaneshiro Y, et al. Regression of nephropathy developed in diabetes by (pro)renin receptor blockade. J. Am. Soc. Nephrol. 2007;18:2054–2061. doi: 10.1681/ASN.2006080820. [DOI] [PubMed] [Google Scholar]
- 16.Yagi S, Morita T, Katayama S. Combined treatment with an AT1 receptor blocker and angiotensin converting enzyme inhibitor has an additive effect on inhibiting neointima formation via improvement of nitric oxide production and suppression of oxidative stress. Hypertens. Res. 2004;27:129–135. doi: 10.1291/hypres.27.129. [DOI] [PubMed] [Google Scholar]
- 17.Padia SH, Howell NL, Siragy HM, Carey RM. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension. 2006;47:537–544. doi: 10.1161/01.HYP.0000196950.48596.21. [DOI] [PubMed] [Google Scholar]
- 18.Awad AS, Webb RL, Carey RM, Siragy HM. Renal nitric oxide production is decreased in diabetic rats and improved by AT1 receptor blockade. J. Hypertens. 2004;22:1571–1577. doi: 10.1097/01.hjh.0000133718.86451.6a. [DOI] [PubMed] [Google Scholar]
- 19.Siragy HM, Xue C, Abadir P, Carey RM. Angiotensin subtype-2 receptors inhibit renin biosynthesis and angiotensin II formation. Hypertension. 2005;45:133–137. doi: 10.1161/01.HYP.0000149105.75125.2a. [DOI] [PubMed] [Google Scholar]
- 20.Kalantarinia K, Awad AS, Siragy HM. Urinary and renal interstitial concentrations of TNF-alpha increase prior to the rise in albuminuria in diabetic rats. Kidney Int. 2003;64:1208–1213. doi: 10.1046/j.1523-1755.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- 21.Siragy HM, Huang J. Renal (pro)renin receptor upregulation in diabetic rats through enhanced angiotensin AT1 receptor and NADPH oxidase activity. Exp. Physiol. 2008;93:709–714. doi: 10.1113/expphysiol.2007.040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carey RM, Siragy HM. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends. Endocrinol. Metab. 2003;14:274–281. doi: 10.1016/s1043-2760(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 23.Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, Bryer-Ash M. Increased plasma inactive renin in diabetes mellitus. A marker of microvascular complications. N. Engl. J. Med. 1985;312:1412–1417. doi: 10.1056/NEJM198505303122202. [DOI] [PubMed] [Google Scholar]
- 24.Price DA, Porter LE, Gordon M, et al. The paradox of the low-renin state in diabetic nephropathy. J. Am. Soc. Nephrol. 1999;10:2382–2391. doi: 10.1681/ASN.V10112382. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson-Berka JL. Prorenin and the (Pro)renin Receptor in Ocular Pathology. Am. J. Pathol. 2008;173:1591–1594. doi: 10.2353/ajpath.2008.080757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schefe JH, Menk M, Reinemund J, et al. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ. Res. 2006;99:1355–1366. doi: 10.1161/01.RES.0000251700.00994.0d. [DOI] [PubMed] [Google Scholar]
- 27.Onozato ML, Toji A, Goto A, Fujita T, Wilcox CS. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int. 2002;61:186–194. doi: 10.1046/j.1523-1755.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 28.Privratsky JR, Wold LE, Sowers JR, Quinn MT, Ren J. AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension. 2003;42:206–212. doi: 10.1161/01.HYP.0000082814.62655.85. [DOI] [PubMed] [Google Scholar]
- 29.Satofuka S, Ichihara A, Nagai N, Noda K, Ozawa Y, Fukamizu A, Tsubota K, Itoh H, Oike Y, Ishida S. (Pro)renin receptor-mediated signal transduction and tissue renin-angiotensin system contribute to diabetes-induced retinal inflammation. Diabetes. 2009;58:1625–1633. doi: 10.2337/db08-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalender B, Oztürk M, Tunçdemir M, et al. Renoprotective effects of valsartan and enalapril in STZ-induced diabetes in rats. Acta. Histochem. 2002;104:123–130. doi: 10.1078/0065-1281-00643. [DOI] [PubMed] [Google Scholar]
- 31.Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. The inflammatory process in type 2 diabetes. The role of cytokines. Ann. N. Y. Acad. Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa G, Nakano K, Sawada M, et al. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 1991;40:1007–1012. doi: 10.1038/ki.1991.308. [DOI] [PubMed] [Google Scholar]
- 33.Vilcek J. The cytokines: An overview. In: Thomson AW, Lotze MT, editors. The Cytokine Handbook. 4th edn. London: Academic Press; 2003. Ch. 1. [Google Scholar]
- 34.Feldt S, Batenburg WW, Mazak I, et al. Prorenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptide. Hypertension. 2008;51:682–688. doi: 10.1161/HYPERTENSIONAHA.107.101444. [DOI] [PubMed] [Google Scholar]
- 35.Sakoda M, Ichihara A, Kaneshiro Y, et al. (Pro)renin receptor-mediated activation of mitogen-activated protein kinases in human vascular smooth muscle cells. Hypertens. Res. 2007;30:1139–1146. doi: 10.1291/hypres.30.1139. [DOI] [PubMed] [Google Scholar]
- 36.Saris J, ‘tHoen P, Garrelds I, et al. Prorenin induces intracellular signaling in cardiomyocytes independently of angiotensin II. Hypertension. 2008;48:564–571. doi: 10.1161/01.HYP.0000240064.19301.1b. [DOI] [PubMed] [Google Scholar]
- 37.He M, Zhang L, Shao Y, et al. Inhibition of renin/prorenin receptor attenuated mesangial cell proliferation and reduced associated fibrotic factor release. Eur. J. Pharmacol. 2009;606:155–161. doi: 10.1016/j.ejphar.2008.12.050. [DOI] [PubMed] [Google Scholar]