Abstract
Epithelial-mesenchymal transition (EMT) is a phenotypic change in which epithelial cells detach from their neighbors and become motile. Whereas soluble signals such as growth factors and cytokines are responsible for stimulating EMT, here we show that gradients of mechanical stress define the spatial locations at which EMT occurs. When treated with transforming growth factor (TGF)-β, cells at the corners and edges of square mammary epithelial sheets expressed EMT markers, whereas those in the center did not. Changing the shape of the epithelial sheet altered the spatial pattern of EMT. Traction force microscopy and finite element modeling demonstrated that EMT-permissive regions experienced the highest mechanical stress. Myocardin-related transcription factor (MRTF)-A was localized to the nuclei of cells located in high-stress regions, and inhibiting cytoskeletal tension or MRTF-A expression abrogated the spatial patterning of EMT. These data suggest a causal role for tissue geometry and endogenous mechanical stresses in the spatial patterning of EMT.
Keywords: ECM, biomechanics, force, micropatterning, mechanotransduction
Introduction
The spatial patterning of cellular behaviors plays an important role during tissue development, differentiation, and wound healing. Localized patterns may result from a variety of stimuli, including concentration gradients of diffusible factors (morphogens), adhesion to the extracellular matrix, and mechanical forces [Nelson, 2009]. Endogenous (cell-generated) mechanical stresses arising from isometric cytoskeletal tension are transmitted within tissues between cells, their neighbors, and the surrounding extracellular matrix. In culture, gradients in mechanical stress can arise as a result of the geometry of the tissue, with free edges or areas of high curvature experiencing the greatest stress [Nelson et al., 2005]. Mechanical stress has been increasingly implicated as a key regulator of a wide range of cellular behaviors, including proliferation [Nelson et al., 2005], stem cell lineage commitment [Engler et al., 2006], and the tumorigenic phenotype [Paszek et al., 2005].
Epithelial-mesenchymal transition (EMT) is a phenotypic shift that governs a variety of morphogenetic processes, including gastrulation, neural crest development, and heart valve formation [Thiery et al., 2009]. During EMT, epithelial cells loosen attachments to their neighbors, acquire a mesenchymal-like morphology and become motile. These phenotypic changes are accompanied by alterations in gene expression patterns, including attenuation of epithelial markers (such as epithelial cytokeratins and E-cadherin) and neo-expression of mesenchymal markers (such as vimentin and α-smooth muscle actin (αSMA)) [Zeisberg and Neilson, 2009]. Developmental EMTs occur at specific times and locations to ensure proper patterning of the embryo (reviewed in [Shook and Keller, 2003]). Pathologic EMTs can also be spatially patterned, having been observed at the edges of wounds and the invasive front of metastatic lesions [Arnoux et al., 2005; Oft et al., 1998]. EMT can be induced by soluble stimuli including cytokines, growth factors, and metalloproteinases [Thiery et al., 2009], but mechanisms for spatially patterning EMT are largely unexplored.
Here, we investigated the spatial patterning of EMT in two-dimensional (2D) sheets of epithelial cells. We found that when treated with transforming growth factor (TGF)-β, microfabricated monolayers of mammary epithelial cells of defined shape and size underwent EMT preferentially at specific locations, notably the edges and corners of square sheets. Given that endogenous mechanical stress was concentrated in these regions, we explored how cell-cell contact and the transmission of intercellular tension affected TGFβ-induced EMT. We demonstrated that spatial patterning of EMT can result from endogenous gradients in mechanical stress, suggesting a role for the physical microenvironment in the regulation of EMT.
Materials and Methods
Cell culture and reagents
SCp2 mouse mammary epithelial cells were cultured as previously described [Nelson et al., 2008]. NMuMG mouse mammary epithelial cells (ATCC) were cultured in DMEM supplemented with 10% fetal bovine serum (Atlanta Biologicals), 10 μg/mL insulin and 50 μg/mL gentamicin. Microfabricated epithelial sheets were treated with 10 ng/mL recombinant human TGFβ1 (R&D Systems) for 48 hours and with the following reagents: Y27632 (Tocris; 10 μM); NSC23766 (Tocris; 100 μM); cytochalasin D (Tocris; 200 nM); ML-7 (EMD Chemicals, 25 μM); blebbistatin (Sigma, 25 μM); CCG-1423 (Cayman Chemical, 10μM).
Microfabrication
Microfabricated substrata containing fibronectin-coated islands were created as previously described [Nelson et al., 2008]. Briefly, poly(dimethylsiloxane) (PDMS; Ellsworth Adhesives) stamps were coated with fibronectin (BD Biosciences), rinsed in PBS, and dried under a stream of compressed nitrogen. PDMS-coated glass coverslips were UV-oxidized, stamped with fibronectin, blocked with 1% pluronics F108 (BASF), and rinsed in PBS before seeding cells. Samples were rinsed after 2 hours to remove non-adherent cells.
Transfections and adenoviral transductions
Mouse pLKO.1 lentiviral MKL1 shRNA (shMRTF-A#1 5′-CCCACTCAGGTTCTTTCTCAA-3′ and shMRTF-A#2 5′-CAGATTTCAAAGAGCCACCAT-3′) were obtained from Open Biosystems. Human FLAG-tagged MRTF-A (p3xFLAG-MKL1) and pLKO.1 lentiviral scramble shRNA were obtained from Addgene. For controls, YFP was subcloned into the p3xFLAG-CMV-7.1 vector. MRTF-A-ΔN was generated using site-directed mutagenesis. Cells were transfected with plasmids using Fugene HD (Roche). Recombinant adenovirus encoding human E-cadherin lacking the β-catenin-binding domain (Ad-EΔ) was a gift from Christopher Chen (University of Pennsylvania) [Nelson et al., 2005]; control adenovirus encoding GFP (Ad-GFP) was obtained from Vector Biolabs. High titer preparations of recombinant adenoviruses were generated using the AdEasy virus purification kit (Stratagene). Cells were transduced at an MOI resulting in >99% transduction efficiency.
Real-time PCR
Total RNA was isolated using Trizol reagent followed by cDNA synthesis using a Super Script First-Strand Synthesis kit (Invitrogen). Transcript levels were measured by quantitative real-time PCR using SYBR green chemistry. Amplification was followed by melting curve analysis to verify the presence of a single PCR product. The primers used include: 18s forward primer 5′-TCAGATACCGTCGTAGTTC-3′ and reverse 5′-CCTTTAAGTTTCAGCTTTGC-3′; and MRTF-A forward primer 5′-ATGGAGCTGGTGGAGAAGAATATC-3′ and reverse 5′-GAAGGAGGAACTGTCTGCTACC-3′.
Immunofluorescence analysis
For staining cytoskeletal proteins, samples were fixed with 1:1 methanol:acetone at −20°C, rinsed in PBS, blocked with 10% goat serum (Sigma), and incubated with the following primary antibodies: pan-keratin (Dako), αSMA (Sigma), or vimentin (Sigma). For all other staining, samples were fixed with 4% paraformaldehyde, rinsed in PBS, permeabilized with 0.1% Triton-X-100 in PBS, blocked with 10% goat serum, and incubated with the following primary antibodies: phospho-Smad1/5/8 (Cell Signaling), TGFβRII (Santa Cruz), β-catenin (Sigma), FLAG (Sigma), or SRF (Santa Cruz). Samples were then rinsed and incubated with Alexa-conjugated secondary antibodies (Invitrogen), and nuclei were counterstained with Hoechst 33342 (Invitrogen).
Microscopy and analysis
Samples were imaged using either a 4× (NA = 0.2), 10× (NA = 0.3) or 20× (NA = 0.45) air objective on a Nikon Eclipse Ti-U inverted fluorescence microscope equipped with a Hamamatsu ORCA CCD camera. Frequency maps were created using ImageJ and Photoshop software. First, gray-scale images were converted to black-and-white images using a binarize function. Black-and-white images were then summed to create a composite gray-scale image which was converted into a color-coded frequency map using the indexed color mode in Photoshop.
Traction forces
Finite element method (FEM) models of cell monolayers were generated as previously described [Nelson et al., 2005]. Bead displacements were measured using polyacrylamide (PA) gels. Briefly, PA gels comprised of 5% monomer and 0.03% bis-acrylamide were prepared using an adapted protocol [Pelham and Wang, 1997]. Fluorescent microbeads (1-μm diameter, Invitrogen) were embedded in the gels and fibronectin-coated islands were stenciled onto the surface [Wang et al., 2002]. Cells were plated to confluence onto the fibronectin-coated islands, and bead locations were imaged before and after relaxation with 0.05% Triton-X-100. Displacement maps were generated using Imaris tracking software (Bitplane) and MATLAB.
Results
TGFβ induces EMT at the edges and corners of square epithelial sheets
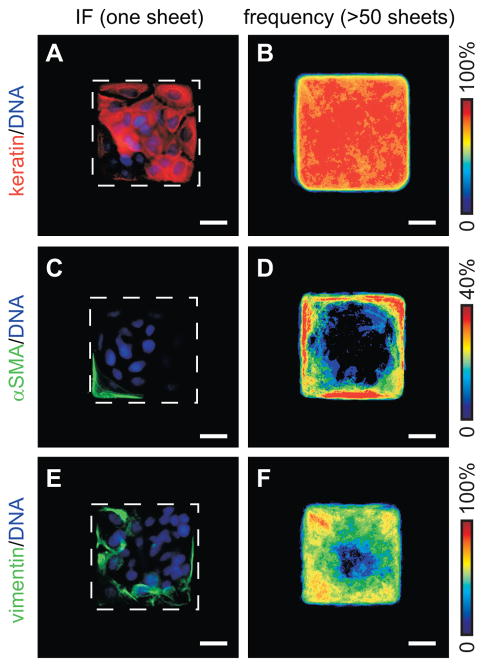
TGFβ-induced EMT is characterized by down-regulation of epithelial cytokeratins and up-regulation of mesenchymal markers including vimentin and αSMA. To investigate the role of tissue geometry in the spatial regulation of EMT, we used a microfabrication technique to generate confluent two-dimensional (2D) epithelial sheets of defined shape and size. SCp2 mouse mammary epithelial cells were cultured on substrata which contained 100 μm-square fibronectin-coated islands surrounded by non-adhesive regions. When treated with TGFβ, the expression of epithelial cytokeratins decreased moderately, with a greater reduction in the cells located along the edges and corners of the square sheets (Fig. 1A,B). Conversely, de novo expression of the mesenchymal markers, αSMA and vimentin, was restricted to the edges and corners of the monolayers (Fig. 1C–F). We observed no spatial differences in marker expression in the absence of treatment with TGFβ (Fig. S1). These data indicate that TGFβ induces EMT preferentially at the edges and corners of square mammary epithelial sheets, suggesting that spatial asymmetries in EMT can arise within these model tissues.
Fig. 1.
TGFβ induces spatial patterning of EMT in 2D epithelial sheets. Immunofluorescence staining and frequency maps for (A,B) cytokeratins, (C,D) αSMA, and (E,F) vimentin for TGFβ-treated tissues. Scale bars, 25 μm.
In the canonical signaling pathway, binding of TGFβ ligand to its type I (TGFβRI) and type II (TGFβRII) receptors stimulates phosphorylation and nuclear translocation of Smad proteins [Shi and Massague, 2003]. Spatial patterning of EMT could thus result from spatial differences in the levels of receptors or their downstream signaling. However, immunofluorescence analysis of TGFβRII indicated that this receptor was evenly localized across the square sheets (Fig. S2). Similarly, we found even nuclear localization of phosphorylated Smad1/5/8 in sheets treated with TGFβ (Fig. S2). Thus, TGFβ signaling to the nucleus through the canonical pathway appears homogeneous across the square sheets, suggesting that the spatial patterning of EMT is due to other factors.
Patterned induction of EMT requires transmission of intercellular isometric tension
Cells located along the edges of tissues simultaneously experience reduced cell-cell adhesion (due to the presence of a neighbor-free edge) and increased mechanical stress (due to propagation of isometric contractile tension) [Keller et al., 2003; Nelson et al., 2005]. Indeed, the square epithelial sheets generated gradients in endogenous mechanical stress, with cells on the corners and edges experiencing the highest magnitudes of stress [Nelson et al., 2005] (Fig. S3). Both of these microenvironmental signals have been shown to independently influence TGFβ signaling. In addition to the canonical signaling pathway, TGFβ can mediate changes in gene expression by activating any of several kinase cascades or by cooperating with cytoplasmic β-catenin or cytoskeletal mediators [Derynck and Zhang, 2003]. Furthermore, the ability of TGFβ to induce αSMA expression in fibroblasts is regulated by cytoskeletal tension [Li et al., 2007; Tomasek et al., 2002].
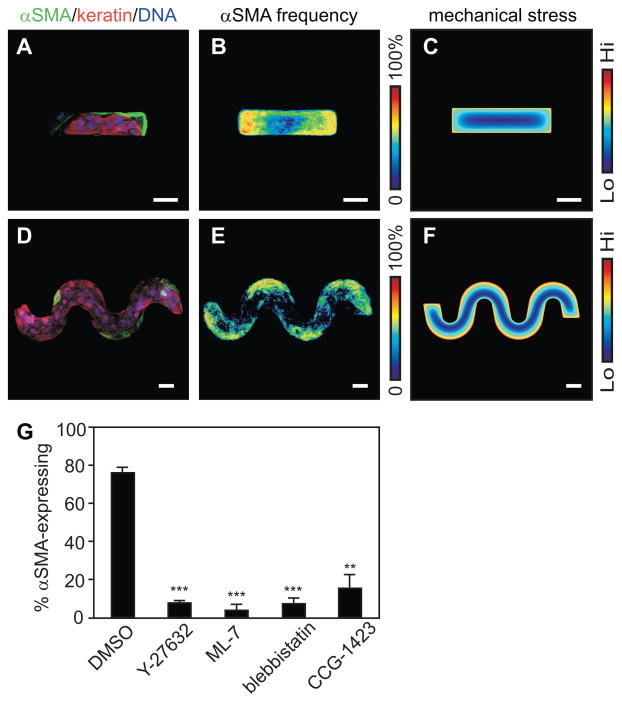
To distinguish between effects due to cell-cell contact and those due to gradients of mechanical stress, we varied the geometry of the epithelial sheets. In rectangular monolayers, the TGFβ-mediated expression of αSMA was concentrated on the short edges (Fig. 2A,B), regions predicted to experience the highest mechanical stress (Fig. 2C). Similarly, alterations in the expression of EMT markers were restricted to the high-stress convex regions of sinusoidal monolayers (Fig. 2D–F) as compared to the low-stress concave regions. These spatial distributions are consistent with a role for mechanical stresses in the spatial patterning of EMT markers. In epithelial sheets, gradients of mechanical stress are generated by intercellular transmission of tension from the actomyosin cytoskeleton, which is regulated in part by signaling through RhoA, its effector Rho kinase (ROCK), and myosin light chain kinase (MLCK). Decreasing contractile tension through treatment with the ROCK inhibitor Y27632, the MLCK inhibitor ML-7, or the non-muscle myosin ATPase inhibitor blebbistatin all reduced the number of epithelial sheets exhibiting spatial patterning of EMT markers (Fig. 2G). Although other Rho family GTPases can affect tissue contractility, inhibiting signaling through Rac by treatment with NSC23766 disrupted formation of lamellipodia but had no effect on TGFβ-induced patterning of EMT (Fig. S4).
Fig. 2.
Spatial patterning of EMT correlates with endogenous gradients of cytoskeletal tension. (A) Immunofluorescence staining and (B) frequency map for αSMA expression in rectangular epithelial sheets treated with TGFβ. (C) Sites of EMT correlate with FEM-predicted endogenous mechanical stress. (D) Immunofluorescence staining and (E) frequency map for αSMA expression, and (F) FEM-predicted distribution of mechanical stress in sinusoidal epithelial sheets treated with TGFβ. (G) Frequency of epithelial sheets with patterned αSMA expression after simultaneous treatment with TGFβ and DMSO vehicle, Y27632, ML-7, blebbistatin, or CCG-1423. Scale bars, 50 μm. (**), p<0.005; (***), p<0.0001 compared to DMSO.
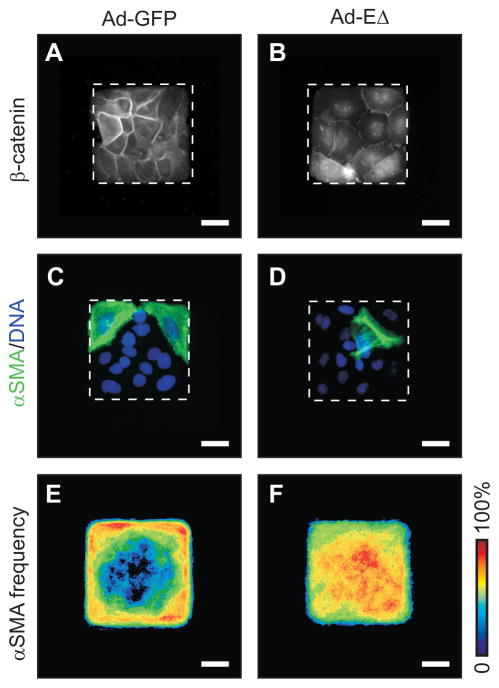
Cells within sheets transmit tension to and from their neighbors through cadherin-mediated intercellular adhesions [Adams and Nelson, 1998]. To determine whether the spatial patterning of EMT is directed by intercellular transmission of cytoskeletal tension, we infected cells with recombinant adenovirus containing a cytoplasmic deletion mutant of E-cadherin (Ad-EΔ) that blocks the connection between E-cadherin and the actin cytoskeleton by inhibiting junctional localization of β-catenin [Nelson et al., 2005] (Fig. 3A,B). Immunofluorescence staining revealed that TGFβ induced spatially uniform expression of αSMA within EΔ-expressing sheets, as compared to patterned expression of αSMA observed in control sheets infected with adenovirus containing GFP (Ad-GFP) (Fig. 3C–F). These data are consistent with the spatial patterning of EMT being regulated by intercellular tension.
Fig. 3.
Spatial patterning of EMT arises from intercellular transmission of stress. Immunofluorescence staining for β-catenin in epithelial sheets transduced with (A) Ad-GFP or (B) Ad-EΔ. Immunofluorescence staining for αSMA (green) and DNA (blue) in epithelial sheets transduced with (C) Ad-GFP or (D) Ad-EΔ and simultaneously treated with TGFβ. Frequency maps of αSMA expression in epithelial sheets transduced with (E) Ad-GFP or (F) Ad-EΔ and simultaneously treated with TGFβ. Scale bars, 25 μm.
Tissue geometry patterns EMT by governing the nuclear localization of MRTF-A
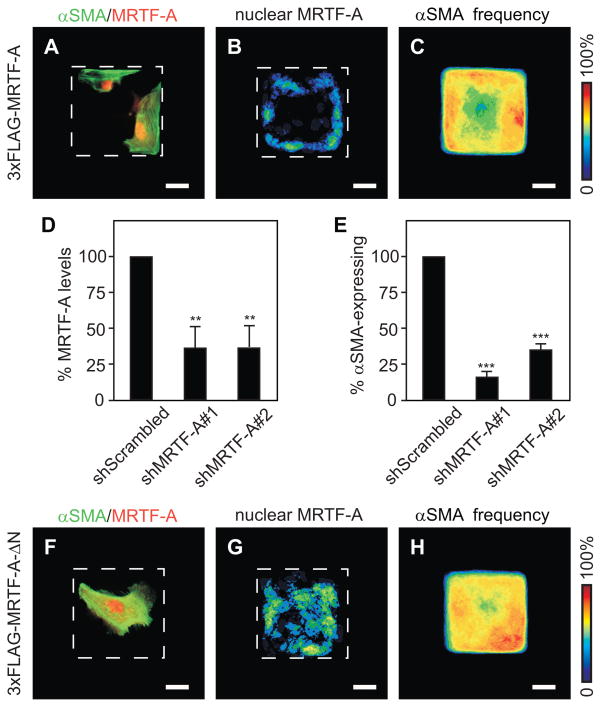
In addition to signaling through Smads, TGFβ has been demonstrated to enhance binding of serum response factor (SRF) to CArG boxes within the promoter regions of genes including αSMA [Hautmann et al., 1999]. SRF regulates the transcription of many genes involved in proliferation and differentiation, including immediate early genes and actin-regulatory proteins (reviewed by [Posern and Treisman, 2006]). A subgroup of SRF-target genes is sensitive to Rho-actin signaling; this differential control is mediated by the SRF cofactors, myocardin-related transcription factors (MRTF)-A and -B (also known as MAL and MKL1/2) [Miralles et al., 2003]. MRTF-A regulates the transcription of αSMA [Elberg et al., 2008] and was also recently shown to effect TGFβ-mediated EMT by associating with Smad3 to enhance the transcription of Slug [Morita et al., 2007]. Immunofluorescence analysis revealed that nuclear localization of SRF was evenly distributed across the epithelial sheets (Fig. S5). However, we found patterned nuclear localization of FLAG-tagged MRTF-A along the edges and corners (Fig. 4A,B). The patterned nuclear localization of MRTF-A was correlated with patterned αSMA expression in these epithelial sheets (Fig. 4C). Down-regulating the expression of MRTF-A with shRNA reduced the number of epithelial sheets exhibiting patterned α-SMA expression in comparison to controls (Fig. 4D,E). Furthermore, treatment with CCG-1423, which inhibits the interaction between SRF and MRTF-A [Evelyn et al., 2007], decreased TGFβ-mediated EMT (Fig. 2G). These data suggest that TGFβ induces patterned EMT by cooperating with SRF/MRTF-A.
Fig. 4.
Spatial patterning of the nuclear localization of MRTF-A governs spatial patterning of EMT. Nuclear localization of FLAG-tagged MRTF-A correlates with αSMA expression. Shown are (A) immunofluorescence staining for αSMA (green) and FLAG (red), (B) frequency map of nuclear FLAG-tagged MRTF-A, and (C) frequency map of αSMA expression in FLAG-tagged MRTF-A-expressing sheets treated with TGFβ. Down-regulating MRTF-A expression blocks patterned EMT. Shown are (D) transcript levels of MRTF-A for cells transfected with shRNA and (E) frequency of shRNA-transfected epithelial sheets with patterned αSMA expression after treatment with TGFβ. Forced nuclear localization of MRTF-A disrupts patterned EMT. Shown are (F) immunofluorescence staining for αSMA (green) and FLAG (red), (G) frequency map of nuclear FLAG-tagged MRTF-A-ΔN, and (H) frequency map of αSMA expression in FLAG-tagged MRTF-A-ΔN-expressing sheets treated with TGFβ. Scale bars, 25 μm. (**), p<0.05; (***), p<0.005 compared to shScrambled.
The activity and nuclear localization of MRTF-A are regulated by its association with monomeric G-actin [Asparuhova et al., 2009; Posern and Treisman, 2006]. Increased cytoskeletal tension causes nuclear translocation of MRTF-A by reducing the available pool of monomeric actin [Posern and Treisman, 2006]. Consistent with the localization patterns, treatment with cytochalasin D, which drives MRTF-A into the nucleus by disrupting its association with actin monomers [Busche et al., 2008], resulted in EMT over the entire surface of the epithelial sheets (Fig. S6). Conversely, over-expressing a constitutively active form of MRTF-A (MRTF-A-ΔN), which lacks the RPEL motifs responsible for binding to actin monomers [Busche et al., 2008; Miralles et al., 2003], resulted in unpatterned nuclear localization of MRTF-A. MRTF-A-ΔN-expressing monolayers treated with TGFβ showed expression of αSMA over the entire epithelial sheet (Fig. 4F–H). These data suggest that tissue geometry patterns EMT by regulating the nuclear localization of MRTF-A.
Discussion
In this study, we found that spatial patterns of EMT can arise within epithelial sheets and that the patterning is consistent with gradients of mechanical stress sculpted by tissue geometry. We observed similar results in TGFβ-treated monolayers of NMuMG mouse mammary epithelial cells (Fig. S7), a widely used model for EMT. Gradients of mechanical stress have previously been reported to yield spatial asymmetries in a diverse array of cellular behaviors, including proliferation [Nelson et al., 2005], motility [Parker et al., 2002], and stem cell differentiation [Ruiz and Chen, 2008]. Our results may give insight into the mechanisms by which mechanical stress is transduced into these phenotypes. Here, TGFβ-mediated EMT is controlled by patterns of nuclear localization of the SRF cofactor, MRTF-A. SRF is a widely expressed transcription factor responsible for regulating a plethora of genes involved in cell proliferation and differentiation. Actin cytoskeletal dynamics modulated by Rho family GTPases control the localization of MRTF-A, which in turn controls binding of SRF to CArG boxes in the promoters of responsive genes. Given the recently uncovered links between EMT and stem cell differentiation [Mani et al., 2008], it will be interesting to determine whether Rho-mediated mechanical gradients influence both differentiation programs through MRTF-A.
Previous studies have implicated contact disassembly in the nuclear accumulation of MRTF-A and subsequent EMT [Busche et al., 2008; Fan et al., 2007; Masszi et al., 2004; Sebe et al., 2008]. Our data suggest that loss of cell-cell contact per se is not sufficient for nuclear translocation of MRTF-A and induction of EMT in monolayers of mammary epithelial cells. That is, not all cells located at the edges of monolayers undergo EMT. The edges refractory to the EMT stimulus are those predicted to be located in the low-stress regions of the monolayer, suggesting that nuclear translocation of MRTF-A is co-regulated by cell-cell contacts (or the lack thereof) and isometric tension within the tissue. This interpretation is validated by previous studies which found that applying exogenous forces induced αSMA expression in fibroblasts [Chan et al., 2009; Zhao et al., 2007], whereas inhibiting isometric tension prevented EMT in sub-confluent cultures of kidney tubular cells [Fan et al., 2007]. These data also suggest that edge-free regions of tissues (such as three-dimensional tissues in vivo) that experience high mechanical stress may also show enhanced EMT.
A number of cytokines and other soluble stimuli can induce EMT during normal and pathological development. Our data add to the growing evidence suggesting a role for the physical microenvironment in the regulation of EMT. Insoluble ECM molecules within the microenvironment impart signaling information and control cell morphology. Indeed, ECM components can regulate EMT in some cell lines [Shintani et al., 2008], and cell morphology regulates the induction of EMT by matrix metalloproteinase-3 [Nelson et al., 2008]. The size and shape of a tumor control the distribution of oxygen within its mass; tumorigenic EMT is affected profoundly by hypoxia (reviewed by [Hill et al., 2009]). Furthermore, spatial segregation of EMT has been observed at the edges of healing wounds [Arnoux et al., 2005] and the leading edge of invasive metastatic cohorts [Brabletz et al., 2001; Oft et al., 1998]. This patterning may be due to geometrically-prescribed intercellular tension such as described here. Dissecting how EMT is patterned may thus help to reveal the mechanisms involved in normal and metastatic development.
Supplementary Material
Epithelial and mesenchymal markers are not spatially patterned in epithelial sheets in the absence of TGFβ treatment. (A) Immunofluorescence image of cytokeratins (red) and DNA (blue). Frequency maps of (B) cytokeratins, (C) αSMA and (D) vimentin. Scale bars, 25 μm.
TGFβ signaling is evenly distributed over epithelial sheets. Shown are TGFβ-treated sheets stained for (A) TGFβRII, (B) DNA, and (C) pSmad1/5/8. Scale bars, 25 μm.
Mammary epithelial sheets generate mechanical forces by cytoskeletal contraction. (A) Epithelial sheet cultured on polyacrylamide gel with embedded fluorescent microbeads (red). (B) Average bead displacement map for five epithelial sheets. Larger displacements correlate with greater stresses. Scale bars, 25 μm.
Inhibiting signaling through the small GTPase Rac has no effect on spatial patterning of TGFβ-mediated EMT. Immunofluorescence images of F-actin in mammary epithelial cells treated with epidermal growth factor (4 nM) and (A) vehicle control or (B) the Rac inhibitor NSC23766 (100 μM). Immunofluorescence images and frequency maps of αSMA expression in (C,E) control and (D,F) NSC23766-treated epithelial sheets. Scale bars, 25 μm.
Nuclear localization of SRF is even across epithelial sheets. (A) Immunofluorescence image and (B) frequency map of SRF in control epithelial sheets. (C) Immunofluorescence image and (D) frequency map of SRF in TGFβ-treated epithelial sheets. Scale bars, 25 μm.
Treatment with cytochalasin D results in EMT over the entire epithelial sheet. Immunofluorescence images and frequency maps for (A,B) cytokeratins and (C,D) αSMA. Scale bars, 25 μm.
EMT is spatially patterned in epithelial sheets of NMuMG epithelial cells. (A) Immunofluorescence image and (B) frequency map of αSMA in TGFβ-treated sheets. Frequency maps of αSMA in NMuMG sheets transduced with (C) Ad-GFP or (D) Ad-EΔ and simultaneously treated with TGFβ. Frequency maps of (E) nuclear localized MRTF-A and (F) αSMA in NMuMG sheets transfected with MRTF-A-ΔN construct and treated with TGFβ. Scale bars, 25 μm.
Acknowledgments
Funded by: • National Institutes of Health (NIH); Grant Numbers: CA128660 and GM083997
• Susan G. Komen for the Cure; Grant Number: FAS0703855
We would like to thank L. Loo for use of her cleanroom and K. Lee, C. Liu, and A. Pavlovich for technical assistance. This work was supported in part by grants from the NIH (CA128660 and GM083997), Susan G. Komen for the Cure (FAS0703855), and the David and Lucile Packard Foundation. C.M.N. holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund. E.W.G. was supported by postdoctoral fellowships from the New Jersey Commission on Cancer Research and Susan G. Komen for the Cure.
Abbreviations
- EMT
epithelial-mesenchymal transition
- FEM
finite element method
- MRTF
myocardin-related transcription factor
- SMA
smooth muscle actin
- SRF
serum response factor
- TGF
transforming growth factor
- TGFβRII
TGFβ receptor type II
- 2D
two-dimensional
References
- Adams CL, Nelson WJ. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr Opin Cell Biol. 1998;10:572–7. doi: 10.1016/s0955-0674(98)80031-8. [DOI] [PubMed] [Google Scholar]
- Arnoux V, Come C, Kusewitt D, Hudson L, Savagner P. Cutaneous wound reepithelialization: a partial and reversible EMT. In: Savagner P, editor. Rise and fall of epithelial phenotype: concepts of epithelial-mesenchymal transition. Berlin: Springer; 2005. pp. 111–134. [Google Scholar]
- Asparuhova MB, Gelman L, Chiquet M. Role of the actin cytoskeleton in tuning cellular responses to external mechanical stress. Scand J Med Sci Sports. 2009;19:490–9. doi: 10.1111/j.1600-0838.2009.00928.x. [DOI] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356–61. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche S, Descot A, Julien S, Genth H, Posern G. Epithelial cell-cell contacts regulate SRF-mediated transcription via Rac-actin-MAL signalling. J Cell Sci. 2008;121:1025–35. doi: 10.1242/jcs.014456. [DOI] [PubMed] [Google Scholar]
- Chan MW, Arora PD, Bozavikov P, McCulloch CA. FAK, PIP5KI{gamma} and gelsolin cooperatively mediate force-induced expression of {alpha}-smooth muscle actin. J Cell Sci. 2009;122:2769–81. doi: 10.1242/jcs.044008. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Elberg G, Chen L, Elberg D, Chan MD, Logan CJ, Turman MA. MKL1 mediates TGF-beta1-induced alpha-smooth muscle actin expression in human renal epithelial cells. Am J Physiol Renal Physiol. 2008;294:F1116–28. doi: 10.1152/ajprenal.00142.2007. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Evelyn CR, Wade SM, Wang Q, Wu M, Iniguez-Lluhi JA, Merajver SD, Neubig RR. CCG-1423: a small-molecule inhibitor of RhoA transcriptional signaling. Mol Cancer Ther. 2007;6:2249–60. doi: 10.1158/1535-7163.MCT-06-0782. [DOI] [PubMed] [Google Scholar]
- Fan L, Sebe A, Peterfi Z, Masszi A, Thirone AC, Rotstein OD, Nakano H, McCulloch CA, Szaszi K, Mucsi I, Kapus A. Cell contact-dependent regulation of epithelial-myofibroblast transition via the rho-rho kinase-phospho-myosin pathway. Mol Biol Cell. 2007;18:1083–97. doi: 10.1091/mbc.E06-07-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautmann MB, Adam PJ, Owens GK. Similarities and differences in smooth muscle alpha-actin induction by TGF-beta in smooth muscle versus non-smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:2049–58. doi: 10.1161/01.atv.19.9.2049. [DOI] [PubMed] [Google Scholar]
- Hill RP, Marie-Egyptienne DT, Hedley DW. Cancer stem cells, hypoxia and metastasis. Semin Radiat Oncol. 2009;19:106–11. doi: 10.1016/j.semradonc.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46:1246–56. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masszi A, Fan L, Rosivall L, McCulloch CA, Rotstein OD, Mucsi I, Kapus A. Integrity of cell-cell contacts is a critical regulator of TGF-beta 1-induced epithelial-to-myofibroblast transition: role for beta-catenin. Am J Pathol. 2004;165:1955–67. doi: 10.1016/s0002-9440(10)63247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–42. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- Morita T, Mayanagi T, Sobue K. Dual roles of myocardin-related transcription factors in epithelial mesenchymal transition via slug induction and actin remodeling. J Cell Biol. 2007;179:1027–42. doi: 10.1083/jcb.200708174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM. Geometric control of tissue morphogenesis. Biochim Biophys Acta. 2009;1793:903–10. doi: 10.1016/j.bbamcr.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Jean RP, Tan JL, Liu WF, Sniadecki NJ, Spector AA, Chen CS. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci U S A. 2005;102:11594–9. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Khauv D, Bissell MJ, Radisky DC. Change in cell shape is required for matrix metalloproteinase-induced epithelial-mesenchymal transition of mammary epithelial cells. J Cell Biochem. 2008;105:25–33. doi: 10.1002/jcb.21821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oft M, Heider KH, Beug H. TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol. 1998;8:1243–52. doi: 10.1016/s0960-9822(07)00533-7. [DOI] [PubMed] [Google Scholar]
- Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. Faseb J. 2002;16:1195–204. doi: 10.1096/fj.02-0038com. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–5. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posern G, Treisman R. Actin’ together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16:588–96. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Ruiz SA, Chen CS. Emergence of Patterned Stem Cell Differentiation within Multicellular Structures. Stem Cells. 2008;26:2921–2927. doi: 10.1634/stemcells.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebe A, Masszi A, Zulys M, Yeung T, Speight P, Rotstein OD, Nakano H, Mucsi I, Szaszi K, Kapus A. Rac, PAK and p38 regulate cell contact-dependent nuclear translocation of myocardin-related transcription factor. FEBS Lett. 2008;582:291–8. doi: 10.1016/j.febslet.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shintani Y, Maeda M, Chaika N, Johnson KR, Wheelock MJ. Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signaling. Am J Respir Cell Mol Biol. 2008;38:95–104. doi: 10.1165/rcmb.2007-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120:1351–83. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Wang N, Ostuni E, Whitesides GM, Ingber DE. Micropatterning tractional forces in living cells. Cell Motil Cytoskeleton. 2002;52:97–106. doi: 10.1002/cm.10037. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–37. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XH, Laschinger C, Arora P, Szaszi K, Kapus A, McCulloch CA. Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J Cell Sci. 2007;120:1801–9. doi: 10.1242/jcs.001586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Epithelial and mesenchymal markers are not spatially patterned in epithelial sheets in the absence of TGFβ treatment. (A) Immunofluorescence image of cytokeratins (red) and DNA (blue). Frequency maps of (B) cytokeratins, (C) αSMA and (D) vimentin. Scale bars, 25 μm.
TGFβ signaling is evenly distributed over epithelial sheets. Shown are TGFβ-treated sheets stained for (A) TGFβRII, (B) DNA, and (C) pSmad1/5/8. Scale bars, 25 μm.
Mammary epithelial sheets generate mechanical forces by cytoskeletal contraction. (A) Epithelial sheet cultured on polyacrylamide gel with embedded fluorescent microbeads (red). (B) Average bead displacement map for five epithelial sheets. Larger displacements correlate with greater stresses. Scale bars, 25 μm.
Inhibiting signaling through the small GTPase Rac has no effect on spatial patterning of TGFβ-mediated EMT. Immunofluorescence images of F-actin in mammary epithelial cells treated with epidermal growth factor (4 nM) and (A) vehicle control or (B) the Rac inhibitor NSC23766 (100 μM). Immunofluorescence images and frequency maps of αSMA expression in (C,E) control and (D,F) NSC23766-treated epithelial sheets. Scale bars, 25 μm.
Nuclear localization of SRF is even across epithelial sheets. (A) Immunofluorescence image and (B) frequency map of SRF in control epithelial sheets. (C) Immunofluorescence image and (D) frequency map of SRF in TGFβ-treated epithelial sheets. Scale bars, 25 μm.
Treatment with cytochalasin D results in EMT over the entire epithelial sheet. Immunofluorescence images and frequency maps for (A,B) cytokeratins and (C,D) αSMA. Scale bars, 25 μm.
EMT is spatially patterned in epithelial sheets of NMuMG epithelial cells. (A) Immunofluorescence image and (B) frequency map of αSMA in TGFβ-treated sheets. Frequency maps of αSMA in NMuMG sheets transduced with (C) Ad-GFP or (D) Ad-EΔ and simultaneously treated with TGFβ. Frequency maps of (E) nuclear localized MRTF-A and (F) αSMA in NMuMG sheets transfected with MRTF-A-ΔN construct and treated with TGFβ. Scale bars, 25 μm.