Abstract
People frequently make decisions under stress. Understanding how stress affects decision making is complicated by the fact that not all stress responses are created equal. Challenge states, for example, occur when individuals appraise a stressful situation as demanding, but believe they have the personal resources to cope, and are characterized by efficient cardiovascular reactivity and approach motivation. Threat states, in contrast, occur when situational demands are perceived to outweigh resources and are characterized by less efficient cardiovascular reactivity and withdrawal motivation. We randomly assigned participants to social-feedback conditions (i.e., positive or negative feedback) designed to engender challenge or threat, or a no-stress condition. Participants then completed an anchoring-and-adjustment questionnaire. Those assigned to the challenge condition adjusted more from self-generated anchors than those assigned to the threat condition. Cardiovascular responses mediated the relationship between condition and adjustment. This study demonstrates the importance of considering profiles of cardiovascular reactivity when examining the influence of stress on decision making.
On October 27, 2007, Chase Sampson appeared on the television show “Who Wants to Be a Millionaire?” He had successfully completed the show's extensive vetting process, including the written test, interview, and “fastest finger” competition. After some idle banter, host Meredith Vieira asked the first question: “Homeowners buy surge protectors to protect their possessions from unexpected surges of what?” Chase wasted little time in answering: “B: Water Flow—my final answer.”
Chase's appearance lasted less than 90 s, but his 15 min of fame (or infamy) would continue. After the episode aired, YouTube videos of his defeat were posted on numerous blogs and discussion forums, accompanied by comments on Chase's stupidity. Though his question was easy, answering it in that situation may have been more difficult than bloggers acknowledged. Chase had flown the night before from Nashville for the taping, had not slept, found himself being watched by a live audience of hundreds, and knew that tens of thousands of viewers would see him later on TV. Unlike the people who would comment on his performance from the comfort of their homes, Chase was visibly nervous.
Chase is 1 of 75 people to leave “Millionaire” without a dime, but not all contestants react as poorly to the pressures of “the hot seat.” Eleven have walked away with the million-dollar prize, 25 have cashed in for $500,000, and many more have made more than $100,000. Undoubtedly, all contestants feel some stress when the cameras begin to roll, but their bodies and brains may be reacting to the stress in different ways. Could these divergent stress responses contribute to their disparate performances?
Previous research on the relationship between stress and decision making has yielded conflicting results. On the one hand, epinephrine levels, an index of the sympathetic nervous system's stress response, have been found to correlate positively with performance on academic exams (Jamieson, Mendes, Blackstock, & Schmader, in press; Johansson, Frankenhaeuser, & Magnusson, 1973; Rauste-von Wright, von Wright, & Frankenhaeuser, 1981), showing little evidence for the inverted-U relationship anticipated by the Yerkes-Dodson law (Dienstbier, 1989; Sapolsky, 2004). On the other hand, stress induced through annoying sounds (Schaeffer, 1989), social pressures (Balmer et al., 2007), and social evaluation (Lovallo & Thomas, 2000), and stress naturally induced through participants' circadian rhythm (Bodenhausen, 1990), has been found to impair memory, to increase people's reliance on intuition, and to decrease the use of conscious reasoning.
This conflict may result from the oversimplification of stress as a construct. Frequently, researchers have focused on only one of the two primary stress systems—either the sympathetic-adrenal-medullary (SAM) axis or the hypothalamic-pituitary-adrenal (HPA) axis—rather than considering how they combine to influence cognition. One valuable framework for considering complex stress responses comes from the literature on challenge versus threat (Blascovich & Tomaka, 1996; see also Frankenhaeuser, 1986; Henry, 1980). This research differentiates “good stress” from “bad stress” and considers the activation of both stress systems during active, goal-relevant tasks.1 The states of challenge and threat can be identified by individuals' appraisals of how demanding a stressful situation will be, as well as their appraisals of their personal resources to cope with the situation, and have divergent consequences for both short-term behavior and long-term health. Challenge occurs when individuals perceive that they have sufficient personal resources to cope with the demands of a task at hand, whereas threat occurs when demands outweigh perceived resources.
Challenge and threat states can also be differentiated by examining cardiovascular (CV) changes. In challenge, people show increased cardiac efficiency (i.e., increased cardiac output) and decreased vascular resistance (i.e., lower total peripheral resistance), which enables more blood to be supplied to the periphery. The body moves blood more quickly to effector muscles and to the brain, preparing for action and signaling approach motivation. In contrast, threat is characterized by less efficiency in the cardiac cycle and increased resistance in the vasculature. The body moves blood more slowly, and less blood reaches the periphery and the brain— changes that can lead to immobilization and may serve to prepare the body for damage or defeat (Mendes, Blascovich, Hunter, Lickel, & Jost, 2007).
In the experiment reported here, we placed participants in a stressful situation in order to manipulate challenge and threat states and thus allow for causal inferences regarding the effects of these stress profiles on decision making. After the stress induction, participants completed a quantifiable measure of conscious reasoning, adjustment from self-generated anchors (Epley & Gilovich, 2001). Anchoring and adjustment is a prominent mode of dual-process reasoning, whereby beliefs, decisions, judgments, or attitudes generated through automatic processes are fine-tuned by controlled processes (Gilbert, 1999).
For example, how long is the gestation period of an African elephant? People not well versed in the obstetrics of the Loxodonta africana probably do not know the answer. However, they can provide a fairly good guess by starting with the fact that humans have a 9-month pregnancy and then adjusting upward from 9 months because elephant calves are larger than human newborns. More adjustment on these types of questions tends to result in more accurate guesses. However, the ability to adjust from self-generated anchors (e.g., getting from 9 months to 22 months—the correct answer in this case) requires controlled processing that can be diminished for any number of reasons (Epley, 2004; Gilbert, 2002). Adjustment can be decreased by alcohol consumption, time pressure, and cognitive load. Thus, adjustment away from self-generated anchors depends on the expenditure of mental effort, and adjustments tend to be insufficient when mental resources are diminished.
Participants in our study completed an anchoring-and-adjustment questionnaire after engaging in a task designed to engender a challenge or threat stress response, or after a low-arousal (control) manipulation. We predicted that participants who were assigned to the threat condition would show less adjustment than those assigned to the challenge condition and that CV reactivity to the stressor would mediate the relationship between condition and adjustment.
METHOD
One hundred three participants (71 females, 32 males; mean age = 22.14 years, SD = 3.41) were recruited through newspaper ads and the university study pool. They were paid $25 or received study-pool credit.
After application of physiological sensors, participants sat quietly for a 5-min baseline period. They then completed a modified version of the Trier Social Stress Test (Kirschbaum, Pirke, & Hellhammer, 1993)—a mock job interview consisting of speech and question-and-answer tasks. Participants were randomly assigned to one of three conditions: two designed to activate the sympathetic nervous system (stress conditions) and a control condition. In the stress conditions, participants were asked to imagine that they were interviewing for a desirable job. They were given 5 min to prepare a speech describing their strengths and weaknesses and then delivered this speech to two interviewers. The interviewers then asked participants to answer a series of questions similar to those asked in job interviews. In between the speech and question-and-answer tasks, participants completed demand and resource appraisals (Blascovich & Tomaka, 1996). On the basis of previous research, we manipulated challenge and threat states using positive and negative feedback during the interview (Akinola & Mendes, 2008). Approximately 30 s into the speech, the interviewers for participants assigned to the positive-feedback condition began to express positive nonverbal feedback by nodding, smiling, and leaning forward. In the negative-feedback condition, interviewers expressed negative nonverbal feedback by shaking their heads, furrowing their brows, and crossing their arms.
Participants in the control condition completed the same tasks but were alone in the room; they gave the speech and answered questions aloud, but with no evaluation. Questions were provided on index cards that the subjects read and then answered aloud. We instructed the control participants that we were interested in physiological changes associated with speaking, but we would not be watching them during the task.
Immediately after the interview, all participants were given 2 min to provide their best guesses in response to nine anchoring-and-adjustment questions labeled “Trivia” (Epley & Gilovich, 2001).
Throughout the experiment, the following physiological data were obtained: electrocardiography (ECG, Biopac, Goleta, CA), impedance cardiography (HIC-2000, Instrumentation for Medicine, Chapel Hill, NC), and continuous blood pressure (Colin 7000, Colin Medical Instruments, San Antonio, TX). All signals were integrated with Biopac MP 150 hardware. Signals were examined off-line; data were scored manually using Mindware software (Mindware Technologies, Lafeyette, OH; see Mendes, 2009, for details), and a subsample was rescored to assure reliability.
RESULTS
Manipulation Checks
We first tested whether we had engendered a stressful situation by examining changes in participants' preejection period (PEP) and differences in their self-reported appraisals. PEP, the amount of time between left ventricular contraction and the opening of the aortic valve, provides a direct measure of sympathetic nervous system activity (Brownley, Hurwitz, & Schneiderman, 2000). Decreases in PEP from baseline indicate that the sympathetic nervous system is activated and can be used to identify a motivated-performance situation, which is a precondition for analysis of challenge and threat CV responses (Mendes, Reis, Seery, & Blascovich, 2003). During the speech task, both stress conditions resulted in significant decreases in PEP from baseline, whereas the control condition did not—positive feedback: M = −5.33, t(27) = −5.18, prep > .996; negative feedback: M = −6.35, t(27) = −4.84, prep > .996; control: M = −1.07, t(32) = −1.02, prep = .633.
Cognitive appraisals were assessed by probing perceived demands and resources for coping with the interview task. Six questions tapped demand appraisals (α = .76), and five assessed resource appraisals (α = .79). As intended, the negative-feedback condition resulted in higher demand (M = 4.6) and lower resource (M = 38) appraisals than the positive-feedback condition (demands: M = 3.9; resources: M = 4.3), F(1, 66) = 6.63, prep = .945, and F(1, 66) = 4.51, prep = .897, respectively.
Adjustment
To create an index of adjustment, we followed the procedure outlined by Epley and Gilovich (2001), subtracting the appropriate anchor from each answer. Statistical outliers (values more than 2.5 SD above or below the mean) were removed, and adjustments were standardized and averaged, creating a single index of the amount of adjustment for each participant.
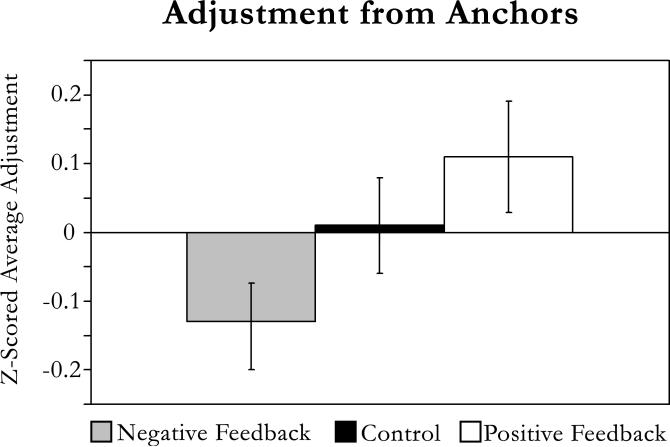
A one-way analysis of variance with feedback condition (positive, negative, control) as the independent variable and adjustment as the dependent variable yielded a marginally significant effect, F(2, 95) = 2.78, prep = .852. A planned comparison revealed significantly more adjustment in the positive-feedback condition (M = 0.11, SD = 0.45) than in the negative-feedback condition (M = −0.13, SD = −0.32), F(1, 95) = 5.45, prep = .923, as predicted (see Fig. 1).
Fig. 1.
Adjustment from self-generated anchors as a function of condition. Error bars represent standard errors of the mean.
CV Profiles
We then examined whether the CV responses engendered by our manipulations mediated the link between condition and adjustment. Because challenge and threat profiles assume sympathetic activation, only the two stress conditions were appropriate for examination (Mendes et al., 2003). We created an index of CV responses by summing cardiac output and vascular reactivity after standardizing the indicators and reverse-coding cardiac output; higher numbers indicate increased threat (Blascovich, Seery, Mudridge, Norris, & Weisburch, 2004). As is standard in challenge-and-threat research, we focused on the 1st minute of the question-and-answer task, which occurred after the feedback manipulation. We used the CV threat index to test whether physiological changes during the stressor mediated the effect of feedback condition on adjustment, following the strategy outlined by Baron and Kenny (1986). To control for individual differences in physiological reactivity (Stern, Ray, & Quigley, 2001), we used the CV threat index from the speech-preparation period, which preceded the feedback manipulation, as a covariate.2
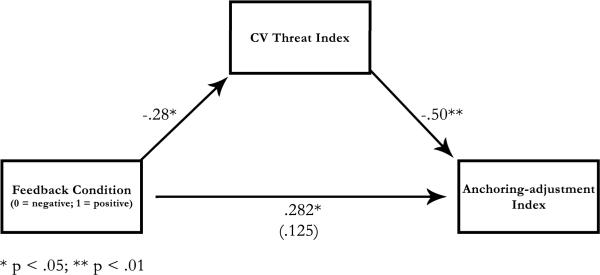
The first regression3 tested the link between feedback condition and adjustment, t(45) = 1.98, prep = .872—as described earlier, participants who had received positive feedback (challenge condition) adjusted more than those who had received negative feedback (threat condition; see Fig. 2). A second regression tested the link between feedback condition and CV responses. This regression equation yielded a significant effect, t(44) = −2.30, prep = .915. Participants who had received positive feedback had lower threat reactivity than those who had received negative feedback. A third regression predicted adjustment using feedback condition and CV reactivity. CV reactivity predicted adjustment, t(43) = −3.05, prep = .970, with increased CV threat resulting in decreased adjustment. The bootstrap-estimated indirect effect (Preacher & Hayes, 2008) was .099 (SE = .051), prep = .888, indicating that inclusion of the CV threat index resulted in a reliable decrease in the relationship between feedback condition and the adjustment index.
Fig. 2.
Results of the regression analysis testing the cardiovascular (CV) threat index as a mediator of the relation between condition and adjustment from self-generated anchors. Paths are represented as standardized betas; the path in parenthesis represents the condition-to-adjustment path controlling for CV threat. Asterisks indicate the significance of the path coefficients, *p < .05, **p < .01.
Ancillary Analyses: Parasympathetic Influences
We also examined the role of parasympathetic nervous system (PNS) activity in predicting adjustment. Challenge and threat responses are mediated by sympathetic influences, and as a result the extant literature on challenge and threat has largely ignored the role of PNS activity (cf. Quigley, Barrett, & Weinstein, 2002). However, PNS withdrawal has been associated with tasks such as target shooting and mental arithmetic, and these findings suggest a possible link with attention and mental effort (Tattersall & Hockey, 1995). It was therefore important to examine PNS activity in the context of adjustment in our study.
Parasympathetic influences, operating through the vagus nerve, result in characteristic high-frequency variation in the heart's rhythm. One method of indexing the level of parasympathetic activity is by quantifying the amount of that variation, in particular, by measuring the amplitude of variation in the frequency band defined by respiration—that is, respiratory sinus arrhythmia (RSA; Porges, 2007). Across all conditions and controlling for respiration rate, we observed a significant negative correlation between the adjustment index and RSA reactivity during the anchoring-and-adjustment task, r(83) = −.33, prep = .976; decreases in RSA activity were associated with greater adjustment. The magnitude of the correlation did not differ by condition. The significant correlation observed provides preliminary evidence that parasympathetic withdrawal might be linked to greater conscious control.
DISCUSSION
Type of stress, and not just the amount of stress, can have a significant impact on people's abilities to excel on cognitive tasks. Participants who were placed in a stressful situation and received positive feedback cognitively adjusted more than those who were placed in the same situation but experienced negative feedback, an effect that was mediated by CV reactivity. Participants who exhibited CV responses consistent with challenge (increased cardiac output and decreased vascular reactivity) showed greater cognitive adjustment than those who exhibited CV responses consistent with threat (decreased cardiac output and increased vascular reactivity).
There are at least two possible explanations for how challenge and threat states might have influenced adjustment as observed in this study. One possibility is that the stress profiles created differences in mental and physical resources that led to differences in adjustment. Challenge states are characterized by greater resources than threat states (Mendes, Gray, Mendoza-Denton, Major, & Epel, 2007), so participants who experienced more challenge may have had greater cognitive resources available, and thus an increased ability to adjust. A second possibility is that challenge and threat states provided bodily signals to approach and avoid, respectively (Niedenthal, Barsalou, Winkielman, Krauth-Gruber, & Ric, 2005). In previous research (e.g., Mendes, Blascovich, et al., 2007), challenge states were correlated with approach body positions, and threat states were correlated with withdrawal body positions. It may be the case that challenge and threat states and their concomitant body positions provide internal cues to individuals to adjust more or less, respectively. Future research will be needed to determine which of these mechanisms account for the effects observed. Also, it is important to point out that these findings are limited to a highly specific context—specifically, a highly stressful situation—and we would not anticipate observing the same results in low- or no-arousal situations.
These findings have important implications for recent research showing that incidental emotions can influence decision making. For example, anger has been shown to increase optimism and risk taking relative to fear (Lerner & Keltner, 2000), and sadness affects people's willingness to buy and sell consumer goods (Lerner, Small, & Loewenstein, 2004). As work in this area continues to develop, it will be interesting to see if changes in specific emotions influence decision making by way of physiological changes, as we have shown here for challenge and threat.
Acknowledgments
This research was funded in part by a National Heart, Lung, and Blood Institute grant (RO1 HL079383) to W.B.M.
Footnotes
Although challenge-and-threat theory explicitly considers the role of the two stress systems, typically SAM activation is measured, and HPA activation is inferred (though see Mendes, Gray, Mendoza-Denton, Major, & Epel, 2007). Other researchers, however, have done the opposite: assessed HPA activation and inferred SAM activation (Dickerson & Kemeny, 2004).
We also ran these analyses without controlling for individual responses preceding feedback. The results yielded effect sizes similar to those of the models with the covariate.
We were unable to calculate the threat index for 16 participants because of various problems with the physiological data; therefore, the mediational analysis is based on an N of 47. The threat index requires multiple sources of information—continuous blood pressure, impedance cardiography, and electrocardiography—and cannot be calculated if any one of those elements is missing. The number of participants with missing threat-index data did not differ significantly by condition, χ2(1, N = 63) = 0.67, n.s.
REFERENCES
- Akinola M, Mendes WB. The dark side of creativity: Biological vulnerability and negative mood leads to greater artistic creativity. Personality and Social Psychology Bulletin. 2008;34:1677–1686. doi: 10.1177/0146167208323933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer NJ, Nevill AM, Lane AM, Ward P, Williams AM, Fairclough SH. Influence of crowd noise on soccer refereeing consistency in soccer. Journal of Sport Behavior. 2007;30:140–145. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Blascovich J, Seery MD, Mudridge CA, Norris KR, Weisburch M. Predicting athletic performance from cardiovascular indexes of challenge and threat. Journal of Experimental Social Psychology. 2004;40:683–688. [Google Scholar]
- Blascovich J, Tomaka J. The biopsychosocial model of arousal regulation. In: Zanna MP, editor. Advances in experimental social psychology. Vol. 28. Academic Press; San Diego, CA: 1996. pp. 1–51. [Google Scholar]
- Bodenhausen GV. Stereotypes as judgmental heuristics: Evidence of circadian variations in discrimination. Psychological Science. 1990;1:319–322. [Google Scholar]
- Brownley KA, Hurwitz BE, Schneiderman N. Cardiovascular psychophysiology. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2nd ed. Cambridge University Press; New York: 2000. pp. 224–264. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dienstbier RA. Arousal and physiological toughness: Implications for mental and physical health. Psychological Review. 1989;96:84–100. doi: 10.1037/0033-295x.96.1.84. [DOI] [PubMed] [Google Scholar]
- Epley N. A tale of tuned decks? Anchoring as accessibility and anchoring as adjustment. In: Koehler DJ, Harvey N, editors. Blackwell handbook of judgment and decision making. Blackwell; Malden, MA: 2004. pp. 240–257. [Google Scholar]
- Epley N, Gilovich T. Putting adjustment back in the anchoring and adjustment heuristic: Differential processing of self-generated and experimenter-provided anchors. Psychological Science. 2001;12:391–396. doi: 10.1111/1467-9280.00372. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser M. A psychobiological framework for research on humans' stress and coping. In: Appley MH, Trumbull R, editors. Dynamics of stress: Physiological, psychological, and social perspectives. Plenum; New York: 1986. pp. 101–116. [Google Scholar]
- Gilbert DT. What the mind's not. In: Chaiken S, Trope Y, editors. Dual process theories in social psychology. Guilford; New York: 1999. pp. 3–11. [Google Scholar]
- Gilbert DT. Inferential correction. In: Gilovich T, Griffin DW, Kahneman D, editors. Heuristics and biases: The psychology of intuitive judgment. Cambridge University Press; Cambridge, England: 2002. pp. 167–184. [Google Scholar]
- Henry JP. Present concept of stress theory. In: Usdin E, Kvetnansky R, Kopin IJ, editors. Catecholamines and stress: Recent advances. Elsevier/North-Holland; New York: 1980. pp. 557–571. [Google Scholar]
- Jamieson J, Mendes WB, Blackstock E, Schmader T. Turning the knots in your stomach into bows: Reappraising arousal improves performance on the GRE. Journal of Experimental Social Psychology. doi: 10.1016/j.jesp.2009.08.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson G, Frankenhaeuser M, Magnusson D. Catecholamine output in school children as related to performance and adjustment. Scandinavian Journal of Psychology. 1973;14:20–28. doi: 10.1111/j.1467-9450.1973.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, Hellhammer DH. The 'Trier Social Stress Test': A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Lerner JS, Keltner D. Beyond valence: Toward a model of emotion-specific influences on judgment and choice. Cognition and Emotion. 2000;14:473–493. [Google Scholar]
- Lerner JS, Small DA, Loewenstein G. Heart strings and purse strings: Carryover effects of emotions on economic decisions. Psychological Science. 2004;15:337–341. doi: 10.1111/j.0956-7976.2004.00679.x. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Thomas TL. Stress hormones in psychophysiological research: Emotion, behavioral, and cognitive implications. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2nd ed. Cambridge University Press; New York: 2000. pp. 342–367. [Google Scholar]
- Mendes WB. Assessing the autonomic nervous system. In: Harmon-Jones E, Beer J, editors. Methods in social neuroscience. Guilford Press; New York: 2009. pp. 118–147. [Google Scholar]
- Mendes WB, Blascovich J, Hunter SB, Lickel B, Jost JT. Threatened by the unexpected: Physiological responses during social interactions with expectancy-violating partners. Journal of Personality and Social Psychology. 2007;92:698–716. doi: 10.1037/0022-3514.92.4.698. [DOI] [PubMed] [Google Scholar]
- Mendes WB, Gray HM, Mendoza-Denton R, Major B, Epel ES. Why egalitarianism might be good for your health: Physiological thriving during stressful intergroup encounters. Psychological Science. 2007;18:991–998. doi: 10.1111/j.1467-9280.2007.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes WB, Reis HT, Seery MD, Blascovich J. Cardiovascular correlates of emotional expression and suppression: Do content and gender context matter? Journal of Personality and Social Psychology. 2003;84:771–792. doi: 10.1037/0022-3514.84.4.771. [DOI] [PubMed] [Google Scholar]
- Niedenthal PM, Barsalou LW, Winkielman P, Krauth-Gruber S, Ric F. Embodiment in attitudes, social perception, and emotion. Personality and Social Psychology Review. 2005;9:184–211. doi: 10.1207/s15327957pspr0903_1. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Quigley KS, Barrett LF, Weinstein S. Cardiovascular patterns associated with threat and challenge appraisals: A within-subjects analysis. Psychophysiology. 2002;39:292–302. doi: 10.1017/s0048577201393046. [DOI] [PubMed] [Google Scholar]
- Rauste-von Wright M, von Wright JM, Frankenhaeuser M. Relationships between sex-related psychological characteristics during adolescence and catecholamine excretion during achievement stress. Psychophysiology. 1981;18:362–370. doi: 10.1111/j.1469-8986.1981.tb02467.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and cognition. In: Gazzaniga M, editor. The cognitive neurosciences. 3rd ed. MIT Press; Cambridge, MA: 2004. pp. 1031–1040. [Google Scholar]
- Schaeffer MH. Environmental stress and individual decision-making: Implications for the patient. Patient Education and Counseling. 1989;13:221–235. [Google Scholar]
- Stern RM, Ray WJ, Quigley KS. Psychophysiological recording. 2nd ed. Oxford University Press; New York: 2001. [Google Scholar]
- Tattersall AJ, Hockey GRJ. Level of operator control and changes in heart rate variability during simulated flight maintenance. Human Factors. 1995;37:682–698. doi: 10.1518/001872095778995517. [DOI] [PubMed] [Google Scholar]