Abstract
Many laboratory models used in aging research are inappropriate for understanding senescence in mammals, including humans, because of fundamental differences in life history, maintenance in artificial environments, and selection for early aging and high reproductive rate. Comparative studies of senescence in birds and mammals reveal a broad range in rates of aging among a variety of taxa with similar physiology and patterns of development. These comparisons suggest that senescence is a shared property of all vertebrates with determinate growth, that the rate of senescence has been modified by evolution in response to the potential life span allowed by extrinsic mortality factors, and that most variation among species in the rate of senescence is independent of commonly ascribed causes of aging, such as oxidative damage. Individuals of potentially long-lived species, particularly birds, appear to maintain high condition to near the end of life. Because most individuals in natural populations of such species die of aging-related causes, these populations likely harbor little genetic variation for mechanisms that could extend life further, or these mechanisms are very costly. This, and the apparent evolutionary conservatism in the rate of increase in mortality with age, suggests that variation in the rate of senescence reflects fundamental changes in organism structure, likely associated with the rate of development, rather than physiological or biochemical processes influenced by a few genes. Understanding these evolved differences between long-lived and short-lived organisms would seem to be an essential foundation for designing therapeutic interventions with respect to human aging and longevity.
Introduction
Most organisms with determinate body size exhibit senescence—a decrease in functional capacity with age—and have finite life spans (Finch, 1990; Carnes, Staats & Sonntag, 2008). We are well aware of this quality in humans and the domesticated and laboratory animals with which we are most familiar. Long-term studies of age-related mortality in natural populations have confirmed that senescence is a shared characteristic of all birds and mammals (Finch, 1990; Austad & Fischer, 1991; Gaillard, Allaine, Pontier et al., 1994; Holmes & Austad, 1995; Ricklefs, 1998; Loison, Festa-Bianchet, Gaillard et al., 1999). Humans understandably would like to extend their lives and reduce the consequences of aging-related decline in condition and function. To this end, we have provided substantial resources for research on aging in animal models, with new results appearing every week in the most prominent professional journals. Work on animals as different as roundworms, flies, and mice has revealed commonalities in the aging process, which offer the promise of understanding factors that cause senescence and suggest possible interventions (Smith, Tsuchiya, Fox et al., 2008). Several hypotheses concerning controls over the rate of aging have received particular attention, among which oxidative damage (Barja, 2004; Monaghan, Metcalfe & Torres, 2009), telomere shortening (Haussmann, Winkler & Vleck, 2005; Monaghan & Haussmann, 2006; Finkel, Serrano & Blasco, 2007), stress resistance (von Zglinicki, Burkle & Kirkwood, 2001; Gems & Partridge, 2008), and IGF-1 signaling pathways (Selman, Lingard, Choudhury et al., 2008) play a prominent role. Age at death has a modest genetic heritability in humans and other mammal populations (McGue, Vaupel, Holm et al., 1993; Yashin, De Benedictis, Vaupel et al., 1999; Reale & Festa-Bianchet, 2000; Christensen, Frederiksen, Vaupel et al., 2003; Ricklefs & Cadena, 2008; Wilson, Charmantier & Hadfield, 2008), and so the pattern of aging-related mortality apparently can be selected and is subject to evolutionary modification. Many genes appear to profoundly influence life span (Bartke, Coshigano, Kopchick et al., 2001; Finch & Ruvkun, 2001), further suggesting that gene therapy might prove to be effective.
In spite of considerable progress in understanding the causes of senescence, there are still some caveats. Many animal models are poor comparators for humans, and for species with long life spans in general, and it is unclear how easily knowledge gained from these model systems will transfer to human aging (Ricklefs, 2008). For practical considerations, animal models have short life spans, and furthermore have been selected for early reproduction, often resulting in accelerated senescence (Carnes & Olshansky, 2001; Miller, Harper, Dysko et al., 2002). Many have profoundly different life histories compared to mammals. For example, fruit flies (Drosophila) undergo a complete metamorphosis, which separates early development and adult life by a complete remodeling of the organism. Many animal models are so highly inbred, and are maintained under such unnaturally favorable conditions, that genetic effects become difficult to interpret in the context of “normal” aging (e.g., Van Voorhies, Fuchs & Thomas, 2005).
Evolutionary and comparative biologists have argued that the study of aging in natural populations can provide insights into the mechanisms responsible for senescence and suggest new approaches for laboratory studies (Promislow, Fedorka & Burger, 2006; De Magalhaes, Costa & Church, 2007; Wilson, Charmantier & Hadfield, 2008; Holmes & Martin, 2009). The rate of aging varies widely among animals (Finch, 1990) and is presumed to be under selective pressure (Kirkwood & Rose, 1991; Rose, 1991; Ricklefs, 1998; Kirkwood, 2002; Reznick, Bryant & Holmes, 2006). Accordingly, the observed rate of aging would reflect the evolutionary optimization of conflicting demands on the individual through its life. An important concept has been that longer life comes at the expense of reproductive success early in life (Westendorp & Kirkwood, 1998; Kirkwood & Westendorp, 2000; Lycett, Dunbar & Voland, 2000; Zera & Harshman, 2001; Robinson, Pilkington, Clutton-Brock et al., 2006) because resources that would otherwise be used to produce offspring are needed to prevent or repair damage to the parent individual and maintain parental condition (Kirkwood, 1990; Kirkwood & Austad, 2000). This type of thinking would apply to mechanisms that reduce oxidative damage, prevent autoimmune reactions in old age, or control tumor formation through telomere shortening and oncogene repression. Clearly, if we are to understand the mechanisms that enable some organisms to achieve life spans similar to our own, we should try to understand what these organisms have in common.
Actuarial senescence
Information about condition, reproductive success, and causes of death in natural populations of long-lived organisms is gained only with difficulty. Although field studies, many of them continuing for several decades, use increasingly sophisticated methods to assess health, the basic data chronicling senescence in the wild are reproductive success and age at death. Because an individual dies only once, death cannot portray changes in condition with age. However, because individuals tend to decline in health as they become older, their probability of death increases with age. This relationship can be used to describe the rate of aging in a population, sometimes called actuarial senescence (Holmes & Austad, 1995), and it provides a basis for the comparative analysis of aging in natural populations. Where comparisons can be made, actuarial senescence and reproductive senescence generally go together (Ricklefs, Scheuerlein & Cohen, 2003), so that one can interpret changes in the probability of death with age as a general manifestation of senescence. Equally important, the relative temporal patterns of mortality causes appear to be conserved across mammal species with highly divergent rates of actuarial senescence, including humans, dogs, and mice (Carnes & Olshansky, 1997; Carnes, Holden, Olshansky et al., 2006).
Several mathematical functions have been used to characterize the change in mortality rate with age based on ages at death within a population. Rates of actuarial senescence estimated in this manner are complex indices reflecting both the general pattern of aging within a population and heterogeneity among individuals in the rate of aging (Vaupel, Manton & Stallard, 1979). Regardless, the various aging functions span a single important contrast in the interpretation of aging-related deaths (Gavrilov & Gavrilova, 1991; Wilson, 1994; Carnes, Olshansky & Grahn, 1996; Ricklefs & Scheuerlein, 2002; Lynch & Fagan, 2009). The commonly applied Gompertz function can be expressed as
| (1) |
where mx is mortality at age x, m0 is the mortality rate of young adults, generally the minimum mortality rate experienced by a population, and γ (gamma) is the exponential rate of increase in the mortality rate with age. γ is expressed in units of 1/time, or time−1. In this model, the rate of mortality increases exponentially with age, and its level at any given age is directly proportional to the initial level m0. The Gompertz function can be interpreted as describing the increasing vulnerability of an individual to causes of mortality experienced throughout life, including early adulthood.
Alternatively, the Weibull function describes aging-related mortality as independent of, and added to, the initial mortality suffered by young adults. The function can be expressed as
| (2) |
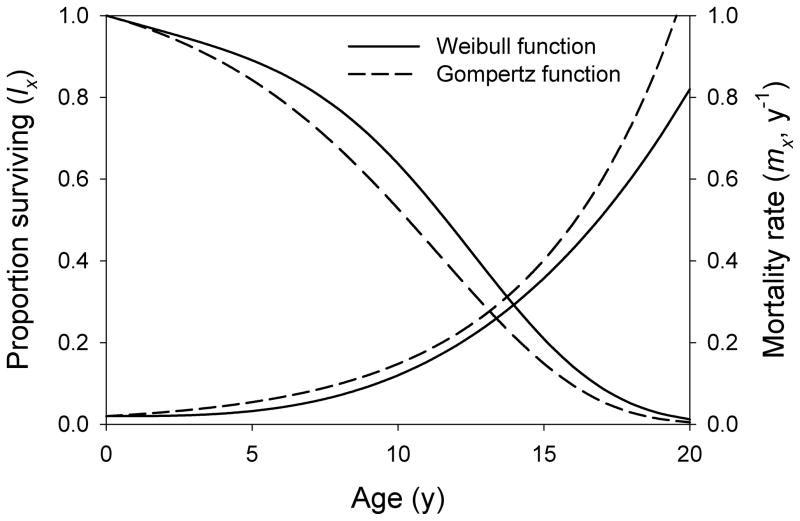
where β (beta) describes the acceleration of aging-related mortality with age and α (alpha) is a scaling factor influencing the rate of mortality at all ages; the age-related component of mortality at age x = 1 is equal to α. The parameters α and β can be combined to form a variable (omega, ω) related to the overall rate of aging by ω = α1/(β+1), which, like γ, has units of time−1 (Ricklefs, 1998). The Gompertz and Weibull functions describe actuarial senescence in animal populations about equally well (Figure 1) (Ricklefs & Scheuerlein, 2002). However, the parameters have different interpretation depending on whether aging-related mortality is dependent on (Gompertz) or independent of (Weibull) the initial mortality rate.
Figure 1.
Examples of mortality rate and survival as a function of age for Weibull and Gompertz functions with parameters chosen to match closely (Weibull: m0 = 0.02, α = 0.0001, β = 3; Gompertz: m0 = 0.02, γ = 0.02).
As a reasonable approximation, the initial mortality m0 can be interpreted as death caused by extrinsic factors, including predators, pathogens, food shortages, and inclement weather, that affect healthy young individuals. If aging-related mortality reflects increasing vulnerability to such factors because of declining condition, then reducing the impact of these factors (reducing m0), for example by bringing a population into captivity, should reduce aging-related mortality. In this case, the Gompertz function would provide a biologically useful description of actuarial senescence. In contrast, if older animals die of intrinsic consequences of aging not experienced by younger individuals, including degenerative vascular disease and most types of cancer, then protecting them from extrinsic causes of death should have no effect on aging-related mortality and the Weibull function, which partitions extrinsic and intrinsic causes, would be appropriate.
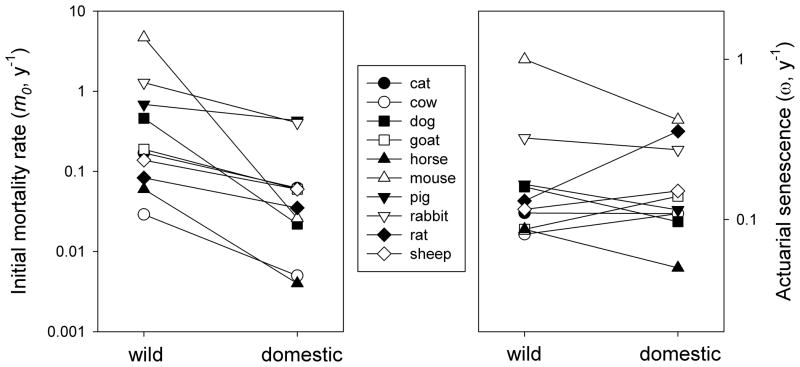
Where animals have been brought into captivity, for example in zoological gardens, estimates of m0 typically decrease dramatically, as one would expect, but ω (as well as maximum reported longevity) tends to remain unchanged on average (Ricklefs, 2000; Ricklefs & Scheuerlein, 2001). The comparison between domesticated animals and populations of related wild species is more compelling because domesticated animals are bred to live in captivity and are generally well cared for, minimizing mortality from extrinsic and stress-related causes. As shown in Figure 2, the estimated extrinsic mortality (m0) of the domesticated forms is only a quarter, on average, of that in populations of related species in nature, but the aging-related component of mortality is about the same.
Figure 2.
Initial mortality rate (m0, left) and rate of increase in mortality rate (ω, right) in wild and domesticated representatives of 10 lineages of mammals. Lineage is a significant effect for ln(ω) (F9,9 = 5.6, P = 0.008) but not for ln(m0) (F9,9 = 2.2, P = 0.13); the difference between wild and domesticated is significant for ln(m0) (F1,9 = 6.3, P = 0.03; means, −1.30 vs. −0.67), but not for ln(ω) (F1,9 = 0.3, P = 0.57; means, −0.79 vs. −0.84). m0 decreases with domestication in all 10 species (P = 0.001), but ω increases in half the species and decreases in the other half. The correlation coefficient (r) between m0 and ω was 0.77 (P = 0.01) for the wild populations and 0.37 (P = 0.29) for the domesticated populations (Ricklefs and Scheuerlein, unpubl. data).
Comparisons between captive or domesticated populations and populations in nature suggest that the Weibull function describes actuarial senescence in a biologically more interesting way than the Gompertz function. As we shall see, the parameters of the Weibull function allow us to relate actuarial senescence to extrinsic mortality as independent measures. An inescapable implication of the choice between these two functions is that aging-related mortality is more closely associated with intrinsic, catastrophic failure of organ systems than it is with increasing vulnerability to extrinsic causes of mortality (Ricklefs, 2008). No doubt an arthritic gazelle is easier prey for a lion than a healthy, young individual, but even if were to escape predation the older individual might soon die of an intrinsic catastrophic cause. Without knowing what kills most animals in natural populations, it is difficult to evaluate the validity of different models of aging-related mortality directly.
Aging and the life history
Being able to quantify the rate of actuarial senescence with a single parameter, such as ω, makes it possible to compare rates of aging across many taxa. Comparative studies demonstrate that the rate of aging decreases, and maximum longevity increases, with adult mass (Promislow, 1991; Promislow, 1993; Ricklefs, 1998; De Magalhaes, Costa & Church, 2007; Ricklefs, 2007), as is the case with many physiological functions (Calder, 1984; Calder, 1985). For example, in a sample of 62 studies of natural populations representing 30 species of mammal in 17 families and 9 orders, and 12 species of bird from 9 families in 3 orders, log-transformed values of ω were inversely related to log-transformed body mass with a slope of −0.150 ± 0.026 SE. However, body size does not tell the whole story because, for the same body size, rates of aging were 0.319 ± 0.096 log10 units (a factor of 2.1) higher in mammals than in birds (Figure 3, left).
Figure 3.
Left: Logarithmic relationship between the rate of actuarial senescence in natural populations of birds (open symbols) and mammals (filled symbols) as a function of adult body mass. The relationship, including the difference between birds and mammals, accounts for 37% of the variance in ω. Numbered species of mammals that lie below the regression line for birds are: 1, Common Pipistrelle Bat Pipistrellus pipistrellus; 2, Ermine Mustela erminea; 3, Senegal Bushbaby Galago senegalensis; 4, Rhesus Macaque Macaca mulatta; 5, Chimpanzee Pan troglodytes; 6, Common or Harbor Seal Phoca vitulina. Right: Logarithmic relationship of the rate of actuarial aging to the initial (extrinsic) mortality rate. The regression accounts for 42% of the total variance in ω. Data from various sources, mostly summarized by Ricklefs (1998) and by Lynch and Fagan (2009).
A prominent evolutionary theory proposes that the rate of aging should be directly related to the extrinsic mortality suffered by a population (Williams, 1957; Kirkwood, 1977; Rose, 1991; Charlesworth, 1993; Kirkwood, 2002). The reasoning is that if an individual’s probability of surviving to reproduce in the future is low, it should allocate resources to current offspring even if this sacrifices longevity. Comparative studies bear this out (Figure 3, right). The rate of aging (ω) in the same sample of birds and mammals increases in proportion to the 0.402 ± 0.061 SE power of m0, and birds are not statistically distinguishable from mammals in this regard. The substantial variation about the regression line reflects, to an unknown extent, other considerations that affect the rate of aging, and errors in estimating the parameters m0 and ω.
Because the rate of actuarial senescence is related more closely to extrinsic adult risk than to body mass, a connection between aging and metabolic rate is not evident in comparative studies, assuming that metabolism and body mass are closely tied (De Magalhaes, Costa & Church, 2007). In other words, the chain of causality appears to be not body size→metabolic rate→rate of aging, but rather body size→safety→rate of aging. To the extent that this is true, anti-oxidant theories of aging could not explain evolutionary diversification in potential longevity among species (Holmes & Martin, 2009), although oxidative damage might account for some of the unexplained variance in these relationships. The observation that many of the more active mammals, including bats and carnivores (as well as birds), have relatively low rates of aging for their body size (Austad & Fischer, 1991), yet relatively high rates of metabolism, throws further doubt on the hypothesis that aging is a simple byproduct of metabolism. The comparative data suggest, therefore, that the consequences of oxidative damage for aging-related mortality are controlled by other processes subject to evolutionary modification.
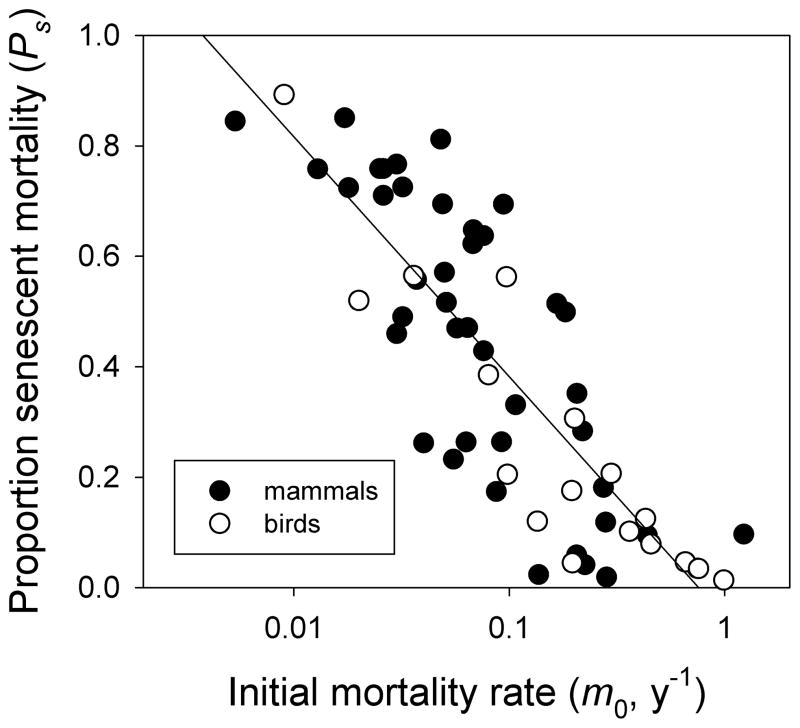
Evolutionary responses depend on both genetic variation in a population and selection acting on that variation. For the rate of aging, the potential strength of selection on genetic factors that could modify this rate is directly related to the proportion of aging-related mortality (Ps) in a population estimated from the fitted parameters of the Weibull function (Ricklefs, 1998). This represents the proportion of adult deaths that are due to aging-related causes and which would be reduced by slowing the rate of actuarial senescence. Although the rate of senescence (ω) declines with increasing potential longevity allowed by extrinsic causes (1/m0, see Figure 3, right), this decline is not fully compensating. As a result, the proportion of aging-related mortality increases with the average life span in a population, as seen in Figure 4. That is, few individuals in populations with high extrinsic mortality live long enough to experience aging-related causes of death, whereas low extrinsic mortality allows individuals to reach an age at which mortality rate increases sharply. In fact, in populations for which m0 is less than 10% per year, senescent causes appear to account for 40% or more of all deaths. This proportion exceeds 80% for the species with the longest recorded life spans.
Figure 4.
Decrease in the proportion of aging-related mortality in natural populations of birds and mammals as a function of increasing extrinsic mortality (m0). The slope of the relationship is −0.435 ± 0.037 proportion of deaths per 10-fold increase in m0 (F1,57 = 136, P < 0.0001, r2 = 0.704). Birds and mammals are not distinguishable statistically. The species with the greatest longevity (upper left) are the African Bush Elephant Loxodonta africana and the Wandering Albatross Diomedea exulans. PS based on Weibull functions fitted to survival data compiled by Ricklefs (1998) and Lynch and Fagan (2009).
The remarkable conclusion from the estimates of Ps in Figure 4 is that potential selection on any mechanism that might extend life in long-lived organisms (including humans) is, and has been, very strong. By implication, genetic variation for such mechanisms simply does not exist in natural populations. Alternatively, mechanisms to prolong life beyond old ages might be available, but are very costly in terms of evolutionary fitness. Thus, long-lived birds and mammals appear to have exhausted evolutionary possibilities, by means of physiological and biochemical mechanisms, to further extend life.
For a given rate of acceleration of mortality in actuarial senescence (β), which typically is ca. 3 (Ricklefs, 1998), one can calculate the strength of selection on the scaling factor α, which influences mortality rate at all ages. The characteristic equation (Euler’s equation) based on age specific birth (bx) and survival (lx) in a population, can be used to estimate fitness, which can be defined as the growth rate of the population of individuals with a particular phenotype. In this context, fitness is expressed as the symbol λ for geometric growth in the relationship
| (3) |
Most natural populations experience density-dependent feedbacks on births and deaths and, on average, population size neither increases nor decreases, in which case λ = 1 and the sensitivity of λ to a change in α is expressed by
| (4) |
where
| (5) |
(Hamilton, 1966). Because α varies over many orders of magnitude among populations, it makes more sense to calculate the change in λ in response to a proportional change in α, i.e., αdλ/dα (Figure 5). What is striking in Figure 5 is that the selection on absolute changes in α is strongest in those populations having the lowest value of α, that is, those with the slowest actuarial senescence. Selection on relative (proportional) changes in α is about equally strong across the range of m0.
Figure 5.
The increase in fitness (λ) as a function of absolute and relative decreases in the scaling parameter (α) of the Weibull aging function, shown as a function of the initial mortality rate in the population, given the relationship between m0 and ω portrayed by the regression line in Figure 3.
Aging-related mortality
The fact that the pattern of aging-related mortality in a particular population is independent of extrinsic mortality factors in the environment (Figure 2) implies that causes of aging-related death are largely intrinsic to the organism. Although an individual might lose condition through gradual deterioration of structure and function with age, supposed increasing vulnerability to extrinsic mortality factors apparently is paralleled by an increasing probability of death from intrinsic causes. Indeed, to the extent that intrinsic causes of death might be catastrophic, individuals might actually maintain a high level of condition until they die, whether by extrinsic causes or intrinsic aging-related causes (Ricklefs, 2000; Coulson & Fairweather, 2001; Ricklefs, 2008).
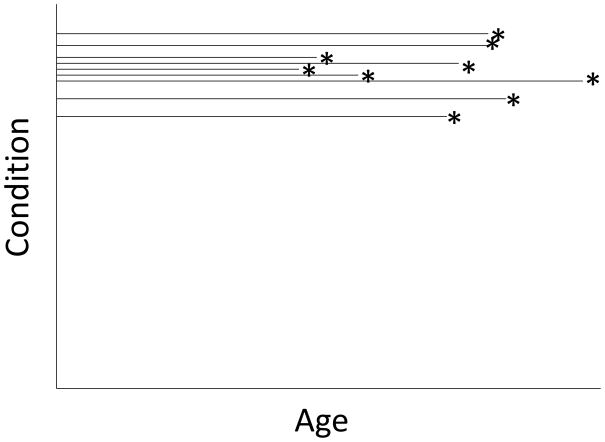
If an individual’s condition were to deteriorate gradually with age, one would expect to find that measures of function, including reproductive success, might also decrease with age. The left-hand panel in Figure 6 portrays a population in which individuals decline in condition with age at an accelerating pace until they die. If extrinsic causes exerted little mortality pressure on the population, most individuals would die of “old age” simply because they no longer functioned well. Alternatively (Figure 6, right-hand panel), individuals could maintain a high level of function until late in life, when the probability of catastrophic death increases. The age-related pattern of mortality in these two populations might be similar, but one would expect reproductive success to be maintained at a high level until near the end of life in the second population.
Figure 6.
Left: Individuals decline in condition until death (*), which is often a direct consequence of reduced performance in old age. Right: Individuals maintain a high level of condition into old age, but die with increasing probability at older ages from catastrophic causes. Ages at death are the same in both panels.
Longitudinal studies of individual performance in wild populations support both scenarios. In most species of mammal that have been investigated (primarily ungulates), condition and reproductive performance deteriorate noticeably among individuals that reach advanced ages (Clutton-Brock, Albon & Guinness, 1988; Gaillard, Allaine, Pontier, Yoccoz & Promislow, 1994; Clutton-Brock & Isvaran, 2007). Similar patterns have been detected in very large samples of small songbirds that include old-age individuals (McCleery & Perrins, 1989; Sternberg, 1989; Reid, Bignal, Bignal et al., 2003a). In contrast, several studies on long-lived seabirds have failed to detect significant declines in physiological markers (Nisbet, Finch, Thompson et al., 1999) or reproductive success (Coulson & Fairweather, 2001; Nielsen & Drachmann, 2003; Catry, Phillips, Phalan et al., 2006; Reed, Kruuk, Wanless et al., 2008) until close to the end of an individual’s life, regardless of its age at death. Thus, in Kittiwake Gulls (Rissa tridactyla), for example, Coulson and Fairweather (2001) observed a decrease in reproductive success only in the last year before an individual died. These observations imply that long-lived seabirds, and perhaps other species, maintain high levels of function throughout their lives, until they finally succumb to catastrophic death, whether intrinsic or extrinsic. Nonetheless, such species clearly exhibit actuarial senescence in the sense that the mortality rate increases with age, and a large proportion of the adults in such populations die of aging-related causes. In this case, however, aging is not accompanied by a decrease in overall condition. Of course, little is known about the causes of death in these populations.
The evolution of delayed senescence
Birds and mammals appear to differ with respect to the maintenance of condition into old age, and perhaps seabirds also are special in this respect. Birds are rather remarkable for their longevity compared to mammals of similar body size, particularly considering their high rates of metabolism and other unfavorable (from a mammalian point of view) physiological markers, such as high blood glucose levels (Holmes & Martin, 2009). Possibly this difference is related to flight, which allows populations to avoid many causes of extrinsic mortality and thus establishes selection for postponed senescence. Among mammals, bats, many carnivores, and such social types as primates also exhibit low extrinsic mortality and correspondingly low rates of actuarial senescence. Thus, the overall difference between birds and mammals with respect to aging might simply reflect their different environments and evolution leading to different resolution of a life span-reproduction trade-off.
The apparent maintenance of youthful condition by birds until late in life also might be related to the stringencies of flight. An earth-bound animal might walk or run a little slower, but still could get around. An organism that depends on flight for feeding, escaping enemies, or migrating to wintering grounds cannot function at less than a high level of condition because flight is physiologically demanding. Many pelagic seabirds—species, such as albatrosses, which forage over great distances—might have an additional constraint on senescence. Because they depend on such a sparsely distributed resource base, these birds rear only a single young each year (or every other year in some cases). When many offspring are produced at one time, a decline in parental condition might result in lower reproductive success; with a single offspring, a decline in condition results in none. Thus, selection to maintain condition in such species must be very strong.
The genetic basis of senescence
Both the phylogenetic conservatism in the evolved rate of aging and the stability of the age-related component of mortality between natural and domesticated populations (Figure 2) imply that variation in the rate of aging among taxa has a strong genetic component. Although many genes have been shown to have strong effects on life span in model systems (Finch & Ruvkun, 2001; Arking, 2006), these probably are not the genes that differentiate evolved rates of aging among natural populations. The genetic basis of evolved variation in aging is poorly understood. Three ideas have gained prominence (Rose, 1991; Charlesworth, 1993). (1) Mutation accumulation: because fewer individuals survive to advanced age, genes expressed at older ages are less frequently exposed to selection, and deleterious germ line mutations are likely to accumulate and result in a decline in condition with age. (2) Antagonistic pleiotropy: certain genes that benefit individuals early in life but alter their expression to become deleterious later (so called pleiotropic genes) are maintained in populations because the strength of selection declines with age and positive selection at young ages predominates (Rose, 1991). (3) Disposable soma: a subtle variation of (2) in which certain genes influence processes throughout life that affect fitness differently early and late in life, although gene expression need not change with age (Kirkwood, 1981; Kirkwood, 1990). For example, a damage prevention or repair mechanism might prolong potential life span but exert a cost in terms of annual reproductive success. The optimal expression of such a process would depend on the life span potential set by extrinsic mortality factors.
Although evidence for mutation accumulation (mechanism 1) has been obtained for laboratory populations, I believe that this cannot be responsible for evolved differences between natural populations, simply because the strength of selection to remove deleterious mutations is strong in populations of long-lived as well as short-lived species (Figure 5). Mechanism 2 (antagonistic pleiotropy) is plausible, but few genes appear to change their expression with age to produce contrasting effects in young and old individuals. Oncogenes undergo somatic mutation or change in expression to cause tumors (Cutler & Semsei, 1989; Croce, 2008), but one could argue that the expression of these mutations as aging-related deaths depends on genetically controlled mechanisms of prevention and repair, which are referable to the disposable soma theory. Any gene with opposite effects on different components of evolutionary fitness, for example, that increases fecundity while decreasing survival rate (mechanism 3), is liable to be selected differently in populations with different levels of extrinsic mortality. Kirkwood (1990) has called this mechanism the “disposable soma” theory, recognizing that the individual, or soma, simply represents the way that DNA in the germ line propagates itself through time; that is, the individual has no special status in the eyes of evolution. Evolutionary biologists are generally persuaded that the rate of senescence is subject to selection and that differences between species represent evolutionary optimization of compromises of the sort embodied in genetic mechanisms 2 and 3.
Field studies of natural populations often find trade-offs between reproduction and age at death, or between reproductive success early and late in life (Roff, 1992; Stearns, 1992; Bennett & Owens, 2002; Reid, Bignal, Bignal, McCracken & Monaghan, 2003a; Reid, Bignal, Bignal et al., 2003b). These trade-offs likely reflect constraints on the allocation of limited resources in natural environments. In captive zoo populations of birds and mammals, no relationship was found between age at first reproduction and age at death or between number of offspring produced up to a certain age and survival beyond that age (Ricklefs & Cadena, 2007). Thus, reproduction per se does not appear to interact with longevity through intrinsic mechanisms involving changes in physiological state. Rather, these aspects of the life history are antagonistic only when environmental resources are limited. This line of evidence supports mechanism 3 (the disposable soma) rather than mechanism 2 (antagonistic pleiotropy).
If the rate of senescence were subject to evolutionary modification, and differences between species had a genetic basis, then one would expect to find genetic variation for the rate of senescence in natural populations. Of course, one cannot measure actuarial senescence for individuals, and so studies of inheritance have focused on age at death, which might bear a complex relationship to the rate of aging. Heritabilities, which measure the additive genetic contribution to phenotypic variation, typically are significant, but low, for age at death in populations of humans and in domesticated and laboratory animals (Yashin & Iachine, 1995; Finch & Tanzi, 1997). Significant heritability of age at death has been more difficult to identify in natural populations, perhaps because extrinsic mortality contributes a large component of non-genetic variance.
Ricklefs and Cadena (2008) examined the heritability of age at death in captive populations of wild animals, in which extrinsic causes of death are minimized. Using parent-offspring regression to estimate heritability, they found significant genetic contributions to age at death in several species of mammals, but none in birds (Figure 7). Thus, although difficult to detect, most populations of mammals, at least, possess significant genetic variation in age at death, although these genetic factors need not be the same as those that differentiate rate of actuarial senescence among species. It is also possible, for the same reasons that individual birds might maintain a high level of condition until late in life, that much genetic variation for age at death is removed from populations of birds.
Figure 7.
Heritability of age at death in captive (zoo) populations of birds and mammals plotted as a function of the probability (P) that heritability differs significantly from 0. The six species of mammal with P < 0.10 were, from the lowest value, the Lion (Panthera leo), Addax (Addax nasomaculatus), Golden Lion Tamarin (Leontopithecus rosalia), Domestic Goat (Capra hircus), Red Kangaroo (Macropus rufus), and Cheetah (Acinonyx jubatus). From data in Ricklefs and Cadena (2008).
The rate of actuarial senescence clearly varies among species and represents an evolved, intrinsic quality of individuals. The presence of genetic variation for age at death in populations suggests that rate of aging might easily be altered by selection. Certainly, many populations of laboratory model organisms, including Drosophila flies (Linnen, Tatar & Promislow, 2001) and mice (Miller, Harper, Dysko, Durkee & Austad, 2002), have been selected for early reproduction and rapid development, with a consequent reduction in life span. Selecting for increased longevity might be a different matter, however. Although selection on genetic variation in productive life span potential is strong, particularly in long-lived populations (Figure 5), evolution of longevity appears to be stalled in natural populations. If, on one hand, rate of aging were labile evolutionarily, one would expect to find a large part of the total variation in the rate of aging (ω) distributed among closely related species. If, on the other hand, rate of aging resisted selective pressure, variation would be distributed among higher taxonomic levels, representing more long-term evolutionary change of fundamental variation in body plan, physiology, or life history.
A hierarchical nested analysis of variance of adult mass (M) and ω among mammals based on the taxonomic levels order, family, genus, and species, shows a concentration of the variance at the levels of families within orders and orders within mammals, and a significant correlation between ω and M only among families within orders (Table 1). Although 26% of the variance in ω resides at the level of species within genera, most of this variation probably represents errors in estimating the parameter owing to small sample sizes. From this perspective, particularly considering the small amount of variance among genera within families, actuarial senescence appears to be relatively conservative. Either ω resists selection, or the strength of selection (i.e., related to m0) is conserved among closely related species and genera.
Table 1.
Nested analysis of variance in adult body mass (M) and rate of actuarial senescence (ω) in 160 species of mammal based on analysis of primarily captive populations (R. E. Ricklefs and A. Scheuerlein, unpubl.). The analysis partitions variance (proportion of the total) into components representing species within genera, genera within families, families within orders, and orders within mammals. Correlation coefficients (r) and regression slopes (b) are calculated from partitioned covariance components. Because the data are primarily from captive populations, m0 does not estimate the extrinsic mortality of natural populations and is not reported. Because of the way variance and covariance components are estimated, correlation coefficients can exceed 1 and −1. Bolded values for correlations and regression slopes were significant at P < 0.0001.
| Variance components |
Correlation (r) | Regression slope (b) | |||
|---|---|---|---|---|---|
| Level | df | M | ω | ω vs. M | ω vs. M |
| Total | 159 | 1.000 | 1.000 | −0.441 | −0.088 |
| Order | 14 | 0.583 | 0.340 | −0.173 | −0.026 |
| Family | 35 | 0.293 | 0.355 | −0.911 | −0.201 |
| Genus | 63 | 0.105 | 0.043 | −1.155 | −0.149 |
| Species | 47 | 0.019 | 0.262 | 0.114 | 0.085 |
Note: df = degrees of freedom.
Life history correlates of aging
Although rate of actuarial senescence appears to be evolutionarily conservative, its relationship to other aspects of the life history provide insights into possible mechanistic connections between life span and other traits, including body size and development rate (de Magalhaes & Church, 2005; De Magalhaes, Costa & Church, 2007). I analyzed variation in ω in relation to adult mass, neonate mass, weaning mass, length of gestation period, weaning period, and postnatal growth rate. Among 52 species of mammal for which all variables were available, and including taxonomic order as an effect to examine the relationships among variables within orders (see Table 1), neither neonate mass nor postnatal growth rate was a significant effect. With these variables removed, among 85 species of mammal for which the remaining variables were available, weaning mass also could be deleted as not contributing uniquely to variation in ω. Among the 129 species of mammal included in a final analysis, rate of actuarial senescence was inversely related to adult mass (F1,121=7.3, P = 0.008, b = −0.064 ± 0.024 SE), gestation period (F1,121 = 17.8, P < 0.0001, b = −0.36 ± 0.09 SE), and weaning period (F1,121 = 9.2, P = 0.003, b = −0.16 ± 0.06 SE). In addition, 19.5% of the total variance was related to differences between orders, reflecting variation that is unrelated to the other life history variables. The orders of mammals with the lowest rates of aging were the primates, carnivores, bats, and tree shrews; among the highest were the odd-toed and even-toed ungulates, and the rodents, although cetaceans and elephants also exhibited relatively high rates of aging, considering their large size and slow development. The high proportion of aging-related mortality in these species (see Figure 4) further emphasizes potential constraints on the evolution of life span.
When variation in the average value of ω among 15 orders of mammals was related to average values among orders for the other life history variables, only gestation period was significant, explaining, 68% of the variance and having a regression slope of b = −0.47 ± 0.09 SE (F1,13 = 27.7, P = 0.0002). Thus, much of the variation among orders in rate of aging is also related to the length of the gestation period.
It is difficult to sort out significant relationships when many variables are correlated, but the broad analyses presented here point to links between the length of the development period and length of life (Metcalfe & Monaghan, 2003; de Magalhaes & Church, 2005). These connections are emphasized by the influences of stresses experienced by embryos and infants on adult phenotypes, often referred to as fetal programming (Desai & Hales, 1997; Jennings, Ozanne, Dorling et al., 1999; Metcalfe & Monaghan, 2001; Metcalfe & Monaghan, 2003). With respect to evolved differences between species, slow development might reduce oxidative stress related to embryonic and postnatal growth rate that produces molecular damage with late-life consequences (Gavrilov & Gavrilova, 2001; Gavrilov & Gavrilova, 2003). Some evidence in birds suggests that long embryo development periods are associated with prevention or control of infections by haemosporidian parasites, implying an influence on the immune system (Ricklefs, 1992). Another possibility worth investigating is that slow embryo growth allows greater precision of neural connections during brain development, which could influence the functioning of the nervous system late in life, as individual cells die. Genetic studies and selection experiments demonstrate that both the period of embryonic development and the growth of the brain are extremely conservative traits in birds (Ricklefs & Marks, 1984; Ricklefs, 1993; Ricklefs & Starck, 1998), suggesting that this organ might set the pace of embryo growth more generally.
Implications for research on aging
Comparative analyses of the rate of actuarial senescence in mammals and birds emphasize the following points.
Although an increase in mortality rate with age appears to be a general feature of birds and mammals, many species have long potential life spans in nature. In general, birds (and bats) exhibit greater longevity than terrestrial mammals, although carnivores and primates also achieve relatively great longevity.
Although longevity generally increases with adult body mass, this relationship apparently reflects the relatively low extrinsic mortality suffered by large animals, not physiological aspects of body size per se.
Because longevity appears to be related only indirectly to body size and the associated consequences of size for the overall rate of metabolism, and because many small birds with high rates of metabolism and other supposedly unfavorable indicators for long life can achieve great longevity, evolved variation in the rate of actuarial senescence appears to be independent of the potential for oxidative damage.
The similarity of aging-related patterns of mortality between natural and captive or domesticated populations suggests that the increase in mortality rate with age reflects intrinsic causes of catastrophic failure, including cardiovascular failure and carcinomas, rather than increasing vulnerability to extrinsic causes of mortality (e.g., predation, inclement weather).
The absence in captive and domestic settings of an effect of reproduction on lifespan suggests that physiological changes associated with producing offspring do not influence length of life, and where such trade-offs are observed in nature, they reflect compromises over the allocation of limited resources that feed back on life span through various kinds of stress, or wear and tear.
Populations of mammals, including humans, possess modest genetic variation for the length of life, although this has not been confirmed for populations of birds. It is likely that the maintenance of such variation indicates that it has little consequence for the evolution of life span. That is, it represents genetic variation for trade-offs that represent alternatives with roughly equivalent fitness, and not for the overall pace of senescent change. The nature of these trade-offs is not known, but they might not relate to the constraints optimized in the long-term evolution of potential length of life.
Estimates of the proportion of mortality in natural populations due to aging-related causes indicate that in most populations of potentially long-lived organisms, potential selection on genetic variation to extend life is very strong. An implication is that these populations have exhausted natural biological mechanisms to further extend longevity at reasonable fitness cost.
Analyses of variation in the rate of actuarial senescence among species suggest that the rate of aging is evolutionarily conservative, with relatively little variation among closely related species. This might reflect conservatism in selective factors in the environment, but also might further indicate the resistance of longevity to natural selection.
Among many life history attributes of organisms, potential life span seems most closely related to the rate of development, particularly that of the embryo. Embryo development is one of the most genetically and evolutionarily conservative aspects of an individual’s life history.
Comparative analyses of actuarial senescence in natural populations of mammals and birds suggest that biological mechanisms involved in variation in rates of aging among species probably cannot be exploited to extend life in any particular population. Increasing life span likely will require interventions that are not part of normal phenotypic variation. Increases in survival of the elderly in modern societies (Vaupel, Carey, Christensen et al., 1998; Robine & Vaupel, 2001; Robine, Saito & Jagger, 2003) are difficult to explain, but they clearly are not genetic and might be related to the mother’s condition during pregnancy or to early life influences affected by recent cultural change.
Our understanding of aging clearly would benefit from further research on physiological processes in long-lived animal models. Small birds, primates, and bats would seem to hold keys to understanding longevity owing to their having great potential life spans in spite of their high metabolism (Austad & Fischer, 1991; Holmes & Martin, 2009). Studies of bird populations in the wild suggest, at least in some species, that fitness can be maintained at young adult levels to old age and that aging-related death is due primarily to catastrophic, intrinsic causes. In contrast, fitness in mammals appears to decline throughout adult life (e.g., Bronikowski, Morgan, Garland et al., 2006), suggesting that aging might differ qualitatively between birds and mammals.
Comparative studies have been helpful in demonstrating the full range of aging patterns in birds and mammals, and for indicating potential animal models for understanding the molecular and physiological bases for variation in senescence among species. Comparative studies also suggest that some organisms do not lose fitness with age and that youthful levels of activity can be maintained throughout adult life. Thus, practical approaches to extending human life might be limited to reducing stress during embryo and postnatal development, maintaining fitness as adults, and preventing death from catastrophic causes (Olshansky, Carnes & Grahn, 1997). Although potential life span apparently has been adjusted by evolution independently of changes in body mass and metabolic rate, the modifications responsible for these variations evidently are (a) conservative, exhibiting little genetic variation in natural populations, (b) costly, and (c) possibly are related to fundamental attributes of early development. Thus, we probably will not be able to take advantage of mechanisms responsible for variation in growth rates among species to influence human life span. However, understanding these variations might provide insights for interventions that could reduce the consequences of ordinary life processes for the potential length of life.
Acknowledgments
I have been generously supported by the National Institute on Aging at NIH, the National Science Foundation, the Curators of the University of Missouri, and the Alexander von Humboldt Foundation. Dr. Nate Flesness, Director of the International Species Inventory System (ISIS), provided demographic data on zoo populations. Dr. Alexander Scheuerlein of the Max Planck Institute of Demographic Research, Rostok, Germany, has been involved in many of the analyses described in this article. I am grateful to Stephen Austad, Caleb Finch, and Richard Miller for extended discussions of aging, and particularly to Joao Pedro de Magalhaes and Bruce A. Carnes for insightful and constructive suggestions for revising the manuscript.
Literature cited
- Arking R. Observations and Principles. Oxford University Press; Oxford: 2006. The Biology of Aging. [Google Scholar]
- Austad SN, Fischer KE. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. Journal of Gerontology: Biological Sciences. 1991;46:B47–B53. doi: 10.1093/geronj/46.2.b47. [DOI] [PubMed] [Google Scholar]
- Barja G. Aging in vertebrates, and the effect of caloric restriction: a mitochondrial free radical production-DNA damage mechanism? Biological Reviews of the Cambridge Philosophical Society. 2004;79:235–251. doi: 10.1017/s1464793103006213. [DOI] [PubMed] [Google Scholar]
- Bartke A, Coshigano K, Kopchick J, Chandrashekar V, Mattison J, Kinney B, Hauck S. Genes that prolong life: relationships of growth hormone and growth to aging and life span. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2001;56:B340–B349. doi: 10.1093/gerona/56.8.b340. [DOI] [PubMed] [Google Scholar]
- Bennett PM, Owens IPF. Life Histories, Mating Systems and Extinction. Oxford University Press; Oxford: 2002. Evolutionary Ecology of Birds. [Google Scholar]
- Bronikowski AM, Morgan TJ, Garland T, Carter PA. The evolution of aging and age-related physical decline in mice selectively bred for high voluntary exercise. Evolution. 2006;60:1494–1508. [PubMed] [Google Scholar]
- Calder WAI. Size, Function, and Life History. Harvard University Press; Cambridge, MA: 1984. [Google Scholar]
- Calder WAI. The comparative biology of longevity and lifetime energetics. Experimental Gerontology. 1985;20:161–170. doi: 10.1016/0531-5565(85)90033-6. [DOI] [PubMed] [Google Scholar]
- Carnes BA, Holden LR, Olshansky SJ, Witten MT, Siegel JS. Mortality partitions and their relevance to research on senescence. Biogerontology. 2006;7:183–198. doi: 10.1007/s10522-006-9020-3. [DOI] [PubMed] [Google Scholar]
- Carnes BA, Olshansky SJ. A biologically motivated partitioning of mortality. Experimental Gerontology. 1997;32:615–631. doi: 10.1016/s0531-5565(97)00056-9. [DOI] [PubMed] [Google Scholar]
- Carnes BA, Olshansky SJ. Heterogeneity and its biodemographic implications for longevity and mortality. Experimental Gerontology. 2001;36:419–430. doi: 10.1016/s0531-5565(00)00254-0. [DOI] [PubMed] [Google Scholar]
- Carnes BA, Olshansky SJ, Grahn D. Continuing the search for a law of mortality. Population and Development Review. 1996;22:231–264. [Google Scholar]
- Carnes BA, Staats DO, Sonntag WE. Does senescence give rise to disease? Mechanisms of Ageing & Development. 2008;129:693–699. doi: 10.1016/j.mad.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catry P, Phillips RA, Phalan B, Croxall JP. Senescence effects in an extremely long-lived bird: the grey-headed albatross Thalassarche chrysostoma. Proceedings of the Royal Society of London - Series B: Biological Sciences. 2006;273:1625–1630. doi: 10.1098/rspb.2006.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B. Evolutionary mechanisms of senescence. Genetica. 1993;91:11–19. doi: 10.1007/BF01435984. [DOI] [PubMed] [Google Scholar]
- Christensen K, Frederiksen H, Vaupel JW, Mcgue M. Age trajectories of genetic variance in physical functioning: A longitudinal study of Danish twins aged 70 years and older. Behavior Genetics. 2003;33:125–136. doi: 10.1023/a:1022501817781. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH, Albon SD, Guinness FE. Reproductive success in male and female red deer. Reproductive Success. In: Clutton-Brock TH, editor. Studies of Individual Variation in Contrasting Breeding systems. University of Chicago Press; Chicago: 1988. p. 325. [Google Scholar]
- Clutton-Brock TH, Isvaran K. Sex differences in ageing in natural populations of vertebrates. Proceedings of the Royal Society B: Biological Sciences. 2007;274:3097–3104. doi: 10.1098/rspb.2007.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson JC, Fairweather JA. Reduced reproductive performance prior to death in the black-legged kittiwake: senescence or terminal illness? Journal of Avian Biology. 2001;32:146–152. [Google Scholar]
- Croce CM. Oncogenes and cancer. New England Journal of Medicine. 2008;358:502–511. doi: 10.1056/NEJMra072367. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Semsei I. Development, cancer and aging: possible common mechanisms of action and regulation. Journal of Gerontology. 1989;44:25–34. doi: 10.1093/geronj/44.6.25. [DOI] [PubMed] [Google Scholar]
- De Magalhaes JP, Church GM. Genomes optimize reproduction: aging as a consequence of the developmental program. Review of Medical Physiology. 2005;20:252–259. doi: 10.1152/physiol.00010.2005. [DOI] [PubMed] [Google Scholar]
- De Magalhaes JP, Costa J, Church GM. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. Journal of Gerontology: Biological Sciences. 2007;62A:149–160. doi: 10.1093/gerona/62.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Hales CN. Role of fetal and infant growth in programming metabolism later in life. Biological Reviews. 1997;72:329–348. doi: 10.1017/s0006323196005026. [DOI] [PubMed] [Google Scholar]
- Finch CE. Longevity, Senescence, and the Genome. University of Chicago Press; Chicago: 1990. [Google Scholar]
- Finch CE, Ruvkun G. The genetics of aging. Annual Review of Genomics & Human Genetics. 2001;2:435–462. doi: 10.1146/annurev.genom.2.1.435. [DOI] [PubMed] [Google Scholar]
- Finch CE, Tanzi RE. Genetics of aging. Science. 1997;278:407–411. doi: 10.1126/science.278.5337.407. [DOI] [PubMed] [Google Scholar]
- Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- Gaillard JM, Allaine D, Pontier D, Yoccoz NG, Promislow DEL. Senescence in natural populations of mammals - a reanalysis. Evolution. 1994;48:509–516. doi: 10.1111/j.1558-5646.1994.tb01329.x. [DOI] [PubMed] [Google Scholar]
- Gavrilov LA, Gavrilova NS. The Biology of Life Span: A Quantitative Approach. Harwood Academic Publishers; New York: 1991. [Google Scholar]
- Gavrilov LA, Gavrilova NS. The reliability theory of aging and longevity. Journal of Theoretical Biology. 2001;213:527–545. doi: 10.1006/jtbi.2001.2430. [DOI] [PubMed] [Google Scholar]
- Gavrilov LA, Gavrilova NS. Early-life factors modulating lifespan. In: Rattan SI, editor. Biology of Aging and its Modulation. Kluwer Academic Publ; Dordrecht, Netherlands: 2003. pp. 27–50. [Google Scholar]
- Gems D, Partridge L. Stress-response hormesis and aging: “That which Does Not Kill Us Makes Us Stronger”. Cell Metabolism. 2008;7:200–203. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The moulding of senescence by natural selection. Journal of Theoretical Biology. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- Haussmann MF, Winkler DW, Vleck CM. Longer telomeres associated with higher survival in birds. Biology Letters. 2005;1:212–214. doi: 10.1098/rsbl.2005.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D, Martin K. A bird’s-eye view of aging: what’s in it for ornithologists? Auk. 2009;126:1–23. [Google Scholar]
- Holmes DJ, Austad SN. The evolution of avian senescence patterns: implications for understanding primary aging processes. American Zoologist. 1995;35:307–317. [Google Scholar]
- Jennings BJ, Ozanne SE, Dorling MW, Hales CN. Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. FEBS Letters. 1999;448:4–8. doi: 10.1016/s0014-5793(99)00336-1. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL. Repair and its evolution: survival versus reproduction. In: Townsend CR, Calow P, editors. Physiological Ecology: An Evolutionary Approach to Resource Use. Blackwell Scientific; Oxford: 1981. pp. 165–189. [Google Scholar]
- Kirkwood TBL. The disposable soma theory of aging. In: Harrison DE, editor. Genetic Effects on Aging. Telford Press; Caldwell, NJ: 1990. pp. 9–19. [Google Scholar]
- Kirkwood TBL. Evolution of ageing. Mechanisms of Ageing & Development. 2002;123:737–745. doi: 10.1016/s0047-6374(01)00419-5. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL, Austad SN. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Philosophical Transactions of the Royal Society London B. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL, Westendorp RGJ. Human longevity at the cost of reproductive success: trade-offs in the life history. In: Robine J-M, Kirkwood TBL, Allard M, editors. Sex and Longevity: Sexuality, Gender, Reproduction, Parenthood. Springer; Berlin: 2000. pp. 1–6. [Google Scholar]
- Linnen C, Tatar M, Promislow D. Cultural artifacts: a comparison of senescence in natural, laboratory-adapted and artificially selected lines of Drosophila melanogaster. Evolutionary Ecology Research. 2001;3:877–888. [Google Scholar]
- Loison A, Festa-Bianchet M, Gaillard JM, Jorgenson JT, Jullien JM. Age-specific survival in five populations of ungulates: Evidence of senescence. Ecology. 1999;80:2539–2554. [Google Scholar]
- Lycett JE, Dunbar RIM, Voland E. Longevity and the costs of reproduction in a historical human population. Proceedings of the Royal Society of London - Series B: Biological Sciences. 2000;267:31–35. doi: 10.1098/rspb.2000.0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch HJ, Fagan WF. Survivorship curves and their impact on the estimation of maximum population growth rates. Ecology. 2009;90:1116–1124. doi: 10.1890/08-0286.1. [DOI] [PubMed] [Google Scholar]
- Mccleery RH, Perrins CM. Great tit. In: Newton I, editor. Lifetime Reproduction in Birds. Academic Press; London: 1989. pp. 35–54. [Google Scholar]
- Mcgue M, Vaupel JW, Holm N, Harvald B. Longevity is moderately heritable in a sample of Danish twins born 1870–1880. Journal of Gerontology: Biological Sciences. 1993;48:B237–244. doi: 10.1093/geronj/48.6.b237. [DOI] [PubMed] [Google Scholar]
- Metcalfe NB, Monaghan P. Compensation for a bad start: grow now, pay later? Trends in Ecology & Evolution. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- Metcalfe NB, Monaghan P. Growth versus lifespan: perspectives from evolutionary ecology. Experimental Gerontology. 2003;38:935–940. doi: 10.1016/s0531-5565(03)00159-1. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harper JM, Dysko RC, Durkee SJ, Austad SN. Longer life spans and delayed maturation in wild-derived mice. Experimental Biology & Medicine. 2002;227:500–508. doi: 10.1177/153537020222700715. [DOI] [PubMed] [Google Scholar]
- Monaghan P, Haussmann MF. Do telomere dynamics link lifestyle and lifespan? Trends in Ecology & Evolution. 2006;21:47–53. doi: 10.1016/j.tree.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Monaghan P, Metcalfe NB, Torres R. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecology Letters. 2009;12:75–92. doi: 10.1111/j.1461-0248.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- Nielsen JT, Drachmann J. Age-dependent reproductive performance in Northern Goshawks Accipiter gentilis. Ibis. 2003;145:1–8. [Google Scholar]
- Nisbet I, Finch CE, Thompson N, Russek-Cohen E, Proundman JA, Ottinger MA. Endocrine patterns during aging in the common tern (Sterna hirundo) General and Comparative Endocrinology. 1999;114:279–286. doi: 10.1006/gcen.1999.7255. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Carnes BA, Grahn D. Confronting the boundaries of human longevity. American Scientist. 1997;86:52–61. [Google Scholar]
- Promislow D, Fedorka KM, Burger JMS. Evolutionary biology of aging: future directions. In: Masoro EJ, Austad S, editors. Handbook of the Biology of Aging. Elsevier; Amsterdam: 2006. pp. 217–242. [Google Scholar]
- Promislow DEL. Senescence in natural populations of mammals: a comparative study. Evolution. 1991;45:1869–1887. doi: 10.1111/j.1558-5646.1991.tb02693.x. [DOI] [PubMed] [Google Scholar]
- Promislow DEL. On size and survival - progress and pitfalls in the allometry of life span. Journals of Gerontology. 1993;48:B 115–B 123. doi: 10.1093/geronj/48.4.b115. [DOI] [PubMed] [Google Scholar]
- Reale D, Festa-Bianchet M. Quantitative genetics of life-history traits in a long-lived wild mammal. Heredity. 2000;85:593–603. doi: 10.1046/j.1365-2540.2000.00795.x. [DOI] [PubMed] [Google Scholar]
- Reed TE, Kruuk LEB, Wanless S, Frederiksen M, Cunningham EJA, Harris MP. Reproductive senescence in a long-lived seabird: rates of decline in late-life performance are associated with varying costs of early reproduction. American Naturalist. 2008;171:E89–E101. doi: 10.1086/524957. [DOI] [PubMed] [Google Scholar]
- Reid JM, Bignal EM, Bignal S, Mccracken DI, Monaghan P. Age-specific reproductive performance in red-billed choughs Pyrrhocorax pyrrhocorax: patterns and processes in a natural population. Journal of Animal Ecology. 2003a;72:765–776. [Google Scholar]
- Reid JM, Bignal EM, Bignal S, Mccracken DI, Monaghan P. Environmental variability, life-history covariation and cohort effects in the red-billed chough Pyrrhocorax pyrrhocorax. Journal of Animal Ecology. 2003b;72:36–46. [Google Scholar]
- Reznick D, Bryant M, Holmes D. The evolution of senescence and post-reproductive lifespan in guppies (Poecilia reticulata) Plos Biology. 2006;4:136–143. doi: 10.1371/journal.pbio.0040007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE. Embryonic development period and the prevalence of avian blood parasites. Proceedings of the National Academy of Sciences USA. 1992;89:4722–4725. doi: 10.1073/pnas.89.10.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE. Sibling competition, hatching asynchrony, incubation period, and lifespan in altricial birds. Current Ornithology. 1993;11:199–276. [Google Scholar]
- Ricklefs RE. Evolutionary theories of aging: confirmation of a fundamental prediction, with implications for the genetic basis and evolution of life span. American Naturalist. 1998;152:24–44. doi: 10.1086/286147. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE. Intrinsic aging-related mortality in birds. Journal of Avian Biology. 2000;31:103–111. [Google Scholar]
- Ricklefs RE. Tyrannosaur ageing. Biology Letters. 2007;3:214–217. doi: 10.1098/rsbl.2006.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE. The evolution of senescence from a comparative perspective. Functional Ecology. 2008;22:379–392. [Google Scholar]
- Ricklefs RE, Cadena CD. Lifespan is unrelated to investment in reproduction in populations of mammals and birds in captivity. Ecology Letters. 2007;10:867–872. doi: 10.1111/j.1461-0248.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Cadena CD. Heritability of longevity in captive populations of nondomesticated mammals and birds. Journals of Gerontology: Biological Sciences. 2008;63A:435–446. doi: 10.1093/gerona/63.5.435. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Marks HL. Insensitivity of brain growth to selection of four-week body mass in Japanese quail. Evolution. 1984;38:1180–1185. doi: 10.1111/j.1558-5646.1984.tb05641.x. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Scheuerlein A. Comparison of age-related mortality among birds and mammals. Experimental Gerontology. 2001;36:845–857. doi: 10.1016/s0531-5565(00)00245-x. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Scheuerlein A. Biological implications of the Weibull and Gompertz models of aging. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2002;57:B69–B76. doi: 10.1093/gerona/57.2.b69. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Scheuerlein A, Cohen A. Age-related patterns of fertility in captive populations of birds and mammals. Experimental Gerontology. 2003;38:741–745. doi: 10.1016/s0531-5565(03)00101-3. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Starck JM. Embryonic growth and development. In: Starck JM, Ricklefs RE, editors. Avian Growth and Development. Evolution within the Altricial-Precocial Spectrum. Oxford University Press; New York: 1998. pp. 31–58. [Google Scholar]
- Robine JM, Saito Y, Jagger C. The emergence of extremely old people: the case of Japan. Experimental Gerontology. 2003;38:735–739. doi: 10.1016/s0531-5565(03)00100-1. [DOI] [PubMed] [Google Scholar]
- Robine JM, Vaupel JW. Supercentenarians: slower ageing individuals or senile elderly? Experimental Gerontology. 2001;36:915–930. doi: 10.1016/s0531-5565(00)00250-3. [DOI] [PubMed] [Google Scholar]
- Robinson MR, Pilkington JG, Clutton-Brock TH, Pemberton JM, Kruuk LEB. Live fast, die young: trade-offs between fitness components and sexually antagonistic selection on weaponry in Soay sheep. Evolution. 2006;60:2168–2181. [PubMed] [Google Scholar]
- Roff DA. The Evolution of Life Histories. Chapman and Hall; New York: 1992. [Google Scholar]
- Rose MR. Evolutionary Biology of Aging. Oxford University Press; New York: 1991. [Google Scholar]
- Selman C, Lingard S, Choudhury AI, Batterham RLMC, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Garmgnac D, Robinson ICA, Thornton JM, Gems D, Partridge L, Withers DJ. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB Journal. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- Smith ED, Tsuchiya M, Fox LA, Dang N, Hu D, Kerr EO, Johnston ED, Tchao BN, Pak DN, Welton KL, Promislow DEL, Thomas JH, Kaeberlein M, Kennedy BK. Quantitative evidence for conserved longevity pathways between divergent eukaryotic species. Genome Research. 2008;18:564–570. doi: 10.1101/gr.074724.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC. The Evolution of Life Histories. Oxford University Press; New York: 1992. [Google Scholar]
- Sternberg H. Pied flycatcher. In: Newton I, editor. Lifetime Reproduction in Birds. Academic Press; London: 1989. pp. 55–74. [Google Scholar]
- Van Voorhies WA, Fuchs J, Thomas S. The longevity of Caenorhabditis elegans in soil. Biology Letters. 2005;1:247–249. doi: 10.1098/rsbl.2004.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel JW, Carey JR, Christensen K, Johnson TE, Yashin AI, Holm NV, Iachine IA, Kannisto V, Khazaeli AA, Liedo P, Longo VD, Zeng Y, Manton KG, Curtsinger JW. Biodemographic trajectories of longevity. Science. 1998;280:855–860. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16:439–454. [PubMed] [Google Scholar]
- Von Zglinicki T, Burkle A, Kirkwood TBL. Stress, DNA damage and ageing - an integrative approach. Experimental Gerontology. 2001;36:1049–1062. doi: 10.1016/s0531-5565(01)00111-5. [DOI] [PubMed] [Google Scholar]
- Westendorp RGJ, Kirkwood TBL. Human longevity at the cost of reproductive success. Nature. 1998;396:743–746. doi: 10.1038/25519. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Wilson AJ, Charmantier A, Hadfield JD. Evolutionary genetics of ageing in the wild: empirical patterns and future perspectives. Functional Ecology. 2008;22:431–442. [Google Scholar]
- Wilson DL. The analysis of survival (mortality) data: fitting Gompertz, Weibull, and logistic functions. Mechanisms of Ageing and Development. 1994;74:15–33. doi: 10.1016/0047-6374(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Yashin AI, De Benedictis G, Vaupel JW, Tan Q, Andreev KF, Iachine IA, Bonafe M, Deluca M, Valensin S, Carotenuto L, Franceschi C. Genes, demography, and life span: The contribution of demographic data in genetic studies on aging and longevity. American Journal of Human Genetics. 1999;65:1178–1193. doi: 10.1086/302572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashin AI, Iachine IA. Genetic analysis of durations: correlated frailty model applied to survival of Danish twins. Genetic Epidemiology. 1995;12:529–538. doi: 10.1002/gepi.1370120510. [DOI] [PubMed] [Google Scholar]
- Zera AJ, Harshman LG. The physiology of life history trade-offs in animals. Annual Review of Ecology & Systematics. 2001;32:95–126. [Google Scholar]