Abstract
The Urea Cycle Disorders Consortium (UCDC) was created as part of a larger network established by the National Institutes of Health to study rare diseases. This paper reviews the UCDC’s accomplishments over the first six years, including how the Consortium was developed and organized, clinical research studies initiated, and the importance of creating partnerships with patient advocacy groups, philanthropic foundations and biotech and pharmaceutical companies.
Keywords: urea cycle disorder, rare disease
Introduction
The Rare Diseases Clinical Research Network (RDCRN) was established by the National Institutes of Health (NIH) in 2003 to facilitate collaboration among experts in many different types of rare diseases of childhood and adulthood. Although research networks or consortia have long served to advance our understanding of a number of rare diseases (e.g. PKU), such was not the case with Urea Cycle Disorders (UCD) before 2003. There had never been an attempt to develop a national collaborative approach to researching UCD. Funding of the Urea Cycle Disorders Consortium (UCDC) as part of the RDCRN over the past six years has addressed this gap. There is now a network of 15 clinical and research centers that provide state-of-the-art care and clinical research to patients with UCD.
The UCDC has developed a website, which was visited over 34,000 times by the lay public and by professionals in 2008. The resources available on the website include general information about UCD for lay and professional groups, diagnostic and treatment guidelines, a registry, and information about clinical trials and outcome. In addition, the UCDC has trained eleven geneticists who have now entered the field of inborn errors of metabolism as clinical investigators. The UCDC has established partnerships with the National Urea Cycle Disorders Foundation (NUCDF), philanthropic foundations, and biotech and pharmaceutical companies, which have contributed to the UCDC’s success.
Rare Diseases Clinical Research Network
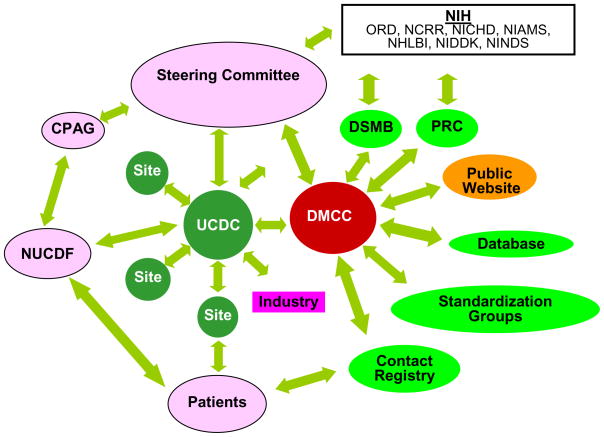
Ten consortia and a Data and Technology Coordinating Center (DTCC, now DMCC, Data Monitoring and Coordinating Center) were selected by the NIH to be a part of the original Rare Diseases Clinical Research Network, including the Urea Cycle Disorders Consortium (UCDC). In the re-competition held in 2008, this network has been expanded to 19 consortia. The RDCRN is governed by a steering committee, comprised of a principal investigator from each consortium, the director of the Data Monitoring and Coordinating Center (DMCC), NIH program and scientific officers, and a representative of the Coalition of Patient Advocacy Groups (CPAG). The steering committee and its standardization working groups develop policies, procedures, and standards for data collection, which reduces the effort each individual consortium must put forth in these efforts. The DTCC provides statistical, study implementation, data management, and monitoring support for the network. It also acts as a liaison between the consortia and the NIH-led Protocol Review Committee (PRC), which provides in depth scientific review of protocols developed by the consortia, and the Data and Safety Monitoring Board (DSMB), which monitors study protocols, ensures the safety of study participants and the integrity of studies (Figure 1).
Figure 1. The Urea Cycle Disorders Consortium (UCDC) within the context of the Rare Diseases Clinical Research Network (RDCRN).
DSMB-data safety and monitoring board; PRC-protocol review committee; CPAG-patient advocacy group; NUCDF-National Urea Cycle Disorders Foundation; DMCC-data monitoring and coordinating center.
Urea Cycle Disorders Consortium
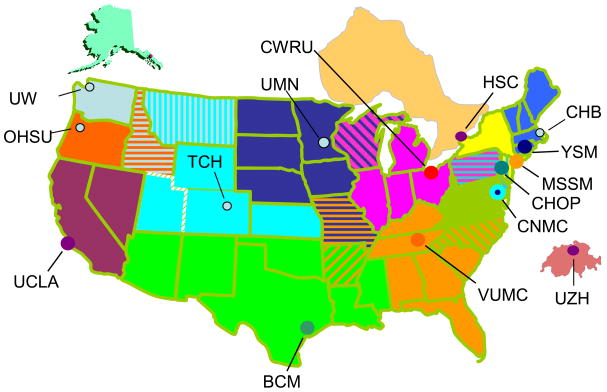
When initially funded, the UCDC consisted of five sites: Children’s National Medical Center in Washington, D.C. (CNMC) (lead institution); Baylor College of Medicine in Houston, Texas; Children’s Hospital of Philadelphia (CHOP) in Pennsylvania; University of California Los Angeles in California; and Vanderbilt University Medical Center in Nashville, Tennessee. Subsequent to receiving NIH grant funding, the UCDC received a five year matching grant from the O’Malley Family Foundation. This gift permitted the addition of two additional sites in 2004, one at Mount Sinai School of Medicine in New York City and a second at Yale University in New Haven, Connecticut. A grant from a second philanthropic foundation, the Kettering Fund, established a site at Case Western Reserve School of Medicine in Cleveland, Ohio in 2005. The UCDC consisted of these eight sites at the time the first study was activated in February 2006. It soon became apparent that the most successful mode of recruitment was by internal referral, i.e. referral to the Longitudinal Study by one of the UCDC investigators. Thus, the UCDC sought to further expand the number of sites and investigators. The O’Malley Family Foundation agreed to fund a site in Zurich, Switzerland which led to the addition of the UCDC’s first international site at the University of Zurich Kinderspital in 2007. That same year, the Hospital for Sick Children in Toronto, which is funded by a Canadian philanthropist, was added to the consortium. In 2008, The O’Malley Family Foundation helped support new UCDC sites at Seattle Children’s Hospital in Washington, Oregon Health & Science University in Portland, The Children’s Hospital of Denver in Colorado, and the Children’s Hospital Boston in Massachusetts, bringing the UCDC to its current fourteen sites. The UCDC is working to activate its 15th site at University of Minnesota in Minneapolis in late 2009, completing geographical coverage of the contiguous United States plus Alaska, Ontario and Switzerland (Figure 2).
Figure 2. UCDC Geographical Coverage.
CHB-Children’s Hospital, Boston; YSM-Yale School of Medicine; MSSM-Mt Sinai School of Medicine; CHOP-Children’s Hospital of Philadelphia; CNMC-Children’s National Medical Center; VUMC-Vanderbilt University Medical Center; CWRU-Case Western Reserve University; UMN-University of Minnesota; TCH-The Children’s Hospital; BCM - Baylor College of Medicine; UCLA-University of California, Los Angeles; OHSU-Oregon Health Sciences University; UW-University of Washington (Seattle Children’s Hospital); HSC-Hospital for Sick Children, Toronto; UZH-University of Zurich Kinderspital
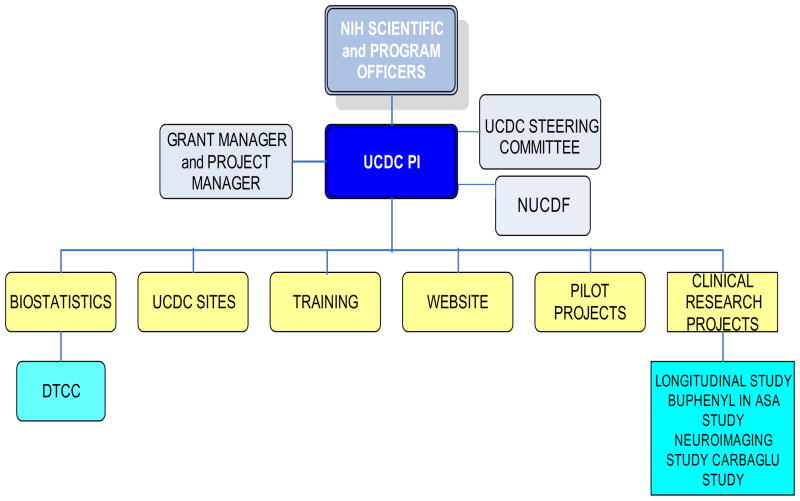
Each Consortium site is led by a principal investigator, who is a board-certified metabolic specialist, with a team consisting of a study coordinator, a neuropsychologist, and at some sites a co-investigator, research fellow, and/or nutritionist. The UCDC also employs several staff members at CNMC for programmatic, grant management, administrative and biostatistical support. The UCDC currently consists of a total of 43 faculty investigators and 26 research staff members. The UCDC’s steering committee is composed of the UCDC directors, the principal investigator from each site, the executive director of the National Urea Cycle Disorders Foundation, the NIH scientific and program officers, the DMCC director, the project manager, and the grant manager (Figure 3).
Figure 3.
Organizational Structure of the Urea Cycle Disorders Consortium
The Urea Cycle Disorders Consortium’s major goals are to: 1) develop better treatments and a deeper scientific understanding of the causes and outcomes of UCD; 2) conduct clinical trials of promising new therapies; 3) develop resources with information on UCD for clinicians, researchers, and patients; and 4) train the next generation of UCD investigators. Other objectives include promoting newborn screening for UCD to aid in identification and treatment of UCD and in establishing a research contact registry for the eight UCD under study.
UCDC Studies
The UCDC initiated a research contact registry, four clinical protocols, and two pilot studies during the initial six-year funding period. The Research Contact Registry and the Longitudinal Study of Urea Cycle Disorders are described in this review article. The other UCDC studies, which are described in separate articles in this symposium issue, include:
Assessing Neural Mechanisms of Injury in Inborn Errors of Metabolism Using Structural MRI, Functional MRI and Magnetic Resonance Spectroscopy (MRS) (Gropman article in this supplement). This project studies cognitive and motor dysfunction in patients who are female carriers of OTC deficiency or are males with late onset presentation of OTC deficiency, utilizing state of the art MRI (magnetic resonance imaging). This project seeks to improve our understanding of the underlying neural mechanisms that contribute to metabolic, cognitive, sensory and motor abnormalities in urea cycle disorders.
Measuring Urea Production in Patients with Urea Cycle Defects (Yudkoff article in this supplement). The overall purpose of this study is to develop a novel method to assess the in vivo rate of ureagenesis by administering [1-13C]acetate to humans and using mass spectrometry to measure the rate of appearance of label in [13C]urea.
A Randomized, Double-Blind, Crossover Study of Sodium Phenylbutyrate (Buphenyl) and Low-Dose Arginine (100 mg/kg/day) Compared to High-Dose Arginine (500 mg/kg/day) Alone on Liver Function, Ureagenesis and Subsequent Nitric Oxide Production in Patients with Argininosuccinate Lyase (AL) Deficiency (Lee article in this supplement). This study focuses on determining if using sodium phenylbutyrate in the treatment of patients with AL deficiency improves outcome as measured by the frequency and severity of hyperammonemic crisis and transaminase levels. This is done by treating patients with sodium phenylbutyrate (Buphenyl-TM), an FDA-approved drug labeled for the treatment of other urea cycle disorders (Ornithine transcarbamylase deficiency and citrullinemia).
N-Carbamylglutamate Treatment of N-acetyl Glutamate Synthetase Deficiency (NAGS) (Tuchman article in this supplement). This study focuses on the effect of N-Carbamylglutamate on the incorporation of [15N]ammonia into urea as a measure of restoration of ureagenesis capacity in patients with inherited NAGS deficiency diagnosed by DNA testing. The hypothesis is that N-Carbamylglutamate will restore deficient ureagenesis capacity and ameliorate the hyperammonemia in patients with inherited NAGS deficiency.
Research Contact Registry
In March 2005, the UCDC, in collaboration with the DTCC, launched an on-line research contact registry. As of July 31, 2009, 295 individuals with UCD have self-registered. Of those, 250 (85%) live in the U.S. Subjects enrolled in the registry receive information from the DTCC about UCDC studies via email bi-annually. The subject can then contact one of the UCDC sites to learn more about the studies and arrange for enrollment if they are deemed eligible to participate. March 2009 estimates show that 34% of the contact registrants are enrolled in one of the DSMB approved UCDC studies. 45% of registrants that live within 200 miles of a UCDC site are enrolled in a study, and 51% of subjects who live within 100 miles of a UCDC site are enrolled in a study. This is slightly above average for the use of contact registries in general and for the RDCRN specifically [1].
Longitudinal Study of Urea Cycle Disorders
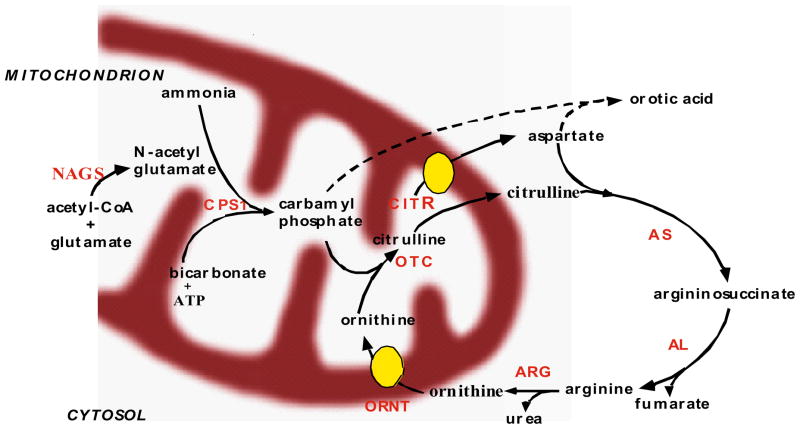
The Longitudinal Study follows the natural history of participants with congenital deficiencies in any of the six enzymes and two membrane transporters involved in urea biosynthesis (see Figure 4). The specific disorders and estimated prevalence [2,3] are noted below:
Figure 4.
The Urea Cycle
Ornithine transcarbamylase deficiency (OTCD) (1:14,000)
Argininosuccinate synthase (AS) deficiency (Citrullinemia) (1:57,000)
Carbamyl phosphate synthase I (CPSI) deficiency (1:62,000)
Argininosuccinate lyase (AL) deficiency (Argininosuccinic aciduria) (1:70,000)
Arginase (ARG) deficiency (Argininemia) (1:350,000)
N-acetylglutamate synthase (NAGS) deficiency (unknown)
Citrullinemia type II (mitochondrial aspartate/glutamate carrier deficiency-CITR) (1 in 21,000 in Japan)
Hyperornithinemia, hyperammonemia, homocitrullinuria (HHH) syndrome (or mitochondrial ornithine carrier deficiency-ORNT) (unknown)
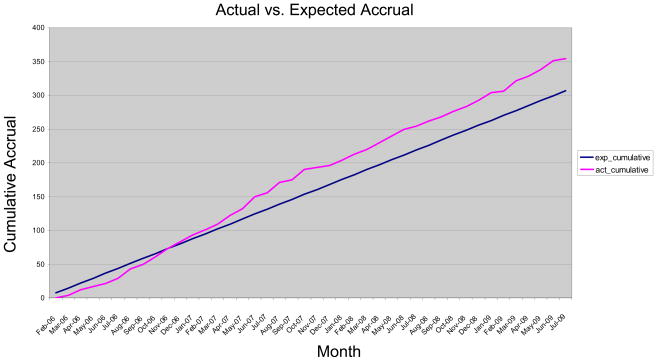
As of July 31, 2009, after 3.5 years of recruitment, the Longitudinal Study has enrolled 352 eligible participants (Figure 5). The recruitment goal is to enroll 440 participants in the study by February 2011. Recruitment from UCDC site metabolic clinic patient populations has proven to be the most effective recruitment mechanism representing 69% of enrolled participants, followed by referrals from the patient advocacy group, NUCDF (13%), referrals from other physicians (8%) and referrals of small numbers of participants from other study participants, the research contact registry, and the internet.
Figure 5.
Actual Accrual vs. Expected Accrual per Longitudinal Study. Data current as of July 31, 2009. Target Enrollment to Date: 294; Enrolled to Date: 352
In early publications [4,5] interim cross-sectional analysis of the first 183 participants enrolled during the initial 22 months of this study were presented. The current publication updates these findings for 352 eligible participants. Using the baseline assessment data provided at the time of enrollment through participant interview and review of medical records, characteristics of each type of UCD and associations with various factors were investigated. The questions posed included the following:
What is the relative frequency of proximal vs. distal UCD defects and of neonatal onset vs. late onset disease?
Table 1 illustrates the relative frequency of the various UCD among enrolled participants and those who have registered in the research contact registry. We examined relative frequencies in OTC deficiency (as a proximal defect) vs. AS/AL deficiencies (as distal defects). Thus far, the distribution of frequency among eligible and registered participants appears to be consistent with prior estimates [6,7]. OTC deficiency is by far the most common disorder among the UCD, accounting for more than half of all participants, followed by AL and AS deficiencies which together account for slightly less than a third of the participants. It is expected that the proportional frequency of these latter two disorders will increase in the near future as infants diagnosed with AS/AL deficiencies by expanded newborn screening using tandem mass-spectrometry [8] are enrolled, while most other UCD, especially CPS1 and OTC deficiencies cannot yet be identified by this screening method.
Table 1.
Frequency of the various urea cycle disorders among eligible participants in the Longitudinal Study and those who have registered with the Research Contact Registry. Data current as of July 31, 2009
| Diagnosis | Registered in Research Contact Registry | Longitudinal Study Participants (Eligible) |
|---|---|---|
| OTC | 162 | 202 |
| AL | 47 | 55 |
| AS | 31 | 52 |
| CPS | 17 | 8 |
| Diagnosis Pending | 13 | 8 |
| ARG | 8 | 12 |
| CITR | 6 | 2 |
| HHH | 4 | 3 |
| NAGS | 4 | 0 |
| Total | 265 | 352 |
Among Longitudinal Study participants with OTC deficiency, 11% are neonatal onset, presenting clinically within the first month of life, and 89% are late onset, presenting after one month of age. Thirty-six percent are asymptomatic heterozygous females; 37% are manifesting heterozygous females; 24% are symptomatic males; and 3% are asymptomatic males. Although the neonatal onset presentation comprises the smallest subgroup for OTC deficiency, the majority of participants with AS and AL deficiencies (56%) are neonatal onset, possibly reflecting greater survival of these participants. Based on prior studies, it was assumed that the proportion of participants with neonatal onset OTC deficiency presentation would be at least equal to those with late onset disease [9]. However, here the proportion of neonatal onset participants is much smaller. This difference may be due to failure to recruit severely affected patients before they died or those who underwent liver transplantation soon after their initial presentation. If this hypothesis is correct, as the study enrolls more participants soon after their initial clinical presentation, the proportion of neonatal cases should increase. As of July 31, 2009, only four out of 352 participants in the Longitudinal Study have died compared to a much higher proportion of deaths reported in earlier studies [10]. A retrospective analysis of data from deceased patients, including those with OTC deficiency, has been added to the study to further evaluate this question.
Are there ethnic/racial differences in UCD prevalence?
Table 2 shows the ethnic/racial origin of the participants. Although still preliminary, a marked difference is observed between the proportion of African-Americans enrolled in the study (4%) and their proportion in the general population (12%) [11]. Hispanics/Latinos who represent a similar proportion of the general population as African-Americans accounted for 15% of enrolled participants. While this could reflect a true difference in disease prevalence, it is more likely to be related to disparity in access to information about the study, location of UCDC sites, and/or mistrust of research due to past abuses [12].
Table 2.
Longitudinal Study Participants by Ethnicity and Race. Data current as of July 31, 2009
| Sex/Gender | ||||
|---|---|---|---|---|
| Category | Females | Males | Unknown/Not Reported | Total |
| Ethnic Categories | ||||
| Hispanic or Latino | 34 | 20 | 0 | 54 |
| Not Hispanic or Latino Origin | 181 | 90 | 0 | 271 |
| Unknown/Not Reported | 10 | 8 | 9 | 27 |
| 225 | 118 | 9 | 352 | |
| Racial Categories | ||||
| American/Alaskan Native | 0 | 0 | 0 | 0 |
| Asian | 11 | 8 | 0 | 19 |
| Native Hawaiian/Pacific Islander | 0 | 0 | 0 | 0 |
| Black/African American | 6 | 7 | 0 | 13 |
| White | 196 | 86 | 0 | 282 |
| More Than One Race | 9 | 6 | 0 | 15 |
| Unknown/Not Reported | 3 | 11 | 9 | 23 |
| 225 | 118 | 9 | 352 | |
What are the triggers for hyperammonemic episodes?
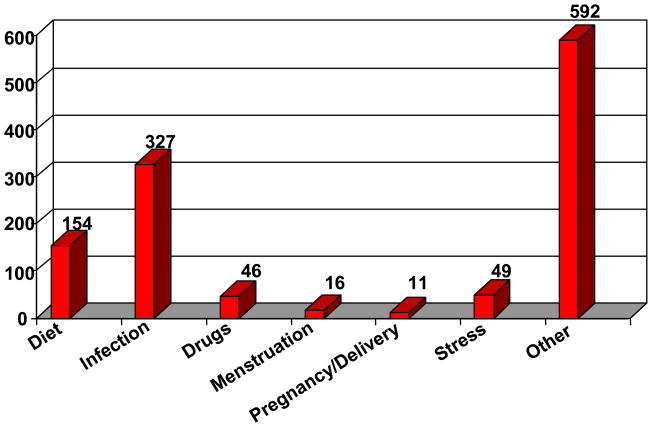
Previous studies show that the number and severity of hyperammonemic episodes impact outcome [13]. As of July 31, 2009, there were a total of 1084 episodes of hyperammonemic crises reported in 189 of the 352 participants. The participants and/or guardians were asked to provide information on perceived triggers that preceded each recorded hyperammonemic episode. They identified several precipitants, noted in Figure 6. Not surprisingly, an intercurrent infection was the most frequently cited trigger, followed by a recent change in diet. There were several dozen other triggers listed, however, none of them was implicated in an appreciable frequency. In the majority of cases there was no specific precipitant identified. As these participants are followed prospectively in the Longitudinal Study triggers will be identified more clearly, which could lead to prevention or amelioration of these crises through early medical intervention.
Figure 6.
Hyperammonemic Episode Triggers (n=1084 episodes). Data current as of July 31, 2009
What is the frequency of developmental disabilities/neurological abnormalities in UCD?
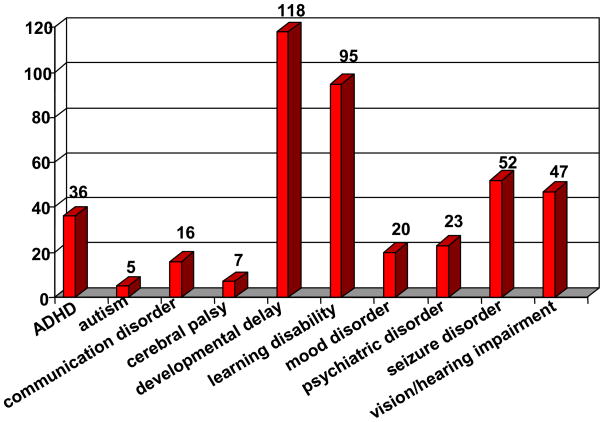
Baseline assessment includes self reporting and medical record review of neurodevelopmental parameters. Of the 352 participants, 177 reported at least one neurodevelopmental disability. As seen in Figure 7, global developmental delay (intellectual disability) was reported most frequently (47%), followed by learning disability (38%). The effects of recurrent hyperammonemia are known to cause irreversible damage to the brain [14], resulting in the effects reported here. Autism and mood disorders have been rarely reported in previous studies [15], as confirmed by Longitudinal Study results.
Figure 7.
Self-reported developmental disabilities and psychiatric disorders in enrolled participants. Data current as of July 31, 2009
What is the pattern of neuropsychological deficits in adults with UCD and its relationship to hyperammonemic episodes?
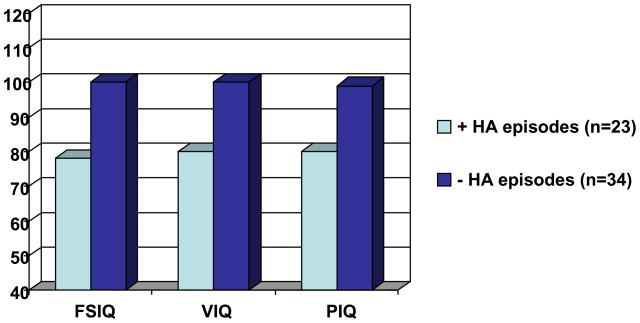
In a preliminary analysis of 60 adult subjects, seven had neonatal onset UCD. This small number reflects the reported high mortality during childhood in this group and a failure to recruit the most severely affected patients. Three of these subjects could not be formally assessed because of the severity of their intellectual disability; 4 were able to undergo neuropsychological testing. In the neonatal onset group, the overall mean IQ was in the range of mild intellectual disability (mean WASI Full Scale IQ (FSIQ)=57, sd=11). In contrast, the mean IQ of the late onset group (n=56) was in the average range (mean FSIQ=94, sd=21). Given the substantial number of individuals in the adult group with either mild UCD or who are asymptomatic carriers, functioning based on a history of hyperammonemic crises was also examined. Adults with no history of a documented hyperammonemic episode (defined as having an ammonia level >100uM and clinical symptoms requiring treatment) had overall intellectual abilities in the average range (n=35, FSIQ =100, SD=18) while those with a history of at least one hyperammonemic episode had an overall intellectual quotient in the borderline range of functioning (n=23, mean FSIQ=78, SD=22) (Figure 8).
Figure 8.
IQ of Adult Participants (17+) Experiencing Hyperammonemic (HA) Episodes vs. No Hyperammonemic Episodes (FSIQ – Full Scale IQ; VIQ – Verbal IQ; PIQ – Performance IQ)
What is the distribution of neuropsychological deficits in children with UCD?
In a sample of 92 children ranging in age from 5 months to 16 years, 9 months (mean = 7.2 years; standard deviation = 4.7), the most prevalent diagnoses were OTCD, AL deficiency, and AS deficiency. Fifty eight percent of the subjects were female, half of whom had partial OTC deficiency.
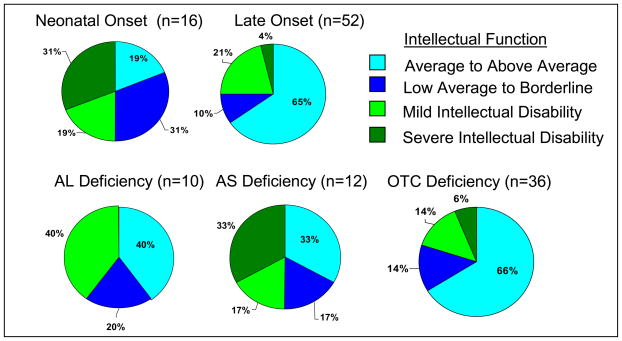
Cognitive outcome was different based on late or early onset of disease, with early onset having more severe deficits; and by diagnosis with AS Deficiency being more severely affected (See Figure 9). For children aged 3–16 years, eight (50%) of the neonatal onset group had FSIQ in the range of intellectual disability, five of whom fell into the moderate to profound classification. In contrast, 13 (25%) of the late onset group were classified as having an intellectual disability, with two (4%) having moderate to profound disabilities.
Figure 9. Cognitive Range Across All Subjects Ages 3–16: Early vs. Late Onset and by Diagnosis*.
*Includes estimated IQ scores for children unable to complete testing in their age range. Excludes lowest functioning children who were unable to complete the test battery in their age range.
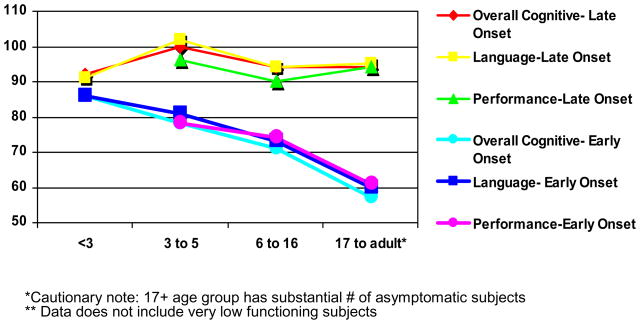
For the neonatal onset cases, there were greater percentages of impairment with age (Figure 10). In children under three, two subjects (8%) of the neonatal onset group and none of the late onset group presented with significant cognitive delay. No significant differences between the groups were evident in either developmental or adaptive functioning (p values ranged from 0.33 to 0.89). However, there is a downward shift in scores, which were half to one standard deviation below the normative mean across all areas -- cognitive development, language, motor skills, and adaptive functioning. This suggests that although most of the young children with UCD do not present with severe impairments, there is evidence that their development is mildly delayed.
Figure 10.
Possible Age Effects on IQ
The overall level of developmental disabilities across both neonatal and late onset groups is less than has been reported previously. In studies of individuals with neonatal onset disease, the percentage with any level of intellectual disability was as high as 80% in prior studies [16,17], as compared to the 50% noted in this study. Similarly, previous research has suggested a higher proportion of developmental disabilities in children with partial UCD. It was previously reported that >50% of school age subjects with a partial UCD were in the range of intellectual disability [18]. Lower rates of intellectual impairment in this study may be attributable to methodological differences or reflect changes in outcomes due to medical advances of children with UCD. The overall sample size in this study is substantially larger and the range of diagnoses is much wider, including more mild presentations, than the sample sizes in previous studies. Improvements in cognitive outcome may also be due to earlier recognition and intervention, and improved treatment protocols. This is suggested by the fewer children with cognitive impairments who are under age three. However, the cross-sectional design is unable to rule out alternative explanations. One possibility is that the majority of the children in this group were tested at either age 6 months or 18 months of age. At those ages, the range of behaviors that can be measured are more limited and the variability of normal developmental timeframes is large. A second possibility is that over time, children with UCD may plateau or even decline in abilities due to recurrent episodes of hyperammonemia. This would be consistent with prior studies [19] that demonstrated a decline in cognitive scores over time in a group of females with OTC deficiency.
Partnerships
The National Urea Cycle Disorders Foundation (NUCDF)
NUCDF, the volunteer health organization for UCD, has been an integral partner and collaborator in the UCDC since its inception. The executive director of NUCDF serves as a voting member on the executive committee of the consortium. One of her key roles has been to continually challenge the existing paradigms and barriers to advancing research in UCD. NUCDF has been especially involved in the research contact registry and longitudinal study enrollment. In terms of study design, NUCDF had a direct involvement in the development of protocols, consents, content evaluations, progress reporting, website content and the training program. NUCDF brought the perspective of the UCD community’s needs into clinical protocols with its unique knowledge of the limitations of the affected community. NUCDF also partnered with the UCDC to develop content for the UCDC website.
NUCDF has facilitated recruitment in UCDC studies in a number of ways, including serving as host for an annual conference during which educational presentations are given to patients and professionals by UCDC investigators. Along with education, enrollment opportunities and information are provided to patients via exhibits and registration centers. NUCDF developed a patient survey to determine attitudes and barriers to study participation and recruited a genetic counseling student to conduct a survey via phone calls to NUCDF. NUCDF collaborated on development of UCDC brochures and distributed the brochures to families, patients and healthcare professionals. It expanded existing website content (www.nucdf.org) to educate patients and families on the importance of participation in research and to inform potential participants about open UCDC trials. NUCDF published focused articles on the UCDC studies in its quarterly newsletter. The UCDC’s experience with the NUCDF points to the importance of engaging patient advocacy groups, especially in studies of rare diseases where recruitment is challenging.
Biotechnology and Pharmaceutical Industry Relations
One of the stated goals of the legislation leading to the establishment of the RDCRN was to bring orphan products more rapidly to market in order to improve the care of individuals with rare disorders in the U.S. In an effort to promote this goal, the UCDC has engaged in a number of collaborations with the pharmaceutical industry that are developing orphan products and biotech firms that are developing diagnostic technology for UCD. It becomes much more attractive for industry to tackle the testing of an orphan product if it has available a large cohort of well-characterized patients who are being followed longitudinally, are being managed according to a clinical pathway and are distributed throughout the U.S. This decreases the cost of performing Phase II/III studies and increases efficiency. These partnerships also potentially provide a source of additional funding to support a treatment network such as the UCDC.
Lessons Learned
Large networks work more slowly than independent, investigator-initiated studies, but the network offers the advantage of several levels of review, guidance from regulatory and statistical experts, and protocol monitoring. Being part of a disorder-specific consortium also offers many advantages, such as creation of tissue, cell, and DNA banks; improved diagnostic facilities; and frequent interaction with professional colleagues, which ultimately benefits patient care. Although the major purpose is research, we have found that patient care has also improved with adherence to clinical diagnostic pathways leading to earlier detection and treatment protocols that have improved outcomes. The UCDC’s experience has shown that collaboration with volunteer health organizations/patient advocacy groups is essential, as these organizations have greater access to patient populations and make significant contributions to protocol development, recruitment, development of appropriate website content, and much more. Seeking partnerships with grateful patients, philanthropic foundations and biotech companies with a shared interest in the disorder-specific patient population can also be mutually beneficial. Finally, while it is tempting to try to answer many questions and hypotheses within a study protocol, it is best to simplify protocols and define hypotheses as much as possible when doing a longitudinal study. Otherwise, data collection becomes cumbersome and difficult to complete accurately. It is also important to be realistic (conservative) when predicting enrollment numbers.
Acknowledgments
Supported by NIH grant (U54RR019453) from the National Institute of Child Health and Human Development (NICHD), the Office for Rare Diseases Research (ORDR) and the National Center for Research Resources (NCRR) and by grants from the Mary Alice and Thomas A. O’Malley Family Foundation and the Kettering Fund. The authors would also like to acknowledge the contributions of the following UCDC site coordinators, neuropsychologists, fellows, co-investigators and program staff: Nicholas Ah-Mew, Vera Anastasoie, Linda Ashford, Talin Babikian, David Brieger, Linnea Brody, Karen Burk-Paull, Susan Caudle, Kristin DeFrancesco, Naghmeh Dorrani, Arlene Gendron, Karalyn Grant, Andrea Gropman, Christina Guzman, Christine Heggie, Kerstin Hildebrandt, Mohammed Hussain, Dorothee Kleiner, Harvey Levy, Yueh-Han Lin, Kara Lord, Robert McCarter, Mary Mullins, Mina Nguyen-Driver, Irma Payan, Shannon Scrivner, Kyle Shattuck, Oleg Shchelechkov, Nina Thomas, Teresa Welch-Burke, Jennifer Woehr.
Footnotes
Conflict of Interest statement
None of the authors on this article have any relevant financial relationships with commercial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richesson RL, Young K, Lloyd J, Adams T, Guillette H, Malloy J, Krischer JP. An Automated Communication System in a Contact Registry for Persons with Rare Diseases: Tools for Retaining Potential Clinical Research Participants. AMIA Annu Symp Proc. 2007 Oct 11;:1094. [PubMed] [Google Scholar]
- 2.Brusilow S, Maestri NE. Urea cycle disorders: diagnosis, pathophysiology, therapy. In: Barness LA, DeVivo DC, Kaback MM, Morrow G, Oski FA, Rudolph AM, editors. Advances in Pediatrics. Vol. 43. Chicago: Mosby; 1996. p. 127. [PubMed] [Google Scholar]
- 3.Nagata N, Matsuda I, Oyanagi K. Estimated frequency of urea cycle enzymopathies in Japan. Am J Med Genet. 1991;39(2):228–229. doi: 10.1002/ajmg.1320390226. [DOI] [PubMed] [Google Scholar]
- 4.Tuchman M, Lee B, Lichter-Konecki U, Summar ML, Yudkoff M, Cederbaum SD, Kerr DS, Diaz GA, Seashore MR, Lee HS, McCarter RJ, Krischer JP, Batshaw ML the Urea Cycle Disorders Consortium of the Rare Diseases Clinical Research Network. Cross-sectional Multicenter Study of Patients with Urea Cycle Disorders in the United States. Mol Genet Metab Aug. 2008;94(4):397–402. doi: 10.1016/j.ymgme.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krivitzky L, Babikian T, Lee HS, Thomas NH, Burk-Paull KL, Batshaw ML. Intellectual, Adaptive, and Behavioral Functioning in Children with Urea Cycle Disorders. Pediatr Res Jul. 2009;66(1):96–101. doi: 10.1203/PDR.0b013e3181a27a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagata N, Matsuda I, Oyanagi K. Estimated frequency of urea cycle enzymopathies in Japan. Am J Med Genet. 1991;39:228–229. doi: 10.1002/ajmg.1320390226. [DOI] [PubMed] [Google Scholar]
- 7.Brusilow S, Maestri NE. Urea cycle disorders: diagnosis, pathophysiology, therapy. In: Barness LA, DeVivo DC, Kaback MM, Morrow G, Oski FA, Rudolph AM, editors. Advances in Pediatrics. Mosby; Chicago: 1996. p. 127. [PubMed] [Google Scholar]
- 8.Sander J, Janzen N, Sander S, Steuerwald U, Das AM, Scholl S, Trefz FK, Koch HG, Haberle J, Korall H, Merquardt I, Korenke C. Neonatal screening for citrullinaemia. Eur J Pediatr. 2003;162:417–420. doi: 10.1007/s00431-003-1177-z. [DOI] [PubMed] [Google Scholar]
- 9.McCullough BA, Yudkoff M, Batshaw ML, Wilson JM, Raper SE, Tuchman M. Genotype spectrum of ornithine transcarbamylase deficiency: Correlation with the clinical and biochemical phenotype. Am J Med Genet. 2000;93:313–319. doi: 10.1002/1096-8628(20000814)93:4<313::aid-ajmg11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Nassogne MC, Héron B, Touati G, Rabier D, Saudubray JM. Urea Cycle Defects: Management and Outcome. J Inherit Metab Dis. 2008;(3):407–414. doi: 10.1007/s10545-005-0303-7. 2005. Review. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Census Bureau. Profiles of General Demographic Characteristics 2000. Issued May 2001.
- 12.Shavers VL, Lynch CF, Burmeister LF. Knowledge of the Tuskegee study and its impact on the willingness to participate in medical research studies. J Natl Med Assoc. 2000;92:563–572. [PMC free article] [PubMed] [Google Scholar]
- 13.Msall M, Monahan PS, Chapanis N, Batshaw ML. Cognitive development in children with inborn errors of urea synthesis. Acta Paediatr Jpn. 1998;30:435–441. doi: 10.1111/j.1442-200x.1988.tb02534.x. [DOI] [PubMed] [Google Scholar]
- 14.Aylward GP. Prediction of functioning from infancy to early childhood: Implications for pediatric psychology. J Pediatr Psychol. 2004;29:555–564. doi: 10.1093/jpepsy/jsh057. [DOI] [PubMed] [Google Scholar]
- 15.Gyato K, Wray J, Huang ZJ, et al. Metabolic and neuropsychological phenotype in women heterozygous for ornithine transcarbamylase deficiency. Ann Neurol. 2003 doi: 10.1002/ana.10794. (in press) [DOI] [PubMed] [Google Scholar]
- 16.Msall M, Batshaw ML, Suss R, Brusilow SW, Mellits ED. Neurologic outcome in children with inborn errors of urea synthesis. N Engl J Med. 1984;310:1500–1505. doi: 10.1056/NEJM198406073102304. [DOI] [PubMed] [Google Scholar]
- 17.Whitington PF, Alonso EM, Boyle JT, Molleston JP, Rosenthal P, Emond JC, Millis JM. Liver transplantation for the treatment of urea cycle disorders. Journal of Inherited Metabolic Disorders. 1998;21(Supp):112–118. doi: 10.1023/a:1005317909946. [DOI] [PubMed] [Google Scholar]
- 18.Batshaw ML, Roan Y, Jung AL, Rosenberg LA, Brusilow SW. Cerebral dysfunction in asymptomatic carriers of ornithine transcarbamylase deficiency. N Engl. 1980;302:483–485. doi: 10.1056/NEJM198002283020902. [DOI] [PubMed] [Google Scholar]
- 19.Maestri NE, Brusilow SW, Clissold DB, Bassett SS. Long-term treatment of girls with ornithine transcarbamylase deficiency. N Engl J Med. 1996;335(12):855–859. doi: 10.1056/NEJM199609193351204. [DOI] [PubMed] [Google Scholar]