Abstract
Rationale: An effective new tuberculosis (TB) vaccine regimen must be safe in individuals with latent TB infection (LTBI) and is a priority for global health care.
Objectives: To evaluate the safety and immunogenicity of a leading new TB vaccine, recombinant Modified Vaccinia Ankara expressing Antigen 85A (MVA85A) in individuals with LTBI.
Methods: An open-label, phase I trial of MVA85A was performed in 12 subjects with LTBI recruited from TB contact clinics in Oxford and London or by poster advertisements in Oxford hospitals. Patients were assessed clinically and had blood samples drawn for immunological analysis over a 52-week period after vaccination with MVA85A. Thoracic computed tomography scans were performed at baseline and at 10 weeks after vaccination. Safety of MVA85A was assessed by clinical, radiological, and inflammatory markers. The immunogenicity of MVA85A was assessed by IFNγ and IL-2 ELISpot assays and FACS.
Measurements and Main Results: MVA85A was safe in subjects with LTBI, with comparable adverse events to previous trials of MVA85A. There were no clinically significant changes in inflammatory markers or thoracic computed tomography scans after vaccination. MVA85A induced a strong antigen-specific IFN-γ and IL-2 response that was durable for 52 weeks. The magnitude of IFN-γ response was comparable to previous trials of MVA85A in bacillus Calmette-Guérin–vaccinated individuals. Antigen 85A–specific polyfunctional CD4+ T cells were detectable prior to vaccination with statistically significant increases in cell numbers after vaccination.
Conclusions: MVA85A is safe and highly immunogenic in individuals with LTBI. These results will facilitate further trials in TB-endemic areas.
Clinical trial registered with www.clinicaltrials.gov (NCT00456183).
Keywords: human, latent TB, vaccine, Koch
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
MVA85A is the first new vaccine to be evaluated in Mycobacterium tuberculosis latently infected subjects.
What This Study Adds to the Field
MVA85A is safe and highly immunogenic in individuals with LTBI. These results will facilitate further trials in TB-endemic areas.
One-third of the world's population is estimated to be infected with Mycobacterium tuberculosis, on the basis of tuberculin skin testing (TST) (1). In individuals with latent tuberculous infection (LTBI), mycobacteria are viable but in a poorly understood state of dormancy (2, 3). They do not cause disease but have the capacity to do so at a later time point. Reactivation of these mycobacteria gives infected immunocompetent individuals a 5 to 10% lifetime risk of developing postprimary disease. This huge reservoir for tuberculosis (TB) disease is an essential group to consider when developing new vaccines against M. tuberculosis, because latent infection may alter the adverse event profile or therapeutic efficacy of vaccination.
An improved vaccine regimen that is more effective than the currently available bacillus Calmette-Guérin (BCG) would have a major impact on the global TB burden. It is conceivable that an effective vaccine could intervene before or after exposure to M. tuberculosis. The aim of a postexposure vaccine, for use in individuals with LTBI, would be to reduce the probability of developing TB disease in the future. Such a vaccine might potentially work by sterilizing bacteria that are residing in a dormant state, by preventing reactivation, and/or by reducing the chance of reinfection by exogenous M. tuberculosis. This is relevant because 19% of individuals are simultaneously infected with multiple strains in areas of high TB endemicity (4). Mycobacteria change their transcription patterns during different clinical conditions (2, 5, 6), but it remains unclear whether vaccines designed primarily as preexposure vaccines could be effective in the infected or active disease setting. The majority of TB vaccine research to date has been in the development of vaccines designed primarily as preexposure vaccines. MVA85A was the first subunit vaccine of this type to enter phase I clinical trials in 2002 (7).
To date, trials with MVA85A have been in BCG-naive and BCG-vaccinated subjects. There have been safety concerns within the field that potent new TB vaccines may induce mycobacteria-specific immunopathology in subjects with LTBI. Furthermore, few vaccines have been effective in the presence of chronic preexposure to the vaccine antigen. This is believed to be the result of poorly defined immunoregulatory mechanisms and greatly complicates the challenge of therapeutic vaccination. Latent infection with M. tuberculosis is one of the most prevalent bacterial infections in humans and could potentially tolerize to subsequent vaccine antigen administration. These concerns have influenced case selection for early clinical trials of new TB vaccines. However, in real clinical practice vaccination of subjects with LTBI will be unavoidable (and perhaps desirable). Therefore, the aim of this open-labeled phase I clinical trial was to evaluate the safety and immunogenicity of MVA85A in subjects with LTBI.
METHODS
Participants
This study was conducted according to a protocol approved by the Oxford Research Ethics Committee (OxREC A; 04/Q1604/61), ClinicalTrials.gov ID NCT00456183. Participants were recruited for this trial from TB contact clinics in the Oxford Radcliffe Hospital (ORH) National Health Service (NHS) Trust and the North West London Hospitals Trust and by poster campaign within hospitals of the ORH NHS Trust. This called for individuals who knew that they had been exposed to TB or who had been told historically that they had LTBI. Written informed consent was obtained from all subjects prior to enrolment in the trial. To be eligible for screening participants needed to be in good health with no clinical evidence of TB disease, be between the ages of 18 and 50 years, have a Heaf test between grade II and IV, and have a normal chest radiograph. Latent infection in this clinical trial was defined using an in-house ex vivo IFN-γ ELISpot assay using the two M. tuberculosis–specific antigens, early-secreted antigenic target 6 kD protein (ESAT-6) and culture filtrate protein 10 (CFP-10), used in the commercially available diagnostic tests (8, 9). To be enrolled into the trial, participants needed to produce at least 50 spot-forming cells (SFC)/million peripheral blood mononuclear cells (PBMCs) for any of six pools of either ESAT-6 or CFP-10 peptides on the in-house ex vivo IFN-γ ELISpot assay. This assay has a sensitivity of 15 SFC/million, so at 50 SFC/million we were ensuring that individuals recruited were well above the sensitivity of the assay. Participants were required to be seronegative for HIV, hepatitis B virus, and hepatitis C virus, and to have normal full blood count, renal, and liver function tests. Females entering the study were required to have a negative pregnancy test prior to entry, no plans for conception during the year of the study, and plans for secure contraception during this period.
Vaccine
The construction of the MVA85A vaccine has previously been described (10). Clinical grade MVA was produced under Good Manufacturing Practice standard by IDT Biologika GmbH (Dessau, Germany). Approval for the study was granted by the Medicines and Healthcare products Regulatory Agency (MHRA), UK, initially under a Doctors and Dentists Exemption Certificate (DDX; MF8000/12078). This was subsequently converted to a Clinical Trial Authorization (CTA; 21,584/0014/001) after the instigation of the European Union Clinical Trials Directive in May 2004.
Enrollment and Follow-up
Participants enrolled in the study were vaccinated with 5 × 107 plaque-forming units administered intradermally. All volunteers were followed up regularly for 12 months with blood samples being taken for inflammatory markers (blood erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]), and immunological assays. Routine hematology and biochemistry assays were performed at Weeks 1 and 12 after vaccination. A diary card was completed by all volunteers recording local and systemic adverse events and body temperature for Days 1 to 7 postvaccination. Throughout the study the local policy for the management of LTBI was followed, with agreement that chemoprophylaxis could be delayed for a year (the duration of the study) for subjects with LTBI who were either new arrivals in the UK or contacts of nonpulmonary TB cases. Contacts of recent smear-positive cases of pulmonary TB were excluded because it was believed that such individuals needed immediate chemoprophylaxis.
High-Resolution Computerized Tomographic Scan
A high-resolution computerized tomographic scan (HRCT) of the thorax was performed prior to vaccination and at 10 weeks postvaccination using a GE Lightspeed computed tomography (CT) scanner (GE Healthcare, Chalfont St. Giles, UK) and a protocol consisting of five 1-mm HRCT sections through the upper lobes. Volumetric CT, through the upper lobes with 1.25-mm contiguous sections, was performed in the subject who developed pulmonary nodules and this subject was subsequently followed up with a low-dose volumetric CT. All scans were reported by one senior respiratory radiology consultant (F.V.G.) who was not blinded to the study.
Immunological Assays
ELISpot assays.
The ex vivo IFN-γ and IL-2 ELISpot assays were performed on blood taken at screening and at Weeks 1, 4, 12, 24, and 52 postvaccination using fresh PBMCs. Tuberculin PPD (20 μg/ml, SSI), recombinant antigen 85A (Ag85A)(10 μg/ml, University of Leiden), seven pools of 9–10 peptides of Ag85A (ABC peptides, Imperial College London), three pools of 5–6 peptides of ESAT-6 (Peptide Protein Research, Hampshire, UK) and three pools of six peptides of CFP-10 (Peptide Protein Research) were used as stimulants for the IFN-γ assays. Stimulation was with Ag85A and the seven pools of 9–10 peptides of Ag85A for the IL-2 assay. All of the peptides used were 15mers overlapping by 10 amino acids, being used at a final concentration of 10 μg/ml in each well. Briefly, for the IFN-γ assay, 300,000 PBMCs were plated per well in 100 μl R10 (RPMI plus 10% fetal calf serum [FCS], 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, and 1 mM sodium pyruvate) directly onto the ELISpot plate (MAIP S4510 Millipore, Billerica, MA) in the presence of antigen and the plate was incubated for 18 hours. Streptokinase (250 U/ml), streptodornase (12.5 U/ml) and phytohemagglutinin (10 μg/ml) were used in all assays as positive controls and R10 and PBMCs were used as a negative control. The IL-2 ELISpot was performed according to the manufacturers instructions (R&D, Abingdon, UK), with the following minor methodological differences: R10 was used for blocking instead of the specified blocking buffer, 50 μl of diluted capture and detection antibodies were used instead of 100 μl, and detection antibody was incubated for 4 hours instead of overnight. For the IL-2 ELISpot assay, 300,000 PBMCs were plated per well in 100 μl R10 directly onto the ELISpot plate.
Assays were performed in duplicate. The ELISpot data were analyzed by subtracting the mean number of SFCs produced from the negative control wells from the mean count from wells with antigens. A well was considered positive if the count was at least twice that in the negative control wells and at least 5 SFC more than the negative control wells. The upper limit of quantification was taken as 500 SFC per well. For the peptide pool wells, the results were summed across all peptide pools for each time point. This potentially resulted in duplicate counting of T cells that responded to any of the 10mer overlap regions, because any 10mer occurred in two pools with adjacent peptides, but allowed direct comparison with immunogenicity data from previous trials (7, 11).
Intracellular cytokine staining assay.
Flow cytometric analysis of Ag85A-specific T-cell responses was performed at screening, and Weeks 1, 4, and 24 after vaccination. Thawed PBMCs were rested overnight in R10 supplemented with 10 U/ml DNase I (DNase I; Ambion, Applied Biosystems, Cheshire, UK). The following morning PBMCs were adjusted to 1 × 106 cells/ml in R10 in the presence of 1 μg/ml αCD28, 1 μg/ml αCD49d (both from BD Biosciences, Oxford, UK) and 10 μg/ml brefeldin A (Sigma-Aldrich, Dorset, UK). PBMCs were incubated at 37°C with 5% CO2 for 6 hours with 2 μg/ml antigen 85A complete peptide pool (66 15-mer peptides overlapping by 10 amino acids; 2 μg/ml final concentration for each individual peptide). Unstimulated PBMCs were used to assess nonspecific cytokine production. After stimulation, PBMCs were washed in FACS buffer (PBS containing 1% FCS and 0.1% sodium azide [Sigma-Aldrich]), then stained with the amine reactive LIVE/DEAD fixable violet dead cell stain kit (Molecular Probes, Invitrogen, Paisley, UK) (12) and fluorochrome conjugated, monoclonal antibodies (mAbs) against CD4 (allophycocyanin [APC], Ebioscience, London, UK) and CD14 (Pacific Blue; Caltag, Invitrogen). Subsequently, PBMCs were washed, permeabilized (Cytofix/cytoperm kit; BD Pharmingen, Oxford, UK) according to the manufacturer's instructions, stained for CD3 (phycoerythrin [PE]-cyanine 5 [Cy5]; Ebioscience), CD8 (APC-Alexafluor 750; Molecular Probes, Invitrogen) and cytokines IFN-γ (fluorescein isothiocyanate; Ebioscience), IL-2 (PE; Ebioscience), and TNF-α (PE-cyanine 7, BD Biosciences) washed and fixed in 1% paraformaldehyde. Cells were analyzed using a CyAn ADP cytometer (Dako Cytomation, Glostrup, Denmark). Antigen-specific cytokine responses and functional profiles were assessed and performed as previously described using FlowJo version 8.4 (Tree Star Inc., Ashland, OR), Pestle version 1.5 and Simplified Presentation of Incredibly Complex Evaluations (SPICE) version 3.1, both provided by Mario Roederer, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health (13).
Statistical Analysis
Vaccine-induced responses at peak (1 wk) and plateau (6 or 12 mo) were compared with baseline responses using the Wilcoxon signed rank test for matched pairs, Stata Statistical Software, Release 9.0. 2005 (Stata Corporation, College Station, TX). Comparisons between vaccine-induced responses in this trial and those from previous trials of MVA85A were made using the Mann-Whitney test, also using Stata.
Role of the Funding Source
This trial was funded by the Wellcome Trust and the sponsor was the University of Oxford. The funder had no role in the design of this study or in the analysis of the data.
RESULTS
Demographics
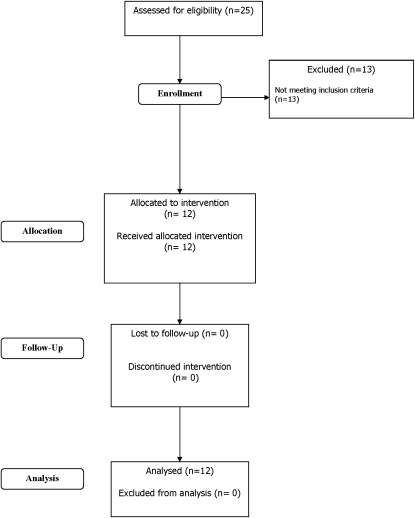
Twenty five individuals were screened between September 2004 and November 2005 to provide 12 participants (see consort diagram in Figure 1). Twelve individuals were excluded because they did not fulfill the ESAT-6/CFP-10 ELISpot criteria. One subject was excluded because of abnormal liver function tests. Five other people were booked for screening but failed to attend.
Figure 1.
The Consort E-Flowchart, August 2005. Consort diagram for study.
The demographics of the 12 participants who entered the trial are shown in Table 1. Nine participants were recruited from TB contact clinics with seven being from countries of high TB endemicity. Three were being screened because they were new arrivals in the UK, and three had had recent contact with a TB case. One participant from a country of high TB endemicity and two others were in the TB clinic at the request of their NHS occupational health departments. The three participants who responded to the poster advertisements had all been told historically that they had LTBI but had never received any treatment.
TABLE 1.
DEMOGRAPHICS AND SCREENING RESULTS OF TRIAL PARTICIPANTS
| Sex | Male | 10 (83.3%) |
| Female | 2 (16.7%) | |
| Median age (range) | 30.5 (20–49) | |
| Country of birth | India | 5 (41.7%) |
| East Africa | 2 (16.7%) | |
| England | 2 (16.7%) | |
| West Africa | 2 (16.7%) | |
| Ireland | 1 (8.3%) | |
| BCG vaccinates | Yes | 10 (83.3%) |
| No | 2 (16.7%) | |
| Heaf result | Grade II | 7 (58.3%) |
| Grade III | 2 (16.7%) | |
| Grade IV | 3 (25%) | |
| Median ESAT-6 (range) | 148 (3–1,773) | |
| Median CFP-10 (range) | 459 (77–2,037) | |
| Baseline computed tomography scans | Normal | 10 (83.3%) |
| Abnormal (hilar calcification) | 2 (16.7%) |
Definition of abbreviations: BCG = bacillus Calmette-Guérin; CFP = culture filtrate protein; ESAT = early-secreted antigenic target
Participants had a range of Heaf test results but there was no correlation between Heaf grade and magnitude of IFN-γ responses to either ESAT-6 or CFP-10 or to the summed ESAT-6 and CFP-10 responses determined by the ELISpot assay (data not shown). All volunteers reached the entry criteria for this study on the basis of CFP-10 responses, whereas only 8 out of 12 of these subjects fulfilled entry criteria from ESAT-6 responses alone.
Two of the three subjects with a grade IV Heaf test had not been vaccinated with BCG, and both of these subjects were white. The two subjects with hilar calcification on baseline thoracic CT scanning had grade IV Heaf tests. The participants with grade IV Heaf results were vaccinated toward the end of the study after participants with grade II and III Heaf tests had been safely vaccinated. This was based on a prior assumption that higher Heaf grades related to higher bacterial loads and to potentially higher risks of developing adverse events due to immunopathology.
Local Adverse Events
Local reactions relating to the MVA vaccine vector occur during the first week after vaccination and have been reported previously (7, 11). The dose of MVA85A used in this trial is the same as that used in the previous trials of MVA85A, 5 × 107 plaque-forming units (7). The local adverse event profile recorded in diary cards is comparable between all of the trials of MVA85A (Table 2). It is also worth noting that no volunteer complained of any increased reactogenicity at the TST site nor was any noticed by the trial clinicians.
TABLE 2.
COMPARISON OF ADVERSE EVENTS BETWEEN TRIALS OF MVA85A IN SUBJECTS WITH AND WITHOUT LATENT TUBERCULOUS INFECTION
| Latency Trial (n = 12) | Previous Trials of MVA85A* (n = 41) | |
|---|---|---|
| Local adverse events | ||
| Redness | 11 (92%) | 41 (100%) |
| Pruritus | 8 (67%) | 35 (85%) |
| Pain | 6 (50%) | 39 (95%) |
| Induration | 11 (92%) | 41 (100%) |
| Systemic adverse events | ||
| Fever | 0 (0%) | 5 (12%) |
| Flu-like | 4 (33%) | 17 (41%) |
| Arthralgia | 1 (8%) | 12 (29%) |
| Headache | 7 (58%) | 17 (41%) |
| Myalgia | 5 (42%) | 20 (49%) |
| Nausea | 0 (0%) | 6 (15%) |
| Tired | 7 (58%) | Not recorded |
| Vasovagal syncope | 0 (0%) | 1 (2%) |
Systemic Adverse Events
Systemic symptoms also occurred during the first week of vaccination and were recorded in the diary cards (see Table 2). Documented fever of more than 37.5°C was the only objective sign in the previous studies and occurred in 2% of subjects. This did not occur in any subjects in this trial. All of the other symptoms were subjective, with similar percentages of subjects experiencing them in this trial compared with previous trials. There was no suggestion from any of the symptoms or signs that there had been induction of any mycobacteria-specific immunopathology.
Inflammatory markers were monitored throughout the study. There were no clinically significant increases in ESR and CRP measures for 11 out of 12 participants throughout the study. One subject fractured his ankle 2 weeks after vaccination and developed an increase in his CRP after this fracture from 8 to 160 mg/L and in ESR from 5 to 28 mm/hour, which persisted until Week 4. This response was considered to be unrelated to vaccination. There were no significantly abnormal biochemistry or hematology blood test results in any subject during the course of the studies.
There were no changes in the HRCT scans at baseline and 10 weeks postvaccination, except in one participant. A 5-mm pulmonary nodule was visualized in the right upper lobe on the 10-week scan, which had not been visualized on the original prevaccination scan. However, the small volume calcified hilar lymphadenopathy that had been detected in the same subject at screening remained unchanged on the 10-week scan. A further scan, performed at 14 weeks, demonstrated complete resolution of this nodule. The patient was asymptomatic throughout this period.
Immunogenicity of Vaccine Determined by IFN-γ and IL-2 ELISpot
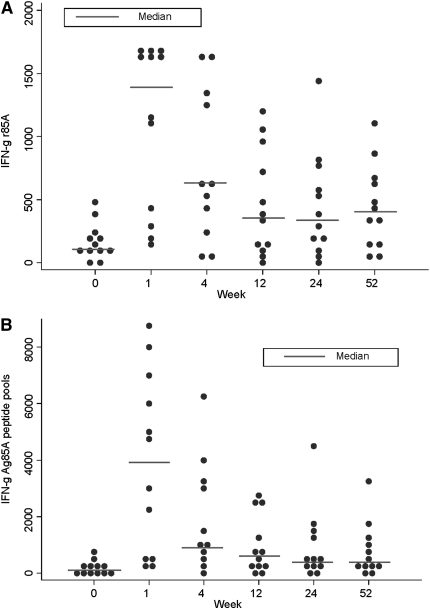
At 1 week postvaccination, the frequency of IFN-γ–secreting T cells responding to Ag85A increased by a median of 933 SFC/million compared with screening (95% confidence interval [CI], 567–1,360; P = 0.002). There remained an increased frequency of Ag85A-specific T cells throughout the trial follow-up. At 52 weeks this response remained significantly higher than baseline (median difference, 275; 95% CI, 103–437; P = 0.006) (Figure 2a). The T-cell responses to the Ag85A peptide pools followed a similar pattern, with a median increase above baseline of 3,637 SFC/million (1,419–5,831; P = 0.002) at Week 1 and of 495 SFC/million (72–1,102; P = 0.017) at 52 weeks (Figure 2B). The kinetics of this Ag85A-specific T-cell response are very similar to previous studies of MVA85A and consistent with understanding of T-cell expansion and contraction to memory phase (14).
Figure 2.
MVA85A vaccination expands antigen 85A-specific IFN-γ–secreting T cells. Median IFN-γ ELISpot responses to (A) Ag85A, and the summed responses to (B) Ag85A peptide pools, for n = 12 for all time points except Week 4, where n = 11 due to subject unavailability. The responses to each antigen at 1 week, 24 weeks, and 52 weeks after vaccination were compared with Week 0 using the Mann-Whitney test.
The median PPD response before vaccination was 959 (range 240–1,650) SFC/million PBMCs, which was significantly higher than that seen in the previous trial of MVA85A in BCG-vaccinated subjects where the baseline PPD response was 60 (0–465) (median difference, −853; 95% CI, −1,242 to −428; P < 0.0001) (7). As a consistent number of PBMCs were added to each well in the ELISpot assay across these trials, this higher baseline response to PPD meant we were unable to detect a significant increase in number of PPD-specific T cells after vaccination in this population at Week 1 (median difference, 135; 95% CI, −15 to 718; P = 0.07) or Week 52 (median difference, 29; 95% CI, −209 to 506; P = 0.39) (7, 11).
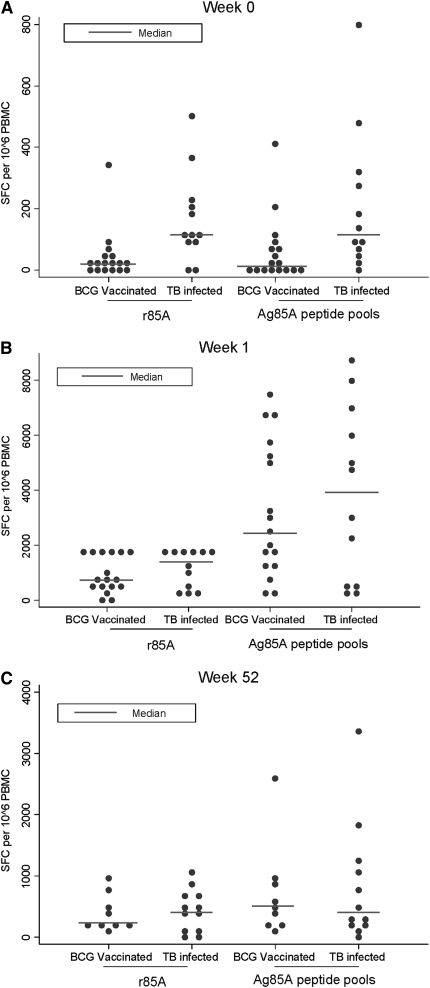
The immunogenicity of MVA85A in this trial in LTBI subjects was compared with the immunogenicity of MVA85A in previous trials in BCG-vaccinated subjects (Figure 3). The magnitude of T-cell response to Ag85A prevaccination was significantly higher in those with LTBI compared with those just vaccinated with BCG at screening, with median difference of 96.3 to rAg85A (95% CI, 47–186; P = 0.003) and of 91 to summed peptide pools (11–251; P = 0.006). However, there was no significant difference in the magnitude of T-cell response to Ag85A or to the 85A peptide pools in those infected with TB compared with those just vaccinated with BCG at either 1 week (median difference, 10; 95% CI, −861 to 228; P = 0.56 for Ag85A and median difference, −351; 95% CI, −3,077 to 1,623; P = 0.66 for Ag85A pools) or 52 weeks (median difference, −129; 95% CI, −416 to 164; P = 0.57 for Ag85A and median difference, 0; 95% CI, −607 to 414; P = 1.00 for Ag85A pools) postvaccination. Ten of the 12 subjects in this LTBI trial had been vaccinated with BCG during childhood. The immunogenicity of MVA85A in the 2 subjects who had not received BCG was not significantly different from the median of the BCG-vaccinated subjects in this trial, suggesting that M. tuberculosis infection can act as a prime for subsequent MVA85A boosting in a similar way to BCG (data not shown).
Figure 3.
MVA85A vaccination is as effective at expanding antigen 85A-specific IFN-γ–secreting T cells in subjects with latent tuberculosis infection (LTBI) as in subjects who have been bacillus Calmette-Guérin (BCG) vaccinated, despite higher baseline antigen 85A–specific responses. Median IFN-γ ELISPOT responses to Ag85A and summed peptide pools in this trial (n = 12) compared with a previous trial of MVA85A in subjects vaccinated with BCG at (A) screening (n = 22), (B) 1 week (n = 17), and (C) 52 weeks (n = 9) after vaccination with MVA85A. The responses to each antigen at each time point for each trial were compared using the Mann-Whitney test.
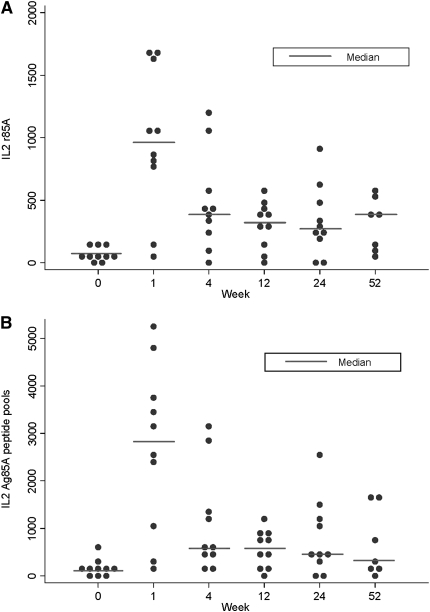
The kinetics of induction of IL-2–producing T cells in response to Ag85A and 85A peptide pools follows a very similar pattern to that of the IFN-γ–producing T cells (see Figure 2, Figure 4). At Week 1 there was a significant increase from baseline in both IL-2–producing T cells in response to Ag85A (median difference, 877; 95% CI, 430–1,320; P = 0.007) and the summed Ag85A peptide pools (median difference, 2,566; 95% CI, 1,236–3,783; P = 0.007), when compared with baseline responses. The Ag85A response remained significantly above baseline at 1 year after vaccination (median difference, 247; 95% CI, 40–460; P = 0.018). The summed peptide pool response just failed to remain significantly above baseline at 1 year (median difference, 461; 95% CI, −3 to 1,191; P = 0.051).
Figure 4.
MVA85A vaccination expands antigen 85A–specific IL-2–secreting T cells. Median IL-2 ELISpot responses to (A) Ag 85A and the summed responses to (B) Ag85A peptide pools for n = 10 for all time points except Week 52, where n = 7 due to reagent unavailability. The responses to each antigen at 1 week, 24 weeks, and 52 weeks after vaccination were compared with Week 0 using the Mann-Whitney test.
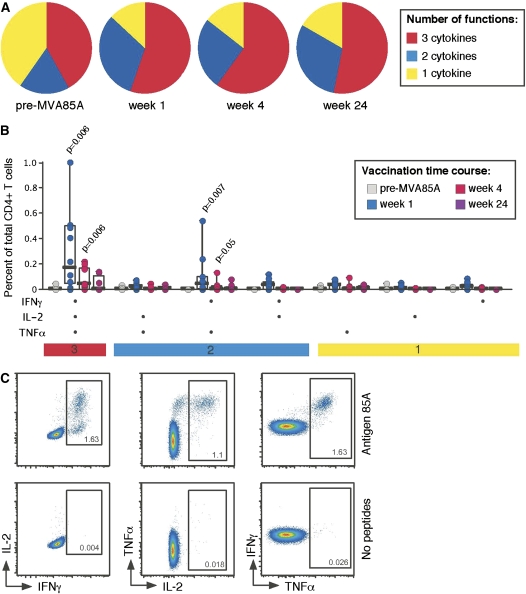
MVA85A Vaccination Expands Multifunctional CD4+ T-cell Populations
Flow cytometric analysis revealed antigen 85A–specific CD4+ T-cell populations, which were able to produce the cytokines IFN-γ, IL-2, and TNF-α prior to vaccination. This is markedly different from the responses observed in non–M. tuberculosis infected, BCG-vaccinated subjects, where at baseline there were lower numbers of Ag85A-specific CD4+ T cells and those detected produced single cytokines only (13) (Figure 5).
Figure 5.
MVA85A vaccination expands multifunctional CD4+ T-cell populations. (A) The group functional profiles of Ag85A-specific CD4+ T cells throughout the vaccination time course are shown (n = 10 for Week 1, 11 for Week 4, and 8 for Week 24). Responding cells (able to produce any of the following cytokines: IFN-γ, IL-2, or TNF-α) were grouped and color coded according to the number of cytokines produced. (B) The absolute Ag85A-specific CD4+ T-cell responses are shown for each of the possible functional species (along the x axis) throughout the vaccination time course. Individual data points are shown with median line, interquartile range boxes, and minimum/maximum whiskers. Significant (P < 0.05) increases above baseline levels are shown (Wilcoxon signed rank test for matched pairs).
Vaccination with MVA85A induced significant changes in the magnitude of IFN-γ+IL-2+TNF-α+ (3+) and the IFN-γ+TNF-α+ (2+) Ag85A-specific CD4+ T cell response at Week 1 (see Figure 5; P = 0.006, P = 0.007, respectively). The functional composition of the Ag85A-specific response was also altered after vaccination with MVA85A. At 1 week more than 50% of the responding CD4+ T cells were producing IFN-γ+IL-2+TNF-α+ (3+). At 24 weeks after MVA85A a larger proportion of the Ag85A-specific response was composed of IFN-γ+TNF-α+ (2+)–responding CD4+ T cells than prior to MVA85A vaccination. No Ag85A-specific CD8+ T-cell responses were detected after vaccination with MVA85A, determined by flow cytometry, which is similar to the non–M. tuberculosis–infected cohort (13). A comparison of the levels of IFN-γ and IL-2 measured by ELISpot and ICS prior to and at the 1-, 4-, and 24-week time points revealed a close correlation between the two assays: IFN-γ P < 0.0000, Spearman's ρ = 0.85 with 41 observations and IL-2 P = 0.0000, Spearman's ρ = 0.84 with 33 observations (data not shown).
Effect of Vaccination on Immune Response to Other Mycobacterial Antigens
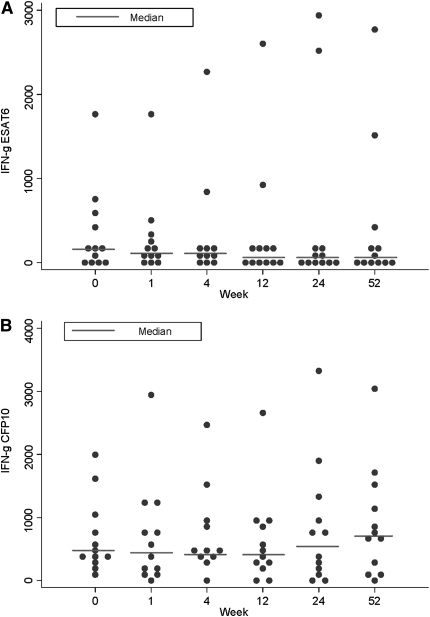
There are no changes after vaccination in the median frequency of T cells responding to the early secreted mycobacterial antigens, ESAT-6 at Week 1 (median difference, −53; 95% CI, −137 to 34; P = 0.39) or Week 52 (median difference, −13; 95% CI, −115 to 386; P = 0.58) and CFP-10 at Week 1 (median difference, −24; 95% CI, −214 to 237; P = 0.53) or Week 52 (median difference, 193; 95% CI, −20 to 480; P = 0.07) (Figure 6). This contrasts with the increased T-cell frequency seen in response to Ag85A, the gene that is encoded within the MVA85A vaccine, over the time course. Even when responses were investigated on an individual level, there were no statistically significant changes in responses to any of these antigens when normalized for frequency of response to streptokinase-streptodornase (data not shown).
Figure 6.
MVA85A vaccination has no effect on early-secreted antigenic target (ESAT)-6– and culture filtrate protein (CFP)-10–specific IFN-γ–secreting T cells. Median IFN-γ ELISpot responses to summed peptide pools of antigens (A) ESAT-6, and (B) CFP-10 for n = 12 for all time points except Week 4, where n = 11 due to subject unavailability. The responses to each antigen at 1 week, 24 weeks, and 52 weeks after vaccination were compared with week 0 using the Mann-Whitney test.
DISCUSSION
The two main findings from this phase I clinical trial are (1) MVA85A is safe in healthy subjects latently infected with M. tuberculosis, and (2) MVA85A is highly immunogenic in this population.
Subjects were defined as having LTBI for this trial using an in-house ex vivo IFN-γ ELISpot assay to ESAT-6 and CFP-10. A modified version of this assay and a whole blood IFN-γ ELISA using the same antigens have been validated and commercialized and are known as the T-SPOT.TB test (Oxford Immunotec, Oxford, UK) and the QuantiFERON-TB Gold (QFT-G) assay, respectively. They are both more specific than the TST and are starting to play a role in TB diagnosis (15). The enhanced specificity of these assays relates to the fact that the antigens ESAT-6 and CFP-10 are not present in BCG or most environmental mycobacteria. The Centers for Disease Control and Prevention (CDC) in the United States now recommend that the TST be replaced by QFT-G for all indications, including screening of contacts, immigrants, and health care workers (8). In the United Kingdom, the National Institute for Health and Clinical Excellence (NICE) recommends continuing with the TST but considering the use of either of the more specific assays in those who are TST-positive or in those in whom the TST may be unreliable (9).
The local and systemic adverse event profile seen in this trial was very similar to that seen in previous trials in naive and BCG-vaccinated subjects. Specifically, we did not see any mycobacteria-specific immunopathology after vaccination, the so-called “Koch phenomenon.” In the 1890s, after preliminary experiments in guinea pigs, Robert Koch administered culture filtrate tuberculosis proteins to patients with active TB in the hope that it would be therapeutic. Unfortunately, this triggered a potent immunological response inducing tissue necrosis in some subjects that was sometimes lethal (16). Murine models have suggested that this Koch phenomenon is more likely to occur in animals with a higher mycobacterial load, because immunopathology developed when DNA vaccination was given to mice with active TB but not to those with latent TB (17). Due to the concern about the possibility of inducing a Koch phenomenon, clinical trials of MVA85A were initially performed in those as mycobacterially naive as possible (PPD negative on skin test, BCG naive), progressing thereafter to subjects vaccinated with BCG (18). MVA85A has been shown to be well tolerated in these populations and highly immunogenic in the context of previous BCG. This trial now extends these reassuring results by showing that vaccination is safe and highly immunogenic in individuals with established LTBI, allowing progression to larger phase II trials in parts of the world where there is a very high prevalence of latent infection and also the greatest need for improved vaccines (7).
It is difficult to be certain whether the transient development of a single pulmonary nodule in one subject in the interval between screening and repeat scanning at 10 weeks was vaccine related. This subject was 29 years of age with a 13 pack-year history of smoking (20 cigarettes per day for 13 yr) and a grade IV Heaf at screening. His nodule may have developed at any stage prior to his 10-week scan, and had fully resolved by 14 weeks. He remained asymptomatic throughout the trial period. It is also possible that the nodule was present prevaccination and was not visualized on the baseline HRCT screening scan, because this was a limited examination of five CT slices (to minimize ionizing radiation exposure). Noncalcified pulmonary nodules are common and frequently identified on thoracic HRCT scan in the absence of any specific disease (19). Such nodules are detected in 5 to 66% of individuals who smoke (as our trial participant did) (20–26). Resolution or reduction in size of these nodules is also well recognized, with nodules of less than or equal to 5 mm being the most likely to resolve (20, 27). Possible diagnoses that have been proposed for resolving nodules include focal inflammatory lesions, mucoid impaction in small bronchi, or intermittent enlargement of benign intrapulmonary lymph nodes.
This trial has demonstrated that MVA85A is equally immunogenic in individuals with LTBI as it is in individuals uninfected with M. tuberculosis but vaccinated with BCG. Vaccine-induced immune responses persisted with significantly more Ag85A protein and peptide pool–specific IFN-γ–secreting T cells present at 1 year after vaccination compared with prevaccination. Interestingly, the kinetic and pattern of IL-2 secretion on ELISpot closely mirrored the IFN-γ secretion and this response was equally durable. Although it is known that IFN-γ is essential for protection, it is increasingly being recognized that alone it is not a good correlate of protection (28–32). Other cytokines are clearly also important in protective immunity against TB. IL-2 is known to be critically important in growth and stimulation of T cells, B cells, and NK cells (33, 34) and is important in the establishment of T-cell memory (35). In addition to effector T-cell responses, it may be important to monitor Th-2 and regulatory T-cell responses, because these may be important in protective immunity and in vaccine efficacy (36).
Induction of a potent antigen-specific T-cell response is likely to be paramount for an effective TB vaccine and there is evidence from a number of systems of the importance of polyfunctional T cells in protective immunity against intracellular pathogens (37, 38). Using polychromatic flow cytometry to investigate the functionality of Ag85A-specific CD4+ T cells induced by MVA85A in this trial revealed similarities and differences to that previously reported for a BCG-vaccinated cohort (13). The functional profile of the cells at baseline was markedly different in the LTBI group compared with the BCG-vaccinated group. At baseline the profile was considerably more polyfunctional than in the BCG-vaccinated group where only monofunctional cells were identified. This is supportive evidence for this population being immunologically different from the non-LTBI population and the fact that these cells are present may suggest that they are an important T-cell subset in natural host immunity against TB disease. As in the previous trial, there was a significant increase in magnitude of 3+ cells compared with baseline after vaccination with MVA85A. It would be interesting to serially investigate the persistence of the polyfunctional T cells in individuals with LTBI to examine whether these 3+ CD4+ T-cell populations decline in frequency in those individuals who go on to develop active TB disease.
It will not be possible to ascertain whether the immune responses induced by MVA85A are of clinical significance until an efficacy trial is completed. Performing extensive immunological analysis in subjects vaccinated in early studies, such as this phase 1 trial, is important because it will eventually facilitate the identification and understanding of protective correlates of immunity against TB.
In this trial no change was detected in either the median or individual frequency of T-cell responses to ESAT-6 or CFP-10 during the year of follow-up after vaccination. Although there is some evidence from animal studies to suggest that the strength of reactivity to these antigens relates to bacterial load and pathology (39, 40), data are more conflicting from human studies. Correlations between T-cell frequency to ESAT-6 and CFP-10 and intensity of exposure to M. tuberculosis have been detected acutely, although no studies have followed this relationship through the passage of time (41, 42). However, another study has suggested an inverse correlation between presumed bacterial load and magnitude of T-cell response to ESAT-6 and CFP-10 in patients not on treatment (43). Furthermore, no correlation has been seen between magnitude of IFN-γ response to ESAT-6 and CFP-10 and radiological disease in patients with active TB or LTBI (44–47).
Because effective antibiotic regimens cure active TB disease and antibiotic chemoprophylaxis reduces the likelihood of individuals developing disease, it seems reasonable to assume that both are associated with a reduction in numbers of viable bacilli (48, 49). A significant decline in ESAT-6 responses has been detected in individuals with active disease on antibiotic treatment (42, 43, 50–52). In subjects with LTBI after point source exposure, those given antibiotic chemoprophylaxis demonstrated a significant decrease in responses to ESAT-6 and CFP-10 at 18 months but not at 6 months, contrasting with the insignificant change in overall frequency of ESAT-6– and CFP-10–specific T cells in the untreated adults (53). However, there is no evidence for a decline in ESAT-6 and CFP-10 results at 3 and 6 months after chemoprophylaxis in subjects exposed over longer periods (54, 55).
Given the lack of clarity as to the relationship between T cell response to ESAT-6 and CFP-10 and bacterial load, and the small number of subjects in this trial, the efficacy of MVA85A in LTBI cannot be evaluated in this study. Further larger and randomized trials are now needed to address this question.
This study is limited by the heterogeneity of the population used and the small sample size. Subjects within this trial were of varying ages, ethnicities, and countries of birth, with varying smoking histories. Although the majority had no clear history of recent M. tuberculosis exposure, it is likely that the interval between M. tuberculosis infection and enrollment in the study was highly variable. In addition, it is likely that each subject had different bacterial burdens of M. tuberculosis and it remains possible that some subjects may have already cleared their infection at the time of vaccination. Consequently, it would have been extremely challenging to have obtained a matched control arm for the study. Some of the heterogeneity could have been minimized by undertaking this study in a highly endemic country. However, such a study is likely to have been confounded to a greater extent by ongoing exposure and infection with M. tuberculosis. It was also believed to be important to undertake this first study of MVA85A in subjects with LTBI in the developed world with the back-up of comprehensive health care facilities. It will be important to investigate safety of MVA85A in subjects who are contacts of individuals with smear-positive pulmonary TB by initially administering the vaccine together with chemoprophylaxis and subsequently comparing the vaccine alone with chemoprophylaxis alone. We recognize that the safety of MVA85A will continue to be monitored in subsequent studies of MVA85A in subjects with LTBI, although using less intensive methodology compared with this study.
This is the first subunit TB vaccine to enter clinical trials in M. tuberculosis–infected subjects since Robert Koch experimented with his “remedy” of culture filtrate protein in 1890 with devastating consequence (16). We have demonstrated that MVA85A is safe in healthy subjects who are latently infected with M. tuberculosis, by close clinical, immunological, and radiological monitoring. Furthermore, we have demonstrated that MVA85A is as immunogenic when administered to subjects infected with M. tuberculosis, as it is when administered to uninfected individuals who have been primed with BCG. This trial has enabled further phase II trials of this vaccine to proceed in an area of high TB endemicity, such as the Western Cape in South Africa. Subsequent trials with MVA85A will continue to monitor safety, but will not require the intensive follow-up performed in this United Kingdom–based phase I trial.
Acknowledgments
The authors thank the TB nurses in Oxford Radcliffe Hospital NHS Trust and Northwest London Hospitals NHS Trust (Northwick Park), Mario Roederer for providing Pestle and SPICE, and Trudie Lang for help with project management.
Supported by funds from the Oxford Biomedical Research Centre. A.V.S.H. is a Wellcome Principal fellow and H.M. is a Wellcome Senior fellow. C.R.S. was funded by AFTBVAC (EU 5th framework).
Originally Published in Press as DOI: 10.1164/rccm.200809-1486OC on January 16, 2009
Conflict of Interest Statement: C.R.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.A.P. is a named inventor on a composition of matter patent for MVA85A filed by the University of Oxford. N.E.R.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. I.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.V.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.V.S.H. is a named inventor on a composition of matter patent for MVA85A filed by the University of Oxford. F.V.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.J.O.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.M. is a named inventor on a composition of matter patent for MVA85A filed by the University of Oxford.
References
- 1.Global tuberculosis control: surveillance, planning, financing. WHO Report 2007 [Internet]. Geneva, Switzerland: World Health Organization; 2007. (WHO/HTM/TB/2007.376). (Accessed 2009 Feb 11). Available from: http://www.who.int/tb/publications/global_report/2007/contents/en/index.html
- 2.Shi L, Jung YJ, Tyagi S, Gennaro ML, North RJ. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc Natl Acad Sci USA 2003;100:241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wayne LG, Sohaskey CD. Nonreplicating persistence of Mycobacterium tuberculosis. Annu Rev Microbiol 2001;55:139–163. [DOI] [PubMed] [Google Scholar]
- 4.Warren RM, Victor TC, Streicher EM, Richardson M, Beyers N, Gey van Pittius NC, van Helden PD. Patients with active tuberculosis often have different strains in the same sputum specimen. Am J Respir Crit Care Med 2004;169:610–614. [DOI] [PubMed] [Google Scholar]
- 5.Cappelli G, Volpe P, Sanduzzi A, Sacchi A, Colizzi V, Mariani F. Human macrophage gamma interferon decreases gene expression but not replication of Mycobacterium tuberculosis: analysis of the host-pathogen reciprocal influence on transcription in a comparison of strains H37Rv and CMT97. Infect Immun 2001;69:7262–7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monahan IM, Betts J, Banerjee DK, Butcher PD. Differential expression of mycobacterial proteins following phagocytosis by macrophages. Microbiology 2001;147:459–471. [DOI] [PubMed] [Google Scholar]
- 7.McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, Fletcher HA, Hill AV. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med 2004;10:1240–1244. [DOI] [PubMed] [Google Scholar]
- 8.Nahid P, Pai M, Hopewell PC. Advances in the diagnosis and treatment of tuberculosis. Proc Am Thorac Soc 2006;3:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institute for Health and Clinical Excellence. Clinical guideline 33. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control [Internet]. London, UK: National Health Service; 2006. (Updated 2008 Sep 23; accessed 2009 Jan 9). Available from: http://www.nice.org.uk/nicemedia/pdf/CG033niceguideline.pdf.
- 10.McShane H, Behboudi S, Goonetilleke N, Brookes R, Hill AV. Protective immunity against Mycobacterium tuberculosis induced by dendritic cells pulsed with both CD8(+)- and CD4(+)-T-cell epitopes from antigen 85A. Infect Immun 2002;70:1623–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pathan AA, Sander CR, Fletcher HA, Poulton I, Alder NC, Beveridge NE, Whelan KT, Hill AV, McShane H. Boosting BCG with recombinant modified vaccinia Ankara expressing antigen 85A: different boosting intervals and implications for efficacy trials. PLoS One 2007;2:e1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perfetto SP, Chattopadhyay PK, Lamoreaux L, Nguyen R, Ambrozak D, Koup RA, Roederer M. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J Immunol Methods 2006;313:199–208. [DOI] [PubMed] [Google Scholar]
- 13.Beveridge NE, Price DA, Casazza JP, Pathan AA, Sander CR, Asher TE, Ambrozak DR, Precopio ML, Scheinberg P, Alder NC, et al. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur J Immunol 2007;37:3089–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badovinac VP, Harty JT. Memory lanes. Nat Immunol 2003;4:212–213. [DOI] [PubMed] [Google Scholar]
- 15.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008;149:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch R. Forsetzung der Mitteilungen uber ein Heilmittel gegen Tuberkulose. Dtsch Med Wochenschr 1891;17:101–102. [Google Scholar]
- 17.Taylor JL, Turner OC, Basaraba RJ, Belisle JT, Huygen K, Orme IM. Pulmonary necrosis resulting from DNA vaccination against tuberculosis. Infect Immun 2003;71:2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McShane H, Pathan AA, Sander CR, Goonetilleke NP, Fletcher HA, Hill AV. Boosting BCG with MVA85A: the first candidate subunit vaccine for tuberculosis in clinical trials. Tuberculosis (Edinb) 2005;85:47–52. [DOI] [PubMed] [Google Scholar]
- 19.Fischbach F, Knollmann F, Griesshaber V, Freund T, Akkol E, Felix R. Detection of pulmonary nodules by multislice computed tomography: improved detection rate with reduced slice thickness. Eur Radiol 2003;13:2378–2383. [DOI] [PubMed] [Google Scholar]
- 20.Diederich S, Thomas M, Semik M, Lenzen H, Roos N, Weber A, Heindel W, Wormanns D. Screening for early lung cancer with low-dose spiral computed tomography: results of annual follow-up examinations in asymptomatic smokers. Eur Radiol 2004;14:691–702. [DOI] [PubMed] [Google Scholar]
- 21.Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, Libby DM, Pasmantier MW, Koizumi J, Altorki NK, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99–105. [DOI] [PubMed] [Google Scholar]
- 22.Kaneko M, Eguchi K, Ohmatsu H, Kakinuma R, Naruke T, Suemasu K, Moriyama N. Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology 1996;201:798–802. [DOI] [PubMed] [Google Scholar]
- 23.Nawa T, Nakagawa T, Kusano S, Kawasaki Y, Sugawara Y, Nakata H. Lung cancer screening using low-dose spiral CT: results of baseline and 1-year follow-up studies. Chest 2002;122:15–20. [DOI] [PubMed] [Google Scholar]
- 24.Pastorino U, Bellomi M, Landoni C, De Fiori E, Arnaldi P, Picchio M, Pelosi G, Boyle P, Fazio F. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet 2003;362:593–597. [DOI] [PubMed] [Google Scholar]
- 25.Sone S, Takashima S, Li F, Yang Z, Honda T, Maruyama Y, Hasegawa M, Yamanda T, Kubo K, Hanamura K, et al. Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet 1998;351:1242–1245. [DOI] [PubMed] [Google Scholar]
- 26.Swensen SJ, Jett JR, Sloan JA, Midthun DE, Hartman TE, Sykes AM, Aughenbaugh GL, Zink FE, Hillman SL, Noetzel GR, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 2002;165:508–513. [DOI] [PubMed] [Google Scholar]
- 27.Diederich S, Hansen J, Wormanns D. Resolving small pulmonary nodules: CT features. Eur Radiol 2005;15:2064–2069. [DOI] [PubMed] [Google Scholar]
- 28.Dorman SE, Picard C, Lammas D, Heyne K, van Dissel JT, Baretto R, Rosenzweig SD, Newport M, Levin M, Roesler J, et al. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet 2004;364:2113–2121. [DOI] [PubMed] [Google Scholar]
- 29.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 1993;178:2249–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med 1996;335:1941–1949. [DOI] [PubMed] [Google Scholar]
- 31.Romano M, D'Souza S, Adnet PY, Laali R, Jurion F, Palfliet K, Huygen K. Priming but not boosting with plasmid DNA encoding mycolyl-transferase Ag85A from Mycobacterium tuberculosis increases the survival time of Mycobacterium bovis BCG vaccinated mice against low dose intravenous challenge with M. tuberculosis H37Rv. Vaccine 2006;24:3353–3364. [DOI] [PubMed] [Google Scholar]
- 32.Vordermeier HM, Huygen K, Singh M, Hewinson RG, Xing Z. Immune responses induced in cattle by vaccination with a recombinant adenovirus expressing Mycobacterial antigen 85A and Mycobacterium bovis BCG. Infect Immun 2006;74:1416–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith KA. Interleukin 2. Annu Rev Immunol 1984;2:319–333. [DOI] [PubMed] [Google Scholar]
- 34.Smith KA. Interleukin-2: inception, impact, and implications. Science 1988;240:1169–1176. [DOI] [PubMed] [Google Scholar]
- 35.Harari A, Vallelian F, Pantaleo G. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur J Immunol 2004;34:3525–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fletcher HA, Pathan AA, Berthoud TK, Dunachie SJ, Whelan KT, Alder NC, Sander CR, Hill AV, McShane H. Boosting BCG vaccination with MVA85A down-regulates the immunoregulatory cytokine TGF-beta1. Vaccine 2008;26:5269–5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007;13:843–850. [DOI] [PubMed] [Google Scholar]
- 38.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006;107:4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langermans JA, Doherty TM, Vervenne RA, van der Laan T, Lyashchenko K, Greenwald R, Agger EM, Aagaard C, Weiler H, van Soolingen D, et al. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine 2005;23:2740–2750. [DOI] [PubMed] [Google Scholar]
- 40.Vordermeier HM, Chambers MA, Cockle PJ, Whelan AO, Simmons J, Hewinson RG. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect Immun 2002;70:3026–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill PC, Brookes RH, Fox A, Fielding K, Jeffries DJ, Jackson-Sillah D, Lugos MD, Owiafe PK, Donkor SA, Hammond AS, et al. Large-scale evaluation of enzyme-linked immunospot assay and skin test for diagnosis of Mycobacterium tuberculosis infection against a gradient of exposure in The Gambia. Clin Infect Dis 2004;38:966–973. [DOI] [PubMed] [Google Scholar]
- 42.Lalvani A, Nagvenkar P, Udwadia Z, Pathan AA, Wilkinson KA, Shastri JS, Ewer K, Hill AV, Mehta A, Rodrigues C. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J Infect Dis 2001;183:469–477. [DOI] [PubMed] [Google Scholar]
- 43.Pathan AA, Wilkinson KA, Klenerman P, McShane H, Davidson RN, Pasvol G, Hill AV, Lalvani A. Direct ex vivo analysis of antigen-specific IFN-γ-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol 2001;167:5217–5225. [DOI] [PubMed] [Google Scholar]
- 44.Joshi R, Patil S, Kalantri S, Schwartzman K, Menzies D, Pai M. Prevalence of abnormal radiological findings in health care workers with latent tuberculosis infection and correlations with T cell immune response. PLoS One 2007;2:e805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pai M, Joshi R, Bandyopadhyay M, Narang P, Dogra S, Taksande B, Kalantri S. Sensitivity of a whole-blood interferon-gamma assay among patients with pulmonary tuberculosis and variations in T-cell responses during anti-tuberculosis treatment. Infection 2007;35:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seah GT, Scott GM, Rook GA. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J Infect Dis 2000;181:385–389. [DOI] [PubMed] [Google Scholar]
- 47.Sodhi A, Gong J, Silva C, Qian D, Barnes PF. Clinical correlates of interferon gamma production in patients with tuberculosis. Clin Infect Dis 1997;25:617–620. [DOI] [PubMed] [Google Scholar]
- 48.American Thoracic Society/Centers for Disease Control. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000;161:S221–S247. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y. The magic bullets and tuberculosis drug targets. Annu Rev Pharmacol Toxicol 2005;45:529–564. [DOI] [PubMed] [Google Scholar]
- 50.Carrara S, Vincenti D, Petrosillo N, Amicosante M, Girardi E, Goletti D. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin Infect Dis 2004;38:754–756. [DOI] [PubMed] [Google Scholar]
- 51.Nicol MP, Pienaar D, Wood K, Eley B, Wilkinson RJ, Henderson H, Smith L, Samodien S, Beatty D. Enzyme-linked immunospot assay responses to early secretory antigenic target 6, culture filtrate protein 10, and purified protein derivative among children with tuberculosis: implications for diagnosis and monitoring of therapy. Clin Infect Dis 2005;40:1301–1308. [DOI] [PubMed] [Google Scholar]
- 52.Aiken AM, Hill PC, Fox A, McAdam KP, Jackson-Sillah D, Lugos MD, Donkor SA, Adegbola RA, Brookes RH. Reversion of the ELISPOT test after treatment in Gambian tuberculosis cases. BMC Infect Dis 2006;6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ewer K, Millington KA, Deeks JJ, Alvarez L, Bryant G, Lalvani A. Dynamic antigen-specific T-cell responses after point-source exposure to Mycobacterium tuberculosis. Am J Respir Crit Care Med 2006;174:831–839. [DOI] [PubMed] [Google Scholar]
- 54.Pai M, Joshi R, Dogra S, Mendiratta DK, Narang P, Dheda K, Kalantri S. Persistently elevated T cell interferon-gamma responses after treatment for latent tuberculosis infection among health care workers in India: a preliminary report. J Occup Med Toxicol 2006;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilkinson KA, Kon OM, Newton SM, Meintjes G, Davidson RN, Pasvol G, Wilkinson RJ. Effect of treatment of latent tuberculosis infection on the T cell response to Mycobacterium tuberculosis antigens. J Infect Dis 2006;193:354–359. [DOI] [PubMed] [Google Scholar]