Abstract
To evaluate the effectiveness of the human umbilical cord blood (HUCB) transplantation for the treatment of intrinsic sphincter deficiency (ISD), we analyzed the short term effects of HUCB mononuclear cell transplantation in rats with induced-ISD. ISD was induced in rats by electro-cauterization of periurethral soft tissue with HUCB mononuclear cell injection after 1 week. The sphincter function measured by mean leak point pressure was significantly improved in the experimental group compared to the control group at 4 weeks. (91.75±18.99 mmHg vs. 65.02±22.09 mmHg, P=0.001). Histologically, the sphincter muscle was restored without damage while in the control group it appeared markedly disrupted with atrophic muscle layers and collagen deposit. We identified injected HUCB cells in the tissue sections by Di-I signal and Prussian blue staining. HUCB mononuclear cell injection significantly improved urethral sphincter function, suggesting its potential efficacy in the treatment of ISD.
Keywords: Urinary Incontinence, Stress; Intrinsic Sphincter Deficiency; Leak Point Pressure; Human Umbilical Cord Blood Mononuclear Cells; Stem Cells
INTRODUCTION
Stress urinary incontinence (SUI), defined as the involuntary leakage of urine on effort or exertion, or on sneezing or coughing, is a common embarrassing condition that primarily affects women. The two epidemiologic factors most strongly associated with the development of SUI in women are parity and advanced age (1, 2). Although the exact mechanism of SUI in elderly postpartum women is poorly understood, two principal causes of SUI have been postulated: 1) lack of sufficient support around the urethra that can lead to increased movement or 'hypermobility' and 2) a weakness in the urethral sphincter that controls the passage of urine, known as 'intrinsic sphincter deficiency' (ISD) due to both neuromuscular and connective tissue injuries (3). Especially ISD is known to be closely related to the injury to the internal rhabdosphincter, such as obstetric trauma or surgical injury. A direct correlation has recently been noted between the frequency of urinary incontinence and apoptosis of the urethral sphincter cells. Decreased muscle cell numbers caused by age-dependent spontaneous apoptosis of muscle cells may also be an important factor in stress urinary incontinence in both men and women. Thus, ISD can be regarded as a type of degenerative disease with decreased ability to recover or regenerate the damaged sphincter that may result in the failure of proper tissue reconstruction (4-6).
The notion of ISD as a degenerative disease provided the theoretical ground for stem cell therapy in ISD in some animal model studies with the prospect that the stem cells may play a role in regenerating the damaged sphincter. Stem cell application in the treatment of ISD has been tried with muscle-derived progenitor cells or satellite cells, the alleged stem cells for skeletal muscle. In addition, tissue engineering using muscle cells is also an area of active research that may present exciting treatment options for urologic diseases (7-10). However, previous stem cell treatments were not entirely successful due to some issues. First, cultivated and extracted stem cells from the aged patient's own muscle do not grow well after transplantation. Second, the muscle extraction process is invasive and may cause complications of bleeding or infection at the surgery site.
We expected that human umbilical cord blood (HUCB) can be a better source of adult stem cells as it is not associated with the problems of other stem cell therapies mentioned above and it can be safely used without significant side effects. Here, we tried HUCB stem cell transplantation in rat ISD model and investigated the changes of sphincter muscle function and structure to evaluate its efficacy in the treatment of ISD.
MATERIALS AND METHODS
Human umbilical cord blood sample collection and animal handling for this experiment were performed in compliance with laws and institutional guidelines approved by the Institutional Animal Care and Use Committee from CHA Gangnam Medical Center and School of Medicine, CHA University. This study was carried out from June 2005 to September 2006 at the CHA Gangnam Medical Center and School of Medicine, CHA University.
Animals
All experiments were performed on normal female, 5-6 week-old Sprague-Dawley rats (Orient Bio Inc. Seongnam, Korea), weighing 200 to 250 g. ISD was induced by electrocauterization in all animals. One week after electro-cauterization, the animals were injected with either HUCB mononuclear cells (experimental group, n=28) or normal saline (control group, n=22). The rats were kept in individual cages and had free access to water and food. Eight and 14 rats from the control group and 9 and 19 rats from the experimental group were studied at 2 and 4 weeks after injection, respectively.
Induction of ISD by electro-cauterization
ISD was induced by electro-cauterization as previously described (8, 11). Briefly, rats were anesthetized by intraperitoneal injection of ketamine hydrochloride (100 mg/kg) and xylazine hydrochloride (15 mg/kg). Under anesthesia, they were placed in the supine position with the lower legs abducted. The bladder and urethra were exposed through a lower midline abdominal incision. Tissues, 1 cm lateral to the mid-urethra were cauterized on both sides to produce sphincter injury. A fine tip, high-temperature cautery (Aaron Medical, St. Petersburg, FL, USA) was used. Each side was cauterized for 30 sec.
Human umbilical cord blood mononuclear cell separation
After thawing at 37℃, cord blood cells were separated into a low-density mononuclear fraction (<1,077 g/mL) by Ficoll-Paque Plus (GE Healthcare AB, Uppsala, Sweden, http://www.amersham.com). Briefly, after adding and mixing the same amount of normal saline into the cord blood, poured the diluted solution on Ficoll in 15 mL tubes (Ficoll: diluted blood=3:4), and centrifuged at 1,800 rpm for 30 min. After carefully extracting the mononuclear cells from the mononuclear cell layer on the Ficoll, gathered the cells in 50 mL tubes and added a 3×volume of normal saline and collected the target cells by centrifugation at 1,800 rpm for 10 min. After removing the upper layer solution, 50 mL of normal saline was added and mixed into the remaining cell pellet and two more centrifugation processes at 1,000 rpm for 10 min were performed. Finally, the cell pellet with 50 mL of normal saline was elutriated and cell counting in 10 µL of the suspension was performed.
Labeling of human umbilical cord blood mononuclear cells
To track the migration and engraftment of HUCB mononuclear cells at the site of injection, cells were double labeled with iron oxides and the fluorescent cell tracker 1,10-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI) dye according to the method developed by Arbab et al. (12). Briefly, HUCB mononuclear cells were grown in 75 cm2 culture flasks to 80-90% confluence before labeling with iron oxides (Feridex®, Berlex Laboratories Inc., Wayne, NJ, USA) and fluorescent dye DiI (Molecular Probes, Eugene, OR, USA). On the day of labeling, the old media was discarded, replaced by 50% of fresh complete BMSC media and 50% serum free media containing iron oxides-protamine sulfate complexes (50 and 2 µg/mL, respectively), and incubated overnight. Cells were harvested and resuspended in serum free media at a concentration of 1-2 million/mL. 1×106 iron-labeled cells were further labeled with fluorescent tracker DiI (Cell tracker CM-DiI, C-7000; Molecular Probes) dye for 30 min at 37℃ in 1 mL of serum-free medium containing 10 mL of DiI stock solution. After labeling with DiI, cells were washed twice with PBS before injection. Cell viability was established by the Trypan blue exclusion method before and after fluorescent labeling.
Human umbilical cord blood mononuclear cell injection
One week after electro-cauterization, the experimental rats underwent ketamine/xylazine anesthesia and a low midline incision was made to expose the bladder and urethra. A 30 µL Hamilton syringe was used to inject a total of 20 µL of HUCB mononuclear cell suspension in saline solution (2×106 cells per 20 µL). Two injections per rat (10 µL each) were performed on each lateral wall of the mid-urethra and then the abdomen was sutured. Control rats were injected with normal saline only.
Leak point pressure measurement
At 2 and 4weeks after HUCB mononuclear cells injection the leak point pressure (LPP) were measured using the vertical tilt/intravesical pressure clamp model of stress urinary incontinence (11). The animals were anesthetized with urethane (1.2 g/kg) injected subcutaneously. And all rats underwent spinal cord transection at the T9-T10 level following laminectomy in order to eliminate spontaneous bladder activity in response to increasing intravesical pressures. This manipulation does not interfere with the spinal continence reflexes of the bladder neck and urethra. The overlying skin was then closed with a suture. Next, through a midline abdominal incision the distal colon was cleared of feces by gentle massage, and a loose suture was secured around the proximal end of the distal colon to prevent further fecal migration to the pelvic floor during the course of the experiment. A transvesical catheter with a fire-flared tip (PE-90, Clay Adams, Parsippany, NJ, USA) was inserted into the dome of the bladder and secured with a ligature for bladder filling and pressure recording. The abdominal wall and overlying skin were closed with a suture. The rat was then mounted on a tilt table with the axis of rotation positioned for constant bladder height in relation to the pressure transducer, either in the supine or vertical position. To measure the intravesical pressure, normal saline was infused through the polyethylene catheter that was inserted into the animal's bladder. The catheter is connected to the microsyringe pump (KD Scientific, Holliston, MA, USA) and the pressure measurement system via a 3-way stopcocks. Data generated by the pressure sensor (ADInstruments, Colorado-springs, CO, USA) and bridge amplifier (ADInstruments, Powerlab® 2/25, Coloradosprings, CO, USA) were analyzed.
All bladder pressure was referenced to air pressure at the level of bladder. The rats were placed at the level of zero pressure while bladders were filled with room temperature saline at 5 mL/hr through the bladder catheter. The rats were then mounted on a tilt table and placed in the vertical position. The pressure at leak point was taken as the LLP. The average of three consecutive LPP was taken as a data point for each animal.
Tissue preparation and histology
Immediately after the LPP measurement, the rats were euthanized, and the proximal urethra was removed. The tissue samples for fluorescent microscopy were prepared by snap freezing of the urethra, embedding in OCT compound (Tissue Tek; Sakura Finekek USA, Torrance, CA, USA). Sections were cut (5 µm) and used for fluorescent dye staining. Specimens for light microscopy were fixed in 10% formaldehyde, embedded in paraffin, and cut to obtain 5 µm thick sections. These sections were deparaffinated, hydrated, and stained with hematoxylin-eosin and Masson's trichrome. All staining were performed using standard methods, and all reagents were from Sigma-Aldrich (St.Louis, MO, USA).
Fluorescent microscopy and prussian blue staining
Tissues were cut into 10 mm pieces (axial) and snap frozen for sectioning at 5 µm thickness. Tissue sections were cover slipped using aqueous mounting media containing 40, 6-diamidino-2-phenylindole (DAPI; Vector Shield, Vector Laboratories Inc., Burlingame, CA, USA) to outline the nucleus. Fluorescent-labeled cells were detected and photographed using a fluorescent microscope. Following fluorescent photomicrography, slides were dismounted and tissue sections were stained for iron using Prussian blue staining technique. Bright field photomicrographs were obtained from the same sites with same magnification to determine whether fluorescent positive cells were also iron positive.
All immunofluorescent labeling and dyes were examined using a Carl Zeiss LSM 510 Meta confocal laser scanning microscope equipped with 488 nm argon ion, 543 nm green helium-neon, and 633 nm red helium-neon laser lines. Three-dimensional images were reconstructed by several consecutive optical sections of various thicknesses (0.25-0.38 µm).
Immunohistochemistry for detecting desmin
Formalin-fixed, paraffin-embedded tissues were sectioned at 5 µm thickness. These sections were deparaffinated, rehydrated, and pretreated with 3% H2O2 for 10 min at room temperature. Then antigen retrieval was performed in boiling 10mM citrate acid buffer (pH6.0) for 10 min followed by cooling at room temperature for 20 min. Then the sections were incubated in 10% normal goat serum in PBS for 1 hr at room temperature followed by incubation with primary antibody (mouse anti-desmin monoclonal antibody, abcam ab17156, Abcam Inc, Cambridge, MA) at 4℃ overnight. Sections were incubated with horseradish peroxidase-conjugated goat-anti mouse IgG (Vector Laboratory Inc. Burlingame, CA, USA) for 15 min at room temperature. DAB chromogen was used for color development under the light microscope and the sections were counterstained with hematoxylin.
Statistical analysis
LPP data are presented as means±SD. Overall comparisons between groups were performed using a one-way analysis of variance for within time-point analyses and two-way analysis of variance for across time-point analyses. Individual comparisons were made using Student's t-test. A P value of less than 0.05 was accepted as significant.
RESULTS
The LPP in HUCB mononuclear cell-injected rats
Fig. 1 shows the LPP values measured using vertical tilt/intravesical pressure clamp technique at 2 and 4 weeks after the injection. The LPP values were slightly higher in the experimental group, but not statistically different at 2 weeks. The mean value for the control group (n=8) was 46.13±12.14 mmHg, and the value for the experimental group (n=9) was 55.72±9.99 mmHg at 2 weeks. But, the LPP values were significantly increased in the experimental group at 4 weeks while the control group did not show significant change in LPP value (mean LPP for control group (n=14): 65.02±22.09 mmHg, for the experimental group (n=19): 91.75±18.99 mmHg, P=0.001, t=-3.728, within 95% validity interval).
Fig. 1.
Comparative effect of HUCB mononuclear cell injection on leak point pressure between groups at 2 and 4 weeks. *is significantly different from each other (P<0.05) at each time. Compared with control group, the leak point pressure in the experimental group at 4 week after HUCB mononuclear cell injection was significantly increased.
HUCB mononuclear cells in the urethral wall in the experimental group at 2 weeks and 4 weeks
Iron-stained, fluorescent-labeled HUCB mononuclear cells were found in the lamina propria and in the muscular urethral sphincter in the experimental group at 2 weeks (Figs. 2, 3). The human cells were mainly found in the lamina propria just beneath the urethral mucosa. A few cells were also noted in the sphincter skeletal muscle layer. Some of the cells were small, round cells with scant cytoplasm and some were bigger with abundant cytoplasm. A few of the cells just beneath the mucosa were spindle shaped. We could not find any iron-stained or fluorescent-labeled cells in the rat urethral tissue at 4 weeks. The control group rats did not show any iron-stained or fluorescent-labeled human cells at 2 weeks and 4 weeks.
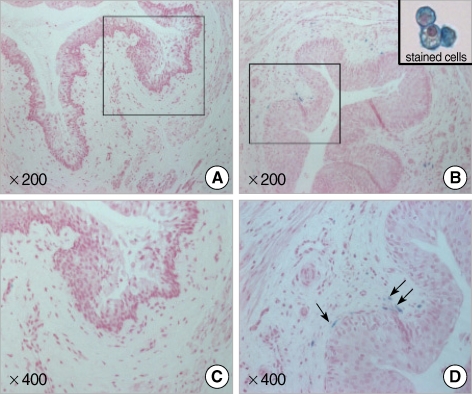
Fig. 2.
Photographs show Feridex®-labeled HUCB mononuclear cells in urethral tissue at 2 weeks post-injection. A number of cells considered human cells within the tissue sections were detected by blue-colored signals. (A, C) Control group; (B, D) Experimental group (Magnification ×200, 400).
Fig. 3.
Photographs show Di-I labeled HUCB mononuclear cells in urethral tissue at 2 weeks post-injection. A number of cells considered human cells within the tissue sections were detected by red-colored Di-I signals (A, B, Di-I stained cells; C, Control group; D-F, Experimental group. A, D are DIC images. B and E are fluorescence images).
The morphological integrity of the internal urethral sphincter
No significant morphological difference in the urethral sphincter muscle was observed at 2 weeks between the experimental group and control group. At 4 weeks after the electro-cauterization, however, obvious disruption of the urethral sphincter muscle was apparent in the control group, while the sphincter muscle in the experimental group injected with HUCB mononuclear cells was largely restored to the morphological integrity of the intact urethral sphincter (Figs. 2-4). The immunohistochemical staining to desmin showed the disrupted arrangement of skeletal muscle layer of the urethral sphincter in the control group while in the experimental group, the sphincter remained well-organized (Fig. 5). In the control group, collagen was deposited at the disrupted area, which was confirmed by the blue color on trichrome staining. On the other hand, collagen deposit was not observed in the experimental group injected with HUCB mononuclear cells (Fig. 5).
Fig. 4.
Photographs show sphincter muscle disruption and collagen deposit in the control group (A) at 4 weeks after injection. Sphincter muscles in the experimental group (B) were well preserved and intact, and with no collagen deposit (H&E, ×400).
Fig. 5.
The immunohistochemical stainings for desmin and trichrome stain show apparent sphincter muscle disruption and collagen deposit in the control group (A&C, control group, B&D, experimental group, ×400).
DISCUSSION
Various types of tissue have been tried as the source of stem cells in the stem cell therapy for ISD in animal studies. Autologous bone marrow-derived stem cells have been one of the most common sources used for the mesenchymal or potentially myogenic stem cells, but harvesting of mesenchymal stem cells from bone marrow aspirate is difficult with low yield and the procedure of bone marrow procurement is painful (13). Muscle-derived stem cells, myoblasts, or satellite cells, alleged muscle progenitor cells, have also been tried. Yiou et al. reported regeneration of an injured rat rhabdomyosphincter with intrinsic satellite cells (5). Yokoyama et al. (14) injected muscle-derived progenitor cells, MDPCs, in the normal rat urethra and observed better durability than obtained by treatment with bovine collagen. Cannon et al. (9) injected allogenic muscle-derived progenitor cells in the urethra of a rat with severed nerves and reported enhanced contractability of the sphincter.
Nevertheless, the use of umbilical cord blood stem cells for treatment of urinary incontinence had never been attempted before. Since Rocha et al. (15) successfully performed cord blood transplantation for Fanconi anemia in 1988, cord blood transplantation has been performed frequently as a substitute for bone marrow transplantation. It also has been investigated in many animal models of various degenerative diseases, such as spinal cord injury model and cerebral ischemia model, with some promising results (16-18). Umbilical cord blood is more easily applicable as a donor because of its less stringent HLA matching requirements due to its immunologic inertness and subsequent lower chance of graft versus host disease (GVHD). Furthermore, the specimen collection is easier and the risk of viral contamination is lower.
In this study, we induced ISD in rats with electro-cauterization and injected mononuclear cells isolated from human umbilical cord blood to investigate the efficacy of HUCB mononuclear cells as a source of stem cell therapy in ISD. LPP values measured in 4 weeks by vertical tilt/intravesical pressure clamp model were significantly higher in the experimental group. The urethral rhabdosphincter of the experimental group remained well-organized and intact while the saline-treated control group showed severe degeneration and disruption of the sphincter muscle layers. No animals showed clinical or histological features of rejection, GVHD or infection. All these results showed that HUCB mononuclear cells may be an effective and safe treatment option for ISD, suggesting the role of umbilical cord blood as an alternative source for stem cell therapy.
The mechanism how the injected HUCB mononuclear cells act on the urethral sphincter is not yet understood. It was speculated that the injected stem cells might migrate to the demanded site and differentiate to skeletal muscular cells and repair the injured sphincter muscle. HUCB stem cells have been shown to differentiate into diverse cell lineages, including myogenic, chondrogenic, and insulin-producing cells (19-21). In our study, the injected HUCB mononuclear cells were noted to be localized around the urethral sphincter. The majority of them were noted in the lamina propria beneath the urethral mucosa but a few of them were found between the muscle layers of the sphincter, implicating that the cells play roles in repairing the injured muscle and maintaining the sphincter muscle intact. The injected stem cells in this study might also have differentiated into skeletal muscle cells. Interestingly a few of the iron positive cells noted around the urethral tissue were spindle shaped rather than round, suggesting mesenchymal differentiation. However, unfortunately, we failed to identify the cellular lineage of the iron-positive cells from the tissue sections due to absence of myoid antibody specific to human. Four weeks after the transplantation, we could no longer detect any human-derived iron-positive cells around the urethral tissue, despite the significant histological difference observed. The iron in the injected cells might have been already metabolized at that point. It is also possible that the injected cells might have been phagocytosed and lost with only very few of them engrafted and able to function. Identification of grafted stem cells in host organs has always been a matter of debate in animal model studies of stem cell therapy (22, 23). Some demonstrated grafted cells viable after quite a long period of time while others could not or could only prove very few in numbers (22, 24-26).
Another postulated mechanism is that in stem cell therapy, it is not the direct transdifferentiation of injected stem cells that actually affect the recovery of the injured tissue. Instead, the injected cells might rather stimulate the intrinsic stem cells in a paracrine way to help them proliferate and differentiate (27). It has also been speculated that it is the effect of growth factors or cytokines used in the culture media that might have been injected together with the cells. In this study, we could exclude the possibility of exogenous cytokines as we used only normal saline for cell suspension. However, the possibility of de novo paracrine effects from injected cells cannot be completely ruled out. Actually in recent years, the opinion that it is the paracrine effect, not the replacement of injured tissue by transdifferentiation of stem cells which promotes the recovery/regeneration of damaged tissue is gaining more support with repeated laboratory observations (28).
While it is still in debate how stem cells work in recovery of the damaged tissue, numerous studies have shown that stem cell therapy can improve the functional capacity of the damaged organs, at least temporarily (28). Some even demonstrated functional improvement in long term period (23, 29). Although we cannot positively guarantee the long term effect from our study alone, which remains to be proved, the results from these long term studies are encouraging.
We believe that HUCB mononuclear cell is worth as an alternative source of stem cells as it is easier to obtain than other sources and safely applicable without significant adverse effects, yet with noticeable therapeutic efficacy, which we demonstrated in our study.
ACKNOWLEDGMENTS
We thank I-Cord Public Umbilical Cord Blood Stem Cell Bank for allowing us to use the samples for research purpose.
Footnotes
This study was supported by the Research Fund from CHA University.
References
- 1.Turan C, Zorlu CG, Ekin M, Hancerliogullari N, Saracoglu F. Urinary incontinence in women of reproductive age. Gynecol Obstet Invest. 1996;41:132–134. doi: 10.1159/000292059. [DOI] [PubMed] [Google Scholar]
- 2.Brown JS, Seeley DG, Fong J, Black DM, Ensrud KE, Grady D. Urinary incontinence in older women: who is at risk? Study of Osteoporotic Fractures Research Group. Obstet Gynecol. 1996;87:715–721. doi: 10.1016/0029-7844(96)00013-0. [DOI] [PubMed] [Google Scholar]
- 3.Summitt RL, Jr, Bent AE, Ostergard DR, Harris TA. Stress incontinence and low urethral closure pressure. Correlation of preoperative urethral hypermobility with successful suburethral sling procedures. J Reprod Med. 1990;35:877–880. [PubMed] [Google Scholar]
- 4.Strasser H, Tiefenthaler M, Steinlechner M, Eder I, Bartsch G, Konwalinka G. Age dependent apoptosis and loss of rhabdosphincter cells. J Urol. 2000;164:1781–1785. [PubMed] [Google Scholar]
- 5.Yiou R, Lefaucheur JP, Atala A. The regeneration process of the striated urethral sphincter involves activation of intrinsic satellite cells. Anat Embryol (Berl) 2003;206:429–435. doi: 10.1007/s00429-003-0313-x. [DOI] [PubMed] [Google Scholar]
- 6.Feki A, Faltin DL, Lei T, Dubuisson JB, Jacob S, Irion O. Sphincter incontinence: is regenerative medicine the best alternative to restore urinary or anal sphincter function? Int J Biochem Cell Biol. 2007;39:678–684. doi: 10.1016/j.biocel.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Deasy BM, Huard J. Gene therapy and tissue engineering based on muscle-derived stem cells. Curr Opin Mol Ther. 2002;4:382–389. [PubMed] [Google Scholar]
- 8.Lee JY, Cannon TW, Pruchnic R, Fraser MO, Huard J, Chancellor MB. The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:31–37. doi: 10.1007/s00192-002-1004-5. [DOI] [PubMed] [Google Scholar]
- 9.Cannon TW, Lee JY, Somogyi G, Pruchnic R, Smith CP, Huard J, Chancellor MB. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003;62:958–963. doi: 10.1016/s0090-4295(03)00679-4. [DOI] [PubMed] [Google Scholar]
- 10.Cannon TW, Sweeney DD, Conway DA, Kamo I, Yoshimura N, Sacks M, Chancellor MB. A tissue-engineered suburethral sling in an animal model of stress urinary incontinence. BJU Int. 2005;96:664–669. doi: 10.1111/j.1464-410X.2005.05702.x. [DOI] [PubMed] [Google Scholar]
- 11.Conway DA, Kamo I, Yoshimura N, Chancellor MB, Cannon TW. Comparison of leak point pressure methods in an animal model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:359–363. doi: 10.1007/s00192-004-1263-4. [DOI] [PubMed] [Google Scholar]
- 12.Arbab AS, Yocum GT, Kalish H, Jordan EK, Anderson SA, Khakoo AY, Read EJ, Frank JA. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004;104:1217–1223. doi: 10.1182/blood-2004-02-0655. [DOI] [PubMed] [Google Scholar]
- 13.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama T, Huard J, Chancellor MB. Myoblast therapy for stress urinary incontinence and bladder dysfunction. World J Urol. 2000;18:56–61. doi: 10.1007/s003450050010. [DOI] [PubMed] [Google Scholar]
- 15.Rocha V, Garnier F, Ionescu I, Gluckman E. Hematopoietic stem-cell transplantation using umbilical-cord blood cells. Rev Invest Clin. 2005;57:314–323. [PubMed] [Google Scholar]
- 16.Dasari VR, Spomar DG, Li L, Gujrati M, Rao JS, Dinh DH. Umbilical cord blood stem cell mediated downregulation of fas improves functional recovery of rats after spinal cord injury. Neurochem Res. 2008;33:134–149. doi: 10.1007/s11064-007-9426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, Sanberg CD, Sanberg PR, Willing AE. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- 18.Lee HH, Kim HG, Jang SK, Choi OH. Cord blood-derived CD34 (+) cells promotes functional recovery in transient middle cerebral artery occlusion model of rat. Korean J Obstet Gynecol. 2007;50:1521–1531. [Google Scholar]
- 19.Kong KY, Ren J, Kraus M, Finklestein SP, Brown RH., Jr Human umbilical cord blood cells differentiate into muscle in sjl muscular dystrophy mice. Stem Cells. 2004;22:981–993. doi: 10.1634/stemcells.22-6-981. [DOI] [PubMed] [Google Scholar]
- 20.Chung HW, Won JH, Choi DH, Kim SJ, Lim MS, Park HK. Human umbilical cord blood-derived cells generate insulin-producing cells in vitro. J Korean Soc Transplant. 2007;21:31–37. [Google Scholar]
- 21.Jung MH, Yang SE, Jin HJ, Lee MK, Song HS, Yang JY, Yang YS, Ha CW. Chondrogenic differentiation of mesenchymal stem cells from human umbilical cord blood. J Korean Orthop Assoc. 2004;39:607–613. [Google Scholar]
- 22.Yoshioka T, Ageyama N, Shibata H, Yasu T, Misawa Y, Takeuchi K, Matsui K, Yamamoto K, Terao K, Shimada K, Ikeda U, Ozawa K, Hanazono Y. Repair of infarcted myocardium mediated by transplanted bone marrow-derived CD34+ stem cells in a nonhuman primate model. Stem Cells. 2005;23:355–364. doi: 10.1634/stemcells.2004-0200. [DOI] [PubMed] [Google Scholar]
- 23.van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard D, Korving J, Tertoolen LG, van Echteld CJ, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1:9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 25.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 26.Peyromaure M, Sebe P, Praud C, DeRocle G, Potin N, Pinset C, Sebille A. Fate of implanted syngenic muscle precursor cells in striated urethral sphincter of female rats: perspectives for treatment of urinary incontinence. Urology. 2004;64:1037–1041. doi: 10.1016/j.urology.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 27.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 28.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 29.Mitterberger M, Pinggera GM, Marksteiner R, Margreiter E, Fussenegger M, Frauscher F, Ulmer H, Hering S, Bartsch G, Strasser H. Adult stem cell therapy of female stress urinary incontinence. Eur Urol. 2008;53:169–175. doi: 10.1016/j.eururo.2007.07.026. [DOI] [PubMed] [Google Scholar]