Abstract
Coronary artery disease remains the leading cause of early death and graft loss in renal transplant patients. The aim of this study was to identify clinical and echocardiographic parameters independently associated with the angiographically-determined severity of coronary atherosclerosis in long-term kidney transplant patients. Fifty-two kidney transplant recipients who underwent elective coronary angiography were reviewed retrospectively. Angiographic severity was evaluated using the modified Gensini index (MGI). The mean age at coronary angiography was 52.5±7.9 yr with a mean prior transplant duration of 118.1±58.8 months. Pearson correlation analysis demonstrated a positive correlation of MGI with transplant duration before coronary angiography and chronic allograft nephropathy, whereas an inverse correlation was demonstrated with ejection fraction and statin use. On subsequent multivariate linear regression analysis, transplant duration before coronary angiography, statin use, and ejection fraction were independently associated with the severity of coronary atherosclerosis in long-term kidney transplant patients. In summary, our study demonstrates that statin use, ejection fraction, and transplant duration before coronary angiography are independent parameters associated with the severity of coronary atherosclerosis in long-term kidney transplant patients. Further investigation is required to reduce the atherosclerotic burden in kidney transplant patients.
Keywords: Coronary Artery Disease, Coronary Angiography, Kidney Transplantation
INTRODUCTION
Kidney transplantation (KT) is the treatment of choice for most patients with end-stage renal disease (ESRD). The survival of patients who undergo KT has improved considerably over the past three decades. Although successful kidney transplantation is accompanied by a 10- to 20-yr increase in life expectancy (1), the survival of kidney transplant recipients is significantly shortened by atherosclerotic cardiovascular disease, which accounts for 40% to 55% of all deaths in these patients (2).
It is well established that angiographically-determined coronary atherosclerosis scored by the modified Gensini index (MGI) has a high predictive value for mortality in both the general population (3) and ESRD patients (4). Moreover, in kidney transplant patients, coronary atherosclerosis is considered to be influenced by a wide range of more complicated conditions. Despite this, correlates of the degree of coronary atherosclerosis in long-term kidney transplant patients have not been documented. Therefore, the aim of this study was to identify clinical and echocardiographic parameters independently associated with the angiographically-determined severity of coronary atherosclerosis, as determined by MGI, in long-term kidney transplant patients.
MATERIALS AND METHODS
Study design and patients
We retrospectively analyzed 52 kidney transplant patients with functioning grafts who underwent both elective coronary angiography and echocardiography at the same hospitalization due to suspected coronary artery disease at Severance Hospital, Yonsei University Health System, Seoul, Korea between July 1997 and July 2007 under approval from the institutional review board (IRB number 4-2009-0383). Subjects were excluded if they had a past history of coronary artery bypass graft surgery, coronary angioplasty, carotid artery surgery, or a cerebrovascular accident.
Immunosuppressive regimes
Most patients were treated with a triple regime of a calcineurin inhibitor (cyclosporine or tacrolimus) plus a purine synthesis inhibitor (azathioprine or mycophenolate mofetil) and prednisolone.
Clinical and laboratory parameters
We reviewed the clinical characteristics (age, gender, smoking habits, type of primary renal disease, history of preexisting hypertension, history of biopsy-proven acute rejection or chronic allograft nephropathy, pre- or post-transplantation diabetes mellitus, the duration and type of dialysis prior to transplantation, and concurrent medications) of all patients prior to coronary angiography. Anthropometric parameters, such as body mass index, blood pressure, and biochemical indices (hemoglobin, total protein, albumin, calcium, phosphorus, lipid profile, blood urea nitrogen, creatinine, C-reactive protein [CRP] level, and presence or absence of proteinuria on dipstick test) were recorded as the values documented at the hospitalization for coronary angiography. Since blood pressure and low-density lipoprotein (LDL) cholesterol were considered established cardiovascular risk factors in general population, they were analyzed as both continuous (mean arterial pressure and serum LDL cholesterol level) and categorical variables (moderate blood pressure control group, blood pressure <140/90 mmHg vs. intensive blood pressure control group, blood pressure <130/80; moderate LDL cholesterol lowering group, LDL cholesterol <100 mg/dL vs. intensive LDL cholesterol lowering group, LDL cholesterol <70 mg/dL), respectively. Renal function was estimated using the abbreviated Modification of Diet in Renal Disease (MDRD) study equation (5).
Assessment of severity of coronary atherosclerosis and echocardiographic studies
The participants in the study were referred to our institution for elective coronary angiography due to suspected coronary artery disease (complaints of chest discomfort, high risk profiles, or abnormal test results). The coronary arteries were cannulated using the Judkins technique (6) with five F catheters and recorded on cine film. At least six standardized projections of the left coronary artery and two of the right coronary artery were obtained routinely. Using the results of the coronary artery cine film, the severity of coronary atherosclerosis was evaluated numerically by MGI. This index assigns a heavier weight to the more severe luminal narrowings. Weights are also assigned to each segment depending on vessel size and importance; segments serving larger regions of myocardium are more heavily weighted (3, 7). Echocardiographic examinations and measurements of cardiac indices were performed at the hospitalization for coronary angiography using M-mode and two-dimensional ultrasonography with color Doppler, as recommended by the American Society of Echocardiography (8).
Statistical analysis
Data are expressed as mean±SD. Pearson correlation analyses were performed to analyze the association of clinical and echocardiographic parameters with the extent of coronary atherosclerosis scored by MGI. Subsequently, stepwise multivariate linear regression analysis was performed to determine the factors independently associated with the severity of coronary atherosclerosis. P values of less than 0.05 were considered statistically significant. Because CRP levels were not normally distributed in this study, these data were normalized by base-10 log transformation before entering regression analyses. All statistical analyses were performed using SPSS for Windows software, version 12.0 (SPSS Inc, Chicago, IL, USA).
RESULTS
Patient characteristics
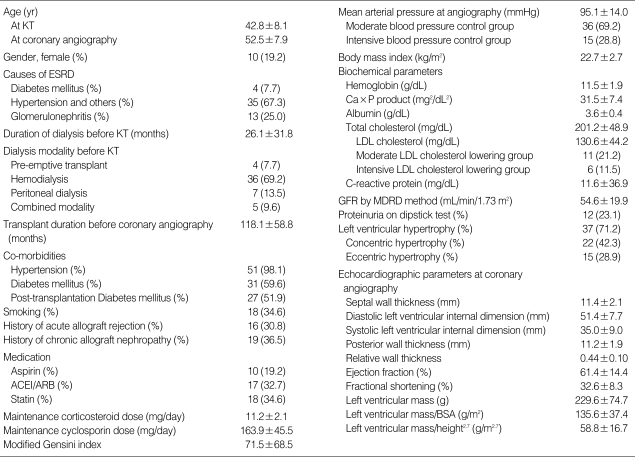
The clinical characteristics of the 52 patients are listed in Table 1. The mean age at coronary angiography was 52.5±7.9 yr, with a mean prior transplant duration of 118.1±58.8 months. Of the 52 patients, 42 (80.8%) were male. The mean age at KT was 42.8±8.1 yr, with a mean prior dialysis duration of 26.1±31.8 months. Thirty-six (69.2%) of the patients had been on hemodialysis prior to transplantation. MGI values ranged from 0 to 344 (median, 48; mean, 71.5±65.8).
Table 1.
Patient characteristics*
*Characteristics are expressed as mean±SD or number (%).
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BSA, body surface area; ESRD, end-stage renal disease; GFR, glomerular filtration rate; KT, kidney transplantation; LDL, low-density lipoprotein; MDRD, Modification of Diet in Renal Disease.
Univariate analysis of factors correlated with modified Gensini index
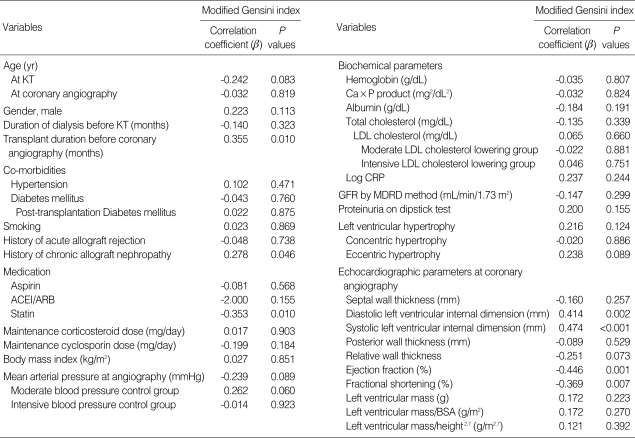
Univariate Pearson correlation analyses of the associations of clinical and echocardiographic left ventricular indices with the extent of coronary atherosclerosis as scored by MGI are shown in Table 2. Chronic allograft nephropathy (r=0.278, P=0.046) was significantly related to higher MGI, whereas statin use was significantly related to lower MGI (r=-0.353, P=0.010). There were also significantly positive correlations of MGI with diastolic left ventricular internal dimension (r=0.414, P=0.002), systolic left ventricular internal dimension (r=0.474, P<0.001), and transplant duration before coronary angiography (r=0.355, P=0.010). In contrast, there were significantly negative correlations of MGI with ejection fraction (r=-0.446, P=0.001) and fractional shortening (r=-0.369, P=0.007).
Table 2.
Pearson correlations of clinical parameters with modified Gensini indices at coronary angiography
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BSA, body surface area; CRP, C-reactive protein; GFR, glomerular filtration rate; KT, kidney transplantation; LDL, low-density lipoprotein; MDRD, Modification of Diet in Renal Disease.
Multivariate analysis of factors independently associated with modified Gensini index
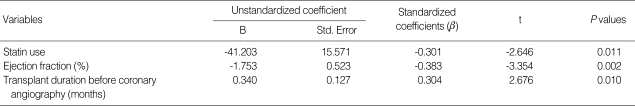
Stepwise multivariate linear regression analysis, Adjusted for age at coronary angiography, gender, diabetes mellitus, history of chronic allograft nephropathy, renal function measured by MDRD equation, and duration of dialysis before KT, revealed that statin use, ejection fraction, and transplant duration before coronary angiography were independently correlated with the severity of coronary atherosclerosis scored by MGI (r2=0.386) (Table 3).
Table 3.
Stepwise multivariate linear regression analysis of factors significantly associated with modified Gensini indices (r2=0.386*)
*Adjusted for age at coronary angiography, gender, diabetes mellitus, history of chronic allograft nephropathy, renal function measured by MDRD equation, and duration of dialysis before KT.
KT, kidney transplantation; MDRD, Modification of Diet in Renal Disease.
DISCUSSION
A number of factors contribute to cardiovascular atherosclerosis in kidney transplant patients. These fall into the categories of traditional factors identified by the Framingham study, including age, male gender, diabetes, hypertension, hyperlipidemia, and smoking (9), and non-traditional factors, including renal insufficiency, dialysis treatment (10), and transplant state per se along with the treatment course (11, 12).
While decreased renal function has been shown to be not only a well established risk factor for adverse cardiovascular outcomes in both the general population (13) and kidney transplant patients (14), but also an independent correlate of angiographically-determined coronary atherosclerosis in the transplant-naïve general population (15), our study showed that renal allograft function at coronary angiography was not correlated with the severity of coronary atherosclerosis. However, biopsy-proven chronic allograft nephropathy was shown to be significantly correlated with the extent of coronary atherosclerosis. In support of this finding, advanced glycation end products and oxidative stress, a major culprit in atherosclerosis, have been reported to be increased in chronic allograft nephropathy, and this cannot be explained solely by the decline in renal function (16). Rather, this was postulated to be mediated by overt and subclinical inflammatory rejection episodes. Statin use showed an independent negative correlation with MGI. Consistent with this, Holdaas et al. revealed that renal transplant patients who received fluvastatin had a 21% reduction in major adverse cardiac events and a 29% reduction in cardiac death or definite non-fatal myocardial infarction (17). It has also been reported that renal transplant patients receiving pravastatin had less severe extracoronary atherosclerosis in the carotid and femoral arteries than the control group, as measured by intima-media thickness (18). In contrast, either absolute serum LDL cholesterol levels or degree of lipid lowering were not correlates of coronary arthrosclerosis. Taken together, this can be explained by previously reported vessel-protective effects of statin even in the absence of lipid lowering (19). While hypertension was another traditional cardiovascular risk factor in non-transplant patients, a consistent relationship between hypertension and posttransplant vascular event in transplant patients has failed to be established (20, 21). In line with this, we did not find a significant correlation between mean arterial pressure or degree of blood pressure control and the severity of coronary atherosclerosis.
Although early clinical reports on the association between the degree of coronary arterial narrowing and left ventricular dysfunction are inconsistent (22), it has been recognized that myocardial infarcts result in alterations in ventricular architecture involving both the infarcted and non infarcted zones through ventricular remodeling (23) and in about 20% of patients following myocardial infarction, left ventricular dilatation tends to be progressive and is associated with a deterioration of global left ventricular function (24). Moreover, Anversa et al. demonstrated experimentally that a fixed reduction in luminal diameter of the coronary arteries clearly leads to deterioration in ventricular pump performance (25). Consistent with this, we showed that left ventricular systolic dysfunction determined by echocardiography was independently associated with coronary atherosclerosis. Notably in our study, coronary atherosclerosis was more exclusively associated with the degree of left ventricular systolic dysfunction accompanied by left ventricular cavity dilatation than left ventricular mass in long-term kidney transplant patients.
Kidney transplant patients are exposed to an increased atherosclerotic burden at each separate stage of renal disease and with each treatment modality. As previously mentioned, renal insufficiency with resulting retention of uremic solutes is considered a pathologic condition characterized by accelerated atherosclerosis, which tends to be more accentuated in patients undergoing maintenance dialysis (10). It has been demonstrated that dialysis duration is a correlate of coronary calcification (26) and an independent predictor of cardiovascular disease and mortality in stable renal transplant recipients (27). Moreover, restoration of renal function by transplantation is only capable of modulating or slightly improving, but not completely stabilizing, the metabolic abnormalities associated with uremia. While one study using spiral computed tomography demonstrated that KT stabilized coronary artery calcification in ESRD patients during the first 15-20 months after KT (26), multiple sources of evidence indicate that the kidney transplant state itself is implicated in the etiology of several pro-atherosclerotic changes. Serum levels of homocysteine were significantly increased in renal transplant patients compared to both healthy individuals and transplant-naïve patients with comparable degrees of renal failure (28). In addition, serum levels of advanced glycation end-products were higher in renal transplant patients than healthy subjects (29). Furthermore, it has been reported that after KT, the inflammatory cytokines, tumor necrosis factor-α and interleukin-6, after initially decreasing at 6 months, increased significantly from 12 months to 18 months. These results may be explained by a low-grade subclinical immune response against the allograft or occult infection (30). Consistent with this, we found that in long-term renal transplant patients, transplant duration before coronary angiography was a more powerful independent predictor of the severity of coronary atherosclerosis than duration of dialysis before KT or age at coronary angiography.
This study has some limitations, including the small number of participants and a retrospective single center cross-sectional design; however, to the best of our knowledge, this is the first report to explore the relationship between non-invasive clinical parameters and the angiographically-determined severity of coronary atherosclerosis in long-term kidney transplant patients. The lack of available reports may be due to the small number of angiographic studies performed in kidney transplant patients, which may be related to fear of the potential damage that can be caused by angiography or contrast nephropathy.
In summary, our study demonstrates that statin use, ejection fraction, and transplant duration before coronary angiography are independent parameters associated with the severity of coronary atherosclerosis in long-term kidney transplant patients. Further investigation is required to reduce the atherosclerotic burden in kidney transplant patients.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Fazelzadeh A, Mehdizadeh AR, Ostovan MA, Raiss-Jalali GA. Predictors of cardiovascular events and associated mortality of kidney transplant recipients. Transplant Proc. 2006;38:509–511. doi: 10.1016/j.transproceed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Ringqvist I, Fisher LD, Mock M, Davis KB, Wedel H, Chaitman BR, Passamani E, Russell RO, Jr, Alderman EL, Kouchoukas NT, Kaiser GC, Ryan TJ, Killip T, Fray D. Prognostic value of angiographic indices of coronary artery disease from the Coronary Artery Surgery Study (CASS) J Clin Invest. 1983;71:1854–1866. doi: 10.1172/JCI110941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joki N, Hase H, Takahashi Y, Ishikawa H, Nakamura R, Imamura Y, Tanaka Y, Saijyo T, Fukazawa M, Inishi Y, Nakamura M, Yamaguchi T. Angiographical severity of coronary atherosclerosis predicts death in the first year of hemodialysis. Int Urol Nephrol. 2003;35:289–297. doi: 10.1023/b:urol.0000020356.82724.37. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 6.Judkins MP. Selective coronary arteriography. I. A percutaneous transfemoral technic. Radiology. 1967;89:815–824. doi: 10.1148/89.5.815. [DOI] [PubMed] [Google Scholar]
- 7.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Kasiske BL, Chakkera HA, Roel J. Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol. 2000;11:1735–1743. doi: 10.1681/ASN.V1191735. [DOI] [PubMed] [Google Scholar]
- 10.Horl WH, Cohen JJ, Harrington JT, Madias NE, Zusman CJ. Atherosclerosis and uremic retention solutes. Kidney Int. 2004;66:1719–1731. doi: 10.1111/j.1523-1755.2004.00944.x. [DOI] [PubMed] [Google Scholar]
- 11.Kasiske BL. Risk factors for accelerated atherosclerosis in renal transplant recipients. Am J Med. 1988;84:985–992. doi: 10.1016/0002-9343(88)90302-6. [DOI] [PubMed] [Google Scholar]
- 12.Chueh SC, Kahan BD. Dyslipidemia in renal transplant recipients treated with a sirolimus and cyclosporine-based immunosuppressive regimen: incidence, risk factors, progression, and prognosis. Transplantation. 2003;76:375–382. doi: 10.1097/01.TP.0000074310.40484.94. [DOI] [PubMed] [Google Scholar]
- 13.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 14.Meier-Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation. 2003;75:1291–1295. doi: 10.1097/01.TP.0000061602.03327.E2. [DOI] [PubMed] [Google Scholar]
- 15.Na KY, Kim CW, Song YR, Chin HJ, Chae DW. The association between kidney function, coronary artery disease, and clinical outcome in patients undergoing coronary angiography. J Korean Med Sci. 2009;24(Suppl 1):S87–S94. doi: 10.3346/jkms.2009.24.S1.S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raj DS, Lim G, Levi M, Qualls C, Jain SK. Advanced glycation end products and oxidative stress are increased in chronic allograft nephropathy. Am J Kidney Dis. 2004;43:154–160. doi: 10.1053/j.ajkd.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Holdaas H, Fellstrom B, Cole E, Nyberg G, Olsson AG, Pedersen TR, Madsen S, Gronhagen-Riska C, Neumayer HH, Maes B, Ambuhl P, Hartmann A, Staffler B, Jardine AG. Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: the ALERT extension study. Am J Transplant. 2005;5:2929–2936. doi: 10.1111/j.1600-6143.2005.01105.x. [DOI] [PubMed] [Google Scholar]
- 18.Cofan F, Gilabert R, Zambon D, Nunez I, Ros E, Cofan M, Campistol JM, Bru C, Oppenheimer F. Effect of pravastatin treatment on the evolution of extracoronary atherosclerosis in renal transplant patients. Transplant Proc. 2002;34:384–388. doi: 10.1016/s0041-1345(01)02813-5. [DOI] [PubMed] [Google Scholar]
- 19.Wilson SH, Simari RD, Best PJ, Peterson TE, Lerman LO, Aviram M, Nath KA, Holmes DR, Jr, Lerman A. Simvastatin preserves coronary endothelial function in hypercholesterolemia in the absence of lipid lowering. Arterioscler Thromb Vasc Biol. 2001;21:122–128. doi: 10.1161/01.atv.21.1.122. [DOI] [PubMed] [Google Scholar]
- 20.Florijn KW, Chang PC, van der Woude FJ, van Bockel JH, van Saase JL. Long-term cardiovascular morbidity and mortality in autosomal dominant polycystic kidney disease patients after renal transplantation. Transplantation. 1994;57:73–81. doi: 10.1097/00007890-199401000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Kasiske BL, Guijarro C, Massy ZA, Wiederkehr MR, Ma JZ. Cardiovascular disease after renal transplantation. J Am Soc Nephrol. 1996;7:158–165. doi: 10.1681/ASN.V71158. [DOI] [PubMed] [Google Scholar]
- 22.Roeske WR, Savage RM, O'Rourke RA, Bloor CM. Clinicopathologic correlations in patients after myocardial infarction. Circulation. 1981;63:36–45. doi: 10.1161/01.cir.63.1.36. [DOI] [PubMed] [Google Scholar]
- 23.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 24.Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation. 1993;87:755–763. doi: 10.1161/01.cir.87.3.755. [DOI] [PubMed] [Google Scholar]
- 25.Anversa P, Zhang X, Li P, Capasso JM. Chronic coronary artery constriction leads to moderate myocyte loss and left ventricular dysfunction and failure in rats. J Clin Invest. 1992;89:618–629. doi: 10.1172/JCI115628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moe SM, O'Neill KD, Fineberg N, Persohn S, Ahmed S, Garrett P, Meyer CA. Assessment of vascular calcification in ESRD patients using spiral CT. Nephrol Dial Transplant. 2003;18:1152–1158. doi: 10.1093/ndt/gfg093. [DOI] [PubMed] [Google Scholar]
- 27.Ducloux D, Motte G, Challier B, Gibey R, Chalopin JM. Serum total homocysteine and cardiovascular disease occurrence in chronic, stable renal transplant recipients: a prospective study. J Am Soc Nephrol. 2000;11:134–137. doi: 10.1681/ASN.V111134. [DOI] [PubMed] [Google Scholar]
- 28.Arnadottir M, Hultberg B, Vladov V, Nilsson-Ehle P, Thysell H. Hyperhomocysteinemia in cyclosporine-treated renal transplant recipients. Transplantation. 1996;61:509–512. doi: 10.1097/00007890-199602150-00034. [DOI] [PubMed] [Google Scholar]
- 29.Franke S, Muller A, Sommer M, Busch M, Kientsch-Engel R, Stein G. Serum levels of total homocysteine, homocysteine metabolites and of advanced glycation end-products (AGEs) in patients after renal transplantation. Clin Nephrol. 2003;59:88–97. doi: 10.5414/cnp59088. [DOI] [PubMed] [Google Scholar]
- 30.Cueto-Manzano AM, Morales-Buenrostro LE, Gonzalez-Espinoza L, Gonzalez-Tableros N, Martin-del-Campo F, Correa-Rotter R, Valera I, Alberu J. Markers of inflammation before and after renal transplantation. Transplantation. 2005;80:47–51. doi: 10.1097/01.tp.0000164348.16689.03. [DOI] [PubMed] [Google Scholar]