Abstract
This study examined urinary cotinine levels and self-reported smoking among pregnant women in Korea and the factors associated with smoking during pregnancy. The subjects were selected from pregnant women who visited 30 randomly sampled obstetric clinics and prenatal care hospitals in Korea in 2006. Smoking status was determined by self-reporting and urinary cotinine measurement. A total of 1,090 self-administered questionnaires and 1,057 urine samples were analyzed. The percentage of smoking revealed by self-reporting was 0.55% (95% confidence interval [CI], 0.11-0.99) and that revealed by urinary cotinine measurement (>100 ng/mL) was 3.03% (95% CI, 1.99-4.06). The kappa coefficient of agreement between self-reported smoking status and urinary cotinine measurement was 0.20 (95% CI, 0.03-0.37). Multiple logistic regression analysis revealed that early gestational period, low educational level, and being married to a smoker were significant risk factors for smoking during pregnancy. Smoking among pregnant women in Korea is not negligible, and those who are concerned to maternal and child health should be aware of this possibility among pregnant women in countries with similar cultural background.
Keywords: Smoking, Pregnancy, Cotinine
INTRODUCTION
Smoking during pregnancy is associated with adverse reproductive outcomes and health problems in the fetus and neonate (1). It appears to be an independent risk factor for conduct disorder in male offspring (2). A meta-analysis for the effects of smoking on pregnancy complications revealed that smoking during pregnancy is a significant and preventable factor affecting ectopic pregnancy, placental abruption, placenta previa, and premature rupture of the membrane (3). Maternal smoking during pregnancy increases the risk of having a child with a congenital anomaly (4, 5).
In 2003, the prevalence of smoking during pregnancy was reported at 10.7% in the United States (6). The US Department of Health and Human Services estimated that if all pregnant women in the United States quit smoking, there would be an 11% reduction in stillbirths and a 5% reduction in newborn deaths (7).
According to a telephone survey conducted in September 2006 by the Korean Association of Smoking and Health, the prevalence of smoking among women of all ages was 2.8%. The prevalence of smoking among women in their 20s and 30s were 3.4 and 2.2%, respectively (8). The reported prevalence of smoking among Korean women was thus much lower than that in America or European countries. However, methodological limitations should be considered when interpreting these data. Most surveys on the prevalence of smoking in Korean women have depended on self-reporting or telephone interviews; however, respondents are often unwilling to disclose their smoking habits in these situations. Nicotine is a highly addictive substance, as potent as narcotics such as heroin or cocaine (9). Therefore, despite widespread recognition of the harmful effects of smoking, many addicted women are unable to quit even when they are pregnant (10, 11).
Little information is available regarding smoking and its associated factors among pregnant women in Korea. Therefore, we investigated smoking status via two different methods, examined the relationship between self-reported habits and clinically validated results, and analyzed associated factors of smoking in pregnant women in Korea.
MATERIALS AND METHODS
Subjects
This study was conducted from 22 May to 28 July 2006. Subjects were selected from pregnant women who visited 30 randomly sampled obstetric clinics and hospitals in Korea for prenatal care. According to the delivery number of each administrative division (seven metropolitan cities and eight provinces), we assigned the predicted number of study populations. With the aid of Health Insurance Review and Assessment Service, we got the annual delivery number of hospitals which had over 500 deliveries per year. After sorting the hospital by the number of delivery, we contacted each hospital for participation of this study and we stopped further contact when the expected number of enrollment of each province was reached. We visited at least one clinic or hospital in each of the administrative divisions that were included in this study.
Smoking habits among pregnant women were ascertained by two methods: voluntary disclosure through self-reporting and urinary cotinine measurement. Subjects were asked to respond to a self-administered questionnaire that inquired about demographic data (age, marital status, educational level, and duration of pregnancy in weeks), current smoking status at the time of the survey, smoking habits for current smokers (age at onset, duration, number of cigarettes per day), and environmental tobacco smoke (ETS) exposure (spousal smoking and the frequency of ETS exposure at home).
After the subjects completed the questionnaire, they were asked to provide a urine sample. Urine samples were transported immediately and analyzed within 1-2 days. A clinical laboratory researcher without knowledge of the subject's smoking history analyzed urinary cotinine concentration. Urinary cotinine concentrations were determined by the DRI cotinine assay for urine (Microgenics Corp., Fremont, CA, USA) using a Toshiba 200FR automated clinical chemistry analyzer (Toshiba Lab Medical, Tokyo, Japan). Briefly, 125 µL of reagents containing a monoclonal antibody to cotinine and an enzyme substrate were added to 20 µL of urine, and then incubated with 125 µL of cotinine-conjugated glucose-6-phosphate dehydrogenase (G6PDH) at 37℃. The absorbance was measured at 340 nm. Urinary cotinine concentrations were determined without an adjustment for urinary creatinine.
We collected 1,135 questionnaires and 1,099 urine samples during the study period. We excluded those who did not provide information on age, gestational period, their last menstrual period, or smoking status. Consequently, 1,090 questionnaires and 1,057 urine samples were analyzed.
In the questionnaire, current smokers were defined as those who were currently smoking or had smoked at least one cigarette during the previous week. Ex-smokers were those who reported quitting by the time of the survey. Non-smokers were those who had never smoked.
In urinary cotinine analysis, current smokers were defined as those whose urinary cotinine levels exceeded 100 ng/mL. Passive smokers and non-smokers showed urinary cotinine levels of 40-100 ng/mL and <40 ng/mL, respectively. These cut-off values were based on a recent study on the usefulness of the urinary cotinine test to distinguish between smokers and non-smokers (12). The Institutional Review Board of Seoul National University Hospital approved the study protocol (SNUH-IRB 0602-070-168), and all subjects provided informed consent.
Statistical analysis
The kappa coefficient was calculated to evaluate the agreement between self-reported smoking status and urinary cotinine levels in women who participated in both the questionnaire and urine analysis. Based on self-reported data, non-smokers and ex-smokers were grouped into the non-smoking category, and current smokers were placed in the smoking category. Likewise, patients were divided into smoking and non-smoking (passive and non-smokers) groups based on the urine analysis data.
The Wilcoxon rank-sum test or Kruskal-Wallis test was used to compare medians or ranks of urinary cotinine levels according to the categories of explanatory variables. A multiple logistic regression model, using demographic data and ETS exposure as explanatory variables and cotinine-validated smoking status (current smokers versus non-smokers) as dependent variables, was constructed to evaluate the associated factors for smoking during pregnancy. The SAS statistical package (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
RESULTS
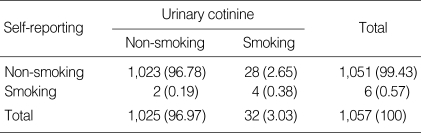
Table 1 shows the self-reported smoking status of pregnant women who participated in the questionnaire survey. Only six women reported that they were current smokers or had smoked at least one cigarette during the previous week. Therefore, self-identified smokers composed only 0.55% (95% confidence interval [CI], 0.11-0.99) of the study population. Among the 182 self-reported ex-smokers, 104 women reported that they had quit before they became aware of being pregnant, and 78 women reported that they had quit after the fact. In self-reporting questionnaires, some items were not responded.
Table 1.
Self-reported smoking status in pregnant women who responded to the questionnaire
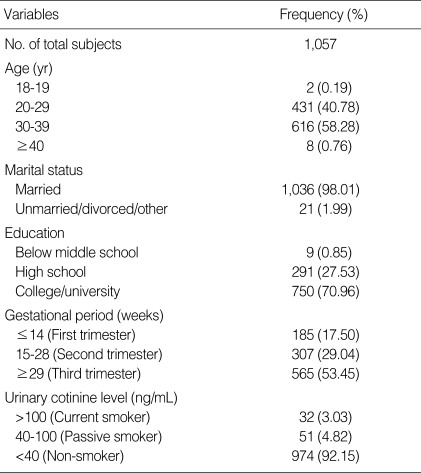
Table 2 shows the general characteristics and urinary cotinine-validated smoking status of pregnant women who participated in the urine analysis. The proportion of 30-39-yr-old women was the largest (58.25%) among all participants. Almost all women were married (98.01%). Most women had graduated from college or university (70.96%) and over half were in their third trimester (53.45%). Thirty-two women showed urinary cotinine levels that exceeded 100 ng/mL, identifying them as current smokers. The percentage of smoking as determined by urinary cotinine measurement was 3.03% (95% CI, 1.99-4.06). Self-reported smoking status and urinary cotinine did not agree in 30 women (2.84%; Table 3). The kappa coefficient for these data was 0.20 (95% CI, 0.03-0.37).
Table 2.
General characteristics and urinary cotinine-validated smoking status in pregnant women who participated in the urine analysis
Table 3.
Agreement between self-reported and cotinine-validated smoking status
Based on self-reported data, non-smokers and ex-smokers were grouped into the non-smoking category, and current smokers were placed in the smoking category; Based on urinary cotinine analysis, non-smokers and passive smokers were grouped into the non-smoking category, and current smokers were placed in the smoking category. Kappa coefficient, 0.20 (95% CI, 0.03-0.37).
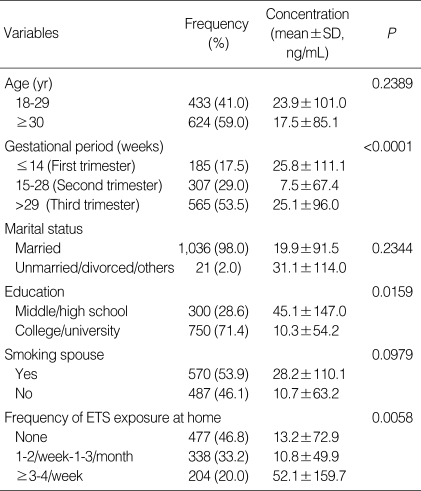
Urinary cotinine concentrations of women in the second trimester were lower than those in the first or third trimester (P<0.01). Urinary cotinine concentrations were also lower in those who graduated from college or university compared to those who graduated from middle or high school (P<0.05). Urinary cotinine concentrations were higher in those exposed to ETS 3-4 times per week or more compared to those exposed to ETS less frequently (P<0.01). No significant differences in urinary cotinine concentrations were observed with regard to age, marital status, or smoking spouses (Table 4).
Table 4.
Urinary cotinine concentrations in relation to demographic data and environmental tobacco smoke (ETS) exposure
The Wilcoxon rank-sum test or Kruskal-Wallis test was used to compare medians or ranks among the categories of selected variables.
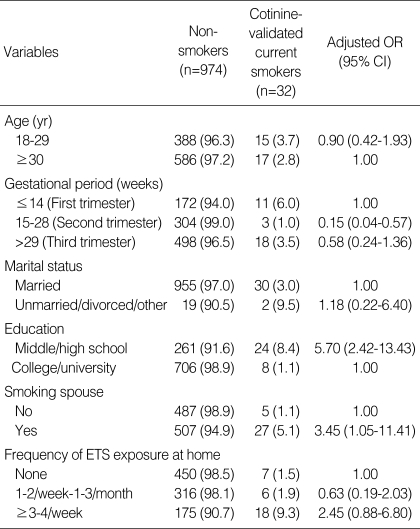
Multiple logistic regression analysis revealed that women in their second trimester (0.15; 0.04-0.56) were less likely to smoke than women in their first trimester. Women who had graduated from middle or high school (5.70; 2.42-13.43) or lived with a smoking spouse (3.45; 1.05-11.41) were more likely to smoke than college or university graduates or women married to non-smokers (Table 5).
Table 5.
Adjusted odds ratios (OR) of demographic data and environmental tobacco smoke (ETS) exposure for cotinine-validated smoking in pregnant women
Cut-off values for urinary cotinine: current smoker, >100 ng/mL; non-smoker, <40 ng/mL.
CI, confidence interval.
DISCUSSION
There is an overwhelming belief that smoking during pregnancy in Korea is negligible. This may be the result of the continuously reported low prevalence of smoking among women (13). In addition, there is no previous research regarding the prevalence of smoking among pregnant Korean women. The self-reported smoking status (0.55%) in this study appears to concur with the common opinion; however, the percentage of smoking validated by urinary cotinine measurement (3.03%) was higher than expected and higher than the results of smoking rate in Korean women reported in the telephone survey conducted by the Korean Association of Smoking and Health (8). Our results cast doubt on the reportedly low prevalence of smoking among Korean women.
We assured anonymity in our questionnaire. However, the discrepancy between self-reported smoking and urinary cotinine levels suggests that the majority of pregnant Korean women who smoke may not choose to reveal their smoking status, even anonymously. General disapproval for women, who smoke, especially while pregnant, may hinder truthful self-reporting. West et al. (14) showed that self-reported smoking prevalence underestimated the true value when comparing self-reported smoking habits and cotinine-validated data from England, the United States, and Poland. This discrepancy may be exaggerated in East Asian countries, where Confucianism has had a heavy traditional influence. Prenatal care physicians must be mindful of this possibility when providing care for pregnant women.
Smoking biomarkers including urine cotinine are generally considered as useful measures to assess objectively the smoking status. However, urine cotinine concentration may be under cut-off value, even undetectable after a few days of abstinence because it has a relative short half-life of 12-24 hr. Therefore, urine cotinine is possible to underestimate smoking status, especially in case of an irregular smoker. Two women reported themselves as current smokers in the questionnaire, but their urinary cotinine levels were less than 100 ng/mL: urinary cotinine was 0 in the first subject and 48 ng/mL in the second. Therefore, they were classified as a non-smoker and a passive smoker, respectively. Dempsey et al. (15) examined the metabolism of nicotine and cotinine in ten healthy pregnant volunteers who smoked. The smokers received infusions of deuterium-labeled nicotine and cotinine during pregnancy and again after birth. The experiment revealed that the clearance of nicotine and cotinine was significantly higher (60 and 140%, respectively) during pregnancy, and that the half-life of cotinine was much shorter (8.8 vs. 16.6 hr). These results suggest that low cotinine levels observed during pregnancy do not necessarily reflect less smoke exposure, and that cut-off levels used to classify smoking habits need to be established for pregnant women.
This study showed that early gestational period, low education level, and smoking spouses were significant risk factors for smoking during pregnancy. Although a smoker was aware of her pregnancy, she sometimes required an extended period to quit. This may explain why the first trimester (early gestational period) was a risk factor for smoking during pregnancy. However, smoking prevalence of the 3rd trimester returned to that of the 1st trimester. This means maternal motivation of abstinence may be weakened because of relatively less effects of smoking to fetus in the 3rd trimester and highly addictive nature of smoking. We found that low educational level was also a risk factor, which is consistent with previous studies (16, 17). Smoking spouses may also raise urinary cotinine levels in pregnant women through ETS exposure. Furthermore, a tendency to select a spouse with similar socioeconomic and behavioral factors might explain why being married to a smoker is a risk factor for smoking during pregnancy.
Several limitations should be considered when interpreting our results. First, more than 70% of the subjects in this study were college or university graduates. According to the Korean National Census, the proportion of 20-39-yr-old women who graduated from college or university was 56.7% (18). Although no data were available regarding the educational level of pregnant Korean women, it appeared that the overall educational level of our subjects was higher than could be expected. To find eligible subjects, we selected clinics and hospitals in which more than 500 deliveries per year (i.e., 1.4 deliveries per day) were recorded. Because these clinics and hospitals were inevitably located in medium-sized or large cities, women with higher education might have been selected more than those with lower education. It is possible that real smoking rate may be higher than that of this study. Second, among the 1,057 pregnant women who participated in both the questionnaire and urinary cotinine analysis, only six reported that they were current smokers. Therefore, we could not include the respondents' smoking characteristics in the multiple logistic regression model for the analysis of factors associated with smoking during pregnancy. Finally, because this was a cross-sectional study, we could not predict smoking behavior for women throughout their pregnancies.
Smoking among pregnant women in Korea is not negligible, and those who are concerned to maternal and child health should be aware of this possibility among pregnant women in countries with similar cultural background.
ACKNOWLEDGMENT
The authors thank Eun-Ah Lee and Sun-Hee Park for their assistance in data collection.
We also extend our gratitude to obstetricians and gynecologists who participated in this study. They are; Prof. MY Kim, Cheil Hospital, Seoul; Dr. JH Chang, Jang's Women Hospital, Seoul; Dr. HS Kim, Areum Hospital, Busan; Dr. KH Kang & Dr. HS Ahn, Ilsin Christian Hospital, Busan; Dr. TS Kim, Mirae Women's Hospital, Daegu; Dr. JS Lee, Medimore Women's Clinic, Daegu; Dr. JN Koo & Dr. IH Oh, Incheon Seoul Women's Hospital, Incheon; Prof. YH Kim, Chonnam National University Hospital, Gwangju; Dr. J Hur, Eden Hospital, Gwangju; Dr. DS Choi, Mizpia Hospital, Gwangju; Dr. KH Ahn, Mirae Lady's Hospital, Daejeon; Dr. BK Lee, Daejun Eulji University Hospital, Daejeon; Dr. MH Lee, Fraumedi Hospital, Ulsan; Dr. ST Choi & Dr. SS Kim, Bombit Women's Hospital, Anyang; Dr. SS Han, Bundang Cheil Women's Hospital, Seongnam; Prof. SS Shim, Bundang Cha Hospital, Seongnam; Prof. KH Park, Bundang Seoul National Hospital, Seongnam; Dr. WH Lee, Charm Women's Clinic, Seongnam; Dr. SH Kim, Dongwon Women's Hospital, Ilsan; Dr. SH Park, MIRAE Women's Hospital, Chuncheon; Dr. CS Ahn, Motaean Women's Hospital, Cheongju; Dr. CM Lee, EHWA Women's Hospital, Cheonan; Dr. KE Hong, Haesung Obstetrics Gynecology, Cheonan; Dr. YH Lee, Andong Hospital, Andong; Dr. MS Jeon, Pohang Women's Hospital, Pohang; Dr. DH Ahn, Soonahn Hospital, Jinju; Dr. CH Lee, Changwon Fatima Hospital, Changwon; Park YB, Hannah Women's Hospital, Jeonju; Dr. KS Kim, Mokpo Hansarang Hospital, Mokpo.
Footnotes
This study was supported by the Health Promotion Research Fund, Ministry for Health, Welfare and Family Affairs, Republic of Korea (05-57).
References
- 1.Wen CP, Cheng TY, Lin CL, Wu HN, Levy DT, Chen LK, Hsu CC, Eriksen MP, Yang HJ, Tsai SP. The health benefits of smoking cessation for adult smokers and for pregnant women in Taiwan. Tob Control. 2005;14(Suppl 1):i56–i61. doi: 10.1136/tc.2004.007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wakschlag LS, Lahey BB, Loeber R, Green SM, Gordon RA, Leventhal BL. Maternal smoking during pregnancy and the risk of conduct disorder in boys. Arch Gen Psychiatry. 1997;54:670–676. doi: 10.1001/archpsyc.1997.01830190098010. [DOI] [PubMed] [Google Scholar]
- 3.Castles A, Adams EK, Melvin CL, Kelsch C, Boulton ML. Effects of smoking during pregnancy. Five meta-analyses. Am J Prev Med. 1996;16:208–215. doi: 10.1016/s0749-3797(98)00089-0. [DOI] [PubMed] [Google Scholar]
- 4.Li DK, Mueller BA, Hickok DE, Daling JR, Fantel AG, Checkoway H, Weiss NS. Maternal smoking during pregnancy and the risk of congenital urinary tract anomalies. Am J Public Health. 1996;86:249–253. doi: 10.2105/ajph.86.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Man LX, Chang B. Maternal cigarette smoking during pregnancy increases the risk of having a child with a congenital digital anomaly. Plast Reconstr Surg. 2006;117:301–308. doi: 10.1097/01.prs.0000194904.81981.71. [DOI] [PubMed] [Google Scholar]
- 6.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2003. Natl Vital Stat Rep. 2005;54:1–116. [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services. The health consequences of smoking: A report of the Surgeon General-2004. Atlanta, Georgia: Office on Smoking and Health, Centers for Disease Control and Prevention; 2004. [PubMed] [Google Scholar]
- 8.A survey on smoking status among Korean adults in September 2006. Korean Association of Smoking and Health. [accessed 15 February 2009]. Available at http://www.kash.or.kr/user_new/pds_list.asp.
- 9.Royal College of Physicians. Nicotine Addiction in Britain. London: Royal College of Physicians; 2000. [Google Scholar]
- 10.Håkansson A, Lendahls L, Petersson C. Which women stop smoking? A population-based study of 403 pregnant smokers. Acta Obstet Gynecol Scand. 1999;78:217–224. [PubMed] [Google Scholar]
- 11.Lawrence T, Aveyard P, Croghan E. What happens to women's self-reported cigarette consumption and urinary cotinine levels in pregnancy? Addiction. 2003;98:1315–1320. doi: 10.1046/j.1360-0443.2003.00485.x. [DOI] [PubMed] [Google Scholar]
- 12.Kang YH, Lee YJ, Kim HK, Yun YH, Jeong SY, Lee JS, Park JG. Usefulness of urinary cotinine test to distinguish smokers from nonsmokers. Korean J Lab Med. 2003;23:92–97. [Google Scholar]
- 13.Jhun HJ, Seo HG. The stages of change in smoking cessation in a representative sample of Korean adult smokers. J Korean Med Sci. 2006;21:843–848. doi: 10.3346/jkms.2006.21.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West R, Zatonski W, Przewozniak K, Jarvis MJ. Can we trust national smoking prevalence figures? Discrepancies between biochemically assessed and self-reported smoking rates in three countries. Cancer Epidemiol Biomarkers Prev. 2007;16:820–822. doi: 10.1158/1055-9965.EPI-06-0679. [DOI] [PubMed] [Google Scholar]
- 15.Dempsey D, Jacob P, 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301:594–598. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- 16.Phung HN, Bauman AE, Young L, Tran MH, Hillman KM. Ecological and individual predictors of maternal smoking behaviour. Looking beyond individual socioeconomic predictors at the community setting. Addict Behav. 2003;28:1333–1342. doi: 10.1016/s0306-4603(02)00255-1. [DOI] [PubMed] [Google Scholar]
- 17.Johnson IL, Ashley MJ, Reynolds D, Goettler F, Lee-Han H, Stratton J, Yim C, Murray J. Prevalence of smoking associated with pregnancy in three Southern Ontario Health Units. Can J Public Health. 2004;95:209–213. doi: 10.1007/BF03403651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korea National Statistical Office. Korea Census 2005: Women by age, education, and number of children. Daejeon: Korea National Statistical Office; 2007. [Google Scholar]