Abstract
Purpose
Laparoscopic donor nephrectomy is associated with less postoperative pain and faster recovery times in living kidney donors. However, pneumoperitoneum, which is required in laparoscopic donor nephrectomy, can result in adverse effects on renal function in donors and recipients. We compared renal function in donors and recipients after hand-assisted laparoscopic donor nephrectomy (HALDN) and open donor nephrectomy (ODN).
Materials and Methods
Between January 1997 and January 2008, 241 live donor nephrectomies were performed by either HALDN (n=118) or ODN (n=123). Preoperative patient characteristics were not significantly different between the donors and recipients. We monitored the changes in serum creatinine levels of the donors and recipients preoperatively and on postoperative days 1, 5, 28, 84, and 365.
Results
The mean operative times of HALDN and ODN were 171 and 163 minutes (p=0.284), and the mean warm ischemic times were 292 and 236 seconds (p=0.207), respectively. The mean serum creatinine level in the recipients on postoperative day 1 was significantly higher after HALDN than after ODN (3.48 vs. 2.62 mg/dl, p=0.003). However, from postoperative day 5 to 1 year, there was no significant difference between the two groups. The mean serum creatinine level in the donors was not significantly different between the HALDN and ODN groups throughout the study period.
Conclusions
Renal function recovery in the donors was similar with both HALDN and ODN. Graft renal function recovery after HALDN was comparable with that after ODN, except immediately after surgery (postoperative day 1).
Keywords: Creatinine, Laparoscopy, Living donors, Transplant recipients
INTRODUCTION
Kidney transplantation is the preferred treatment for end-stage renal failure; however, there is still a lack of transplantable organs [1]. In addition to the increased utilization of marginal cadaveric donors, living organ donation has become an alternative means to increase the number of available organs. Before the laparoscopic technique was introduced in the urologic field, open donor nephrectomy (ODN) had been the standard method for live donor nephrectomy (LDN). However, a rapid postoperative recovery and return to an active social life have always been concerns, because most donors are healthy and socially active people.
Since the first laparoscopic LDN was performed in 1995 by Ratner et al [2], several studies have shown shorter hospital stays, less postoperative pain, and faster times to return to work after LDN than after ODN. However, LDN has its limitations, including potentially longer operating times, longer warm ischemic times, and longer learning curves for the surgeons compared with ODN [3-5]. It also has been reported that laparoscopic surgery causes more ureteral complications and that pneumoperitoneum has adverse effects, which can trigger rejection or delayed graft function as well as poor recovery of the remaining kidney in the donor [1,6,7]. However, hand-assisted laparoscopic donor nephrectomy (HALDN) has achieved widespread success because it is a safer procedure for both recipient and donor than are the open or the pure laparoscopic procedures [8-10]. Although several recent studies reported that pure LDN had similar or better results than HALDN [11,12], HALDN is generally easier to perform than is pure LDN. Despite the advantages of HALDN, the impact of pneumoperitoneum on kidney function in the donor and recipient remains controversial. Some studies have shown a decrease in short-term graft function in recipients of laparoscopically procured kidneys [13,14], whereas other studies have shown no differences [3,15,16]. We compared renal function in both donors and recipients after HALDN and ODN.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of donors and recipients after 118 HALDNs and 123 ODNs performed between January 1997 and February 2008. All donors underwent a routine preoperative evaluation, including a renal scan with glomerular filtration rate (GFR) measurement, three-dimensional magnetic resonance angiography, and/or aortography. The rationale for donor kidney selection for HALDN was identical to the standard principles used for ODN. When the kidneys were equal, the left kidney was selected to take advantage of the longer renal vein. However, if the left renal vascular anatomy was unfavorable compared with that of the right, the right kidney was selected. HALDN was performed transperitoneally, and ODN was performed retroperitoneally through a flank incision. Mannitol was given before renal vascular clamping in all patients. Surgery in the recipient was performed through a Gibson incision with creation of standard vascular anastomoses and extravesical ureteroneocystostomy.
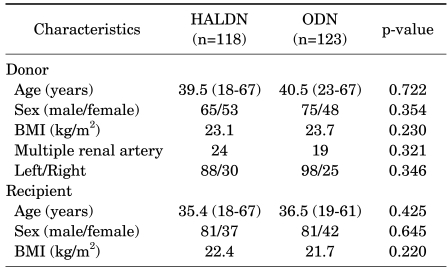
Preoperative demographic data of the donors and recipients are shown in Table 1. Although this was a nonrandomized and retrospective study, characteristics at baseline (e.g., age, sex, and body mass index) were not significantly different between the donors and the recipients. There were 24 and 19 patients with multiple renal arteries in the HALDN and ODN groups, respectively (p=0.321). A right side nephrectomy was performed in 30 patients in the HALDN group and in 25 patients in the ODN group (p=0.346) (Table 1). Serum creatinine levels were measured preoperatively and on days 1, 5, 28, 84, and 365 postoperatively. The GFR measured by renal scan was checked preoperatively and on days 84 and 365 postoperatively. The warm ischemic time was defined as the time from renal artery occlusion to kidney reperfusion. Categorical variables were compared with the chi-square test. Continuous variables were compared with Student's t-test. All analyses were conducted by using SPSS 12.0 for Windows, and statistical significance was accepted at a p-value less than 0.05.
TABLE 1.
Preoperative characteristics of the donors and recipients
HALDN: hand-assisted laparoscopic donor nephrectomy, ODN: open donor nephrectomy, BMI: body mass index
RESULTS
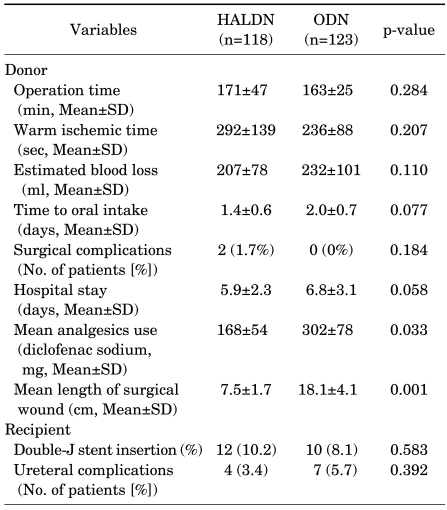
The mean operative times of HALDN and ODN were 171 and 163 minutes (p=0.284), and the mean warm ischemic times were 292 and 236 seconds (p=0.207), respectively. There was less use of analgesics and shorter lengths of surgical wounds in the HALDN group than in the ODN group. If a short ureter length or swelling at the anastomosis site was found, a double-J stent was inserted in the ureter of the recipient (HALDN: 12; ODN: 10; p=0.583). Four ureteral complications occurred in the HALDN group, and seven occurred in the ODN group (p=0.392) (Table 2). There were no significant differences in preoperative baseline serum creatinine levels between the donors and the recipients of either the HALDN or the ODN group.
TABLE 2.
Comparison of operative parameters between the HALDN and the ODN groups
HALDN: hand-assisted laparoscopic donor nephrectomy, ODN: open donor nephrectomy, SD: standard deviation
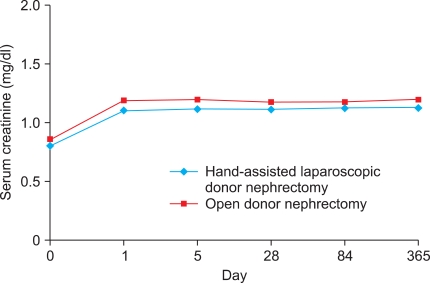
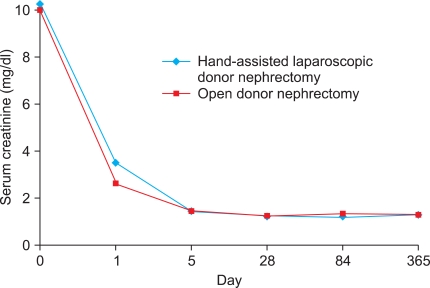
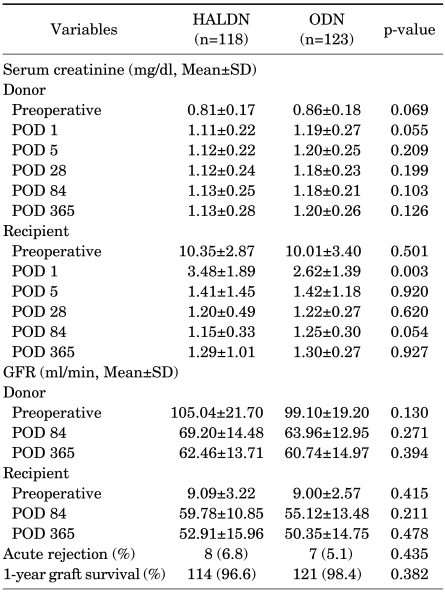
Postoperative changes in serum creatinine levels in both the donors and the recipients are shown in Fig. 1 and Fig. 2, respectively. The mean serum creatinine level in the recipients on postoperative day 1 was significantly higher in the HALDN than in the ODN group (3.48 vs. 2.62 mg/dl, p=0.003). However, from postoperative day 5 to 1 year, there was no significant difference between the two groups. Mean serum creatinine was not significantly different between the two donor groups throughout the study periods. From the third postoperative month, serum creatinine in the HALDN and ODN donor groups remained 39% (0.81→1.13 mg/dl) and 37% (0.86→1.18 mg/dl) higher than preoperative values, respectively. The mean GFR in the donors and the recipients was not significantly different between the two groups on days 84 and 365 postoperatively. Acute rejection occurred in 8 patients in the HALDN group (6.8%) and in 7 in the ODN group (5.1%) (p=0.435). One-year graft survival rates in the HALDN and ODN groups were 96.6% (114/118) and 98.4% (121/123), respectively (p=0.382) (Table 3).
FIG. 1.
Changes in serum creatinine levels in donors after handassisted laparoscopic donor nephrectomy and open donor nephrectomy.
FIG. 2.
Changes in serum creatinine levels in recipients after hand-assisted laparoscopic donor nephrectomy and open donor nephrectomy.
TABLE 3.
Perioperative mean serum creatinine levels and GFR in donors and recipients
GFR: glomerular filtration rate, HALDN: hand-assisted laparoscopic donor nephrectomy, ODN: open donor nephrectomy, SD: standard deviation, POD: postoperative day
DISCUSSION
Since 1995, when Ratner et al reported their first experience with laparoscopic live donor nephrectomy [2], the advantages for donors have interested many transplant centers worldwide. The advantages include less postoperative pain, shorter hospital stay, faster recovery times, and fewer long-term complications, such as neuralgia and herniation [1,6]. However, the pure laparoscopic technique for living donor nephrectomy is a technically difficult procedure, and the longer operating times and warm ischemic times compared with the open technique are also perceived as disadvantages of LDN. In contrast, the hand-assisted procedure, which was first successfully performed by Wolf et al [17], has more advantages with the early incision that is necessary for intact organ removal. Several studies have reported more advantages of HALDN, such as shorter operating times and warm ischemic times compared with pure LDN [18,19]. In addition to these advantages, HALDN has operating times and warm ischemic times comparable with those of ODN and is associated with less postoperative pain, faster recovery times, and better cosmesis [9,20]. Our study also showed similar results. Although recent studies have reported that pure LDN had similar or better results compared with HALDN [11,12], those studies were performed by experienced laparoscopic surgeons. In general, HALDN is still considered an effective and safe procedure.
It is commonly known that pneumoperitoneum, which is required in laparoscopic procedures, may cause adverse intraoperative effects on the cardiovascular system and renal function [21]. In addition, since the first LDN was performed, adverse effects of donor and graft renal function have been questioned. We investigated renal function in donors and recipients after HALDN and ODN on the basis of serum creatinine. Our data suggested no significant differences in renal functional recovery between the HALDN and ODN groups in donors (p>0.05). One study reported that the decline in renal function was significantly greater in laparoscopic donors than in open donors on the first postoperative day [7]. However, this difference was no longer evident by the third postoperative day. They speculated that this difference may be secondary to the effect of prolonged pneumoperitoneum on the GFR. Few data exist on the effect of pneumoperitoneum on renal histology. In one experimental study in rats, a prolonged period of pneumoperitoneum did not cause any histologic changes in the kidneys [22]. Hazebroek et al also reported that abdominal insufflation does not have a deleterious effect on histomorphology [23]. However, intraabdominal pressure is known to be a significant factor in decreased renal function. McDougall et al and Kirsch et al showed a decrease in urine output and in GFR with increasing intraabdominal pressure in animal models [24,25]. A pneumoperitoneum of 15 mmHg for 4 hours resulted in a decrease in renal blood flow to 70% of baseline. In our series, we maintained an intraabdominal pressure of 10 to 12 mmHg during HALDN. The serum creatinine levels of the donors on the first postoperative day were elevated in both the HALDN (1.11 mg/dl) and the ODN (1.19 mg/dl) groups. However, there was no significant difference between the two groups (p=0.055). Of interest was the similarity in serum creatinine levels between both donor groups, which remained 39% (0.81→1.13 mg/dl) and 37% (0.86→1.18 mg/dl) higher than preoperative levels in the HALDN and ODN groups, respectively. This finding was comparable with that of Goldfarb et al, who found an increase of approximately 30% [26]. Although, in our study, HALDN was performed through a transperitoneal approach and ODN was performed through a retroperitoneal approach, the difference in technique did not seem to have an effect on renal function recovery. A previous study by Dols et al reported that there was no significant difference on renal function between retroperitoneoscopic donor nephrectomy and transperitoneal laparoscopic donor nephrectomy groups [27].
Several previous studies have reported the effects of LDN on recipient graft function [2,13]. Nogueira et al compared graft function in recipients after 132 LDNs and 99 ODNs [13]. The mean serum creatinine was significantly higher in the LDN group during the first week after transplant, but was similar between groups at 3 months. In another study, by Ratner et al, 110 LDN patients were compared with 48 ODN patients [14]. This study also showed a higher serum creatinine level on days 2 and 3 in the LDN group, but no significant difference by day 4. Early graft function was also compared between LDNs and ODNs by using information in the United Network for Organ Sharing database [28]. This study compared 2734 LDNs and 2576 ODNs that were performed over a 13-month period from 1999 to 2000. In this study, significantly more patients in the LDN group than in the ODN group had creatinine levels greater than 1.4 or 2 mg/dl at discharge. However, all subsequent serum creatinine levels and graft survival at 1 year were similar between the two groups. In our study, the mean serum creatinine level in recipients was higher in the HALDN group (3.48 mg/dl) than in the ODN group (2.62 mg/dl) on the first postoperative day (p=0.003). After the fifth postoperative day, however, the mean serum creatinine level was comparable until 1 year after transplant (1.41 vs. 1.42 mg/dl, p=0.920).
Pure LDN might cause relatively long warm ischemic times, mainly because of the long time required to extract the kidney through a small incision. However, HALDN may reduce kidney extraction and warm ischemic times, even for a non-experienced laparoscopic surgeon, because of the early incision that is necessary for intact organ removal. In our study, the mean warm ischemic time was not significantly different between the HALDN and ODN groups. Moreover, in a review of 100 LDNs, only recipients of kidneys with a warm ischemic time greater than 10 minutes had serum creatinine levels greater than 2 mg/dl on postoperative day 7 [5]. In animal models, postoperative serum creatinine levels were significantly increased when the warm ischemic time was greater than 30 minutes [29]. Although the warm ischemic time in our HALDN group (290 seconds) was longer than that reported in other studies [18], it was acceptably low and did not appear to cause any significant renal ischemic injury or affect short-term functional recovery or our longer-term results.
Our 1-year HALDN and ODN graft survival rates were 96.6% and 98.4%, respectively, and were not significantly different between the two groups (p=0.382). Other studies of laparoscopic graft survival have shown results (91-95%) similar to those of our study [14,28,30]. The number of rejection episodes also did not differ significantly between the HALDN (8/118, 6.8%) and the ODN (7/123, 5.1%) groups (p=0.435), nor did other complications, such as ureteral complications (p=0.392).
Recently published data provide additional support for the benefits of laparoscopic living donor nephrectomies. Peritransplantation morbidity and mortality data have been reported after both open and laparoscopic procedures. Our data indicate almost no significant differences in renal function recovery between donors and recipients between the HALDN and ODN groups, although serum creatinine levels in the recipients were higher in the HALDN group than in the ODN group on the first postoperative day. Renal function recovery was comparable between the HALDN and ODN groups.
CONCLUSIONS
This retrospective study showed that, although serum creatinine levels in the recipients were higher in the HALDN group than in the ODN group on the first postoperative day, renal function recovery was similar in donors and recipients in both the HALDN and ODN groups. The results of our study indicate that long-term functional outcomes are not significantly different between kidneys obtained laparoscopically or via the open approach at our institution.
Footnotes
The authors have nothing to disclose.
References
- 1.Giessing M. Laparoscopic living-donor nephrectomy. Nephrol Dial Transplant. 2004;19(Suppl 4):36–40. doi: 10.1093/ndt/gfh1039. [DOI] [PubMed] [Google Scholar]
- 2.Ratner LE, Ciseck LJ, Moore RG, Cigarroa FG, Kaufman HS, Kavoussi LR. Laparoscopic live donor nephrectomy. Transplantation. 1995;60:1047–1049. [PubMed] [Google Scholar]
- 3.Ratner LE, Kavoussi LR, Schulam PG, Bender JS, Magnuson TH, Montgomery R. Comparison of laparoscopic live donor nephrectomy versus the standard open approach. Transplant Proc. 1997;29:138–139. doi: 10.1016/s0041-1345(96)00037-1. [DOI] [PubMed] [Google Scholar]
- 4.Yoo KY, Hong SH, Hwang TK. Donor nephrectomy: comparison of open, hand-assisted and laparoscopic donor nephrectomy. Korean J Urol. 2006;47:1309–1314. [Google Scholar]
- 5.Sasaki TM, Finelli F, Bugarin E, Fowlkes D, Trollinger J, Barhyte DY, et al. Is laparoscopic donor nephrectomy the new criterion standard? Arch Surg. 2000;135:943–947. doi: 10.1001/archsurg.135.8.943. [DOI] [PubMed] [Google Scholar]
- 6.Lennerling A, Blohme I, Ostraat O, Lönroth H, Olausson M, Nyberg G. Laparoscopic or open surgery for living donor nephrectomy. Nephrol Dial Transplant. 2001;16:383–386. doi: 10.1093/ndt/16.2.383. [DOI] [PubMed] [Google Scholar]
- 7.Vats HS, Rayhill SC, Thomas CP. Early postnephrectomy donor renal function: laparoscopic versus open procedure. Transplantation. 2005;79:609–612. doi: 10.1097/01.tp.0000151662.84962.4e. [DOI] [PubMed] [Google Scholar]
- 8.Rawlins MC, Hefty TL, Brown SL, Biehl TR. Learning laparoscopic donor nephrectomy safely: a report on 100 cases. Arch Surg. 2002;137:531–534. doi: 10.1001/archsurg.137.5.531. [DOI] [PubMed] [Google Scholar]
- 9.Slakey DP, Hahn JC, Rogers E, Rice JC, Gauthier PM, Ruiz-Deya G. Single-center analysis of living donor nephrectomy: hand-assisted laparoscopic, pure laparoscopic, and traditional open. Prog Transplant. 2002;12:206–211. [PubMed] [Google Scholar]
- 10.Waller JR, Hiley AL, Mullin EJ, Veitch PS, Nicholson ML. Living kidney donation: a comparison of laparoscopic and conventional open operations. Postgrad Med J. 2002;78:153–157. doi: 10.1136/pmj.78.917.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branco AW, Kondo W, Branco Filho AJ, George MA, Rangel M, Stunitz LC. A comparison of hand-assisted and pure laparoscopic techniques in live donor nephrectomy. Clinics. 2008;63:795–800. doi: 10.1590/S1807-59322008000600015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Percegona LS, Bignelli AT, Adamy A, Jr, Pilz F, Chin EW, Meyer F, et al. Hand-assisted laparoscopic donor nephrectomy: comparison to pure laparoscopic donor nephrectomy. Transplant Proc. 2008;40:687–688. doi: 10.1016/j.transproceed.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Nogueira JM, Cangro CB, Fink JC, Schweitzer E, Wiland A, Klassen DK, et al. A comparison of recipient renal outcomes with laparoscopic versus open live donor nephrectomy. Transplantation. 1999;67:722–728. doi: 10.1097/00007890-199903150-00014. [DOI] [PubMed] [Google Scholar]
- 14.Ratner LE, Montgomery RA, Maley WR, Cohen C, Burdick J, Chavin KD, et al. Laparoscopic live donor nephrectomy: the recipient. Transplantation. 2000;69:2319–2323. doi: 10.1097/00007890-200006150-00016. [DOI] [PubMed] [Google Scholar]
- 15.Philosophe B, Kuo PC, Schweitzer EJ, Farney AC, Lim JW, Johnson LB, et al. Laparoscopic versus open donor nephrectomy: comparing ureteral complications in the recipients and improving the laparoscopic technique. Transplantation. 1999;68:497–502. doi: 10.1097/00007890-199908270-00009. [DOI] [PubMed] [Google Scholar]
- 16.Wolf JS, Jr, Merion RM, Leichtman AB, Campbell DA, Jr, Magee JC, Punch JD, et al. Randomized controlled trial of hand-assisted laparoscopic versus open surgical live donor nephrectomy. Transplantation. 2001;72:284–290. doi: 10.1097/00007890-200107270-00021. [DOI] [PubMed] [Google Scholar]
- 17.Wolf JS, Jr, Tchetgen MB, Merion RM. Hand-assisted laparoscopic live donor nephrectomy. Urology. 1998;52:885–887. doi: 10.1016/s0090-4295(98)00389-6. [DOI] [PubMed] [Google Scholar]
- 18.Gershbein AB, Fuchs GJ. Hand-assisted and conventional laparoscopic live donor nephrectomy: a comparison of two contemporary techniques. J Endourol. 2002;16:509–513. doi: 10.1089/089277902760367476. [DOI] [PubMed] [Google Scholar]
- 19.Kokkinos C, Nanidis T, Antcliffe D, Darzi AW, Tekkis P, Papalois V. Comparison of laparoscopic versus hand-assisted live donor nephrectomy. Transplantation. 2007;83:41–47. doi: 10.1097/01.tp.0000248761.56724.9c. [DOI] [PubMed] [Google Scholar]
- 20.Baik S, Rho J, Kim CS. Comparison of hand-assisted laparoscopic donor nephrectomy with open donor nephrectomy. Korean J Urol. 2005;46:1125–1129. [Google Scholar]
- 21.London ET, Ho HS, Neuhaus AM, Wolfe BM, Rudich SM, Perez RV. Effect of intravascular volume expansion on renal function during prolonged CO2 pneumoperitoneum. Ann Surg. 2000;231:195–201. doi: 10.1097/00000658-200002000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee BR, Cadeddu JA, Molnar-Nadasdy G, Enriquez D, Nadasdy T, Kavoussi LR, et al. Chronic effect of pneumoperitoneum on renal histology. J Endourol. 1999;13:279–282. doi: 10.1089/end.1999.13.279. [DOI] [PubMed] [Google Scholar]
- 23.Hazebroek EJ, de Bruin RW, Bouvy ND, Marquet RL, Bonthuis F, Bajema IM, et al. Long-term impact of pneumoperitoneum used for laparoscopic donor nephrectomy on renal function and histomorphology in donor and recipient rats. Ann Surg. 2003;237:351–357. doi: 10.1097/01.SLA.0000055272.96210.A0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDougall EM, Monk TG, Wolf JS, Jr, Hicks M, Clayman RV, Gardner S, et al. The effect of prolonged pneumoperitoneum on renal function in an animal model. J Am Coll Surg. 1996;182:317–328. [PubMed] [Google Scholar]
- 25.Kirsch AJ, Hensle TW, Chang DT, Kayton ML, Olsson CA, Sawczuk IS. Renal effects of CO2 insufflation: oliguria and acute renal dysfunction in a rat pneumoperitoneum model. Urology. 1994;43:453–459. doi: 10.1016/0090-4295(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 26.Goldfarb DA, Matin SF, Braun WE, Schreiber MJ, Mastroianni B, Papajcik D, et al. Renal outcome 25 years after donor nephrectomy. J Urol. 2001;166:2043–2047. [PubMed] [Google Scholar]
- 27.Dols LF, Kok NF, Terkivatan T, Tran KT, Alwayn IP, Weimar W, et al. Optimizing left-sided live kidney donation: hand-assisted retroperitoneoscopic as alternative to standard laparoscopic donor nephrectomy. Transpl Int. 2009 doi: 10.1111/j.1432-2277.2009.00990.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Troppmann C, Ormond DB, Perez RV. Laparoscopic (vs open) live donor nephrectomy: a UNOS database analysis of early graft function and survival. Am J Transplant. 2003;3:1295–1301. doi: 10.1046/j.1600-6143.2003.00216.x. [DOI] [PubMed] [Google Scholar]
- 29.Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35:198–204. doi: 10.1097/00007890-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs SC, Cho E, Dunkin BJ, Flowers JL, Schweitzer E, Cangro C, et al. Laparoscopic live donor nephrectomy: the University of Maryland 3-year experience. J Urol. 2000;164:1494–1499. [PubMed] [Google Scholar]