Abstract
Increased catecholamine (CA) levels after severe burn are associated with stress, inflammation, hypermetabolism and impaired immune function. The CA secretion profiles in burned patients are not well described. Mechanisms, duration and extent of CA surge are unknown. The purpose of this large unicenter study was to evaluate the extent and magnitude of CA surge following severe burn in pediatric patients. Patients admitted between 1996 and 2008 were enrolled in this study. Twenty-four-hour urine collections were performed during acute hospitalization and up to 2 years post burn. Results from the samples collected from 12 normal, healthy volunteers were compared with the data from the burned patients. Relevant demographic and clinical information was obtained from Medical Records. Student’s t-test and one way ANOVA were used to analyze the data where appropriate. Significance was accepted at p<0.05. Four-hundred thirteen patients were enrolled in this study, 17 patients died during acute hospitalization. Burn caused a marked stress and inflammatory response, indicated by massive tachycardia and elevated pro-inflammatory cytokines. In burned patients, CA levels are consistently and significantly modulated after burn when compared to the levels in normal, healthy volunteers. CA levels were significantly higher in males compared to females, correlated with burn size in burns over 40% and were increased in older children. There were differences over time in survivors vs. non-survivors, with CA levels significantly higher in non-survivors at 2 time points. Inflammatory cytokines show a similar profile during the study period. Our study gives clinicians a useful insight into the extent and magnitude of CA elevation to better design treatment strategies.
Keywords: burn, catecholamine, stress
INTRODUCTION
Catecholamine (CA) are hormones produced in the adrenal medulla and other cell types and released into the circulation in response to stress (1). Upon binding to their cognate receptors, they signal through different pathways to affect a wide variety of physiologic processes, from nerve transmission to energy metabolism and cardiac function. Epinephrine is a direct agonist of α1, α2, β1,β2 adrenergic receptors, norepinephrine binds to α1, α2, β1 adrenergic receptors and dopamine binds to dopamine receptors D1 and D2 and with less affinity to α and β receptors. These receptors signal downstream to different classes of G protein-coupled molecules to affect intracellular Ca2+ or cAMP concentration. The effect of CA in various disease states have been extensively studied, but mechanisms still remain unknown. There is an intensive ongoing effort to decipher the mechanism of CA action in individual tissues or cell types and to design effective treatments to modulate their effects in the body (1).
Burn/trauma-related sympathetic activity likely contributes to the activation of stress cascades, leading to increased secretion of cytokines. Sympathetic activity may function as an upstream trigger for activation of p38 mitogen-activated protein kinase (MAPK), JNK and nuclear factor-kappa B (NF-κB) pathways (2), leading to inflammation, immunosuppression and organ dysfunction (2). Sympathetic activation following injury is further associated with impaired immune function, by inhibiting CCL3 production and increasing CCL2 secretion. These chemokines have been shown to contribute to increased susceptibility to infection in severely burned patients (3, 4).
We hypothesize that CA play a central role in the aftermath of severe burn. We are currently trying to decipher and identify what are the specific cellular and metabolic consequences of CA post-burn.
CA actions in critically ill patients are an important opportunity for pharmacological manipulation, but the secretion profiles in pediatric burned patients are not well described. The aim of this large unicenter study is to evaluate the magnitude and extent of CA surge in pediatric burned patients when compared to the CA levels in healthy, non-burned children. This is the first study to elucidate the pattern and physiology of CA post-burn in a large, prospective design.
METHODS
Patients admitted from 1996 to 2008 to our burn center, with informed consent, were enrolled in the study (n=413). Twenty-four-hour urine collections were performed during acute hospitalization and at 3, 6, 9, 12, 18, 24 months post burn. Urine was also collected from healthy non-burned volunteers (n=12). All specimens with total volume below 0.5 ml/kg body weight/hour were excluded to avoid incomplete collection and all specimens with creatinine clearance below 40 to avoid patients on dialysis. During acute hospitalization, 24-hour urine collection was collected via Foley catheters, starting at 6:00 AM ± 2 hours. The urine collection after discharge from the ICU (3, 6, 9, 12, 18, 24 months) started at random times during the day. The study was approved by the Institutional Review Board of the University of Texas Medical Branch and informed consent was obtained from patients, parents, or legal guardians prior to enrollment.
Relevant demographic and clinical information was obtained from Medical Records. Urine samples were acidified to pH=2 with hydrochloric acid and stored at 4–8°C until analyzed. Epinephrine, norepinephrine and dopamine were extracted using Bio-Rad Urinary Catecholamine Kit (BioRad Laboratories, Hercules, CA) according to manufacturer’s instructions and analyzed on a Discovery HS F5 (567516-U) (Sigma-Aldrich, St. Louis, MO) column. Mobile phase A consisted of 25 mM potassium phosphate monobasic in water HPLC grade, pH=2.7 with phosphoric acid and mobile phase B comprised Methanol HPLC grade pH=2.7 with phosphoric acid. A gradient 0–12% B over 20 minutes at 0.7 ml/min flow rate at room temperature achieved complete separation and good resolution between peaks. We used both UV detection at 280 nm and electrochemical detection (0.75 V, filter 2s, sensitivity 10nA/V) for a wider dynamic range of the method.
Heart rates were continuously monitored during acute hospitalization and later admissions for surgical interventions.
Cytokines were measured using Bio-Rad’s human cytokine multiplex kits according to manufacturer’s instructions.
Student’s t-test and one way ANOVA were used to analyze the data where appropriate. Significance was accepted at p<0.05.
RESULTS
Study subjects
Four-hundred thirteen patients were enrolled in this study, 284 males and 129 females, average total body surface area burn (TBSA) 59±17%, third-degree burn 45±25%, age distribution 8±5 years, 17 patients died during acute hospitalization. Results from the samples collected from 12 normal, healthy volunteers, 8 boys, 4 girls, 12±3 years, were compared with the data from the burned patients.
Physiologic changes
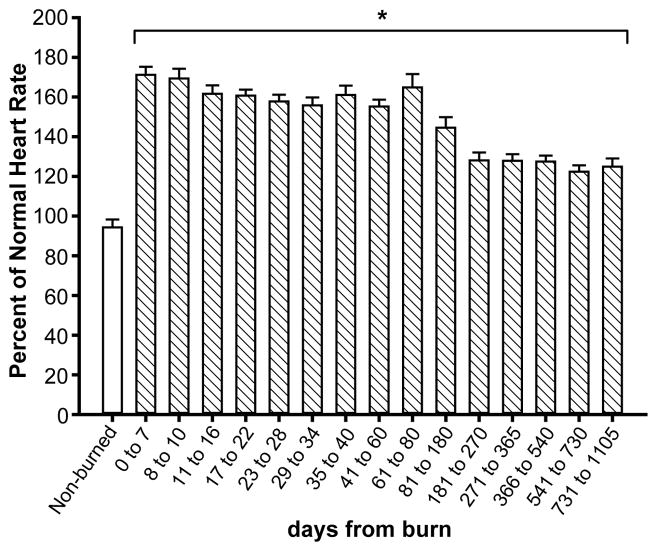
One of the first clinical symptoms of sympathetic stimulation is tachycardia. As shown in Figure 1, predicted heart rates according to age during acute hospitalization are significantly increased, which leads to profound alterations of cardiac function.
Figure 1.
Predicted heart rate according to age during acute hospitalization: burned patients have a significantly increased heart rate compared to norms for age.
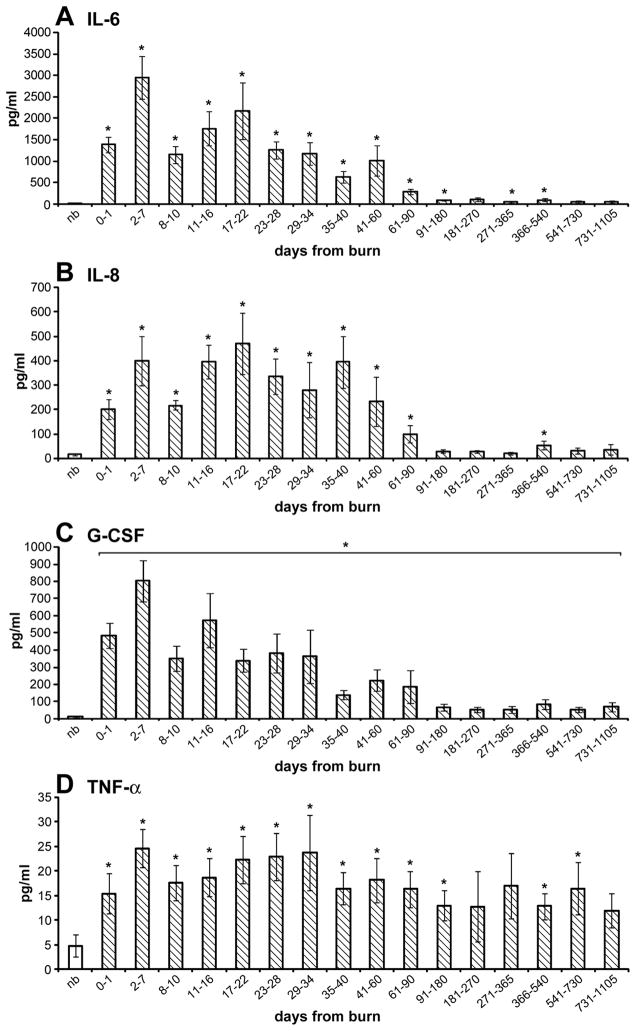
The increase in the secretion of pro-inflammatory mediators is a hallmark in burns. As shown in Figure 2, there is similarity between expression profiles of inflammatory cytokines, such as IL-6, IL-8, G-CSF, TNF-α, and CA secretion.
Figure 2.
Cytokine expression profiles over time in burned children: inflammatory cytokines are increased for a prolonged period of time after burn injury when compared to non-burned children.
CA secretion profiles
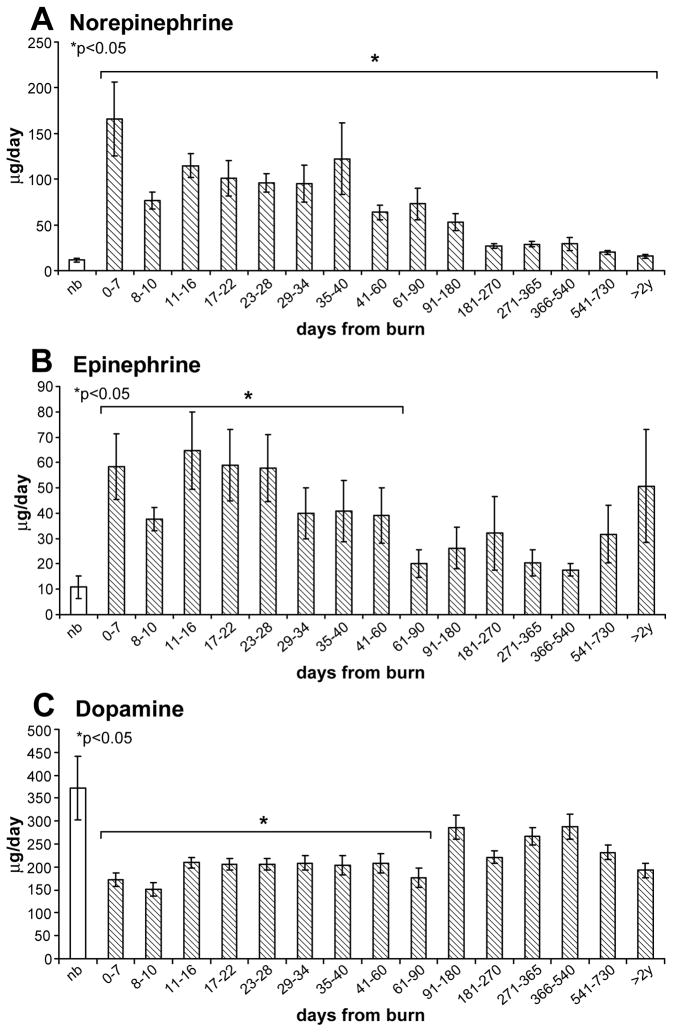
In Figure 3A–C, the levels of urinary norepinephrine (A), epinephrine (B) and dopamine (C) are depicted over time. In burned patients, norepinephrine levels (A) are consistently and significantly elevated for up to 2 years when compared to the levels in normal, healthy volunteers (p<0.05). Epinephrine levels (B) vary over time and statistical significance is reached up to 60 days post-burn. Dopamine levels (C) are consistently lower than in normal healthy volunteers and significance is reached up to 90 days post-burn (p<0.05).
Figure 3.
Urine CA levels over time in burned patients compared to normal, healthy volunteers: A) Norepinephrine levels are significantly higher in burn patients than in healthy volunteers for up to 2 years post burn. B) Epinephrine levels are higher in burned patients when compared to healthy volunteers for up to 60 days post burn. C) Dopamine levels are lower in burn patients than in healthy volunteers.
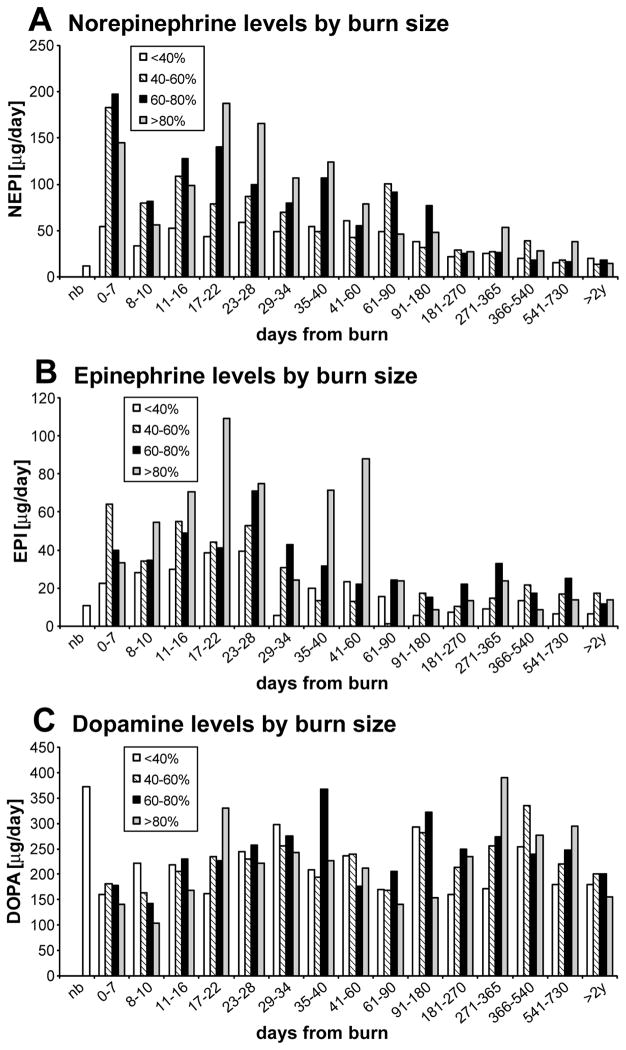
Patients were stratified by burn size. The previous hypothesis that CA levels correlate with burn size held true in our patient population only for burns over 40% (Figure 4). Patients with burns over 80% TBSA have the highest and longest secretion of CA.
Figure 4.
Urine CA levels by burn size: there is no identifiable pattern of secretion of CA related to burn size.
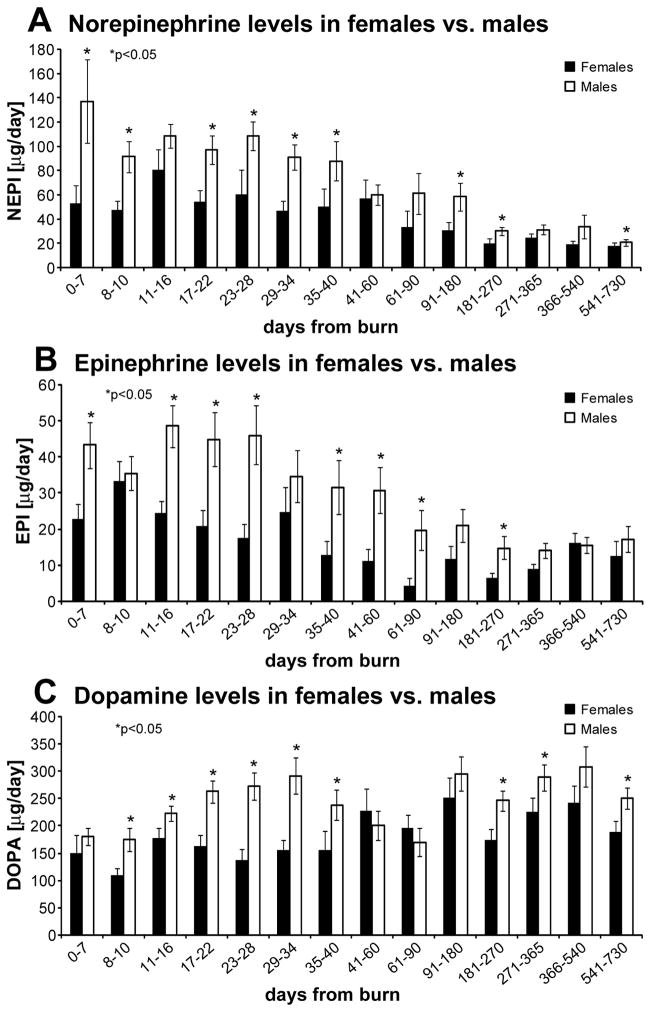
To further assess if gender affects CA, we divided our patient population into males and females. All three CA levels were significantly higher in males compared to females at multiple time points (Figure 5), (p<0.05).
Figure 5.
Urine CA levels in female vs males: CA levels are significantly higher in males than in females.
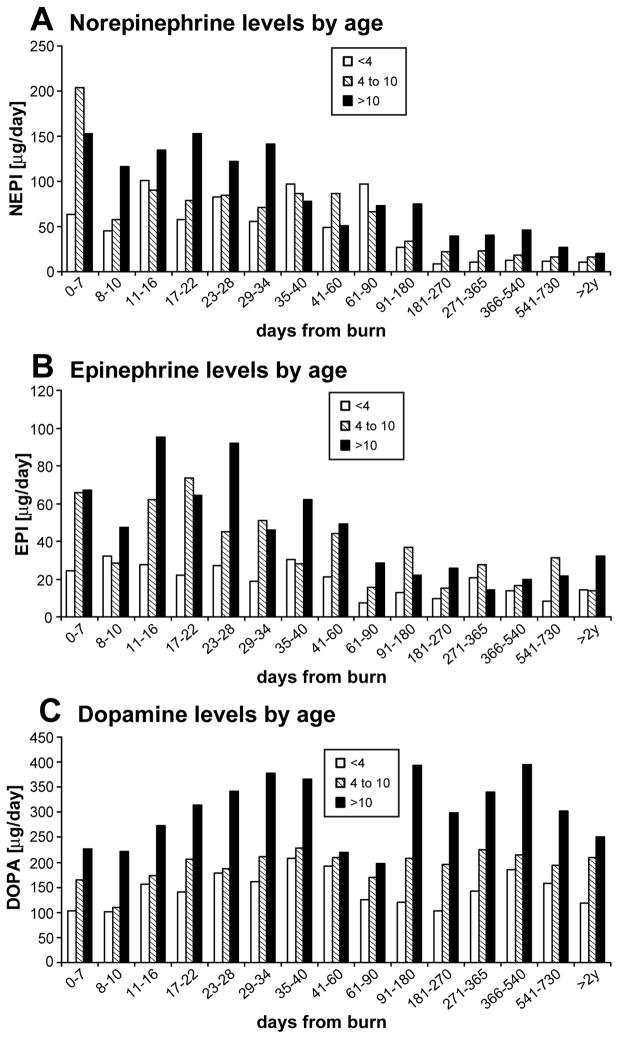
Our data implies that age has a big impact on CA levels after severe burn over time (Figure 6). Norepinephrine and dopamine patterns of secretion show that there is a direct relationship between the levels and age, older children having higher CA levels. Epinephrine data did not reveal a pattern of secretion with regard to age.
Figure 6.
Urine CA levels by age: Older patients have higher levels of CA over time.
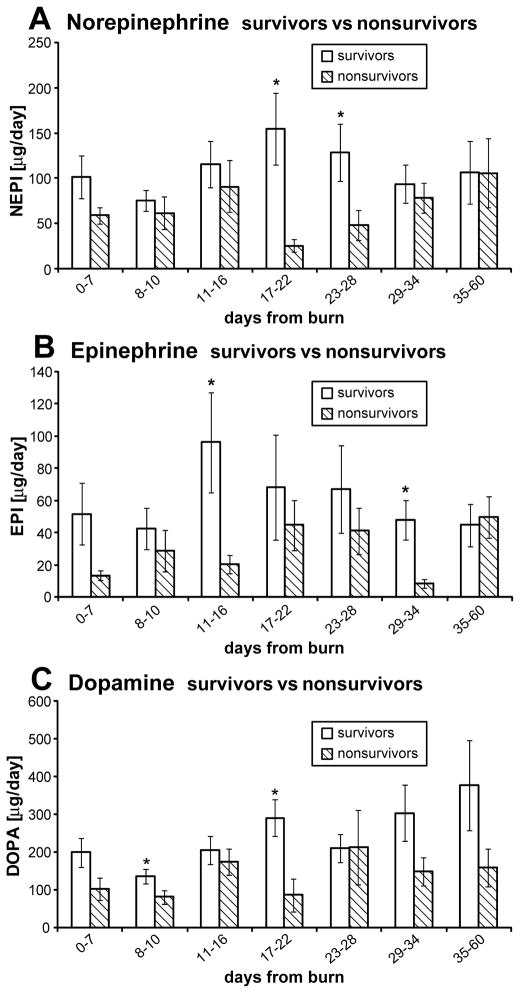
In a subgroup of patients, we compared CA levels in non-survivors with matched survivors. We found significant differences for 2 time points (Figure 7), (p<0.05). We could not identify a pattern of secretion for epinephrine, norepinephrine or dopamine as the time of death approached, therefore, we believe that the amount of CA released into the circulation does not directly impact survival, but rather the ability of each individual patient to respond to the effects of CA surge is responsible for mortality. Our group has shown previously that severe infection episodes are associated with increased CA levels (5).
Figure 7.
CA levels in survivors and non-survivors: there is no discernible pattern of CA secretion.
DISCUSSION
In our institution, CA levels are measured in a 24-hour urine collection. We believe that urine CA levels are a better indicator of adrenal function than plasma levels. Plasma levels give a snapshot of CA secretion and they cannot be used as a reliable biomarker of catecholaminergic system.
There are few large cohort studies to compare CA levels of burned children with normal volunteers and the time course of secretion profiles after injury. In most cases, the plasma CA is analyzed. These measurements are problematic because of epinephrine administration during skin grafting or burn surgery to reduce blood loss and plasma levels reflect only a snapshot of CA secretion. A better indicator of hormone levels is the 24-hour urine amount.
The knowledge of action of CA in severe trauma has increased tremendously in the past 10 years worldwide. A study by Smith et al. in 1997 (6) found that the magnitude of stress response to burn in children is closely related to the size of burn surface area. The pathophysiologic response to severe burn is mediated through chemical mediators, such as CA, that are interrelated with inflammatory responses, such as increases in cytokine production (7). Differences between the stress response in children and in adults have been noted (8), suggesting that the management of burn-injured children has to be designed according to their particular needs.
Our finding that females have lower CA levels than males correlates with the lower hypermetabolic response in females found in a previous study (9).
One of the big improvements in burn care over the past 15–20 years has been the use of CA intraoperatively to minimize blood loss during surgery (10, 11). Topical or subcutaneous administration of epinephrine during burn wound excision had been shown to have transient effects on cardiac function (12) because of its short life time.
CA has profound catabolic effects in the muscle. Decrease in lean body mass and muscle protein wasting are well documented in critically ill patients. Our group has shown that beta-blockade is a useful tool in the arsenal for fighting post-burn muscle proteolysis and to ameliorate the physical sequelae of severe burn (13, 14).
Gosain et al. (15) have shown that CA modulate the inflammatory and proliferative phases of wound healing in a temporally defined, cell specific manner. Norepinephrine appears to have a protective role in defense against infection by recruiting innate immune cells and expediting wound closure. In another study, the group found that norepinephrine has an immunosuppressive effect on wound macrophage function that is tissue specific and appears to be mediated through adrenergic receptors and their downstream signaling pathway (15). This might provide an explanation for the reduced morbidity and mortality associated with beta blockade.
CA surge after severe illness has been involved in insulin resistance, due to the effects on fat metabolism (16) and liver function (17). In a large study analyzing the insulin resistance post burn, we found a strong correlation between insulin resistance and increased CA levels (18). Patients had an Oral Glucose Tolerance Test performed at multiple time points up to 3 years post initial injury and at each return to clinic, 24 hour-urine collection was analyzed. There was a strong correlation between increased CA levels and insulin resistance, reflected specially in peripheral insulin sensitivity indices.
An unexpected association between CA levels and the severity of traumatic stress disorder (both acute stress disorder [ASD] and long-term post-traumatic stress disorder [PTSD]) has emerged in the past few years. Studies in acute and long-term survivors of multiple trauma, burn or myocardial infarction have demonstrated a clear and vivid recall of different categories of traumatic memory such as nightmares, anxiety, respiratory distress, or pain with little or no factual recall of the events, and the number of traumatic memory recalls was proportional to the levels of CA (19). In our patient population, the incidence of ASD is about 8% and the incidence of PTSD is about 7% (20–22). These values are lower than other institutions have reported and are a good indicator of the efforts by our psychiatrists and psychologists and the value the programs they are conducting for our patients. We are currently investigating the correlation between CA levels and incidence of ASD and/or PTSD.
The effects of septic episodes on the outcome of a critically ill patient are well documented. Our group has shown that septic episodes are accompanied by an enormous increase in CA levels (5).
Beta-blockade treatment has been shown to be beneficial in the treatment of severe burns, resulting in decreased energy expenditure and muscle catabolism (23). Supportive therapy and pharmacological manipulations with inhibitors and antagonists of adrenergic receptors can ameliorate hypermetabolic response, immune deficiency, peripheral lipolysis and bone metabolism (24).
CA play a key role in orchestrating the response to stress (1). Production and secretion of CA is crucial to handle emergency situations. When the exposure to stress stimuli is prolonged, adaptation of the organism becomes a big burden, increasing the susceptibility for a wide range of serious diseases. Catecholaminergic systems are responsive to activation of the hippocampal-pituitary-adrenal axis and corticosteroid hormones may have a direct effect. Different signaling pathways and transcription factors are activated depending on the frequency and intensity of exposure to stressful stimuli. Most of the animal studies investigating these processes were performed in males, however, there is evidence that females respond differently to stress. It has been shown that estradiol can regulate the expression of genes involved in CA biosynthesis, such as tyrosine hydroxylase or modulate their response to second messengers, such as cAMP.
CA surge may cause activation of transcription factors such as CREB and c-Fos, leading to induction of MAPK family: ERK1/2, JNK1/2/3 and p38 (25), thus contributing to the hyperinflammatory response after severe burn. Inflammatory cytokines are increased for a prolonged period of time after severe injury. These findings further strengthen our hypothesis that the inflammatory response and the humoral response after burn are linked. We believe that the initial hormonal response after severe injury occurs within 15 minutes, followed by the inflammatory response, which occurs in 3 to 6 hours (26). They are part of the fight-or-flight response. The mechanism remains to be determined, but we can hypothesize that stress response through G protein-coupled receptors and adrenergic receptors are linked to activation of NF-κB through MEK protein, which is a member of the MAPK family downstream from the adrenergic receptor and G protein-coupled receptor and in the cytoplasm forms a complex with NIK (inducible NF-κB kinase). The link between sympathetic nervous system and activation of stress responsive pathways in cardiomyocytes was hypothesized by Jureta Horton and her group. Their study aimed to determine the effects of α1-adrenergic receptor antagonists on the activation of stress response pathways (2).
Summary and conclusion
In this study, we show that CA levels are increased for up to 2 years following severe burn. We found correlation between CA levels and burn size over time. Our data shows that females have lower CA levels over time and older patients have higher levels over time. This is the first cohort study to examine the patterns of CA secretion over a 2-year period after burn. It gives clinicians a useful insight into the extent and magnitude of CA elevation to better design treatment strategies.
Acknowledgments
This study was supported by grants from Shriners Hospitals for Children (8660, 8760, and 9145), National Institutes of Health (R01-GM56687, R01-HD049471, T32 GM008256, and P50 GM60338), and National Institute on Disability and Rehabilitation Research (H133A020102).
The authors would like to thank Maricela Pantoja for her support in analyzing the specimens and clinical research nurses for collecting the samples. The authors would also like to thank Eileen Figueroa and Steve Schuenke for their expert support in editing the manuscript.
Footnotes
Authors have no disclosures to declare.
References
- 1.Sabban EL. Catecholamines in stress: molecular mechanisms of gene expression. Endocr Regul. 2007;41:61–73. [PubMed] [Google Scholar]
- 2.Ballard-Croft C, Maass DL, Sikes P, White J, Horton J. Activation of stress-responsive pathways by the sympathetic nervous system in burn trauma. Shock. 2002;18:38–45. doi: 10.1097/00024382-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi H, Kobayashi M, Tsuda Y, Herndon DN, Suzuki F. Contribution of the sympathetic nervous system on the burn-associated impairment of CCL3 production. Cytokine. 2005;29:208–214. doi: 10.1016/j.cyto.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi H, Tsuda Y, Kobayashi M, Herndon DN, Suzuki F. Increased norepinephrine production associated with burn injuries results in CCL2 production and type 2 T cell generation. Burns. 2004;30:317–321. doi: 10.1016/j.burns.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Norbury WB, Jeschke MG, Herndon DN. Metabolism modulators in sepsis: propranolol. Crit Care Med. 2007;35:S616–620. doi: 10.1097/01.CCM.0000278599.30298.80. [DOI] [PubMed] [Google Scholar]
- 6.Smith A, Barclay C, Quaba A, Sedowofia K, Stephen R, Thompson M, Watson A, McIntosh N. The bigger the burn, the greater the stress. Burns. 1997;23:291–294. doi: 10.1016/s0305-4179(96)00137-4. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda H, Kobayashi K. [Pathophysiologic changes in patients with severe burns: role of hormones and chemical mediators] Nippon Geka Gakkai Zasshi. 1998;99:2–7. [PubMed] [Google Scholar]
- 8.Sedowofia K, Barclay C, Quaba A, Smith A, Stephen R, Thomson M, Watson A, McIntosh N. The systemic stress response to thermal injury in children. Clin Endocrinol (Oxf) 1998;49:335–341. doi: 10.1046/j.1365-2265.1998.00553.x. [DOI] [PubMed] [Google Scholar]
- 9.Jeschke MG, Przkora R, Suman OE, Finnerty CC, Mlcak RP, Pereira CT, Sanford AP, Herndon DN. Sex differences in the long-term outcome after a severe thermal injury. Shock. 2007;27:461–465. doi: 10.1097/01.shk.0000238071.74524.9a. [DOI] [PubMed] [Google Scholar]
- 10.McQuitty CK, Berman J, Cortiella J, Herndon D, Mathru M. beta-adrenergic desensitization after burn excision not affected by the use of epinephrine to limit blood loss. Anesthesiology. 2000;93:351–358. doi: 10.1097/00000542-200008000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Missavage AE, Bush RL, Kien ND, Reilly DA. The effect of clysed and topical epinephrine on intraoperative catecholamine levels. J Trauma. 1998;45:1074–1078. doi: 10.1097/00005373-199812000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Cartotto R, Kadikar N, Musgrave MA, Gomez M, Cooper AB. What are the acute cardiovascular effects of subcutaneous and topical epinephrine for hemostasis during burn surgery? J Burn Care Rehabil. 2003;24:297–305. doi: 10.1097/01.BCR.0000085847.47967.75. [DOI] [PubMed] [Google Scholar]
- 13.Pereira C, Murphy K, Jeschke M, Herndon DN. Post burn muscle wasting and the effects of treatments. Int J Biochem Cell Biol. 2005;37:1948–1961. doi: 10.1016/j.biocel.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Pereira CT, Jeschke MG, Herndon DN. Beta-blockade in burns. Novartis Found Symp. 2007;280:238–251. doi: 10.1002/9780470059593.ch16. [DOI] [PubMed] [Google Scholar]
- 15.Gosain A, Jones SB, Shankar R, Gamelli RL, DiPietro LA. Norepinephrine modulates the inflammatory and proliferative phases of wound healing. J Trauma. 2006;60:736–744. doi: 10.1097/01.ta.0000196802.91829.cc. [DOI] [PubMed] [Google Scholar]
- 16.Cree MG, Wolfe RR. Postburn trauma insulin resistance and fat metabolism. Am J Physiol Endocrinol Metab. 2008;294:E1–9. doi: 10.1152/ajpendo.00562.2007. [DOI] [PubMed] [Google Scholar]
- 17.Jeschke MG, Micak RP, Finnerty CC, Herndon DN. Changes in liver function and size after a severe thermal injury. Shock. 2007;28:172–177. doi: 10.1097/shk.0b013e318047b9e2. [DOI] [PubMed] [Google Scholar]
- 18.Gauglitz GG, Herndon DN, Kulp GA, Meyer WJ, 3rd, Jeschke MG. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab. 2009;94:1656–1664. doi: 10.1210/jc.2008-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schelling G. Post-traumatic stress disorder in somatic disease: lessons from critically ill patients. Prog Brain Res. 2008;167:229–237. doi: 10.1016/S0079-6123(07)67016-2. [DOI] [PubMed] [Google Scholar]
- 20.Ratcliff SL, Brown A, Rosenberg L, Rosenberg M, Robert RS, Cuervo LJ, Villarreal C, Thomas CR, Meyer WJ., 3rd The effectiveness of a pain and anxiety protocol to treat the acute pediatric burn patient. Burns. 2006;32:554–562. doi: 10.1016/j.burns.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg L, Rosenberg M, Perry J, Sharp S, Richardson L, Holzer C, Meyer W., 3rd Long-term psychosocial adjustment of pediatric burn survivors previously diagnosed and treated for acute stress disorder. J Burn Care Res. 2009;30:S80. (abstract #74) [Google Scholar]
- 22.Sharp S, Thomas C, Rosenberg L, Rosenberg M, Meyer W., 3rd Propranolol does not reduce risk for acute stress disorder in pediatric burn trauma. J Burn Care Res. 2009;30:S145. doi: 10.1097/TA.0b013e3181a8b326. (abstract #204) [DOI] [PubMed] [Google Scholar]
- 23.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 24.Murphy KD, Lee JO, Herndon DN. Current pharmacotherapy for the treatment of severe burns. Expert Opin Pharmacother. 2003;4:369–384. doi: 10.1517/14656566.4.3.369. [DOI] [PubMed] [Google Scholar]
- 25.Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89:535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- 26.Gauglitz GG, Song J, Herndon DN, Finnerty CC, Boehning D, Barral JM, Jeschke MG. Characterization of the inflammatory response during acute and post-acute phases after severe burn. Shock. 2008;30:503–507. doi: 10.1097/SHK.0b013e31816e3373. [DOI] [PMC free article] [PubMed] [Google Scholar]