Abstract
Predictors of successful virologic, immunologic, and clinical response with combined antiretroviral therapy (cART) containing a boosted protease inhibitor or a nonnucleoside reverse transcriptase inhibitor were analyzed among an antiretroviral naive (ARV-naive) urban cohort. Measures of success included virologic suppression [HIV-1 viral load (VL) <400 copies/ml], an increase in CD4+ T cells from baseline of >100 cells/μl, and lack of development of an AIDS-defining illness at 24 and 48 weeks after cART initiation. Two hundred and eighty-seven ARV-naive patients were included in this cohort, of which 76.7% were male and 86.8% were nonwhite. At the time of cART initiation their median age was 39 years, the geometric mean CD4+ count was 42 cells/μl, and the mean viral load was 5.3 log10 copies/ml. At 48 weeks, 72% of patients achieved virologic suppression, with ≥90% adherence and high school graduation predicting viral undetectability at 48 weeks. Baseline VL ≤100,000 copies/ml and a CD4+ cell count >100 cells/μl were associated with viral suppression at 24 weeks [OR (95% CI) = 3.55 (1.29–9.81) and 3.96 (1.19–13.15), respectively]; female gender was associated with a greater increase in CD4+ cell counts [OR (95% CI) = 7.41 (2.48–22.1)]. CDC stage A1–C2 at baseline predicted lack of clinical progression at 48 weeks. The results of this analysis of an ARV-naive cohort comprised predominantly of indigent, minority patients suggest that men who did not have a high school education and who had advanced HIV infection are less likely to have therapeutic success after cART initiation.
Introduction
With the widespread use of highly combined antiretroviral therapy (cART) since 1996, AIDS-related death has decreased dramatically in the United States.1,2 To optimize therapeutic success, and minimize morbidity, timely identification of patients who are at risk of failing cART is essential. Previous literature describing predictors of success to highly active antiretroviral therapy (HAART) have the limitation of including patients who were taking regimens not recommended by current antiretroviral guidelines [nonboosted protease inhibitors (PI)].3–12 Furthermore, the majority of patients in these studies are males of European descent, which does not represent current trends in the U.S. AIDS epidemic. The U.S. HIV-1 epidemic has became more concentrated in the southern states, and has an increasingly large number of female and minority patients who present with advanced immune suppression.13,14
We conducted a retrospective cohort analysis of patients with HIV infection who started antiretroviral therapy for the first time (antiretroviral naive) between 2003 and 2005 at an urban clinic located in Atlanta, Georgia. Our objective was to evaluate individual patient characteristics and their association with successful response to therapy, defined as having an HIV-1 RNA viral load less than 400 copies/ml at 24 and 48 weeks. In addition, we also explored the effect of specific antiretroviral combinations on viral load suppression, CD4+ cell counts change, and lack of AIDS clinical progression.
Materials and Methods
This study was conducted at the Grady Infectious Disease Clinic, a Ryan White funded clinic that provides comprehensive HIV care to over 4000 HIV-1-infected patients annually. Among all patients seen in the clinic 72% of the patients are African American and 71% are below 300% of the federal poverty level. Additionally, the majority of patients seen in this clinic are severely immunosuppressed (CD4+ cell counts of less than 200 cells/μl).
Patients
We used pharmacy records to identify potential treatment-naive patients who had started either a boosted-protease inhibitor (PI/r) or a nonnucleoside reverse transcriptase inhibitor (NNRTI)-based cART regimen between January 2, 2003 and December 31, 2005. During this time period our clinic pharmacy served as the Georgia State pharmacy for the AIDS Drug Assistance Program (ADAP) and for patients receiving medications through Medicaid or the Ryan White grant program. In later years a variety of pharmacies supplied medications for these programs. Between 2003 and 2005 we were therefore able to verify exact dates of prescription refill for all patients served in our program. We confirmed that patients were cART naive by reviewing the patient's chart, where providers were required to document the date and type of specific ART that the patient had ever taken. Those patients who were 18 years old and older, were not pregnant, had a CD4+ count and viral load at baseline, and at least 24 ± 3 weeks follow up were included in this analysis. Collected data included demographics, continent of origin (for immigrants), highest level of education attained, type and date of initial cART, body mass index (BMI), and active drug use (cocaine, amphetamine, and intravenous drug use) use documented by health care provider, HIV risk behaviors [men who have sex with men (MSM), heterosexual, injection drug users (IDUs)], opportunistic infections (OI) at baseline and follow up, clinical stage of HIV disease (as by CDC1993 modified classification), major psychiatric illnesses (schizophrenia and bipolar disease depression documented by health care provider), hepatitis B surface antigen, hepatitis C antibody, CD4+ cell count, and HIV plasma viral load at baseline and during follow up.
Due to the retrospective nature of this study, patient adherence was assessed through monthly pharmacy antiretroviral (cART) pick up. Pharmacy records for the majority of the clinic patients were monitored by a centralized pharmacy located at the Grady Health system. The pharmacy provides only 1 month of medication after which the patient has to return for a refill. Pharmacy records include data on patients who did not pick up cART for the month or picked up medications later than the expected duration of pills dispensed for the prior month. A late pick up was considered if 15 days or more had passed from due date of pick up. Adherence was calculated at 24 and 48 weeks by dividing the number of months individuals picked up medications on time by the total number of months of follow up then multiplied by 100.15–17
Outcomes
The primary end point was virologic treatment success, defined as an HIV-1 RNA less than 400 copies/ml at 24 and 48 weeks. Secondary end points included increase of baseline CD4+ T cells by 100 or more cells/μl at 24 and 48 weeks, respectively, and lack of AIDS clinical progression (presentation of AIDS defining illness) during the 48 weeks of follow up.
Statistical analysis
Due to their highly skewed distribution, HIV-1 viral load and CD4+ counts were transformed (log10 and geometric mean, respectively). Using Fisher's exact test and t test, patient characteristics were compared between those with successful response and those who did not achieve a successful response. Subsequently, logistic regression analysis of outcome predictors was conducted. The following variables were included a priori in all models: type of cART (PI/r vs. NNRTI), gender (female vs. male), age (≤50 vs. >50 years), ethnicity (whites vs. African Americans, black Africans, and Latinos), baseline HIV-1 viral load (≤5 vs. >5 log10 copies/ml), baseline CD4+ count (≤100 vs. >100 cells/μl), and adherence (100–90% vs. <90%). Additionally, variables reaching a p value of <0.10 in univariate analyses were entered into multivariate models, and those still statistically significant at 0.05 were kept in the final multivariate model.
All analysis used a two-tailed p value with a significance level of <0.05. Statistical analyses were performed using SAS statistical package version 9 (SAS Institute, Cary, NC).
This study was approved by the Institutional Review Board (IRB) of Emory University and the Grady Research Oversight Committee. The IRB committee waived the need for informed consent.
Results
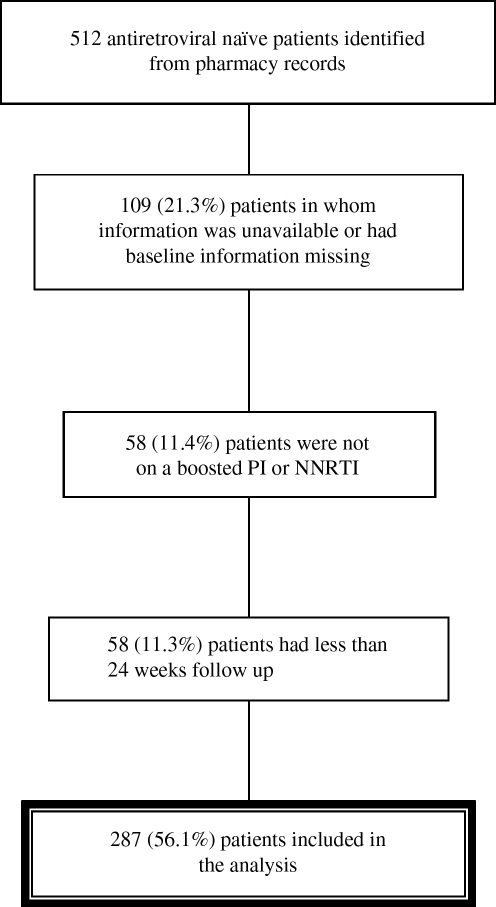
From pharmacy records 512 potential patients were identified, of whom 287 patients (56%) met criteria to be included in this study. A diagram showing the percent and reasons for exclusion of patients is found in Fig. 1.
FIG. 1.
Total patients included in the study and reasons for exclusions.
Baseline characteristics stratified by baseline cART regimen are shown in Table 1. The cohort consisted of mostly males (77%) and nonwhites (87%) with a median age of 39 years (standard deviation 9.7). Fifteen percent had active hepatitis B infection and 11% had positive serology for hepatitis C. At the time of initiation of antiretroviral therapy the geometric mean CD4+ count was 42 cells/μl (range 1–354 cells/ μl) with 67% having a CD4+ count <100 cells/μl. The mean log10 HIV-1 viral load was 5.3 copies/ml, with 63.5% having an HIV-1 viral load >100,000 copies/ml. Fifty-six percent of the individuals were started on an NNRTI-based regimen and 44% with a PI/r-based regimen. Patients who were prescribed a boosted PI were more likely to have had a baseline CD4+ count <100 cells/μl than were those prescribed an NNRTI (75% vs. 60%, respectively, p = 0.003). Table 1 presents baseline characteristics by gender. Women had a higher baseline BMI and were less likely to be prescribed an NNRTI regimen (18% in females vs. 81% in males, p = 0.02).
Table 1.
Baseline Characteristics of the Study Patients by Type of cART and by Gender
| Characteristics | NNRTI (n = 161) | PI/r (n = 126) | p | Males (n = 220) | Females (n = 67) | p |
|---|---|---|---|---|---|---|
| Male gender (%) | 130 (81) | 85 (68) | 0.02 | |||
| Female gender (%) | 29 (18) | 37 (30) | ||||
| cART = NNRTI | 130 (60) | 29 (44) | 0.02 | |||
| High school or higher education (%) | 113 (70) | 91 (72) | 0.92 | 166 (76) | 42 (63) | 0.14 |
| Mean age (SD) | 39 (9.7) | 40 (9.6) | 0.88 | 38 (9.5) | 41 (9.9) | 0.06 |
| Mean BMI (SD) | 24 (4.4) | 24 (4.1) | 0.43 | 23 (4.1) | 25 (5.3) | 0.02 |
| Ethnicity | 0.18 | <0.0001 | ||||
| African American (%) | 103 (64) | 74 (59) | 142 (65) | 39 (59) | ||
| White (%) | 18 (11) | 20 (16) | 33 (15) | 5 (8) | ||
| Black African (%) | 14 (9) | 15 (12) | 13 (6) | 17 (26) | ||
| Latino (%) | 24 (15) | 11 (9) | 31 (14) | 4 (6) | ||
| HIV transmission risk factor | 0.47 | <0.0001 | ||||
| MSM (%) | 74 (46) | 50 (40) | 124 (57) | NA | ||
| IVDU (%) | 14 (9) | 15 (12) | 19 (9) | 10 (15) | ||
| Heterosexual (%) | 70 (44) | 57 (46) | 71 (33) | 56 (84) | ||
| HIVRNA <100,000 copies/ml (%) | 36 (22) | 37 (29) | 0.4 | 54 (27) | 19 (28) | 0.89 |
| Mean CD4+ cells/mm3 | 47 | 37 | 0.65 | 38 | 53 | 0.06 |
| CD4+ <100 cell/mm3 (%) | 96 (60) | 94 (75) | 0.003 | 148 (68) | 42 (63) | 0.29 |
| Drug abuse (%) | 30 (19) | 31 (25) | 0.19 | 43 (20) | 21 (32) | 0.04 |
| Major psychiatric illness (%) | 7 (4) | 4 (3) | 0.76 | 10 (5) | 1 (2) | 0.26 |
| Depression (%) | 36 (23) | 34 (27) | 0.32 | 51 (24) | 20 (30) | 0.24 |
| Hepatitis C antibody positive (%) | 17 (11) | 15 (12) | 0.64 | 21 (10) | 11 (17) | 0.12 |
| Hepatitis B surface antigen positive (%) | 28 (18) | 17 (14) | 0.4 | 38 (17) | 7 (11) | 0.17 |
| OI at baseline (%) | 81 (50) | 66 (53) | 0.6 | 107 (50) | 40 (60) | 0.14 |
| CDC Stage C3 (%) | 80 (50) | 64 (51) | 0.67 | 107 (49) | 38 (57) | 0.23 |
| NRTI used | 0.7 | 0.19 | ||||
| Zidovudine/lamivudine (%) | 73 (46) | 66 (53) | 101 (46) | 38 (57) | ||
| Tenofovir/emtricitabine (%) | 65 (41) | 34 (27) | 82 (37) | 17 (26) | ||
| Other (%) | 21 (13) | 22 (18) | 38 (17) | 11 (17) |
Of the 287 patients included in the study cohort, follow-up data at 48 weeks were available for 238 (83%). Those with information at 48 weeks were more likely to have had adherence of 90–100% in the first 24 weeks of follow up (p = 0.04).
At 24 weeks, 73% of the patients achieved an HIV-1 viral load of <400 copies/ml and 72% achieved virologic suppression (<400 copies/ml) at 48 week. If all 58 patients who did not return for follow-up visits are assumed to meet the criteria for virologic failure, then the proportion of the cohort who achieved 48 week virologic suppression would fall to 61%. CD4+ cell recovery by more than 100 cells/μl from baseline was observed in slightly over half (56%) of the patients at 24 weeks and in 72% of patients at 48 weeks. Clinical progression to a new or recurrent AIDS-defining opportunistic infection developed in 34 (12%) of the study cohort; 29 of these events occurred by week 24 of follow up. No patient who at baseline had achieved a high school or higher level of education developed a new opportunistic infection.
Univariate analysis
There was an association between viral suppression (<400 copies/ml) and having a baseline CD4+ cells >100 cells/μl [OR (95% CI) = 3.96 (1.19–13.15)] and a baseline viral load <100,000 copies/ml [OR (95% CI) = 3.13 (1.52–6.44)]. Virologic suppression was also associated with being Latino [OR (95% CI) = 4.79 (1.24–18.55)] or black African [OR (95% CI) = 4.58 (1.28–17.79)] as well as adherence with medication pick up of more than 90% [OR (95% CI) = 2.63 (1.80–6.15)]. A higher percentage of Latinos and patients immigrating from Africa achieved virologic suppression at 48 weeks compared to African Americans (85% Latinos, 86% black Africans, 71% African Americans, p = 0.02 for trend).
Lack of clinical progression was observed among those with CDC stage A1–C2 disease at initiation of therapy, having at least a high school education, and a viral load of <100,000 copies/ml
Multivariate analysis
The type of cART regimen, gender, age less than 50 years, and ethnicity were not found to be significant predictors for virologic suppression by multivariate analysis at 24 or 48 weeks (Table 2). Significant predictors of virologic suppression at 24 weeks were a baseline viral load of <100,000 copies/ml [OR (95% CI) = 3.55 (1.29–9.81)] and baseline CD4+ ≥100 cells/μl count [OR (95% CI) = 3.96 (1.19–13.15)] and at 48 weeks adherence of 90–100% [OR (95% CI) = 2.70 (1.17–6.24)] and having at least a high school education [OR (95% CI) = 4.98 (1.11–22.40)].
Table 2.
Multivariate Logistic Regression for Undetectable Viral Load (<400 Copies) among the Cohort Patientsa
| Variable | OR | 24 weeks | OR | 48 weeks |
|---|---|---|---|---|
| Baseline ART regimen | ||||
| NNRTI versus bPI | 1.08 | 0.46–2.54 | 0.85 | 0.34–2.18 |
| Baseline viral load | ||||
| <100,000 copies/ml | 3.55 | 1.29–9.81b | 0.87 | 0.34–2.23 |
| Baseline CD4+ count | ||||
| ≥100 cells/μl | 3.96 | 1.19–13.15b | 2.34 | 0.76–7.20 |
| Gender | ||||
| Female | 0.68 | 0.23–1.99 | 1.34 | 0.44–4.09 |
| Age | ||||
| <50 | 2.79 | 0.91–8.6 | 0.73 | 0.16–3.21 |
| Ethnicity | ||||
| African American | — | — | — | — |
| White | 2.51 | 0.61–10.3 | 0.30 | 0.07–1.18 |
| Black African | 3.16 | 0.60–16.73 | 1.34 | 0.44–4.09 |
| Latino | 0.91 | 0.23–3.57 | 1.18 | 0.22–6.30 |
| Adherence | ||||
| 100–90% | 1.75 | 0.74–4.14 | 3.47 | 1.36–8.88c |
| Education | ||||
| High school or higher education | 0.59 | 0.10–3.41 | 4.98 | 1.11–22.4b |
NNRTI, nonnucleoside reverse transcriptase inhibitor; ART, antiretroviral treatment; bPI, boosted protease inhibitor.
Significant at 0.01.
Significant at 0.001.
Female gender and high school education or above were associated with increased CD4+ cell count by ≥100 cells/μl [OR (95% CI) = 7.41 (2.48–22.1) and 4.98 (1.01–24.7), respectively]. Patients of black African ethnicity compared to African Americans were less likely to have CD4+ cell recovery at 48 weeks [OR (95% CI) = 0.23 (0.06–0.97)] (Table 3).
Table 3.
Multivariate Logistic Regression for Change in Baseline CD4 Counts by More Than 100 CD4 Cells among the Cohort Patientsa
| Variable | OR | 24 weeks | OR | 48 weeks |
|---|---|---|---|---|
| Baseline ART regimen | ||||
| NNRTI | 0.72 | 0.31–1.65 | 1.03 | 0.40–2.63 |
| Baseline viral load | ||||
| <100,000 | 0.57 | 0.24–1.38 | 0.81 | 0.32–2.09 |
| Baseline CD4+ count | ||||
| <100 | 1.97 | 0.74–5.29 | 3.53 | 1.30–9.56b |
| Gender | ||||
| Female | 7.38 | 2.25–24.17c | 2.20 | 0.69–7.08 |
| Age | ||||
| <50 | 1.82 | 0.56–5.93 | 0.99 | 0.24–4.06 |
| Ethnicity | ||||
| African American | — | — | — | — |
| White | 0.76 | 0.23–2.56 | 0.34 | 0.09–1.29 |
| Black African | 0.09 | 0.02–0.45c | 0.23 | 0.06–0.97d |
| Latino | 7.57 | 1.14–50.13b | 2.02 | 0.33–12.3 |
| Adherence | ||||
| 100–90% | 0.91 | 0.39–2.15 | 1.39 | 0.54–3.58 |
| Education | ||||
| High school or higher education | 4.98 | 1.01–24.70d | 1.55 | 0.31–7.78 |
NNRTI, nonnucleoside reverse transcriptase inhibitor; ART, antiretroviral treatment.
Significant at 0.01.
Significant at 0.001.
Significant at 0.05.
For the analysis of clinical progression, only CDC stage A1–C2 remained significant in the multivariate model [OR (95% CI) = 7.72 (1.47–40.6)] (Table 4). However, there was a trend that favored NNRTI-based regimens in decreasing likelihood of clinical progression [OR (95% CI) = 2.57 (0.76–8.71)]. Patients having a concordant response (virologic suppression and an increase in CD4+ count) were 6.88 (95% CI 2.28 to 20.8) times less likely to have clinical progression than those who had a discordant response (viral suppression without an increase in CD4+ counts or increase in CD4+ counts without achieving virologic suppression).
Table 4.
Multivariate Logistic Regression of Clinical Progression at 48 Weeksa
| Variable | OR | 95%CI |
|---|---|---|
| Baseline ART regimen | ||
| NNRTI | 2.57 | 0.76–8.71 |
| Baseline viral load | ||
| <100,000 copies/ml | 2.07 | 0.32–3.58 |
| Baseline CD4+ count | ||
| <100 | 0.75 | 0.13–4.34 |
| Gender | ||
| Female | 1.48 | 0.36–6.15 |
| Age | ||
| <50 | 0.42 | 0.05–3.78 |
| Ethnicity | ||
| African American | 0.21 | 0.02–1.96 |
| Black African | 0.09 | 0.01–1.40 |
| Adherence | ||
| 100–90% | 1.08 | 0.32–3.71 |
| Stage | ||
| A1–C2 | 6.72 | 1.31–34.8b |
NNRTI, nonnucleoside reverse transcriptase inhibitor; ART, antiretroviral treatment.
Significant at 0.01.
Discussion
This study provides insight into patient characteristics that help predict successful response to cART when either boosted protease inhibitor-based or NNRTI-based regimens are used in urban dwelling patients residing in the southeastern United States. Overall, patients included in this cohort started cART in the late stages of the disease (51% had stage C3, 64% had an HIV-1 viral load of >100,000 copies/ml, and 69% had a CD4+ cell count of <100 cells/μl) and at a median age of 39 years. In comparison to similar southern U.S. cohorts, individuals in this study had lower CD4+ cell counts at baseline.11 Remarkably, even with such advanced disease, up to 72% of patients who remained in care achieved virologic suppression at 24 and 48 weeks.
Cohort studies have shown that viral load and CD4+ counts at baseline are associated with HAART outcomes.9,11,18–20 In our study, the degree of immunosuppression as well as the baseline viral load predicted a favorable response to HAART at 24 weeks, while at 48 weeks patients' adherence and a higher education level were the most important predictors of response to HAART. This finding emphasizes the dynamic processes influencing response to HAART and also reflects our clinical practice: in the first period of HAART initiation, patients are followed very closely to monitor for side effects, tolerability, and compliance, while at 48 weeks, the follow up is not as stringent and compliance to medication would play a more important role. Similarly, a cohort of HIV-infected Brazilian individuals with CD4+ counts less than 200 cells/μl who started therapy after 1999 revealed that compliance and number of years of formal education were predictors of successful response to HAART.21
For the outcome of clinical progression, HIV disease stage at baseline was the only predictive characteristic reaching statistical significance in the multivariate model. This result is expected due to the relationship of HIV disease stage with severity and degree of immunosuppression. A remarkable finding was that no clinical events occurred among patients with an education of high school or higher. This finding is in concordance with good outcomes in patients with higher education shown in other studies, and highlights the importance of education in health outcomes.4,21 It also suggests that physicians and clinical treatment programs should assess education and comprehension levels among their patients, and devote additional time for adherence training for those with less educational attainment. As HIV increasingly affects patients of racial or ethnic minorities in whom lower rates of high school education completion may be observed, achieving successful outcomes with cART may become more challenging.
As previous studies have shown, the type of cART regimen used was not a significant predictor of success for any outcome in this study, despite a trend that favored NNRTI-based regimens in decreasing likelihood of clinical progression.4,22–24
Ethnic disparities in access and response to HAART have been previously reported.25,26 In our cohort, Latinos and black Africans were more likely than African Americans and whites to achieve virologic suppression at 24 weeks in univariate analysis, but not in multivariate analysis. Black Africans were also less likely to have an increase of more than 100 CD4+ cells from baseline at 48 weeks compared to African Americans (OR = 0.23; 95% CI, 0.06 to 0.97). This finding could not be attributed to black Africans having had lower initial CD4+ counts at initiation of therapy, since there were no statistical differences in baseline T cells among ethnic groups (CD4+ T cells = 48.8 cells/μl for black Africans vs. 38.4 cells/μl for other racial/ethnic groups; p = 0.25).
Finally, women had a better immunological response at 24 weeks. This might be explained by the fact that at baseline women had higher CD4+ cell counts. Nonetheless, the difference in baseline CD4+ cell counts between males and females did not reach statistical significance (geometric mean for women 37.5 vs. 53.1 in males; p = 0.06), and the multivariate analysis adjusting for baseline CD4+ cell count still favored immunologic recovery in women. Previous literature has recognized that females have a tendency to less clinical progression than males.27
This study has several limitations. Due to the retrospective nature of the study there was a lack of randomization of treatment assignments; this leads to some confounding between groups that cannot be fully accounted for even by logistic regression. Furthermore, patients for whom viral load or CD4+ counts were not available 24 weeks after treatment initiation and patients who failed to return after an initial visit were not included in the analysis. This is important as noncompliance with appointments and treatment follow up has been associated with poor outcomes in other studies.3,5,20,28,29 In addition, not all patients had an HIV-1 viral load and a CD4+ count measured at exactly 24 ± 3 and/or 48 ± 3 weeks and thus could not be analyzed. Third, we did not include a variable for socioeconomic status (SES), which has been related to clinical outcomes.30 However, as a public clinic the SES of the population at our clinic is relatively homogeneous and this reduces the potential confounding of this variable.14,31 Finally, other confounding risk factors could be missing on the analysis, such as family/social support, comorbid diseases, and other pharmacological agents taken (such as prophylactic antimicrobial agents, antivirals other than cART, and lipid-lowering drugs).
In conclusion, this retrospective cohort study suggests that potential predictors of success to currently approved cART combinations for initial therapy in antiretroviral naive patients are a baseline HIV-1 viral load of <100,000 copies/ml, a CD4+ count of >100 cells/μl, a high school or greater education, and female gender. For treatment regimens that include an NNRTI or a boosted PI, 90–100% adherence also predicts long-term virologic success.
Acknowledgments
The project was supported in part by the NIH/FIC/AIDS International Training and Research Program of Emory University (D43 TW01042) and the NIH/NIAID Center for AIDS Research of Emory University (2P30 AI 50409-04A1).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Palella FJ., Jr. Delaney KM. Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ., Jr. Baker RK. Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 3.Rastegar DA. Fingerhood MI. Jasinski DRC. Highly active antiretroviral therapy outcomes in a primary care clinic. AIDS Care. 2003;15(2):231–237. doi: 10.1080/0954012031000068371. [DOI] [PubMed] [Google Scholar]
- 4.Friedl AC. Ledergerber B. Flepp M, et al. Response to first protease inhibitor- and efavirenz-containing antiretroviral combination therapy. The Swiss HIV Cohort Study. AIDS. 2001;15(14):1793–1800. doi: 10.1097/00002030-200109280-00008. [DOI] [PubMed] [Google Scholar]
- 5.Robbins GK. Daniels B. Zheng H. Chueh H. Meigs JB. Freedberg KA. Predictors of antiretroviral treatment failure in an urban HIV clinic. J Acquir Immune Defic Syndr. 2007;44(1):30–37. doi: 10.1097/01.qai.0000248351.10383.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas GM. Chaisson RE. Moore RD. Highly active antiretroviral therapy in a large urban clinic: Risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131(2):81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 7.Paris D. Ledergerber B. Weber R, et al. Incidence and predictors of virologic failure of antiretroviral triple-drug therapy in a community-based cohort. AIDS Res Hum Retroviruses. 1999;15(18):1631–1638. doi: 10.1089/088922299309676. [DOI] [PubMed] [Google Scholar]
- 8.Palella FJ., Jr. Chmiel JS. Moorman AC. Holmberg SD. Durability and predictors of success of highly active antiretroviral therapy for ambulatory HIV-infected patients. AIDS. 2002;16(12):1617–1626. doi: 10.1097/00002030-200208160-00007. [DOI] [PubMed] [Google Scholar]
- 9.Bosch RJ. Bennett K. Collier AC. Zackin R. Benson CA. Pretreatment factors associated with 3-year (144-week) virologic and immunologic responses to potent antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44(3):268–277. doi: 10.1097/QAI.0b013e31802c7e20. [DOI] [PubMed] [Google Scholar]
- 10.Hammer SM. Eron JJ., Jr. Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300(5):555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 11.Mugavero MJ. Pence BW. Whetten K, et al. Predictors of AIDS-related morbidity and mortality in a southern U.S. Cohort. AIDS Patient Care STDS. 2007;21(9):681–690. doi: 10.1089/apc.2006.0167. [DOI] [PubMed] [Google Scholar]
- 12.DHSS: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents: Department of Health and Human Services. 2008. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf <. >.
- 13.Hall HI. Geduld J. Boulos D, et al. Epidemiology of HIV in the United States and Canada: Current status and ongoing challenges. J Acquir Immune Defic Syndr. 2009;51(Suppl 1):S13–20. doi: 10.1097/QAI.0b013e3181a2639e. [DOI] [PubMed] [Google Scholar]
- 14.Epidemiology of HIV/AIDS––United States, 1981–2005. MMWR Morb Mortal Wkly Rep. 2006;55(21):589–592. [PubMed] [Google Scholar]
- 15.Steiner JF. Prochazka AV. The assessment of refill compliance using pharmacy records: Methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 16.Low-Beer S. Yip B. O'Shaughnessy MV. Hogg RS. Montaner JS. Adherence to triple therapy and viral load response. J Acquir Immune Defic Syndr. 2000;23(4):360–361. doi: 10.1097/00126334-200004010-00016. [DOI] [PubMed] [Google Scholar]
- 17.Delgado J. Heath KV. Yip B, et al. Highly active antiretroviral therapy: Physician experience and enhanced adherence to prescription refill. Antivir Ther. 2003;8(5):471–478. [PubMed] [Google Scholar]
- 18.Vaamonde CM. Hoover DR. Anastos K, et al. Factors associated with poor immunologic response to virologic suppression by highly active antiretroviral therapy in HIV-infected women. AIDS Res Hum Retroviruses. 2006;22(3):222–231. doi: 10.1089/aid.2006.22.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egger M. May M. Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: A collaborative analysis of prospective studies. Lancet. 2002;360(9327):119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 20.Mugavero MJ. Lin HY. Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–256. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuboi SH. Harrison LH. Sprinz E. Albernaz RK. Schechter M. Predictors of virologic failure in HIV-1-infected patients starting highly active antiretroviral therapy in Porto Alegre, Brazil. J Acquir Immune Defic Syndr. 2005;40(3):324–328. doi: 10.1097/01.qai.0000182627.28595.01. [DOI] [PubMed] [Google Scholar]
- 22.Waters L. Stebbing J. Jones R, et al. A comparison of the CD4 response to antiretroviral regimens in patients commencing therapy with low CD4 counts. J Antimicrob Chemother. 2004;54(2):503–507. doi: 10.1093/jac/dkh329. [DOI] [PubMed] [Google Scholar]
- 23.Bartlett JA. Fath MJ. Demasi R, et al. An updated systematic overview of triple combination therapy in antiretroviral-naive HIV-infected adults. AIDS. 2006;20(16):2051–2064. doi: 10.1097/01.aids.0000247578.08449.ff. [DOI] [PubMed] [Google Scholar]
- 24.MacArthur RD. Novak RM. Peng G, et al. A comparison of three highly active antiretroviral treatment strategies consisting of non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or both in the presence of nucleoside reverse transcriptase inhibitors as initial therapy (CPCRA 058 FIRST Study): A long-term randomised trial. Lancet. 2006;368(9553):2125–2135. doi: 10.1016/S0140-6736(06)69861-9. [DOI] [PubMed] [Google Scholar]
- 25.Hall HI. Byers RH. Ling Q. Espinoza L. Racial/ethnic and age disparities in HIV prevalence and disease progression among men who have sex with men in the United States. Am J Public Health. 2007;97(6):1060–1066. doi: 10.2105/AJPH.2006.087551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Easterbrook PJ. Farzadegan H. Hoover DR, et al. Racial differences in rate of CD4 decline in HIV-1-infected homosexual men. AIDS. 1996;10(10):1147–1155. [PubMed] [Google Scholar]
- 27.Nicastri E. Angeletti C. Palmisano L, et al. Gender differences in clinical progression of HIV-1-infected individuals during long-term highly active antiretroviral therapy. AIDS. 2005;19(6):577–583. doi: 10.1097/01.aids.0000163934.22273.06. [DOI] [PubMed] [Google Scholar]
- 28.Bangsberg DR. Perry S. Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15(9):1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 29.Mugavero MJ. Lin HY. Allison JJ, et al. Racial disparities in HIV virologic failure: Do missed visits matter? J Acquir Immune Defic Syndr. 2009;50(1):100–108. doi: 10.1097/QAI.0b013e31818d5c37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunningham WE. Hays RD. Duan N, et al. The effect of socioeconomic status on the survival of people receiving care for HIV infection in the United States. J Health Care Poor Underserved. 2005;16(4):655–676. doi: 10.1353/hpu.2005.0093. [DOI] [PubMed] [Google Scholar]
- 31.Ashman JJ. Marconi KM. McKinney MM. O'Neill JF. Who receives Ryan White CARE Act services? A demographic comparison of CARE act clients and the general AIDS population. AIDS Patient Care STDS. 2000;14(10):561–565. doi: 10.1089/108729100750018335. [DOI] [PubMed] [Google Scholar]