Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory disease which is in part mediated by proinflammatory factors produced by RA synovial tissue fibroblasts and macrophages, resulting in monocyte migration from the blood to the synovial tissue. In order to characterize the potential role of IL-17 in monocyte migration, RA synovial fibroblasts and macrophages were activated with IL-17 and examined for the expression of monocyte chemokines. The two potentially important monocyte chemoattractants identified were CCL20/MIP-3α and CCL2/MCP-1, which were significantly induced in RA synovial fibroblasts and macrophages. However, in vivo, only CCL2/MCP-1 was detectable following adenovirus (Ad)-IL-17 injection. We found that IL-17 induction of CCL2/MCP-1 was mediated by PI3K, ERK, and JNK pathways in RA synovial tissue fibroblasts and PI3K and ERK pathways in macrophages. Further, we show that neutralization of CCL2/MCP-1 significantly reduced IL-17-mediated monocyte recruitment into the peritoneal cavity. We demonstrate that local expression of IL-17 in ankle joints was associated with significantly increased monocyte migration and CCL2/MCP-1 levels. Interestingly, we show that RA synovial fluids immunoneutralized for both IL-17 and CCL2/MCP-1 have similar monocyte chemotaxis activity as those immunoneutralized for each factor alone. In short, CCL2/MCP-1 produced from cell types present in the RA joint as well as in experimental arthritis may be in part responsible for IL-17-induced monocyte migration, hence these results suggest that CCL2/MCP-1 is a downstream target of IL-17 that may be important in RA.
Keywords: IL-17, CCL2/MCP-1, macrophages, synovial tissue fibroblasts, monocytes, rheumatoid arthritis
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic systemic disorder that is characterized by infiltration of inflammatory cells that cause synovial hyperplasia and progressive destruction of cartilage and bone. RA was initially considered a TH1-associated inflammatory joint disease, however recent evidence from experimental models of arthritis indicates that IL-17 producing T cells play a major role in the initiation and progression of disease (1–4). IL-17 is produced in the T cell rich areas of RA synovial tissue, and high levels of IL-17 are present in the RA synovial fluid compared to osteoarthritis (OA) synovial fluid (5, 6). We recently demonstrated that the percentage of TH-17 cells is higher in RA synovial fluids compared with normal or RA peripheral blood (7).
Several groups have demonstrated that IL-1β, IL-6, IL-23 and TGF-β are essential for driving human TH-17 cell differentiation from naive CD4+ and CD45RA+ peripheral blood lymphocytes (8, 9). Although it was initially suggested that the differentiation of human TH-17 cells is independent of TGF-β, recently published data demonstrate that the absence of TGF-β mediates a shift from a TH-17 profile to a TH1-like profile (8, 9). Both IL-6 and IL-1 play an important role in human TH-17 cell polarization and are induced by IL-17 in the RA joint, thereby resulting in a positive feedback loop that may lead to self-perpetuating chronic disease. Interestingly, IL-1β is central to human TH-17 differentiation, however TNF-α does not seem to be critical for this process (8, 10). Others have shown that TGF-β and IL-21 uniquely promote polarization of TH-17 cells from human naive CD4+ T cells (11).
In RA synovial tissue, T cells are in close proximity to RA synovial tissue fibroblasts and macrophages, and therefore interaction between the different cell types may occur. Previous studies have shown that IL-17 induces RA synovial tissue fibroblasts to produce neutrophil and T cell chemokines, including IL-8, CCL20/MIP-α, CXCL1/GRO-α and CXCL2/GRO-β (12–15). IL-17 is also capable of activating macrophages to express IL-1, TNF-α, cyclooxygenase (COX)2, prostaglandin (PG)E2 and matrix metalloproteinase (MMP)-9 (16–18). However, the role of IL-17 in the induction of monocyte chemokines from macrophages or RA synovial fibroblasts is not known.
In order to characterize the mechanisms by which IL-17 might contribute to the pathogenesis of RA, we recently demonstrated that IL-17 is directly chemotactic for monocytes (19). In the present study, we examined the hypothesis that IL-17 may also mediate monocyte migration through the induction of monocyte chemokines. For this purpose, we screened IL-17 activated in vitro differentiated macrophages and RA synovial fibroblasts for monocyte chemokines. Although IL-17 did not induce CCL5/RANTES, CCL3/MIP-1α, or CX3CL1/fractalkine in the RA synovial fibroblasts or in macrophages, it was effective in inducing CCL2/MCP-1 and CCL20/MIP-3α from both cell types. Consistent with these observations, microarray studies performed employing RA synovial fibroblasts obtained from 6 different patients demonstrated that IL-17 enhances monocyte chemokines including CCL2/MCP-1 and CCL20/MIP-3α.
In the present study, we also show that IL-17 induces production of CCL2/MCP-1 but not CCL20 from the cells present in the peritoneal cavity. We further demonstrate that induction of CCL2/MCP-1 by IL-17 is regulated by activation of PI3K as well as ERK and/or JNK pathways in the different cell types present in the RA joint, and that this is independent of TNF-α and IL-1β induction. In RA synovial tissue fibroblasts, TNF-α and IL-1β induce significantly higher levels of CCL2/MCP-1 compared to IL-17, however these proinflammatory cytokines have similar ability in induction of CCL2/MCP-1 from macrophages. In an in vivo chemotaxis model, CCL2/MCP-1 plays an important role in IL-17-induced monocyte recruitment, since neutralization of CCL2/MCP-1 significantly reduced IL-17 mediated monocyte chemotaxis into the peritoneal cavity. Further, CCL2/MCP-1 is present in ankles that locally express IL-17 and immunoneutralization of IL-17 and CCL2/MCP-1 in RA synovial fluids demonstrates similar effects on monocyte chemotaxis as immunoneutralization of each one alone, indicating that both factors may induce monocyte migration through the same pathway. These results suggest that IL-17 activated monocyte migration in the RA joint may be in part due to induction of CCL2/MCP-1. Hence, therapies directed against IL-17 and its downstream molecules may be beneficial for RA treatment.
MATERIALS AND METHODS
RA synovial tissue fibroblast and macrophage isolation and culture
The studies were approved by the Northwestern University Institutional Review Board, and all donors gave informed written consent. RA and normal synovial tissue fibroblasts were isolated from fresh synovial tissues by mincing and digesting in a solutionof dispase, collagenase, and DNase (20, 21). Cells were used between passages 3–9. RA and normal synovial tissue fibroblasts were treated with IL-17 for 0–8h for mRNA studies and cell supernatants were harvested after 24h for protein studies. Monocytes were separated from buffy coats (Lifesource, Chicago, IL) obtained from healthy donors. Mononuclear cells, isolated by Histopaque (Sigma-Aldrich, St. Louis, MO) gradient centrifugation, were separated by countercurrent centrifugal elutriation. Monocytes were used for chemotaxis or allowed to differentiate to macrophages as previously described (22, 23). Macrophages were treated for 0–8h for mRNA studies and 24h for protein studies.
Real-time RT-PCR
Total cellular RNA was extracted from macrophages and RA synovial fibroblasts treated with IL-17 (0–8h) using Trizol (Invitrogen, Carlsbad, CA). Both cell types were either untreated or preincubated with IgG, anti-TNF-α (10μg/ml, R&D) or anti-IL-1β antibodies (10μg/ml, R&D) for 1h prior to being stimulated with IL-17 for 4–6h. RA synovial tissue fibroblasts and macrophages were either unstimulated (PBS) or activated with IL-17(50 ng/ml), TNF-α (10 ng/ml) and IL-1β(10 ng/ml) for 4–6h. Subsequently, reverse transcription and real-time RT-PCR were performed as previously described (7, 24). Relative gene expression was determined by the ΔΔCt method, and results were expressed as fold increase above the 0 time point.
IL-17 signaling pathways in RA ST fibroblasts and macrophages
RA synovial tissue fibroblasts and macrophages (2×106/ml) were untreated or treated with IL-17 (50 ng/ml) for 0 to 120 min. Cell lysates were examined by Western blot analysis as previously described (25). Blots were probed with phospho (p)-ERK, p-JNK, p-p38 MAPK, and p-AKT (Cell Signaling; 1:1000 dilution) overnight and after stripping, were probed with ERK, JNK, p38 and AKT (Cell Signaling; 1:3000 dilution) for 1h.
Inhibition of the signaling pathways in RA synovial tissue fibroblasts and macrophages
To define which signaling pathways mediate IL-17-induced CCL2/MCP-1 secretion, RA synovial fibroblasts and macrophages were incubated with inhibitors to p38 (SB203580; 10 μM), JNK (SP600125; 10 μM), ERK (PD98059; 10 μM) and PI3K (LY294002; 10 μM) for 1 hour in 10% RPMI. Cells were subsequently activated with IL-17 (50 ng/ml) for 24h and the media was collected in order to quantify the levels of CCL2/MCP-1 employing ELISA.
siRNA studies in RA synovial tissue fibroblasts and macrophages
To confirm that specific chemical inhibitors suppress IL-17-induced CCL2/MCP-1 production, human ERK, JNK (only in RA synovial tissue fibroblasts), p38 MAPK and AKT specific and non-specific control (non-targeting siRNA pool) silencing RNA oligonucleotides (siRNA) (Dharmacon, Inc., Lafayette, CO) were transfected into RA synovial tissue fibroblasts and macrophages (cultured in 6 well plates) by 4 μl/well of Lipofectamine 2000 (Invitrogen), following the manufacturer’s protocol with several modifications. Immediately prior to transfection, the media was replaced with 2 ml/well fresh RPMI 1640 (no additives). siRNA for ERK, JNK, p38 and AKT or control siRNA were transfected at a final concentration of 100 nM. The transfection reactions were supplemented with 250 μl of FBS at 2h and 2250 μl of 10% FBS in RPMI after 5h. After 48h (RA synovial tissue fibroblasts) or 72h (macrophages), the cells were treated with IL-17 for 0–2h in order to validate the reduction of each specific signaling pathway. Cells were then probed for ERK, JNK, p38 MAPK, or AKT (1:3000 dilution) and reprobed for tubulin (1:3000 dilution). After 24h IL-17 stimulation, CCL2/MCP-1 production was determined by ELISA in RA synovial tissue fibroblasts and macrophages transfected with AKT, ERK, JNK (only for RA synovial tissue fibroblasts), or p38 MAPK specific and non-specific control siRNA.
Ankle homogenization
Ankles were homogenized in a 50-ml conical centrifuge tube containing 1 ml of Complete Mini-protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN) homogenization buffer. Ankle homogenization was completed on ice using a motorized homogenizer, followed by 30 seconds of sonication. Homogenates were centrifuged at 2,000g for 10 minutes, filtered through a 0.45-μm pore-size Millipore filter (Millipore, Bedford, MA), and stored at −80°C until use. The concentration of protein in each tissue lysate was determined by using a bicinchoninic acid assay (Pierce, Rockford, IL) and bovine serum albumin as the standard (26–28).
Cytokine Quantification
Human and mouse CCL2/MCP-1 and mouse IL-17 (R&D Systems, Minneapolis, MN) ELISA kits were used according to the manufacturers’ instructions. The mouse ankle CCL2/MCP-1 and IL-17 levels were normalized to the protein concentration of each ankle.
In vivo study protocol
The animal studies were approved by the Northwestern University Institutional Animal Care And Use Committee. The experiments were performed to examine the effect of IL-17 on monocyte migration in vivo. A recombinant human serotype 5 adenovirus (Ad) expressing murine IL-17 was a kind gift of J. K. Kolls and was constructed as reported previously (29). Six to seven week old C57BL/6 mice were injected intraperitoneally with either 108 PFU murine Ad-IL-17, Ad-CMV control or PBS (only on day 1). Mice were sacrificed on days 1, 2, 3 and 4 (n=5) post injection and the peritoneal lavage was collected in 8 ml PBS for quantifying total number of cells, number of monocyte/macrophages and expression of murine IL-17 and CCL2/MCP-1. In a different experiment, mice were treated with Ad-IL-17 or Ad-CMV control intraperitoneally. The Ad-IL-17 group was treated intraperitoneally with either rabbit serum (Sigma) (n=5) or anti-CCL2/MCP-1 (provided by Dr. Karpus and prepared as reported previously (30)) (n=5) and the Ad-CMV group was treated with rabbit serum (n=5) 2h subsequent to the initial treatment. Mice were sacrificed on days 1 through 3 post injection and the peritoneal lavage was collected for quantifying total number monocyte/macrophages.
For local expression of IL-17 in mouse ankle joints, 4–6 week old C57BL/6 mice were injected intra-articularly with 107 PFU Ad-IL-17 or Ad-CMV control. Ankles were harvested on days 4 and 10 post-Ad-IL-17 injection for ELISA and histological studies. Levels of IL-17 were quantified by ELISA from ankles harvested on day 4 and 10 post Ad-IL-17 injection.
Antibodies and immunohistochemistry
Mouse ankles were decalcified, formalin fixed and paraffin embedded, and sectioned in the pathology core facility of Northwestern University. Mouse ankles were immunoperoxidase-stained using Vector Elite ABC Kits (Vector Laboratories), with diaminobenzidine (Vector Laboratories) as a chromogen. Slides were deparaffinized in xylene for 20 min at room temperature, followed by rehydration by transfer through graded alcohols. Antigens were unmasked by first incubating slides in boiling citrate buffer for 20 min. Endogenous peroxidase activity was blocked by incubation with 3% H2O2 for 5 min. Nonspecific binding of avidin and biotin was blocked using an avidin/biotin blocking kit (Vector Laboratories). Nonspecific binding of antibodies to the tissues was blocked by pretreatment of tissues in DakoCytomation Protein Block for 30 min. Tissues were incubated with rat anti-mouse F480 (1/250 dilution; Serotec), or a rat IgG control antibody (eBioscience). Slides were counterstained with Harris hematoxylin. Each slide was evaluated by a blinded observer (27, 28, 31, 32) (A.M.M.). For F480 immunostaining, synovial tissue was graded by a scale, scored 0–100%, in which 0% indicates no stainingand 100% indicates that all cells were immunoreactive. Score data were pooled, and the mean ± SEM was calculated in each data group, (n=5).
Flow cytometry
These studies were performed to quantify the number of monocyte/macrophages induced by Ad-IL-17 injection in the peritoneum compared to the controls. Cells in the lavage were collected, washed [0.5% bovine serum albumin (BSA) in PBS] and blocked by Fc blocker (CD16/CD32) (Pharmingen, San Jose, CA). Cells were thereafter stained with PE conjugated F480 (Serotec, NC), FITC labeled CD11b (Serotec) and PE-CY7 conjugated Gr1 (eBioscience, San Diego, CA) or isotype control antibodies (eBioscience) for 30 min. Cells were subsequently washed twice and resuspended in 0.5% BSA in PBS. The total number of leukocytes recruited into the peritoneal lavage in each treatment group was also quantified. Final results are shown as the total number of monocytes/macrophages (% monocytes/100 × total number of cells) which are determined by the fraction of total cells that met the criteria of F480 and CD11b positivity and Gr1 negativity.
Monocyte chemotaxis
Monocyte chemotaxis was performed in order to examine the role of IL-17 and/or CCL2/MCP-1 in RA synovial fluid induced monocyte migration. For this purpose, chemotaxis was performed in triplicate for 2 hours in Boyden chambers (Neuroprobe) using Formyl-Met-Leu-Phe (FMLP) (100nM) (Sigma) as the positive control and PBS as negative control (33). Chemotaxis induced by RA synovial fluids was examined following incubation of fluids (n=6 fluids) with control IgG, anti-IL-17, anti CCL2/MCP-1 or both antibodies (10μg/ml) for 1h prior to performing the assay(19).
Statistical Analysis
The data was analyzed using 2-tailed Student’s t tests for paired and unpaired samples or Fisher exact test where appropriate. P values < 0.05 were considered significant.
RESULTS
IL-17 induces CCL20/MIP-3α and CCL2/MCP-1 from RA synovial fibroblasts and macrophages
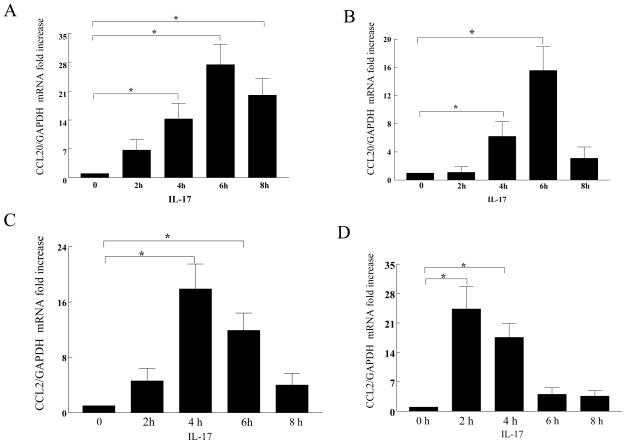
Employing both in vitro and in vivo assays, we observed that IL-17 is chemotactic for monocytes (19). Since not all the in vivo monocyte chemotaxis could be attributed to the direct effect of IL-17, we hypothesized that the indirect effect of IL-17 may be through induction of monocyte chemokines from adjacent cells. Since synovial fibroblasts and macrophages are important in the pathogenesis of RA, synovial fibroblasts and macrophages differentiated in vitro from normal monocytes were employed in order to determine which monocyte chemokines might be induced by IL-17. The mRNA expression for CCL5/RANTES, CCL3/MIP-1α, CX3CL1/Fractalkine, CCL20/MIP-3α and CCL2/MCP-1 was determined in IL-17 treated macrophages and RA synovial fibroblasts. Although IL-17 did not induce CCL5/RANTES, CCL3/MIP-1α, or CX3CL1/fractalkine in RA synovial fibroblasts or normal macrophages, CCL20/MIP-3α (Figure 1A–B) and CCL2/MCP-1 (Figure 1C–D) were induced in both cell types.
Figure 1. CCL20/MIP-3α mRNA is induced in IL-17 activated RA synovial fibroblasts and macrophages.
RA synovial fibroblasts (A,C) and normal macrophages (B,D) were activated with IL-17 (50 ng/ml) for 0 to 8h. Real-time RT-PCR was employed to identify CCL20/MIP-3α (A,B) and CCL2/MCP-1 (C,D), which was normalized to GAPDH. The results are presented as fold increase compared with the 0 time point (untreated cells), and represent the mean ± SE. * represents p <0.05, (n=3–5).
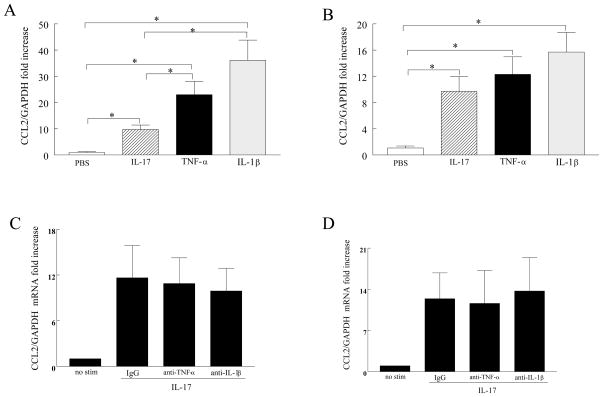
TNF-α and IL-1β are more potent in inducing CCL2/MCP-1 expression in RA fibroblasts compared to IL-17 and IL-17 mediated CCL2/MCP-1 expression is independent of TNF-α and IL-1β production
To compare the potency of IL-17 with TNF-α and IL-1β in induction of CCL2/MCP-1 expression, RA fibroblasts and macrophages were activated with each of the cytokines and the expression of CCL2/MCP-1 was examined by real-time RT-PCR. Results from these experiments demonstrate that while in RA fibroblasts TNF-α and IL-1β induced CCL2/MCP-1 was significantly higher than that detected by IL-17, similar levels of CCL2/MCP-1 were expressed in macrophages that were activated by all three cytokines (Figure 2A–B). Next, we asked whether IL-17-induced TNF-α or IL-1β expression had any effect on CCL2/MCP-1 transcription in RA synovial tissue fibroblasts or normal macrophages. Results from employing neutralizing antibodies to TNF-α and IL-1β demonstrate similar levels of IL-17-induced CCL2/MCP-1 as the IgG control, suggesting that IL-17 mediated CCL2/MCP-1 is independent of TNF-α and IL-1β induction in both cell types (Figure 2C–D).
Figure 2. CCL2/MCP-1 mRNA induced in IL-17 activated RA synovial fibroblasts and macrophages is independent of TNF-α and IL-1β production.
RA synovial fibroblasts (A) and normal macrophages (B) were activated with IL-17 (50 ng/ml), TNF-α (10 ng/ml) or IL-1β (10 ng/ml) for 6 or 4h and CCL2/MCP-1 expression was determined by real-time RT-PCR (n=5–8). RA synovial fibroblasts (C) or normal macrophages (D) were either untreated or treated with IgG, anti-TNF-α (10 μg/ml) or anti-IL-1β (10 μg/ml) prior to being treated with IL-17 (50 ng/ml) for 6 or 4h. CCL2/MCP-1 mRNA was determined by real-time RT-PCR and normalized to GAPDH. The results are presented as fold increase compared with 0 time point (untreated cells) and represent the mean ± SE. * represents p <0.05, (n=3).
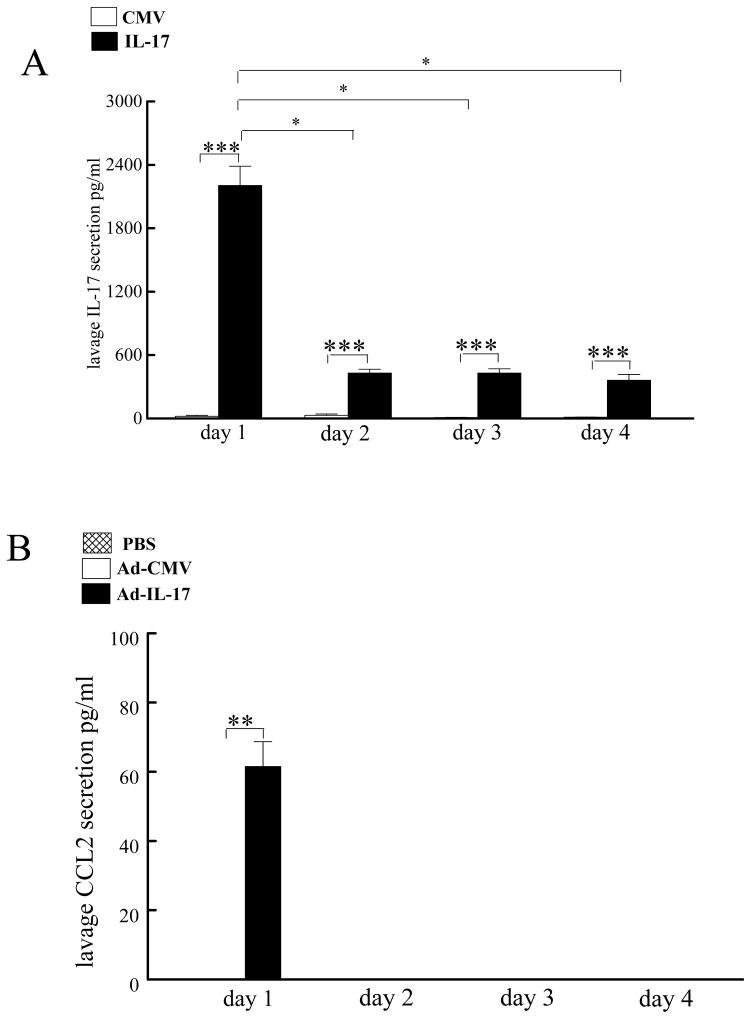
In order to determine which of these chemokines might be relevant in vivo, IL-17 was expressed in the peritoneal spaces of mice (Figure 3A), and the fluids generated were examined by ELISA for the presence of the monocyte chemokines CCL2/MCP-1 (Figure 3B), CCL5/RANTES, CCL3/MIP-1α, or CCL20 (negative data not shown). CCL2/MCP-1 was the only monocyte chemokine detected in the peritoneal lavage following the injection of Ad-IL-17. Since CCL2/MCP-1 was present in vivo following the expression of IL-17 and is a more potent monocyte chemoattractant compared to CCL20/MIP-3α (33), this study is focused on examining the role of CCL2/MCP-1 in IL-17 monocyte chemotaxis as well as investigating the mechanism by which IL-17 activates CCL2/MCP-1 production in cells present in the RA joint.
Figure 3. Adenovirally expressed IL-17 induces CCL2/MCP-1 production from the cells present in the peritoneal cavity.
Ad-IL-17 and/or CMV control (108 PFU) was injected into the peritoneum of mice. Peritoneal lavage was collected to quantify the levels of IL-17 (A) or CCL2/MCP-1 (B) by ELISA. The values represent the mean ± SE. * represents p <0.05 and ** denotes p <0.01 in n= 5 mice per each time point and treatment group.
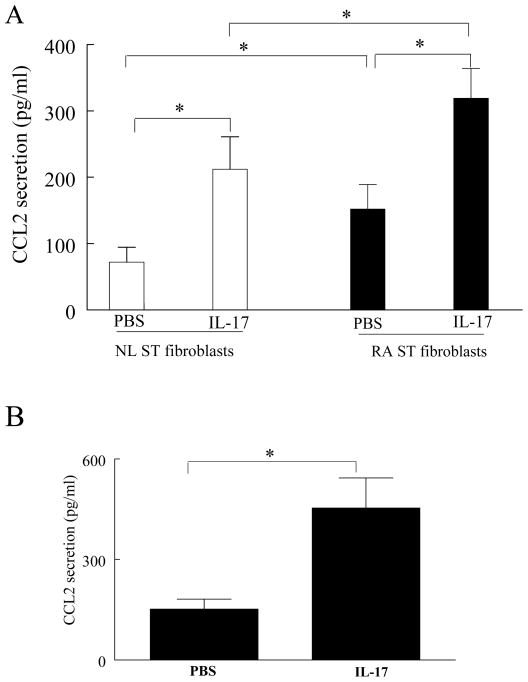
IL-17 induces CCL2/MCP-1 production by RA synovial tissue fibroblasts and macrophages
Based on these results, we asked whether IL-17 induced the secretion of CCL2/MCP-1 in synovial fibroblasts. IL-17 enhanced the secretion of CCL2/MCP-1 from normal and RA synovial fibroblasts. The level of IL-17-induced CCL2/MCP-1 was significantly higher in RA synovial tissue fibroblasts at baseline and following IL-17 treatment compared to normal synovial fibroblasts (Figure 4A).
Figure 4. IL-17 induces CCL2/MCP-1 production in RA synovial tissue fibroblasts and macrophages.
Normal (NL) and RA synovial tissue (ST) fibroblasts (A) or macrophages (B) were treated either with PBS or IL-17 for 24h and the CCL2/MCP-1 levels were detected by ELISA utilizing the conditioned media. Values are the mean ± SE. * represents p <0.05, (n=3–5).
Next, we examined the ability of IL-17 to induce CCL2/MCP-1 in macrophages. IL-17 induced the secretion of CCL2/MCP-1, 2–3 fold (p <0.05) in in vitro differentiated macrophages (Figure 4B), similar to the findings observed with RA synovial fibroblasts. These results demonstrate that IL-17 mediates CCL2/MCP-1 production from synovial fibroblasts and macrophages, cell types important in the pathogenesis of RA.
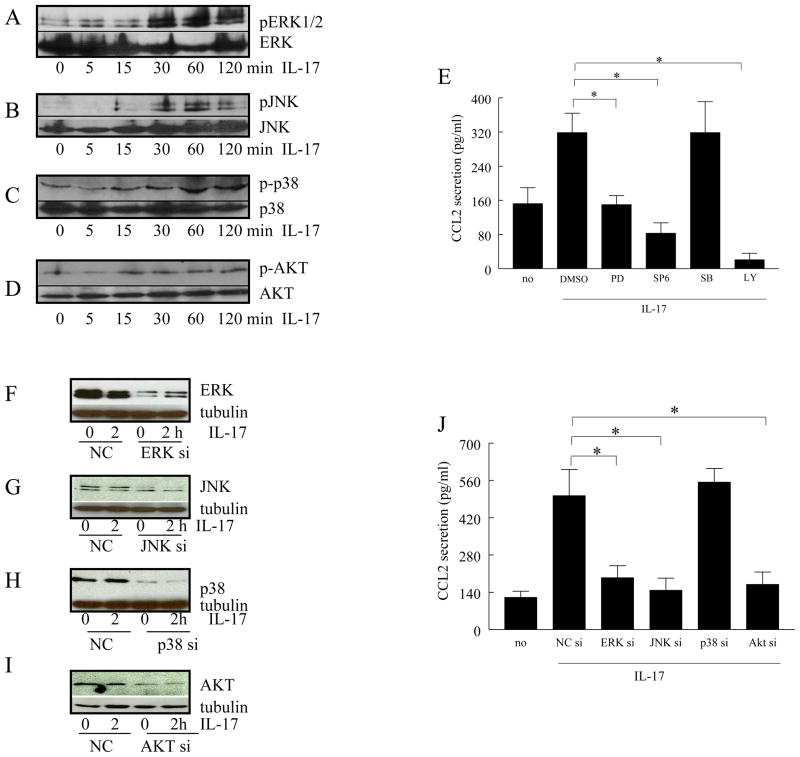
IL-17 induction of CCL2/MCP-1 is mediated by the PI3K, ERK and JNK pathways
To identify the signaling pathways mediating IL-17-induced CCL2/MCP-1, IL-17-activated pathways were examined in RA synovial tissue fibroblasts, by measuring the phosphorylated form of each kinase. The IL-17-mediated activation of the AKT pathway (15 min) occurs prior to that of ERK and JNK (30min) or p38 (60min) (Figure 5A–D). While chemical inhibitors to ERK, JNK, and PI3K suppressed IL-17-induced CCL2/MCP-1 secretion by 50–90% (Figure 5E, p < 0.05), inhibition of p38 did not reduce the levels of CCL2/MCP-1 in RA synovial fibroblasts. Consistent with these observations, the siRNA-mediated forced reduction of AKT-1, ERK and JNK, but not p38, suppressed (p<0.05) IL-17-induced CCL2/MCP-1 production in RA synovial fibroblasts (Figure 5F–J).
Figure 5. In RA synovial tissue fibroblasts, IL-17-induced CCL2/MCP-1 production is modulated by PI3K, ERK and JNK pathways.
In order to determine the mechanism of IL-17 activation in RA synovial tissue fibroblasts, cells were stimulated with IL-17 (50 ng/ml) for 0–120 minutes, and the cell lysates were probed for, p-ERK (A), p-JNK (B), p-p38 (C) or p-AKT (D). To examine which of the signaling pathways were associated with IL-17-induced CCL2/MCP-1 production, RA synovial tissue fibroblasts were untreated or treated with inhibitors to ERK (PD98059; 10 μM), JNK (SP600125; 10 μM), p38 (SB203580; 10 μM), or PI3K (LY294002; 10μM). Cells were subsequently activated with IL-17 (50 ng/ml) for 24h and the conditioned media was collected in order to quantify the levels of CCL2/MCP-1 employing ELISA (E). To determine that siRNA for each signaling pathway could effectively reduce the IL-17 signaling, siRNA for ERK, JNK, p38, AKT, or control siRNA were transfected. After 48h, the cells were treated with IL-17 for 0–2h. Cells were then probed for ERK (F), JNK (G), p38 MAPK (H), or AKT (I) (1:3000 dilution) and reprobed for tubulin (1:3000 dilution). IL-17-induced CCL2/MCP-1 production was determined by ELISA in RA synovial tissue fibroblasts transfected with siRNA against ERK, JNK, p38 MAPK, AKT, or specific or non-specific control siRNA (J). These results are representative of 3 experiments.
Similar studies were performed with macrophages in order to determine which IL-17 activated signaling pathways were responsible for CCL2/MCP-1 production. Unlike RA synovial fibroblasts, in macrophages IL-17 activated both p38 and PI3K pathways as early as 5 min, while ERK was phosphorylated later, at 60min (Figs. 6A–C). Employing chemical inhibitors, suppression of the PI3K and ERK pathways significantly (p<0.05) reduced IL-17- induced CCL2/MCP-1 production in macrophages. Consistent with these observations, the siRNA-mediated reduction of AKT-1 and ERK suppressed IL-17 activation by 70% and 50%, respectively (p<0.05), while the reduction of p38 had no effect (Figs. 6E–H). Collectively, our results suggest that while PI3K and ERK pathways modulate IL-17-induced CCL2/MCP-1 production in both macrophages and RA synovial fibroblasts, activation of JNK by IL-17 plays a role in CCL2/MCP-1 secretion in RA synovial fibroblasts.
Figure 6. PI3K, ERK activation regulates IL-17-induced CCL2/MCP-1 production in macrophages.
In order to determine the mechanism of IL-17 in macrophages, cells were stimulated with IL-17 (50 ng/ml) for 0–120 minutes, and the cell lysates were probed for, p-ERK (A), p-p38 (B) or p-AKT (C). To examine which of the signaling pathways were associated with IL-17-induced CCL2/MCP-1 production, macrophages were untreated or treated inhibitors to ERK (PD98059; 10 μM), p38 (SB203580; 10 μM), or PI3K (LY294002; 10 μM). Cells were subsequently activated with IL-17 (50 ng/ml) for 24h and the conditioned media was collected in order to quantify the levels of CCL2/MCP-1 employing ELISA (D). To determine that siRNA for each signaling pathway could effectively reduce the IL-17 signaling, siRNA for ERK, p38, AKT, or control siRNA were transfected into macrophages. Cells were harvested after 72h and then probed for ERK (E), p38 MAPK (F), or AKT (G) (1:3000 dilution) and reprobed for tubulin (1:3000 dilution). IL-17-induced CCL2/MCP-1 production was determined by ELISA in macrophages transfected with siRNA against ERK, p38 MAPK, AKT, or specific or non-specific control siRNA (H). These results are representative of 3 experiments.
CCL2/MCP-1 production contributes to IL-17-mediated monocyte migration into the peritoneal cavity
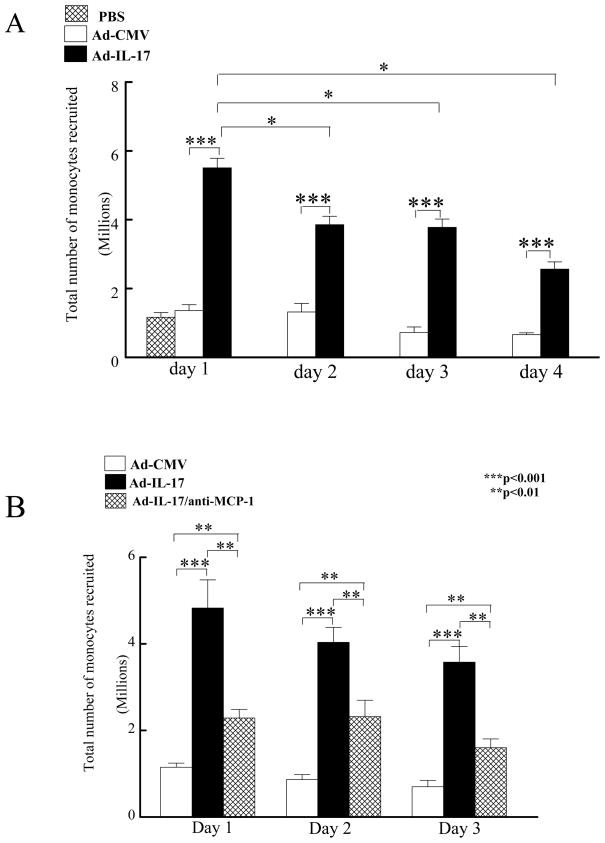
We chose to examine the role of CCL2/MCP-1 in IL-17 induced chemotaxis in an in vivo chemotaxis model in the peritoneal cavity rather than the ankle joints because of the ability to utilize flow cytometry, which is a more quantitative technique compared to histology. For this purpose, mice were injected intraperitoneally with the Ad-IL-17 or the Ad-CMV control vector. Injection of Ad-IL-17 significantly increased (p<0.005) the total number of monocytes recruited into the peritoneum on days 1 through 4 after injection Ad-IL-17 compared to the Ad-CMV and PBS controls (Figure 7A). Of interest, the total number of monocytes migrating to the peritoneum was significantly greater on day 1 post Ad-IL-17 injection (p<0.005) compared with days 2 through 4 (Figure 7A). This observation was consistent with higher levels of peritoneal IL-17 expression on day 1 (5–6 fold increase; p<0.001) compared to days 2–4 (Figure 3A), suggesting that the pattern of monocyte migration is similar to expression levels of IL-17 in the peritoneal cavity.
Figure 7. Neutralization of CCL2/MCP-1 reduces IL-17-mediated peritoneal monocyte recruitment.
PBS, Ad-IL-17 or CMV control (108 PFU) was injected into the peritoneum of mice. Peritoneal lavage was collected to quantify total number of monocytes recruited on days 1, 2, 3 and 4 post injection (A). Next, the Ad-IL-17 group was treated intraperitoneally with either rabbit serum or anti-CCL2/MCP-1 and the CMV group was treated with rabbit serum for 2h subsequent to the initial treatment. Mice were sacrificed on days 1, 2, or 3 post injection and the peritoneal lavage was collected for quantifying the total number monocytes/macrophages (B). The values represent the mean ± SE. * represents p <0.05 and ** denotes p <0.01 and *** denotes p<0.001. n= 5 mice per each time point and treatment group, (n=5 mice).
Experiments were performed to determine the role of CCL2/MCP-1 in IL-17-mediated monocyte migration in vivo. Intraperitoneal injection of neutralizing antibody to CCL2/MCP-1 significantly reduced IL-17-mediated monocyte migration (p<0.05) compared to control injection (Figure 7B). These observations suggest that IL-17-mediated monocyte migration was mediated, in part, through the induction of CCL2/MCP-1.
Local expression of IL-17 in mouse ankles upregulates monocyte migration and CCL2/MCP-1 levels
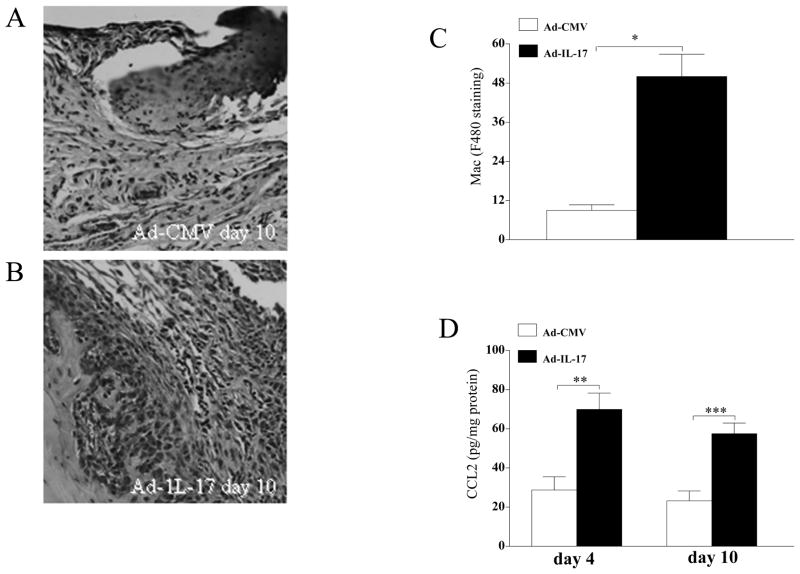
Local expression of IL-17 using an adenoviral vector (107 PFU) resulted in increased inflammation, synovial lining thickness, and bone erosion in the ankles of C57/BL6 mice compared to Ad-CMV-infected controls (107 PFU) (data not shown). The Ad-IL-17-treated group demonstrated significantly greater ankle circumference (data not shown) on days 4 and 10 post-injection compared to the control group. Further, the concentration of IL-17 in the ankles of the IL-17-induced arthritis model was 1200 pg/mg on day 4 and 400 pg/mg on day 10 post Ad injection. F480 staining of ankles harvested from day 10 post-injection showed that Ad-IL-17-treated mice have significantly higher macrophage staining compared with the control group (Figure 8A–C). Further CCL2/MCP-1 levels were markedly increased in the Ad-IL-17 group on days 4 and 10 post injection compared with the control treatment group (Figure 8D). These results suggest that IL-17 may be important for monocyte migration both directly and indirectly through induction of CCL2/MCP-1.
Figure 8. Local expression of IL-17 increases monocyte migration and CCL2/MCP-1 expression in mouse ankles.
Ad-IL-17 or Ad-CMV control was injected intra-articularly into the ankle joints of 4–6 week old C57BL/6 mice. Ankles at day 10 post-Ad injection were harvested, paraffin embedded and decalcified. Macrophages in the ankle were identified employing F480 staining. Control ankles (A) had significantly lower macrophage staining compared to ankles locally expressing IL-17 (B). C. Quantification of F480 staining in the Ad-CMV control and Ad-IL-17 groups. D. CCL2/MCP-1 levels were quantified from ankle homogenates harvested from day 4 and 10 post Ad-IL-17 or Ad-CMV control treatment and normalized to protein concentration. Values express mean ± SE, n=5. * denotes p<0.05 and ** denotes p<0.01.
Neutralization of IL-17 does not enhance the ability of anti-CCL2/MCP-1 to reduce RA synovial fluid-mediated monocyte migration
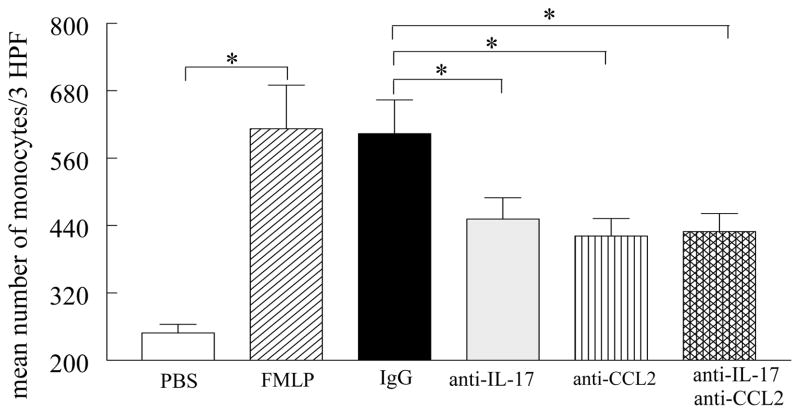
Experiments were performed to determine whether anti-IL-17 would synergize with anti-CCL2/MCP-1 in reducing RA synovial fluid mediated monocyte chemotaxis. The concentrations of IL-17 and CCL2/MCP-1 in the 6 RA synovial fluids utilized for monocyte chemotaxis were 149±27 pg/ml and 1268±314 pg/ml respectively, and our data from these experiments show that immunodepletion of RA synovial fluid for both IL-17 and CCL2/MCP-1 had similar effects on monocyte chemotaxis (Figure 9) as neutralization of each factor alone, suggesting that both of these proinflammatory mediators induce monocyte migration through the same signaling pathway.
Figure 9. IL-17 and/or CCL2/MCP-1 immunodepleted RA synovial fluid had similar effects on monocyte chemotaxis.
RA synovial fluids from 6 patients (1:20 dilution) were incubated with antibodies to IL-17 (10 μg/ml), CCL2/MCP-1 (10 μg/ml), or both as well as isotype control or PBS or FMLP for 1h prior to performing monocyte chemotaxis in response to RA synovial fluids. The values represent the mean ± SE. * represents p <0.05.
DISCUSSION
Recently, we showed that IL-17 mediates monocyte migration in vivo into sponges implanted subcutaneously into severe combined immunodeficient (SCID) mice, and that it is capable of directly mediating monocyte migration in vitro and in rheumatoid arthritis (19). We hypothesized that direct effects of IL-17 might not account for all of its chemotactic ability in vivo. In this study, we demonstrate that IL-17 induces CCL2/MCP-1 production from RA synovial tissue fibroblasts and control macrophages independently of TNF-α and IL-1β, supporting an indirect role of IL-17 in monocyte migration. In macrophages, all three cytokines have similar ability in induction of CCL2/MCP-1 however TNF-α– and IL-1β–induced CCL2/MCP-1 was significantly higher than that induced by IL-17 employing RA fibroblasts. In fibroblasts, activation of PI3K, JNK, and ERK pathways is responsible for IL-17-induced CCL2/MCP-1 production whereas in IL-17 activated macrophages, PI3K and ERK regulate CCL2/MCP-1 production. Further, in this study we demonstrate that CCL2/MCP-1 production contributes to IL-17-mediated monocyte migration into the peritoneal cavity, suggesting that CCL2/MCP-1 plays an important role in IL-17-mediated monocyte migration in vivo. We also show that local expression of IL-17 in mouse ankle joints increases monocyte trafficking and levels of CCL2/MCP-1. Finally, we demonstrate that antibodies against both IL-17 and CCL2/MCP-1 reduce RA synovial fluid mediated monocyte migration to similar levels as each antibody alone.
IL-17 plays a crucial role in regulating neutrophil recruitment. Intra-articular injection of IL-17 enhances neutrophil migration into the joints of mice (3). In the rat airway, IL-17 mediates neutrophil recruitment via induction of IL-8 (CINC-1; CXCL1)(34). Neutrophil chemotaxis caused by conditioned media from IL-17-stimulated gastric epithelial cells was inhibited by neutralizing antibody to IL-8 but not to IL-17 (35). Consistent with these findings, we found that neutrophil migration was increased in the peritoneal cavity following Ad-IL-17 injection on days 1 and 2 (data not shown).
In our in vivo chemotaxis model, adenovirally expressed IL-17 resulted in upregulation of CCL2/MCP-1 on day 1 post-injection. In contrast, other potential monocyte chemoattractants including CCL20/MIP-3α, IL-6, TNF-α, CCL3/MIP-1α, CCL5/RANTES were undetectable in the peritoneal lavage, identifying a key role of CCL2/MCP-1 in IL-17 mediated monocyte chemotaxis in vivo. While CCL20/MIP-3α was undetecteable in the peritoneal cavity following local expression of IL-17, it is possible that this chemokine may have an important role in monocyte migration to RA joints since CCL20 is induced by IL-17 in macrophages and RA fibroblasts.
Our data characterizes the pathways mediating IL-17-induced CCL2/MCP-1 expression. In macrophages and RA synovial fibroblasts, IL-17 activates ERK, p38 and AKT pathways and also JNK in RA fibroblasts only. Suppression of ERK or PI3K in both cell types and JNK in fibroblasts only, employing chemical inhibitors or siRNA, was effective in reducing IL-17-mediated CCL2/MCP-1. Previous studies have shown that PI3K is the major pathway involved in IL-17 induction of proinflammatory mediators such as IL-6, IL-8 and CXCL12/SDF-1 in RA synovial fibroblasts, however activation of p38 was not implicated in the process (36, 37). Similarly, in RA fibroblasts other proinflammatory cytokines such as IL-18 modulate CCL2/MCP-1 production through activation of PI3K and JNK but not through p38 MAPK (38). Consistent with our observations in fibroblasts and macrophages, activation of ERK but not p38 MAPK was essential for M. tuberculosis H37Rv-induced CCL2/MCP-1 secretion by monocytes (39). Collectively, the data suggest that while PI3K, ERK and JNK contribute to IL-17-mediated CCL2/MCP-1 production, p38 does not play a role.
In the present study, we found that the total number of monocytes recruited into the peritoneal cavity was significantly increased by IL-17. However, the total number of monocytes recruited was significantly greater on day 1 compared to day 2–4, and this may be due to higher levels of IL-17 as well as CCL2/MCP-1 in the peritoneal cavity on day 1. It has been shown that intraperitoneal injection of TLR4 ligand, LPS, or thioglycolate can also induce CCL2/MCP-1 production as early as 15h to 24h (40, 41). Our data also suggest that CCL2/MCP-1 plays an important role in IL-17-mediated monocyte migration, since inhibition of CCL2/MCP-1 suppresses this effect. However, no other potential monocyte chemoattractants were detectable in the peritoneal lavage following infection with Ad-IL-17, suggesting that the effects of IL-17 on monocyte chemotaxis may be also due to a direct effect on monocytes as recently described (19). Monocytes in the circulation may be attracted to the higher gradient of IL-17 present in the peritoneal cavity. Monocytes use LFA1 (αLβ2) and VLA4 (α4β1), which bind to intracellular adhesion molecule (ICAM)1 and vascular cell adhesion molecule (VCAM)1 on the endothelial cells for adhesion and migration in vivo (42–44). We have shown that IL-17 can induce VCAM1 from human microvascular endothelial cells (HMVECs) (unpublished data) and others have demonstrated that IL-17 can activate production of ICAM1 (45). Therefore, these adhesion molecules may be important for facilitating IL-17 induced monocyte migration in vivo.
Consistent with the data from the peritoneal cavity, forced expression of IL-17 in the mouse ankle joints upregulated CCL2/MCP-1 concentration, indicating that CCL2/MCP-1 is an important down stream target of IL-17 since it was produced in vitro in cell types present in RA synovium as well as in vivo in the mouse peritoneal cavity and ankle joints. Interestingly, the expression patterns of CCL2/MCP-1 in the peritoneal cavity and the joints were different and this may be due to local differences of cell types and the area for distribution of IL-17. Specifically, the concentration of CCL2/MCP-1 became undetectable in the peritoneal cavity on day 2 to 4, while it remained elevated on days 4 and 10 in the IL-17-induced arthritis model. In the IL-17-induced arthritis model, CCL2/MCP-1 may be produced by synovial fibroblasts and macrophages, allowing for more prolonged expression. However, the structure of the peritoneal cavity is quite different and lacks synovial fibroblasts. The second reason is that the movement of the cells and the area for diffusion of IL-17 is much greater in the mouse peritoneal cavity compared to mouse ankles, as shown by our data IL-17 levels drops from 2000 pg/ml (on day 1) to 400 pg/ml (day 2) in a matter of a day however, in the Ad-IL-17-induced arthritis model, the level of IL-17 is reduced from 1200 pg/mg (day 4) to 400pg/mg (day 10) over a 6 day period.
The Ad-IL-17 induced arthritis model was associated with increased joint macrophage levels, which may be due to expression of IL-17 and/or CCL2/MCP-1 in the mouse ankles. Experiments performed to determine the importance of IL-17 and CCL2/MCP-1 in RA synovial fluid mediated monocyte trafficking revealed that both factors were equally important and there was no synergistic effect when both proinflammatory mediators were neutralized. Interestingly, inhibition of multiple factors in RA synovial fluid does not have additive or synergistic effect on suppressing monocyte chemotaxis and this may be due to the fact that several of the chemotactic mediators utilize the same pathways and inhibition of one can disturb the equilibrium or that inhibition of monocyte migration can not be detected since it is within the bell curve. Similar to our previously reported results with IL-17(19), CCL2/MCP-1 induced monocyte migration is through activation of the p38 pathway (46). A recent paper demonstrates that chemokines competing for similar receptors or using similar signaling pathways do not synergize in monocyte chemotaxis (47). This lack of synergy is totally consistent with other reports (48), which have shown that neutralization of several factors in RA synovial fluid does not reduce RA synovial fluid-mediated migration beyond the effect noted with each factor alone.
In summary, the observations presented here support the role of CCL2/MCP-1 in promoting IL-17 mediated chemotaxis. Together with our recent observation that IL-17 is also directly chemotactic for monocytes (19), these data support monocyte migration as an important mechanism by which IL-17 contributes to chronic inflammation, as observed in patients with RA. Given the importance of monocyte derived macrophages in chronic inflammation (49), these data further support the role of IL-17 as a potential therapeutic target in RA.
Abbreviations used in this paper
- RA
rheumatoid arthritis
- OA
osteoarthritis
- COX2
cyclooxygenase 2
- PG
prostaglandin
- ICAM
intracellular adhesion molecule
- VCAM
vascular cell adhesion molecule
Footnotes
This work was supported in part by awards from the National Institutes of Health (AR056099, AR055240, AR048269 and NS34510), Arthritis National Research Foundation and grants from Within Our Reach from The American College of Rheumatology.
References
- 1.Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, van den Berg WB. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 2.Koenders MI, Lubberts E, van de Loo FA, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Kolls JK, Di Padova FE, Joosten LA, van den Berg WB. Interleukin-17 acts independently of TNF-alpha under arthritic conditions. J Immunol. 2006;176:6262–6269. doi: 10.4049/jimmunol.176.10.6262. [DOI] [PubMed] [Google Scholar]
- 3.Lubberts E, Joosten LA, Oppers B, van den Bersselaar L, Coenen-de Roo CJ, Kolls JK, Schwarzenberger P, van de Loo FA, van den Berg WB. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol. 2001;167:1004–1013. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- 4.Koenders MI, Lubberts E, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Kolls JK, Joosten LA, van den Berg WB. Induction of cartilage damage by overexpression of T cell interleukin-17A in experimental arthritis in mice deficient in interleukin-1. Arthritis Rheum. 2005;52:975–983. doi: 10.1002/art.20885. [DOI] [PubMed] [Google Scholar]
- 5.Stamp LK, James MJ, Cleland LG. Interleukin-17: the missing link between T-cell accumulation and effector cell actions in rheumatoid arthritis? Immunology and cell biology. 2004;82:1–9. doi: 10.1111/j.1440-1711.2004.01212.x. [DOI] [PubMed] [Google Scholar]
- 6.Lubberts E, Koenders MI, van den Berg WB. The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res Ther. 2005;7:29–37. doi: 10.1186/ar1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahrara S, Huang Q, Mandelin AM, 2nd, Pope RM. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R93. doi: 10.1186/ar2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 10.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008 doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz Y, Nadiv O, Beer Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: a possible role as a “fine-tuning cytokine” in inflammation processes. Arthritis Rheum. 2001;44:2176–2184. doi: 10.1002/1529-0131(200109)44:9<2176::aid-art371>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chabaud M, Page G, Miossec P. Enhancing Effect of IL-1, IL-17, and TNF-alpha on Macrophage Inflammatory Protein-3alpha Production in Rheumatoid Arthritis: Regulation by Soluble Receptors and Th2 Cytokines. J Immunol. 2001;167:6015–6020. doi: 10.4049/jimmunol.167.10.6015. [DOI] [PubMed] [Google Scholar]
- 15.Kehlen A, Thiele K, Riemann D, Langner J. Expression, modulation and signalling of IL-17 receptor in fibroblast-like synoviocytes of patients with rheumatoid arthritis. Clin Exp Immunol. 2002;127:539–546. doi: 10.1046/j.1365-2249.2002.01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 17.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Reboul P, He Y, Jolicoeur FC, Pelletier JP. Modulation of TIMP-1 synthesis by antiinflammatory cytokines and prostaglandin E2 in interleukin 17 stimulated human monocytes/macrophages. J Rheumatol. 2001;28:712–718. [PubMed] [Google Scholar]
- 18.Jovanovic DV, Martel-Pelletier J, Di Battista JA, Mineau F, Jolicoeur FC, Benderdour M, Pelletier JP. Stimulation of 92-kd gelatinase (matrix metalloproteinase 9) production by interleukin-17 in human monocyte/macrophages: a possible role in rheumatoid arthritis. Arthritis Rheum. 2000;43:1134–1144. doi: 10.1002/1529-0131(200005)43:5<1134::AID-ANR24>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Shahrara S, Pickens SR, Dorfleutner A, Pope RM. IL-17 induces monocyte migration in rheumatoid arthritis. J Immunol. 2009;182:3884–3891. doi: 10.4049/jimmunol.0802246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Georganas C, Liu H, Perlman H, Hoffmann A, Thimmapaya B, Pope RM. Regulation of IL-6 and IL-8 expression in rheumatoid arthritis synovial fibroblasts: the dominant role for NF-kappa B but not C/EBP beta or c-Jun. J Immunol. 2000;165:7199–7206. doi: 10.4049/jimmunol.165.12.7199. [DOI] [PubMed] [Google Scholar]
- 21.Perlman H, Bradley K, Liu H, Cole S, Shamiyeh E, Smith RC, Walsh K, Fiore S, Koch AE, Firestein GS, Haines GK, 3rd, Pope RM. IL-6 and matrix metalloproteinase-1 are regulated by the cyclin-dependent kinase inhibitor p21 in synovial fibroblasts. J Immunol. 2003;170:838–845. doi: 10.4049/jimmunol.170.2.838. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Ma Y, Pagliari LJ, Perlman H, Yu C, Lin A, Pope RM. TNF-alpha-induced apoptosis of macrophages following inhibition of NF-kappa B: a central role for disruption of mitochondria. J Immunol. 2004;172:1907–1915. doi: 10.4049/jimmunol.172.3.1907. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y, Liu H, Tu-Rapp H, Thiesen HJ, Ibrahim SM, Cole SM, Pope RM. Fas ligation on macrophages enhances IL-1R1-Toll-like receptor 4 signaling and promotes chronic inflammation. Nat Immunol. 2004;5:380–387. doi: 10.1038/ni1054. [DOI] [PubMed] [Google Scholar]
- 24.Shahrara S, Park CC, Temkin V, Jarvis JW, Volin MV, Pope RM. RANTES modulates TLR4-induced cytokine secretion in human peripheral blood monocytes. J Immunol. 2006;177:5077–5087. doi: 10.4049/jimmunol.177.8.5077. [DOI] [PubMed] [Google Scholar]
- 25.Pagliari LJ, Perlman H, Liu H, Pope RM. Macrophages require constitutive NF-kappaB activation to maintain A1 expression and mitochondrial homeostasis. Mol Cell Biol. 2000;20:8855–8865. doi: 10.1128/mcb.20.23.8855-8865.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahrara S, Amin MA, Woods JM, Haines GK, Koch AE. Chemokine receptor expression and in vivo signaling pathways in the joints of rats with adjuvant-induced arthritis. Arthritis Rheum. 2003;48:3568–3583. doi: 10.1002/art.11344. [DOI] [PubMed] [Google Scholar]
- 27.Shahrara S, Proudfoot AE, Woods JM, Ruth JH, Amin MA, Park CC, Haas CS, Pope RM, Haines GK, Zha YY, Koch AE. Amelioration of rat adjuvant-induced arthritis by Met-RANTES. Arthritis Rheum. 2005;52:1907–1919. doi: 10.1002/art.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shahrara S, Proudfoot AE, Park CC, Volin MV, Haines GK, Woods JM, Aikens CH, Handel TM, Pope RM. Inhibition of monocyte chemoattractant protein-1 ameliorates rat adjuvant-induced arthritis. J Immunol. 2008;180:3447–3456. doi: 10.4049/jimmunol.180.5.3447. [DOI] [PubMed] [Google Scholar]
- 29.Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, Spriggs M, Kolls JK. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- 30.Karpus WJ, Kennedy KJ, Kunkel SL, Lukacs NW. Monocyte chemotactic protein 1 regulates oral tolerance induction by inhibition of T helper cell 1-related cytokines. J Exp Med. 1998;187:733–741. doi: 10.1084/jem.187.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruth JH, Volin MV, Haines GK, III, Woodruff DC, Katschke KJ, Jr, Woods JM, Park CC, Morel JCM, Koch AE. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis Rheum. 2001;44:1568–1581. doi: 10.1002/1529-0131(200107)44:7<1568::AID-ART280>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 32.Koch AE, Nickoloff BJ, Holgersson J, Seed B, Haines GK, Burrows JC, Leibovich SJ. 4A11, a monoclonal antibody recognizing a novel antigen expressed on aberrant vascular endothelium. Upregulation in an in vivo model of contact dermatitis. Am J Pathol. 1994;144:244–259. [PMC free article] [PubMed] [Google Scholar]
- 33.Ruth JH, Shahrara S, Park CC, Morel JC, Kumar P, Qin S, Koch AE. Role of macrophage inflammatory protein-3alpha and its ligand CCR6 in rheumatoid arthritis. Lab Invest. 2003;83:579–588. doi: 10.1097/01.lab.0000062854.30195.52. [DOI] [PubMed] [Google Scholar]
- 34.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 35.Luzza F, Parrello T, Monteleone G, Sebkova L, Romano M, Zarrilli R, Imeneo M, Pallone F. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J Immunol. 2000;165:5332–5337. doi: 10.4049/jimmunol.165.9.5332. [DOI] [PubMed] [Google Scholar]
- 36.Hwang SY, Kim JY, Kim KW, Park MK, Moon Y, Kim WU, Kim HY. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-kappaB- and PI3-kinase/Akt-dependent pathways. Arthritis Res Ther. 2004;6:R120–128. doi: 10.1186/ar1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim KW, Cho ML, Kim HR, Ju JH, Park MK, Oh HJ, Kim JS, Park SH, Lee SH, Kim HY. Up-regulation of stromal cell-derived factor 1 (CXCL12) production in rheumatoid synovial fibroblasts through interactions with T lymphocytes: role of interleukin-17 and CD40L-CD40 interaction. Arthritis Rheum. 2007;56:1076–1086. doi: 10.1002/art.22439. [DOI] [PubMed] [Google Scholar]
- 38.Amin MA, Mansfield PJ, Pakozdi A, Campbell PL, Ahmed S, Martinez RJ, Koch AE. Interleukin-18 induces angiogenic factors in rheumatoid arthritis synovial tissue fibroblasts via distinct signaling pathways. Arthritis Rheum. 2007;56:1787–1797. doi: 10.1002/art.22705. [DOI] [PubMed] [Google Scholar]
- 39.Song CH, Lee JS, Lee SH, Lim K, Kim HJ, Park JK, Paik TH, Jo EK. Role of mitogen-activated protein kinase pathways in the production of tumor necrosis factor-alpha, interleukin-10, and monocyte chemotactic protein-1 by Mycobacterium tuberculosis H37Rv-infected human monocytes. J Clin Immunol. 2003;23:194–201. doi: 10.1023/a:1023309928879. [DOI] [PubMed] [Google Scholar]
- 40.Kato S, Yuzawa Y, Tsuboi N, Maruyama S, Morita Y, Matsuguchi T, Matsuo S. Endotoxin-induced chemokine expression in murine peritoneal mesothelial cells: the role of toll-like receptor 4. J Am Soc Nephrol. 2004;15:1289–1299. [PubMed] [Google Scholar]
- 41.Handel TM, Johnson Z, Rodrigues DH, Dos Santos AC, Cirillo R, Muzio V, Riva S, Mack M, Deruaz M, Borlat F, Vitte PA, Wells TN, Teixeira MM, Proudfoot AE. An engineered monomer of CCL2 has anti-inflammatory properties emphasizing the importance of oligomerization for chemokine activity in vivo. J Leukoc Biol. 2008;84:1101–1108. doi: 10.1189/jlb.0108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuluyan HE, Issekutz AC. VLA-4 integrin can mediate CD11/CD18-independent transendothelial migration of human monocytes. J Clin Invest. 1993;92:2768–2777. doi: 10.1172/JCI116895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chuluyan HE, Schall TJ, Yoshimura T, Issekutz AC. IL-1 activation of endothelium supports VLA-4 (CD49d/CD29)-mediated monocyte transendothelial migration to C5a, MIP-1 alpha, RANTES, and PAF but inhibits migration to MCP-1: a regulatory role for endothelium-derived MCP-1. J Leukoc Biol. 1995;58:71–79. doi: 10.1002/jlb.58.1.71. [DOI] [PubMed] [Google Scholar]
- 44.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4:432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 45.Kawaguchi M, Kokubu F, Kuga H, Tomita T, Matsukura S, Hoshino H, Imai T, Adachi M. Effect of IL-17 on ICAM-1 expression of human bronchial epithelial cells, NCI-H 292. Arerugi. 1999;48:1184–1187. [PubMed] [Google Scholar]
- 46.Ayala JM, Goyal S, Liverton NJ, Claremon DA, O’Keefe SJ, Hanlon WA. Serum-induced monocyte differentiation and monocyte chemotaxis are regulated by the p38 MAP kinase signal transduction pathway. J Leukoc Biol. 2000;67:869–875. [PubMed] [Google Scholar]
- 47.Gouwy M, Struyf S, Noppen S, Schutyser E, Springael JY, Parmentier M, Proost P, Van Damme J. Synergy between coproduced CC and CXC chemokines in monocyte chemotaxis through receptor-mediated events. Mol Pharmacol. 2008;74:485–495. doi: 10.1124/mol.108.045146. [DOI] [PubMed] [Google Scholar]
- 48.Koch AE, Volin MV, Woods JM, Kunkel SL, Connors MA, Harlow LA, Woodruff DC, Burdick MD, Strieter RM. Regulation of angiogenesis by the C-X-C chemokines interleukin-8 and Epithelial Neutrophil Activating Peptide 78 in the rheumatoid joint. Arthritis Rheum. 2001;44:31–40. doi: 10.1002/1529-0131(200101)44:1<31::AID-ANR5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 49.Tak PP, Bresnihan B. The pathogenesis and prevention of joint damage in rheumatoid arthritis: advances from synovial biopsy and tissue analysis. Arthritis Rheum. 2000;43:2619–2633. doi: 10.1002/1529-0131(200012)43:12<2619::AID-ANR1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]