Abstract
Background
RBC deformability after trauma/hemorrhagic shock (T/HS) leads to the microcirculatory dysfunction and clinical manifestations of organ failure. However, the cellular mechanism of this phenomenon remains unknown. The spectrins are important for the shape and physical properties of erythrocytes, such as deformability and resistance to mechanical stress. Previous studies have shown that erythrocyte α-spectrin is ubiquitinated. Studies of sickled cells and aged erythrocytes, two conditions known to have decreased RBC deformability, have shown diminished α-spectrin ubiquitination which may contribute to the inability of these cells to change shape. We hypothesized that decreased α-spectrin ubiquitination could participate in the mechanism(s) whereby T/HS erythrocytes become less deformable.
Methods
We determined the level of α-spectrin ubiquitination in erythrocytes isolated from T/HS rats compared to erythrocytes from control sham-shocked (T/SS animals. Following T/SS (n=4) or T/HS (n=7), alpha and beta spectrin subunits were isolated using a low ionic strength buffer at 37°C for 30 minutes. The relative amount of ubiquitinated α-spectrin was evaluated by Western blotting using a monoclonal antibody to ubiquitin.
Results
We found the relative level of α-spectrin ubiquitination (normalized to total α-spectrin in the same preparation) was significantly decreased following T/HS (0.319±0.03) compared to T/SS control erythrocytes (0.485±0.06, p<0.05). To evaluate the content and relative amounts of the other membrane proteins, we also compared the profiles of T/HS and T/SS erythrocytes by SDS-polyacrylamide gel electrophoresis. This did not reveal any significant quantitative differences between T/SS and T/HS spectrin or other membrane proteins.
Conclusion
Our finding of decreased α-spectrin ubiquitination following T/HS suggests that this mechanism could contribute to the increased rigidity of the erythrocyte membrane.
Summary
Ubiquitination of α-spectrin is decreased after trauma/hemorrhagic shock, and this could contribute to the decreased erythrocyte deformability seen post-shock.
Keywords: Erythrocyte, deformability, ubiquitin, ubiquitination
Introduction
While development of the multiple organ dysfunction syndrome (MODS) is relatively common in patients sustaining major trauma especially when complicated by an episode of shock, the various factors contributing to its development remain to be fully defined [1]. However, two lines of evidence have implicated red blood cells as playing a significant role in the development of MODS. The first line of evidence is based on an increasing body of clinical evidence that large volume transfusions of stored blood (> 6 units) is a major risk factor for the development of MODS [2] and that this propensity of stored blood to potentiate MODS is related, at least in part, to red cell-induced impairment of microvascular blood flow [3,4]. A second line of evidence is based on our clinical and laboratory studies showing that red blood cell deformability is impaired after trauma-hemorrhage [5,6] since a decrease in RBC deformability is associated with impaired microvascular (nutrient) blood flow [7] and thus might contribute to the development of MODS [6]. The mechanism by which impaired RBC deformability leads to impaired tissue blood flow is due to the fact that the red cell must change shape as its passes through the microcirculatory bed [8]. Failure of the RBC concave disc, which is 7 microns in diameter, to deform as it passes through the 4 micron capillaries would lead to microvascular blockade and stasis with a resultant decrease in cellular oxygen delivery. This phenomenon is well recognized in other disease states, such as sickle cell anemia, where stress-induced changes in red cell deformability leads to decreased microcirculatory blood flow and clinically apparent vasoactive crises [9].
Although the physiologic effects of impaired RBC deformability is relatively clear, the cellular mechanisms responsible for T/HS-induced loss of deformability remain to be determined. Since the key to RBC deformability lies in the cell membrane and its attachment to the cytoskeleton, we have begun investigating how T/HS influences key red cell membrane proteins, especially spectrin. Spectrin is made up of alpha and beta subunits, which form heterodimers and tetramers, which play an important role in regulating red cell deformability since their location on the inner cytoskeletal portion of the cell membrane results in their forming an intracellular lattice via binding to cytoplasmic actin while at the same time they bind to important structural outer membrane proteins, such as Band 3, Protein 4.1/4.2, Protein 4.9 and ankyrin [10,11]. In fact, the association between spectrin and actin forms a hexagonal lattice, which functions as an intracellular ‘spring’ that aids in restoring the characteristic discoid shape of the RBC following its passage through the capillaries of the microcirculation. Consequently, we have studied the effects of T/HS on the levels of these key RBC membrane proteins. Additionally, because of the important role spectrin plays in regulating RBC deformability and the fact that alpha-spectrin has ubiquitin conjugating and ligating ability [12], we also evaluated the effects of T/HS on spectrin oxidation and phosphorylation as well as whether T/HS reduces spectrin ubiquitin content.
Methods
Male Sprague-Dawley rats weighing between 250 and 300g were housed under barrier-sustained conditions and were allowed to acclimatize for at least 7 days prior to experimentation. All rats were maintained in accordance with the recommendations of the Guide for the care and Use of Laboratory Animals and the experiments were approved by the New Jersey Medical School Unstitutional Animal Care and Use Committee.
Experimental Design
In the first set of experiments, rats subjected to trauma (laparotomy) and hemorrhagic shock (T/HS) or trauma sham shock (T/SS) had their blood volume collected 3 hours after the end of the shock or the sham-shock period, at which time and aliquot of blood was used to measure RBC deformability and morphology (scanning electron microscopy). As the fisrst step in investigating the molecular factors involved in T/HS-induced red cell dysfunction, the remaining red cells were depleted of hemoglobin (ghost formation) and the remaining membrane and cytoskeleton were processed to assess for any quantitative changes in key red blood cell membrane proteins. These included spectrin, ankyrin, band 3, protein 4.1/4.2, protein 4.9 and actin. In subsequent studies, we assessed whether T/HS caused changes in spectrin oxidation, phosphorylation or ubuquitination.
Trauma/hemorrhagic shock model
Male Sprague-Dawley rats were anesthetized with intraperitoneal pentobarbital (50 mg/kg) and the right femoral artery was isolated by minimal dissection and aseptically cannulated with polyethylene (PE-50) tubing containing 0.1 mL heparinized saline. The catheter was connected in-line to a blood pressure recorder and polygraph (Grass Model 79E Data Recorder, Quincy, MA) to allow continuous blood pressure monitoring. After traumatic injury in the form of laparotomy, the abdomen was subsequently closed using a running 4-0 silk suture.
To induce shock, blood was withdrawn from the jugular vein into a syringe containing 10 units of heparin suspended in 0.3 mL 0.9% normal saline to prevent clotting. The mean arterial pressure was reduced to 30–35 mmHg for 90 minutes by the withdrawal or reinfusion of shed blood (kept at 37°C) as needed. After 90 minutes of shock, the animals were resuscitated with their shed blood. At the end of the third hour post-resuscitation, animals were sacrificed by thoracotomy and withdrawal of their total blood volume via cardiac puncture.
Sham-shocked (T/SS) animals had a laparotomy and their blood vessels cannulated, but no blood was withdrawn or given. They were sacrificed as above after 4.5 hours under anesthesia.
Ektacytometry
RBC deformability was determined at various shear stresses by laser diffraction analysis using and ektacytometer (LORCA, RR Mechatronics, Hoorn, The Netherlands) as described by Zaets et al [2]. Briefly, 5 µL RBC were suspended in a highly viscous polyvinylpyrrolidone buffer (viscosity = 30.5 Pa) and rotated for 10 minutes. The sample was loaded into the ektacytometer and was subjected to shear stresses from 0.3 to 29.9 kPa in a concentric glass cylinder. The laser beam diffraction pattern produced by the RBC was analyzed by the microcumputer to yield an elongation index (EI) at each shear stress. Lineweaver-Burk analysis was used to convert these EI values to a single variable, the KEI, which is the shear stress necessary to achieve half-maximal elongation of the red cell. The higher the KEI, the less deformable is the red cell. This variable was used for statistical analyses.
RBC Morphology
Scanning electron microscopic studies of RBC were performed by means of AMRAY 1200B electron microscope. The blood samples were fixed in phosphate-buffered (pH7.2–7.4) 2.5% glutaraldehyde for 1 h, washed two times in 0.1M phosphate buffer (pH 7.2–7.4), and mounted on poly-L-lysine coated glass slides. The slides were kept in a moist atmosphere for 1 h, washed in phosphate buffer, postfixed in 1% osmium tetroxide for 1 h, rinsed in distilled water, and dehydrated n graded ethanol (50%, 70%, 90% and 100%). After critical point drying with liquid CO2 in a vacuum apparatus and covering with a gold-palladium layer, the samples underwent scanning electron microscopic analysis.
Isolation of erythrocyte ghosts
Whole blood was centrifuged at 900 × g for 10 minutes at 4°C. The supernatant (plasma) and buffy coat (WBC) were aspirated and discarded. The remaining RBCs were washed three times with 1X GASP buffer (PBS with 5.5 mM glucose, 0.08% bovine serum albumin), centrifuged at 900 × g for 10 minutes at 4°C, and the supernatant discarded. The final RBC pellet was suspended in an ice-cold 5 mM sodium phosphate buffer, pH 8.0, containing 1 mM EGTA and 1 mM phenylmethylsulfonylflouride (PMSF) and incubated at 4°C for 10 minutes. RBC ghosts were washed three times with the same lysis buffer followed by centrifugation (20,000 × g for 20 mins at 4°C) to obtain “white” ghosts [13].
Spectrin isolation
Spectrin was partially purified from RBC ghost membranes by low-ionic strength extraction as outlined by Pedroni et al [14]. Briefly, ghost residues were resuspended in low-ionic strength buffer (0.3 mM NaPO4, pH 8, 0.1mM EDTA, 0.1 mM PMSF, 0.1 mM 2-mercaptoethanol) and incubated for 30 minutes at 37°C. The solution was centrifuged for 30 minutes at 21,000 × g and the supernatant containing the spectrin extract transferred to fresh tubes.
Protein analysis
Ghosts and/or spectrin extracts were run on a 3–8% Tris/Acetate gel (Invitrogen, Carlsbad, CA) at 150 V for 75 minutes. Sample gels were stained with Coomassie Blue or Proto-Blue and the protein bands were analyzed using densitometry. The areas under the curves for each protein band were compared with the total to obtain the fraction of each protein of the total. Transfer to nitrocellulose was done at 4°C overnight at 75 mA constant. The membranes were removed and stained with MemCode system (Pierce). Non-specific binding sites were blocked with Superblock T-20 (Bio-Rad) for 1 hour at room temperature prior to the addition of antibodies. Membranes were incubated overnight at 4°C in primary antibody diluted in Superblock T-20. Membranes were washed three times in tris-buffered saline containing 0.1% Tween 20 and then incubated for one hour with horseradish peroxidase conjugated secondary antibody (Cell-Signaling), also diluted in Superblock. The membrane was again washed three times in the wash buffer and incubated with West-Pico chemiluminescence system (Pierce) for 5 minutes. Finally, the membrane was exposed to X-ray film and developed.
Spectrin Oxidation
Binding of cDDP, a complex that binds specifically to methionine and cysteine resides of spectrin was performed as described by Schwartz et al [15]. Briefly, 80 µg spectrin was purified and dialyzed against cDDP reaction buffer (20 mM Tris-HCL, pH 7.3, and 80 mM NaCl), then incubated with an equal volume of 350 µg/ML cDDP reaction buffer for 18 hours at 37°C with gentle mixing. After incubation, the reaction tubes were chilled on ice and spectrin was precipitated with ice-cold 10% trichloroacetic acid for 30 minutes. The tubes were then microcentrifuged for 5 minutes. 125 µL of supernatant was collected, to which was added 100 µL concentrated HCL, followed by 250 µL 20% ammonium chloride and 200 µL stannous chloride. The mixture was incubated for 2 hours at room temperature, and the absorbance at 403 nm was measured. These absorbance values were compared to the standard curves with cDDP in the absence of any protein. Results were converted to molar amount of cDDP bound to spectrin by subtraction of the unbound moles of cDDP from the total moles of cDDP added to the reaction mixture.
Spectrin phosphorylation
Western blotting was performed as outlined above, using a monoclonal anti-phosphotyrosine or anti-phosphoserine antibody as primary, and an anti-mouse HRP-conjugated secondary antibody (Cell Signaling). Using densitometry, phosphorylated α-spectrin was normalized to the total α-spectrin assessed using an anti- α-spectrin primary antibody) in the same lane to calculate the proportion of α-spectrin that is phosphorylated. Phosphorylated β-spectrin was normalized to β-actin in the same lane as a loading control.
Spectrin Ubiquitination
Western blotting was performed as outlined above, using a monoclonal anti-ubiquitin antibody (Abcam) and HRP-conjugated anti-rabbit secondary antibody (Cell Signaling). The ratio of ubiquitinated α-spectrin to total α-spectrin was obtained using densitometry as previously stated.
Statistics
Statistical calculations were performed using JMP software (SAS institute; Cary, NC) Results were analyzed using ANOVA followed by t-test for pairwise comparisons. Statistically significant differences were concluded at p < 0.05.
Results
RBC Deformability and Morphology
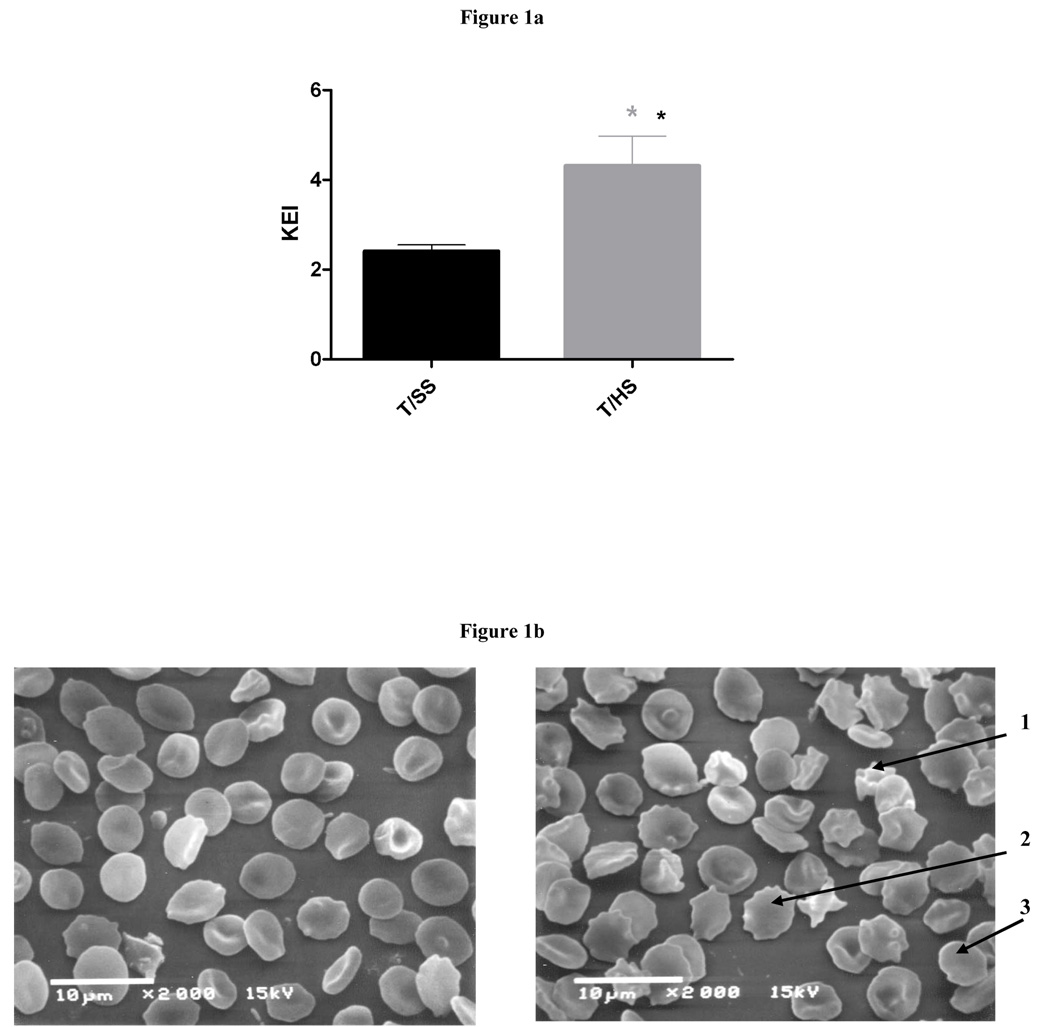
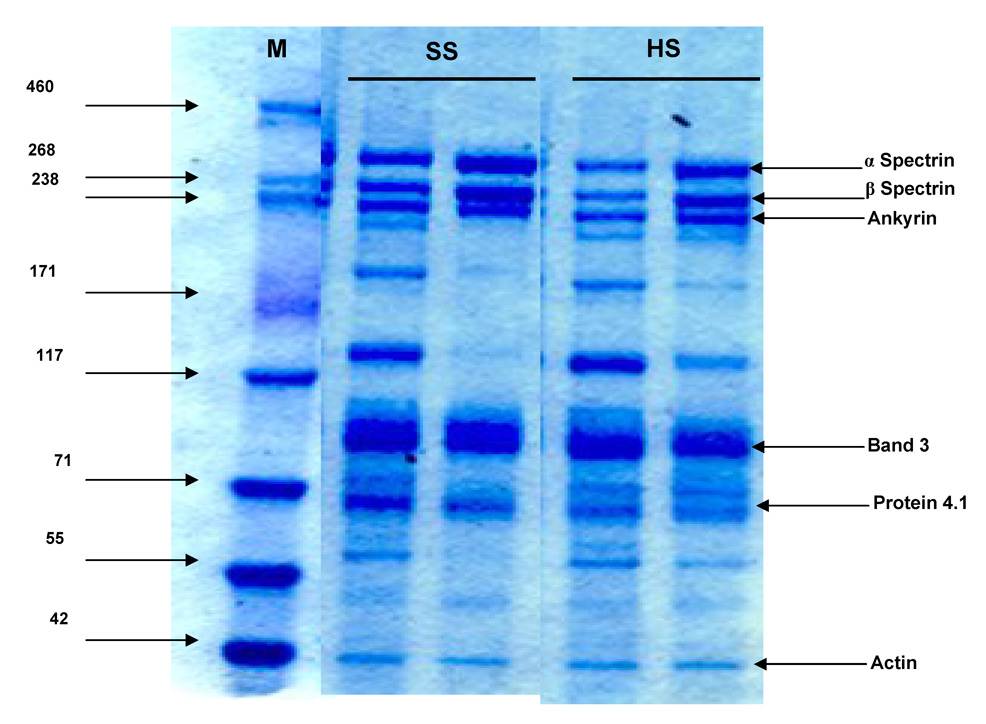
To assess the affects of T/HS on red cell deformability, we compared the KEI values of T/HS to T/SS red cells, since the KEI represents the shear stress necessary to achieve ½ maximal cell elongation. The higher the KEI, the more shear stress is required to deform the red cells. As reflected in a significantly higher KEI, the erythrocytes from the T/HS rats were significantly less deformable (more rigid) than the T/SS erythrocytes (Figure 1a). These T/HS-induced changes in red cell deformability correlated with the increased presence of morphologically abnormal red cells (Figure 1b).
Figure 1.
Figure 1a: T/HS decreases RBC deformability as measured by KEI (n = 5, * = p<0.05).
Figure 1b: Electron micrograph (2000X) showing normal biconcave disc-shaped RBC seen after T/SS (Left panel). Morphologic derangements seen after T/HS (Right panel), including echinocytes (1), spheroechinocytes (2), and spherocytes (3).
Membrane Protein Quantification
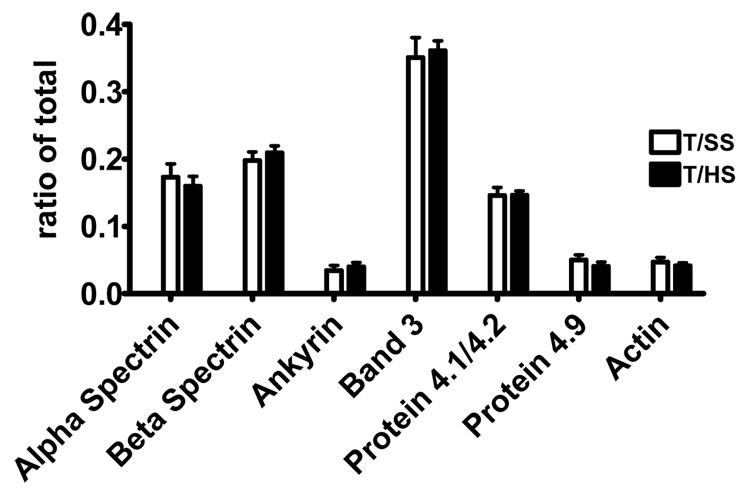
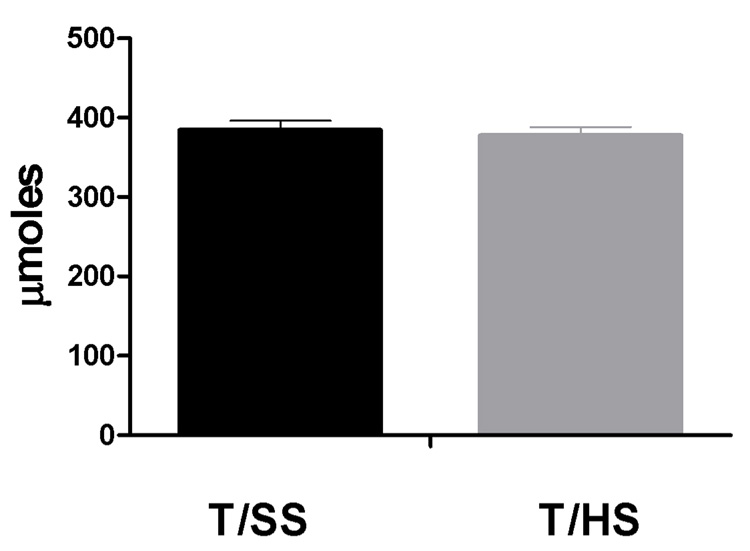
To determine whether the changes in T/HS-induced deformability or morphology was associated with quantitative changes in key membrane proteins, we measured the total amounts of several key membrane proteins in T/HS and T/SS erythrocyte ghosts via Coomassie-blue stained SDS gel electrophoresis (Figure 2). No significant quantitative differences were found between the T/SS ad T/HS for any of the 7 proteins measured (Figure 3).
Figure 2.
ProtoBlue colloid stain of 3–8% Tris-Acetate SDS/PAGE showing RBC membrane proteins and actin. Lane 1 is a molecular weight marker, lanes 2 and 3 are T/SS ghosts, lanes 4 and 5 are T/HS ghosts.
Figure 3.
Comparison of amounts of individual proteins in T/SS and T/HS ghosts. Ratios were obtained comparing the area under the curve of each individual protein with the total protein per sample (n = 5 in each group).
Spectrin Oxidation
Since oxidative stress plays a role in red cell damage in other disease states [15], we investigated if T/HS causes increased oxidation of alpha- spectrin utilizing the cDDP binding assay. In this assay, cDDP binding to the cysteine or methionine residues is reduced when the spectrin molecule is oxidized. Because no differences were found in the degree of cDDP binding between the T/HS and T/SS erythrocytes, it does not appear that T/HS causes red cell alpha spectrin oxidation (Figure 4).
Figure 4.
Oxidation of α-spectrin is not affected by T/HS, as measured by binding of cDDP in µmoles to cysteine and methionine residues (n = 3 in each group).
Spectrin Phosphorylation
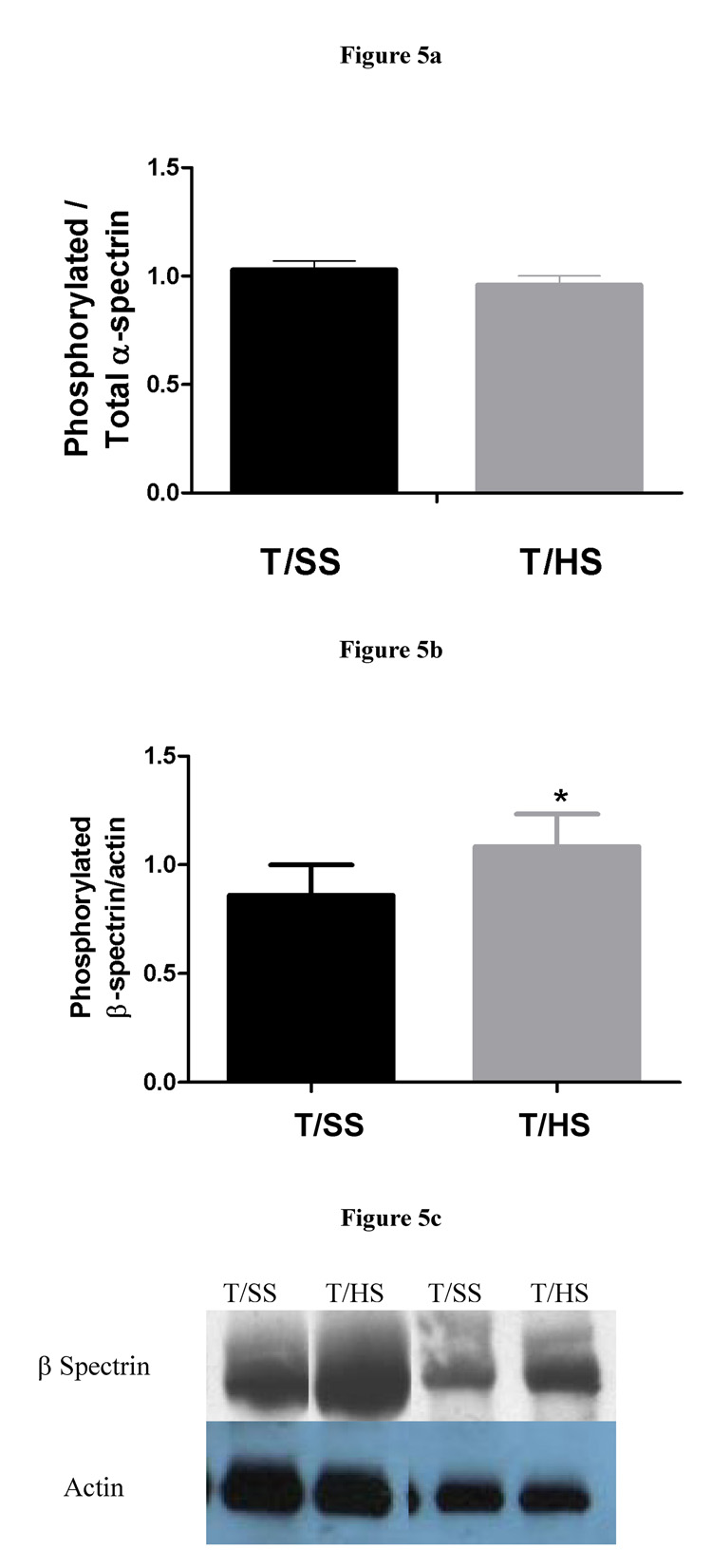
Next, we assessed alpha-spectrin tyrosine phosphorylation and beta-spectrin serine phosphorylation to ascertain the possible contribution of increased spectrin phosphorylation to the decreased red cell deformabitity observed following T/HS. Based on Western blotting of purified spectrin with anti-phosphotyrosine antibody, it appeared that there was no increase in alpha-spectrin phosphorylation in the T/HS red cells (Figure 5A). However, a significant increase in serine phosphorylation of beta-spectrin was observed in the T/HS erythrocytes (Figure 5B and 5C).
Figure 5.
Figure 5a: Tyrosine phosphorylation of α-spectrin is not affected by T/HS (T/SS: n = 5, T/HS: n = 7).
Figure 5b: Serine phosphorylation of β-spectrin increases after T/HS (n = 3, * = p<0.05).
Figure 5c: B: Western blot showing increased serine phosphorylation of β-spectrin using anti-phosphoserine antibody. β-spectrin was confirmed using an anti- β-spectrin antibody (data not shown).
Spectrin Ubiquitination
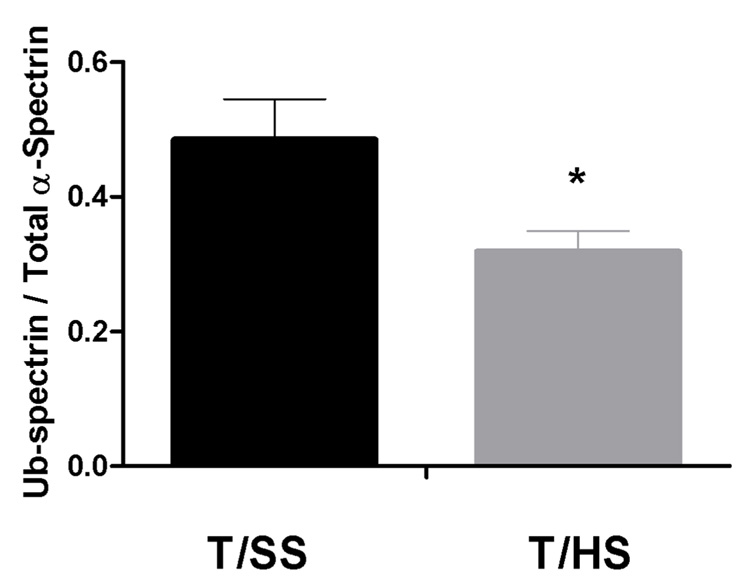
Since recent studies in sickle cell disease have implicated a decrease in alpha-spectrin ubiquitination in the pathogenesis of red cell rigidification [16], we compared the ratio of ubiquinated to non-ubiquinated alpha-spectrin in the T/HS red cells to that of T/SS red cells. These studies revealed a decrease in alpha-spectrin ubiquitination in the erythrocytes of the T/HS animals as compared to the T/SS animals resulting in a decrease in the ratio of ubiquitinated α-spectrin to total α-spectrin (Figure 6).
Figure 6.
Ubiquitination of α-spectrin decreases after T/HS (T/SS: n = 4, T/HS: n = 7, * = p<0.05).
Discussion
The major observations of this study are several-fold. First, that T/HS does not cause a decrease in the amount of the key RBC membrane proteins Ankyrin, Band 3, Protein 4.1/4.2, Protein 4.9 and spectrin or the cytoskeletal protein actin. Thus, T/HS-induced changes in RBC deformability or morphology do not appear to be related to changes in the levels of these proteins. Since T/HS is associated with oxidant-mediated tissue injury [1], oxidant exposure of normal RBC leads to changes in deformability and morphology [17], and abnormal RBC deformability in diabetics is associated with oxidation of spectrin [15], we tested whether T/HS led to increased spectrin oxidation. Since increased RBC spectrin oxidation was not observed after T/HS, it does not appear that the T/HS-induced RBC changes in deformability or morphology are related to spectrin oxidation. However, this observation does not negate the possibility that an oxidant injury to other RBC membrane proteins might contribute to the RBC changes observed after T/HS, since we only measured the level of alpha-spectrin oxidation. Since there is normally very little phosphorylation in RBC of unstressed animals and phosphorylation of membrane proteins has been shown in vitro to impair RBC deformability [18], we next measured the phosphorylation state of both alpha-spectrin and beta-spectrin and found that the phosphorylation of beta- but not alpha-spectrin was increased. From the current studies it is not clear whether the phosphorylation of beta-spectrin has an effect on T/HS RBC deformability, although in vitro studies have shown that increased serine phosphorylation of beta-spectrin decreases erythrocyte membrane stability [19].
Since alpha-spectrin has ubiquitin conjugating (E2) and ligating (E3) activity and the ability to ubiquinate beta-spectrin, ankyrin and Band 3 [12,20,21], plus recent studies on sickle cells have correlated decreased spectrin E2/E3 activity with decreased RBC deformability [16], we tested whether T/HS caused a decrease in the amount of ubiquinated alpha-spectin. Our results documented that there was a decrease in the amount of ubiquitinated alpha-spectrin resulting in a decreased ratio of non-ubiquitinated to total alpha-spectrin. This observation is of potential importance since ubiquiti has been documented to regulate a diverse number of cellular activities. That is, although ubiquitin has been thought of as primarily functioning in the proteosomal degredation of proteins, it is now recognized that ubiquitin plays an important role in many cellular events ranging from protein-protein interactions to transcriptional activation and even apoptosis [22]. The importance of the ubiquinating activity of alpha-spectin in maintaining normal RBC deformability has been recently established in sickle cells with abnormal deformability, where the a decrease in the alpha-spectrin ubiquitin to non-ubiquitin ratio correlated tightly with sickle cell severity and deformability [16]. The proposed mechanism for how decreased alpha-spectrin ubiquitin levels and activity leads to decreased RBC deformability is based on the observation that reduced spectrin ubiquitination leads to tightened spectrin-Protein 4.1-actin and spectrin-adductin-actin complexes that dissociate slowly [16,23,24]. This relationship between low ubiquitinated spectrin and slower membrane protein dissociation under shear stress can result in ‘locked-type’ membrane-cytoskeletal associations and hence decreased RBC deformability. In this context, our observation that the ratio of ubiquitinated to non-ubiquitinated alpha-spectrin is decreased may help explain the cellular mechanism of decreased RBC deformability in T/HS RBC. Further in vitro and in vivo studies testing this notion will be required to ultimately establish the role of decreased ubiquitinated-spectrin in the pathogenesis of T/HS-induced RBC rigidification. Nonetheless, the fact that alpha-spectrin, in addition to being a structural membrane protein, has important enzymatic activity that helps regulate the association of the various RBC membrane proteins to the actin cytoskeleton makes it an attractive target for further study and possible therapy. We plan to modulate ubiquitin and its activity to determine its role in forming a frozen or locked cell membrane after hemorrhagic shock.
In summary, decreased RBC deformability is a common observation in many congenital diseases, such as sickle cell disease, spherocytosis and diabetes as well as a consequence of acquired diseases such as malaria and stressful conditions such as major trauma and sepsis. Since abnormal RBC deformability can impair microcirculatory blood flow and contribute to tissue and cellular ischemia, understanding the mechanisms by which these various conditions and stress states lead to RBC rigidification is a first step in developing potential therapies directed at reversing this RBC defect.
Acknowledgments
Supported by NIH Grant P50 G069790
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deitch EA. Multiple Organ failure: Pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore FA, Moore EE, Sauaia A. Blood transfusion: An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620–625. [PubMed] [Google Scholar]
- 3.Marik PE, Sibbald WJ. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269:3024–3029. [PubMed] [Google Scholar]
- 4.van Bommel J, de Korte D, Lind A, Siegemund M, Trouwborst A, Verhoeven AJ, Ince C, Henny CP. The effect of the transfusion of stored RBCs on intestinal microvascular oxygenation in the rat. Transfusion. 2001;41:1515–1523. doi: 10.1046/j.1537-2995.2001.41121515.x. [DOI] [PubMed] [Google Scholar]
- 5.Zaets SB, Berezina TL, Morgan C, et al., editors. Effect of trauma-hemorrhagic shock on red blood cell deformability and shape. Shock. 2003;19:268–273. doi: 10.1097/00024382-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Machiedo G, Powell R, Rush B, Swislocki N, Dikdan G. The incidence of decreased red cell deformability in sepsis and the association with oxygen free radical damage and multiple system organ failure. Arch Surg. 1989;124:1386–1389. doi: 10.1001/archsurg.1989.01410120032007. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi Y, Da O, Fujino T. Variation in red blood cell deformability and possible consequences for oxygen transport to tissue. Microvasc Res. 1994;47:222–231. doi: 10.1006/mvre.1994.1017. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee R, Nageshwari K, Puniyani R. The diagnostic relevance of red cell rigidity. Clin Hemorheol Microcirc. 1998;19:21–24. [PubMed] [Google Scholar]
- 9.Kaul DK, Fabry ME. In vivo studies of sickle red blood cells. Microcirculation. 2004;11:153–165. [PubMed] [Google Scholar]
- 10.Liu SC, Derick LH, Palek J. Visualization of the hexagonal lattice in the erythrocyte membrane skeleton. J Cell Biol. 1987;104:527–536. doi: 10.1083/jcb.104.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennet V. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev. 1990;70:1029–1065. doi: 10.1152/physrev.1990.70.4.1029. [DOI] [PubMed] [Google Scholar]
- 12.Hsu YJ, Zimmer WE, Goodman SR. Erythrocyte spectrin’s chimeric E2/E3 ubiquitin conjugating/ligating activity. Cell Mol Biol. 2005;51:187–93. [PubMed] [Google Scholar]
- 13.Liu SC, Palek J, Yi SJ, Nichols PE, et al., editors. Molecular basis of altered red blood cell membrane properties in Southeast Asian ovalocytosis: role of the mutant band 3 protein in band 3 oligomerization and retention by the membrane skeleton. Blood. 1995;86:349–358. [PubMed] [Google Scholar]
- 14.Pedroni S, Lecomte MC, Gautero H, Dhermy D. Heterogeneous phosphorylation of erythrocyte spectrin beta chain in intact cells. Biochem J. 1993;294:841–846. doi: 10.1042/bj2940841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz RS, Masden JW, Rybicki AC, Nagel RL. Oxidation of spectrin and deformability defects in diabetic erythrocytes. Diabetes. 1991;40:701–708. doi: 10.2337/diab.40.6.701. [DOI] [PubMed] [Google Scholar]
- 16.Chang TL, Kakhniashvili, Goodman SR. Ubiquitin conjugating/ligating activity is diminished in sickle cells. Am J Hematol. 2005;79:89–96. doi: 10.1002/ajh.20351. [DOI] [PubMed] [Google Scholar]
- 17.Snyder LM, Fortier FJ, Trainor J, Jacobs J, Leb L, Lubin B, Chiu D, Shohet S, Mohandas N. Effect of hydrogen peroxide exposure on normal human erythrocyte deformability, morphology, surface characteristics and spectrin-hemoglobin cross-linking. J Clin Invest. 1985;76:1971–1977. doi: 10.1172/JCI112196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbul A, Zipser Y, Nachles A, Korenstein R. Deoxygenation and elevation of intracellular magnesium induce tyrosine phosphorylation of band 3 in human erythrocytes FEBS Letters. 1999;455:87–91. doi: 10.1016/s0014-5793(99)00822-4. [DOI] [PubMed] [Google Scholar]
- 19.Manno S, Takakuwa Y, Nagao K, Mohandas N. Modulation of erythrocyte membrane mechanical function by beta-spectrin phosphorylation and dephosphorylation. J Biol Chem Mar. 1995;270(10):5659–5665. doi: 10.1074/jbc.270.10.5659. [DOI] [PubMed] [Google Scholar]
- 20.Chang TL, Cubillos F, Kakhniashvili DG, Goodman SR. Ankyrin is a target of spectrin’s E2/E3 ubiquitin-conjugating/ligating activity. Cell Mol Biol. 2004;50:59–66. [PubMed] [Google Scholar]
- 21.Chang TL, Cubillos F, Kakhniashvili DG, Goodman SR. Band 3 is a target protein of spectrin’s E2/E3 activity: implications for sickle cell disease and normal red blood cell aging. Cell Mol Biol. 2004;50:171–177. [PubMed] [Google Scholar]
- 22.Weissman AM. Themes and variatons on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2:169–178. doi: 10.1038/35056563. [DOI] [PubMed] [Google Scholar]
- 23.Ghatpande SS, Goodman SR. Ubiquitination of spectrin regulates the erythrocyte spectrin-protein 4.1-actin ternary complex dissociation: implication for the sickle cell membrane skeletons. Cell Mol Biol. 2004;50:67–74. [PubMed] [Google Scholar]
- 24.Mishra R, Goodman SR. Ubiquitination of spectrin regulates the dissociation of the spectrin-adducin-F-actin ternary complex in vitro. Cell Mol Biol. 2004;50:75–80. [PubMed] [Google Scholar]