Abstract
Through their capacity to secrete, upon activation, a variety of bioactive molecules brain macrophages (and resident microglia) play an important role in brain immune and inflammatory responses. To test our hypothesis that activated macrophages induce neuronal injury by enhancing neuronal outward K+ current, we studied the effects of lipopolysacharride (LPS)-stimulated human monocytes-derived macrophage (MDM) on neuronal transient A-type K+ current (IA) and resultant neuronal injury in primary rat hippocampal neuronal cultures. Bath application of LPS-stimulated MDM-conditioned media (MCM+) enhanced neuronal IA in a concentration-dependent manner. Non-stimulated MCM (MCM-) failed to alter IA. The enhancement of neuronal IA was recapitulated in neurons co-cultured with macrophages. The link of MCM(+)-induced enhancement of IA to MCM(+)-associated neuronal injury, as detected by propidium iodide (PI) and 4″,6-diamidino-2-phenylindol (DAPI) staining and MTT assay, was demonstrated by experimental results showing that addition of IA blocker 4-aminopyridine to the cultures protected hippocampal neurons from MCM(+)-induced neuronal injury. Further investigation revealed that glutamate was involved in MCM(+)-induced enhancement of neuronal IA. These results suggest that during brain inflammation macrophages (and microglia) might mediate neuronal injury via enhancement of neuronal IA, and that neuronal Kv channel might be a potential target for the development of therapeutic strategies for some neurodegenerative disorders by which immune and inflammatory responses are believed to be involved in the pathogenesis.
Keywords: Neuroinflammation, potassium channels, hippocampus, glutamate, apoptosis
Introduction
Recruited to the site of various insults in the brain, macrophages (and resident microglia) function primarily to eliminate dead cells and pathogens through phagocytosis. However, macrophages, in certain scenarios, appear to injure by-stander cells (Gehrmann et al., 1995; Fordyce et al., 2005; Mattson et al., 2005) and have thereby been proposed to play an active role in neuronal death (Lees, 1993). The co-localization of activated macrophages and damaged neurons observed in brain injury and degenerative brain diseases hints to macrophage-induced neuronal damage. Indeed, studies have shown that macrophages injure neurons both in vitro (Thery et al., 1991; Piani et al., 1992; Arantes et al., 2000) and in vivo (Gelbard et al., 1995; Williams et al., 2005). This is largely through their capacity to secrete soluble neurotoxins in abundance, for instance, tumor necrosis factor alpha and interleukin 1 beta, which then mediate brain immune and inflammatory reactions and ultimately neuronal dysfunction and death (Cotter et al., 1998; Williams and Hickey, 2002). Macrophages may also induce neuronal injury through direct cell-to-cell contact (Cameron and Churchill, 1981; Thery et al., 1993). Whereas many studies have shown these soluble toxins induce neuronal injury via different signaling pathways, increasing evidence indicates that macrophages can induce neuronal dysfunction and apoptosis through activation of neuronal voltage-gated K+ (Kv) channels (Gelman et al., 2004; Judge and Bever, 2006; Keblesh et al., 2009a).
Kv channels play crucial roles in regulating a wide variety of physiological and pathophysiological processes. In the hippocampus, several types of kinetically and pharmacologically distinct K+ currents have been described with whole-cell recordings. Each of these K+ currents contributes to the control of neuronal excitability and activity in a unique way. Collectively, these K+ currents regulate many aspects of neuronal physiology and alteration of these K+ currents is likely to cause neuronal dysfunction and apoptosis. Thus, pharmacological modulation of K+ currents may represent a powerful means of controlling CNS disorders, such as stroke and human immunodeficient virus type 1 (HIV-1)-associated dementia (HAD). Indeed, studies have shown that enhancement of outward K+ currents promotes apoptosis in cortical (Yu et al., 1997; Yu et al., 1998), hippocampal (Nadeau et al., 2000; Chen et al., 2005), basal forebrain cholinergic (Colom et al., 1998) and cerebellar granular neurons (Lauritzen et al., 2003); whereas, administration of Kv channel antagonists prevent neuronal injury (Yu et al., 1997; Wei et al., 2004; Hu et al., 2006). Thus, activation of Kv channels has been considered as an essential pathway in apoptosis (Remillard and Yuan, 2004).
We observed previously that lipopolysacharride (LPS)-stimulated human monocyte-derived macrophages [MDM(+)] enhanced neuronal outward delayed rectifier K+ current in primary rat hippocampal neurons, resulting in neuronal injury (Hu et al., 2009). In the present study we investigated effects of the MDM(+)-conditioned media [MCM(+)] and MDM(+) on transient A-type K+ current (IA) in cultured hippocampal neurons and examined the potential link between the MDM(+)-induced alteration of neuronal IA and MDM(+)-associated neuronal injury in vitro. Our results showed that both MDM(+) and MCM(+) produced a significant enhancement of neuronal IA and consequent neuronal injury.
Materials and Methods
Human monocyte culture and MCM collection
Human monocytes were recovered from peripheral blood mononuclear cells of HIV-1, HIV-2 and hepatitis B virus seronegative donors after leukopheresis and purified by counter-current centrifugal elutriation (Gendelman et al., 1988). Cells were obtained under a protocol approved by University of Nebraska Institutional Review Board. Monocytes were cultured in DMEM (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% heat-inactivated human serum, L-glutamine (2mM), gentamicin (50 μg/ml), ciprofloxacin (10 μg/ml), and macrophage colony-stimulating factor (MCSF-a generous gift from Wyeth Pharmaceutical, Cambridge, MA, USA), allowing them to differentiate into macrophages in vitro. The purity of MDM was confirmed by MAC1 immunocytochemistry. MAC1 was expressed in >95% of cells. FACS analysis revealed that >98 % MDM were CD14+ CD45+ CD11b+ positive cells after 7 days in culture (37°C, 5% CO2) in the presence of MCSF. The MDM were then stimulated with LPS (1μg/ml) for 2 h. Non-stimulated MDM were used as controls. To obtain a “guaranteed” activation effect, LPS was used as a model molecule to stimulate MDM instead of some physiological means (e.g. CD40 ligand or IL-1β, etc). The culture media was then removed, the cells were washed three times with PBS, and serum-free neurobasal media (Invitrogen, San Diego, CA, USA) was placed onto MDM for 24 h prior to MCM collection. In some cases, the MCM was collected “immediately” (2-5 min) after addition of neurobasal media to MDM and this “immediately”-collected MCM was used as one of the controls. The collected MCM were stored in aliquots at -80 C° until use. On the day of the experiment, MCM were thawed, diluted, and added to neuronal culture through bath perfusion. Potential existence of residual LPS in MCM was examined using ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit (Genscript Corp, Piscataway, NJ, USA).
Primary hippocampal neuronal culture
Hippocampal neuronal cultures were prepared from rat embryos using the methods described previously (Flavin et al., 1997). Briefly, female Sprague-Dawley (Charles River Laboratory, Wilmingham, MA) rats with 18-19 days of gestation were anesthetized with isoflurane, and embryonic rat pups were surgically removed and decapitated. Hippocampi were harvested under sterile conditions. The hippocampal tissue was enzymatically dissociated in 0.125% trypsin II (Sigma-Aldrich). Isolated neural cells were placed in poly-D-lysine-coated 35 mm plastic culture dishes containing 2 ml of neurobasal medium to a culture surface cell density of 5 × 105/ml (400-500/mm2). The cultures were maintained in neurobasal medium supplemented with B27 (2%, v/v, Invitrogen), glutamine (0.5mM) and penicillin/streptomycin (100U) for at least 7-10 days before being used for experiments. All animal-use procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Nebraska Medical Center (IACUC # 00-062-07).
MDM and hippocampal neuronal co-culture
After 7-10 days in culture, rat hippocampal neurons were co-cultured with human MDM (with or without LPS stimulation) for 24 h prior to performing electrophysiological recordings on neuronal cells. The MDM were collected via centrifugation (1500rpm/min for 5 min); the cells were then re-suspended in neurobasal media, counted and added to neuronal cultures at a concentration of 2×105 cells/ml. The ratio of MDM to neural cells was 1:2.5.
Examination of hippocampal neural cell viability
Experiments were performed in triplicate and total hippocampal neural cell survival in culture was determined by two approaches: 1) counting the number of cells and 2) MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] optical density (OD) assay. MCM were added to hippocampal neuronal culture (final concentration was 1:30 dilution) for 24 h and then cell injury was assessed by staining with a membrane-impermeable DNA-binding dye propidium iodide (PI, Molecular Probes, Eugene, OR, USA) and counterstaining with membrane-permeable 4″,6-diamidino-2-phenylindol (DAPI). More specifically, after incubation with neurobasal medium containing PI (1 μg/ml) for 15 min, cells were washed three times with phosphate buffered saline (PBS), and fixed with 4% paraformaldehyde (in PBS) for 20 min at room temperature. Cells were then permeabilized with 0.2% Triton X-100 (in PBS) for 5 min on ice and washed twice with PBS containing DAPI (0.1μg/ml). Subsequently, under fluorescent microscopy, red (PI) and blue (DAPI) fluorescent images were captured in order to determine the number of injured cells and the total number of cells, respectively. Five different visual fields per culture dish were evaluated.
Cell viability was also analyzed by MTT assay. After cell cultures were treated with MCM(+), 4-aminopyridine (4-AP) or 4-AP+MCM(+) for 24h, a solution of MTT-PBS (5 mg/ml) was added to the neurobasal medium in a 1:10 ratio by volume and mixed gently. After incubation for 3-4 h, the remaining MTT was removed; the cells were solubilized with dimethyl sulfoxide. The OD values of 100 μl aliquots were measured with a spectrophotometer at 570 nm (Kinetic Microplate Reader, Hewlett Packard). All OD values were obtained by MTT assay unless otherwise stated.
Electrophysiology
Whole-cell voltage-clamp was performed on rat hippocampal neuronal cultures in 35-mm tissue culture dishes on the stage of an inverted Nikon microscope (TE 300) using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA). Patch electrodes, made from borosilicate glass micropipettes (WPI Inc. Sarasota, FL) with a P-97 micropipette puller (Sutter Instruments, Novato, CA), had tip resistance of 3.5 - 5.5 MΩ. The electrodes were advanced towards cells by a PC-5000 Burleigh micromanipulator (EXFO, Mississauga, Canada). After establishment of the whole-cell patch configuration, the cells were allowed to stabilize for 3-5 min before tests. The recorded cells were held at −60mV during voltage clamping. Whole-cell outward K+ currents were induced by voltage steps from the holding potential of -60mV to -40mV in the first step and then stepped to +60mV in increments of 10mV. Junction potentials were corrected, and the cell capacitance was compensated (∼70%) in most cells. Current signals were filtered at 1 kHz by a 4-pole Bessel filter and digitized at 5 kHz using Digidata 1320A digitizer (Molecular Devices). The current and voltage traces were displayed and recorded in a Dell computer using pCLAMP 8.1 data acquisition/analysis system (Molecular Devices).
The pipette solution for voltage-clamp experiments contained (in mM): 108 K2HPO4, 9 HEPES, 9 EGTA, and 2.5 MgCl2 buffered to pH 7.4 with KOH (Sodickson and Bean, 1996). To promote the stability of the recordings, 14 mM creatine phosphate (Tris salt), 1 mM Mg-ATP, and 0.3 mM Tris-GTP were included in the pipette solution. Stocks (10×) of the creatine phosphate, ATP, and GTP were stored at −80°C. The extracellular solution contained (in mM): 150 NaCl, 4 KCl, 2 MgCl2, 2 CoCl2, 10 HEPES, 5 tetraethylammonium (TEA) and 10 glucose, buffered to pH 7.4 with NaOH. In order to block sodium channels, 0.3 μM tetrodotoxin (TTX; Calbiochem, La Jolla, CA) was added. To block calcium-activated K+ currents, nifedipine or cadmium chloride was added to the extracellular solution to block voltage-gated Ca2+ channels, or by replacing extracellular Ca2+ with equimolar (2 mM) Co2+ (Klee et al., 1995). Stock solutions of TEA (1 M), 4-AP (1 M), and TTX (1mM) were prepared in deionized water and either stored at 4°C (TEA and 4-AP) or in aliquots at −20°C (TTX).
All experiments were done at room temperature (22-23°C). During experiments, the neuronal cultures were continuously perfused with oxygenated (bubbled with 95% O2, 5% CO2) extracellular solution at a constant flow rate of 2ml/min. The neuronal cells were identified by their triangular-shaped morphology and their firing of action potentials in response to a depolarizing current injection. MCM and/or chemical reagents were applied through bath perfusion. Data was analyzed by Clampfit 8.1 (Molecular Devices) and graphed using Origin 7.5 (OriginLab, Northampton, MA, USA). For each set of experiments, instantaneous peak outward currents generated by voltage steps from -60mV to +60mV were measured and analyzed. The current densities were calculated and expressed as pA/pF. All data are expressed as mean ± S.E. unless otherwise indicated. Statistical analyses were performed by Student t tests. A minimum p value of 0.05 was estimated as the significance level for all tests.
Results
Enhancement of neuronal IA by MCM(+)
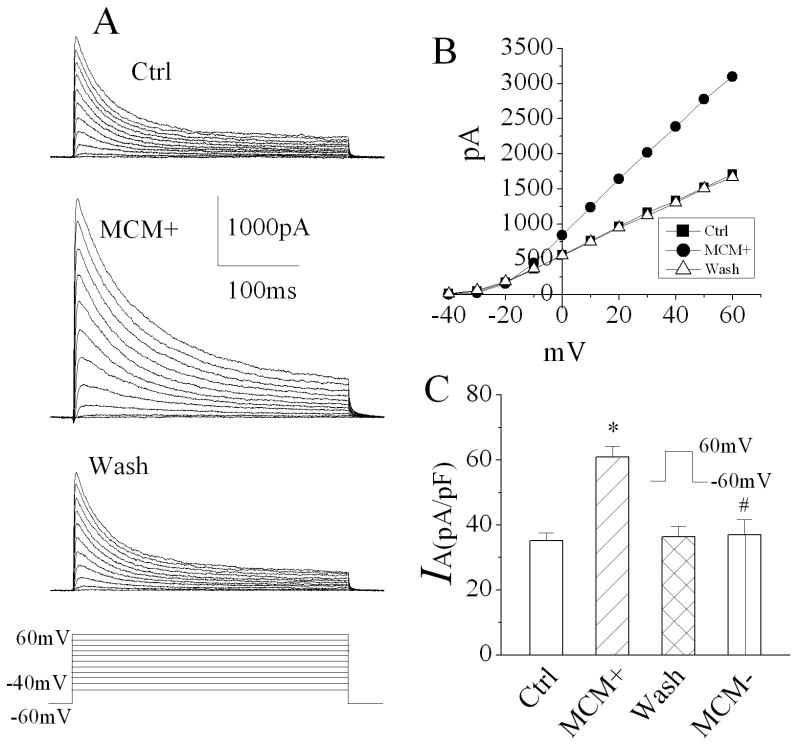
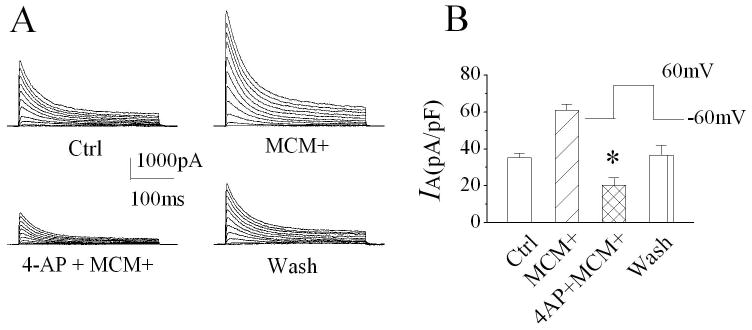
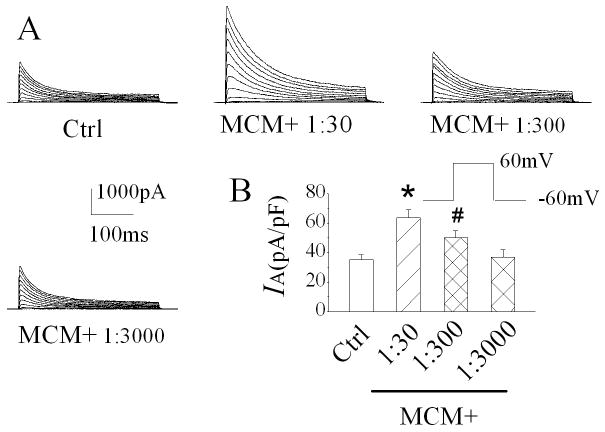
During brain infection/inflammation, monocytes migrate from the periphery into the brain infection/inflammation site and differentiate into macrophages. To investigate whether the infiltrated macrophages alter neuronal function via Kv channels, we studied effects of MCM(+) and MDM(+) on neuronal transient IA in rat primary hippocampal neuronal cultures using the whole-cell configuration of patch clamp techniques. Under voltage clamp conditions when the cell was held at -60mV, the depolarizing pulses more positive to -20mV elicited the outward currents, which peaked in a few milliseconds and inactivated within a few tens of milliseconds (Fig. 1A). The current amplitudes were increased with stronger depolarization as shown in Fig. 1B. When membrane potential depolarized to +60mV, the average instantaneous (peak) outward current density in control was 35.3 ± 2.4 pA/pF (Fig.1C, n=10). Bath application of MCM(+) at a concentration of 1:30 dilution enhanced the transient outward current. The average density of the transient outward current was 60.9 ± 3.5 pA/pF (Fig. 1C, n=10). In comparison with the average current density recorded before bath application of MCM(+) (control), the difference is statistically significant (p≤0.0001), suggesting that MCM(+) enhances neuronal transient outward current. The MCM(+)-induced enhancement of transient outward current was blocked by addition of 4-AP, a specific IA blocker, to the bath solution, indicating that MCM(+) enhances neuronal IA (Fig. 2, n=8). The MCM(+)-mediated increase of neuronal IA was reversible and returned to basal level after 10 -15 min washout. In contrast, bath application of MCM collected from non-stimulated MDM [MCM(-)] had no apparent effect on neuronal IA, with an average current density of 37.1 ± 4.6pA/pF (Fig. 1C, n=10). The difference was statistically significant when compared to the IA recorded during bath application of MCM(+) (p=0.0006), suggesting that LPS-stimulated MDM release soluble factors causing an increase of neuronal IA. The MCM(+)-mediated enhancement of IA was concentration (dilution) dependent. In another set of 11 neuronal cells, the average IA recorded before bath application of MCM(+) (control) was 35.1 ± 2.3 pA/pF. When these cells were superfused with MCM(+) at dilutions of 1:30, 1:300 or 1:3000, the average IA recorded during the bath perfusion were 63.6 ± 5.4 pA/pF, 50.2 ± 4.8 pA/pF and 36.9 ± 5.1 pA/pF, respectively (Fig. 3). These results clearly showed that LPS-stimulated MDM secrete soluble factors causing enhancement of neuronal IA. To rule out the possible presence of residual LPS in MCM(+), we performed LPS detection using ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit (Genscript Corp.) with a high sensitivity of 0.005EU/ml. The results showed that there was no detectable LPS present in the MCM(+)(n=3). To further rule out the possible existence of residual LPS in MCM(+), we tested effects of the “immediately”-collected MCM on neuronal IA. Our results showed that the average IA recorded during bath application of the “immediately”-collected MCM (1:30 dilution) was 38.5 ±5.2 pA/pF (n=5). The difference was statistically significant (p=0.03) when compared with the IA recorded during bath application of MCM(+) (60.9 ± 3.5 pA/pF, n=10), suggesting that the MCM(+)-associated enhancement of neuronal IA was not mediated by residual LPS which might be present, if any, in MCM(+).
Figure 1.
Bath application of MCM(+) significantly enhanced neuronal IA recorded from primary rat hippocampal neuronal cultures. Panels A are exemplary IA traces recorded from a hippocampal neuron before (Ctrl), during (MCM+) and after (wash) bath application of MCM(+) at a concentration of 1:30 dilution. Note that MCM(+) produced a significant increase of IA. Panel B shows the I-V plot of peak IA as shown in Panel A. Panels C is a summary bar graph illustrating the peak IA elicited by a voltage step from -60mv to +60mV at different experimental conditions. * p≤0.0001 vs Ctrl; # p=0.0006 vs MCM(+). Voltage protocol used to generate outward current traces is shown at the bottom in panel A and the same voltage protocol was employed for all voltage clamp experiments.
Figure 2.
Blockade of MCM(+)-induced enhancement of neuronal IA by 4-AP, an IA antagonist. The IA traces recorded during control (Ctrl), bath application of MCM(+) or 4-AP+MCM(+), and during wash period are shown in Panel A. Note the blockade of MCM(+)-induced increase of IA by 4-AP. The bar graph in Panel B exhibits a summary data from 8 cells showing 4-AP blockade of MCM(+) enhancement of IA. * p≤0.0001 vs MCM(+).
Figure 3.
MCM(+) increases IA in a dose-dependent manner. Panel A illustrates IA recorded from a hippocampal neuron before and during bath application of MCM(+) at the concentrations indicated. Panel B summarizes the IA current densities recorded from 11 neurons. * p≤0.0001 vs Ctrl, # p=0.01 vs Ctrl.
Augmentation of neuronal IA by MDM(+)
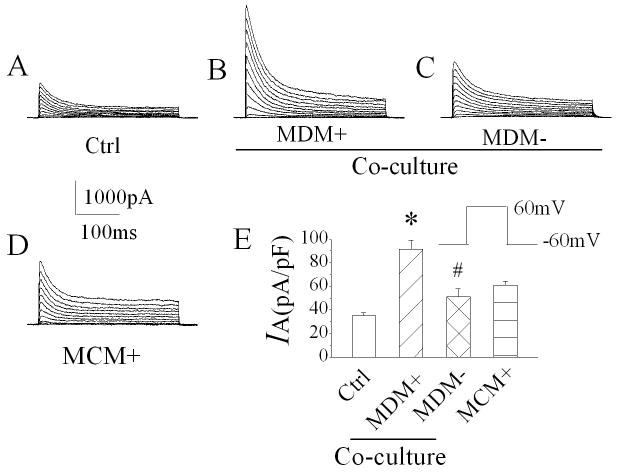
After observation of MCM(+) enhancement of neuronal IA, we further examined whether the activated macrophages, if co-cultured with neurons, have the same augmentative effects on neuronal IA. Using whole-cell voltage clamp techniques, we recorded IA from control neurons (no MDM was added to the culture) and neurons co-cultured with either MDM(+) or MDM(-) for 24 h, respectively. The average peak IA recorded from neurons co-cultured with MDM(+) was 91.4 ± 7.7pA/pF (n=10), compared to 35.3 ± 2.4pA/pF (n=10) recorded in control neurons, the difference was statistically significant (p≤0.0001), indicating that MDM(+) has the same effect on neuronal IA as that produced by MCM(+) (Fig. 4). The peak IA recorded from neurons co-cultured with MDM(-) exhibited an intermediate enhancement, with an average of 51.4 ± 6.5pA/pF (n=10). In comparison with the IA recorded in control neurons, the difference is also statistically significant (p=0.03), suggesting that MDM without LPS stimulation might also secrete soluble factors, resulting in an increase of neuronal IA.
Figure 4.
MDM(+)-mediated enhancement of neuronal IA in a MDM-neuronal co-culture system. MDM(+) or non-stimulated MDM [MDM(-)] were added to neuronal cultures 24 h prior to the recording. Current traces were recorded from four different neurons with different treatments: (A) control (Ctrl), (B) co-cultured with LPS-stimulated MDM (MDM+), (C) co-cultured with non-stimulated MDM (MDM-) and (D) cultured with MCM+. Compared with control, an increase of IA was recorded in neurons co-cultured with MDM+, MDM- and MCM+, respectively (E). Note the strongest effect was produced by MDM+. * p≤0.0001 vs Ctrl, # p=0.03 vs Ctrl.
Involvement of 4-AP sensitive IA in MCM(+)-mediated neuronal injury
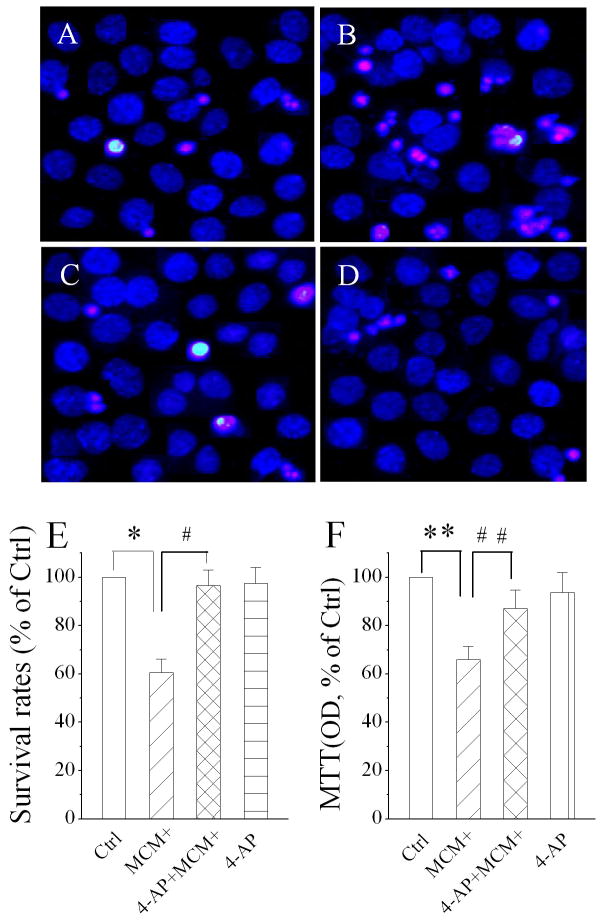
Numerous studies have shown that activated macrophages induce neuronal injury both in vitro and in vivo. To determine whether the MCM(+)-induced enhancement of the 4-AP sensitive IA is involved in MCM(+)-mediated neuronal injury, we examined the neuronal viability by the addition of MCM(+) to the culture in the presence or absence of 4-AP. Twenty-four h after addition of MCM(+)(Fig. 5B), 4-AP+MCM(+)(Fig. 5C) or 4-AP (Fig. 5D), the cell viability was assessed using combined PI and DAPI staining or MTT assay. Studies using PI/DAPI staining showed that the addition of MCM(+) to the culture media produced a significant (∼40%) reduction of cell survival and that this MCM(+)-associated reduction in cell viability was reversed by 4-AP (Fig. 5E). MTT assay revealed an approximately 35% reduction on cell viability, which was significantly blocked by 4-AP (Fig. 5F). 4-AP, however, did not affect cell survival when applied alone (Fig. 5E, 5F).
Figure 5.
Attenuation of MCM(+)-induced neuronal injury by 4-AP, a Kv channel antagonist. Nuclear morphology and membrane integrity of hippocampal neurons were evaluated by fluorescent dyes PI (red) and DAPI (blue). Panels A-D are fluorescent microscopic images showing addition of MCM(+) to neuronal culture induced neuronal injury (B) and the MCM(+)-induced neuronal injury was significantly attenuated by inclusion of 4-AP in the culture media (C). Survival rates were calculated by counting the number of cells from five different visual fields in each dish containing cultured neurons stained by DAPI and PI dyes are shown in Panel E (n=36 visual fields). Panel F illustrates MCM(+)-associated neuronal injury, assayed using MTT assay (n=36 visual fields). Note that the MCM(+)-induced neuronal injury was blocked by 4-AP. *p=0.002 vs Ctrl, # p=0.01 vs MCM(+), ** p=0.03 vs Ctrl, ## p=0.08 vs MCM(+).
Identification of potential active factors for MCM(+)-induced enhancement of neuronal IA
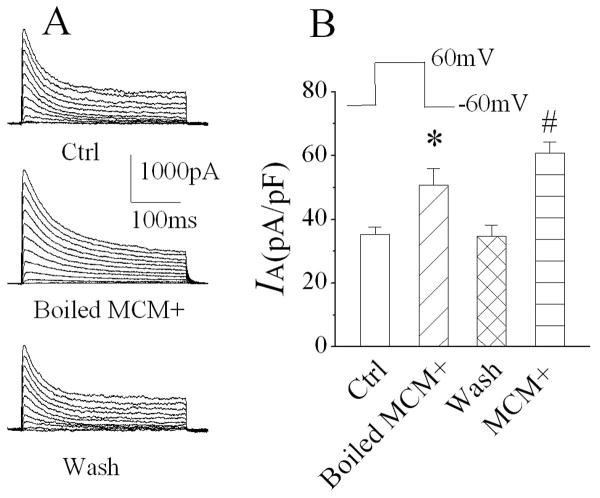
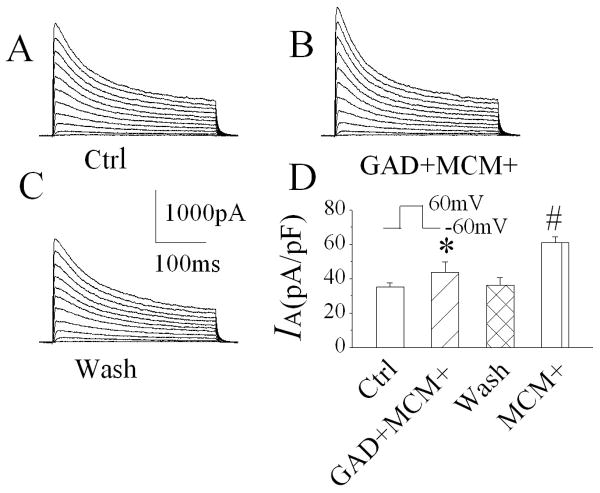
Activated macrophages release a variety of bioactive molecules including, but not limited to, cytokines, chemokines, glutamate, etc. Previously, we have shown that active components underlying the MCM(+)-induced enhancement of neuronal delayed rectifier K+ current was resistant to either heat (boiled) or glutamate decarboxylase (GAD)(Hu et al., 2009). In this study, we examined whether the active factors mediating MCM(+) enhancement of neuronal IA were also resistant to heat or GAD. When applied through bath perfusion at a dilution of 1:30, the heat-treated (boiled for 15 min) MCM(+) produced an increase of IA. The average IA was 50.8 ± 5.3pA/pF (n=8), compared to IA recorded before application of boiled MCM(+) (35.2 ± 2.3pA/pF, n=8), the difference is statistically significant (Fig. 6, p=0.02), suggesting that the proteinous factors released by MDM(+) may not be involved in MCM(+)-mediated enhancement of IA. Studies have shown that immune activated macrophages release glutamate (Jiang et al., 2001), NMDA receptors are coupled with KV channels (Mulholland et al., 2008) and KV channels involved in glutamate-induced neuronal apoptosis (Zhao et al., 2006). To examine whether glutamate is one of the active factors for MCM(+)-associated enhancement of IA, we incubated MCM(+) with GAD for 3 h at 37°C. Bath application of GAD-treated MCM(+) slightly enhanced IA. The average IA was 43.7±6.1 pA/pF (n=8), in comparison with the IA (34.8 ± 3.4pA/pF, n=8) recorded before bath application of GAD-treated MCM(+); the difference was not statistically significant (Fig. 7, p=0.223), suggesting that glutamate released by LPS-stimulated MDM might be the potential factor for enhancing neuronal IA.
Figure 6.
Heating denature of the proteins by boiling the MCM(+) for 15 min failed to abolish the effect of MCM(+) on neuronal IA. Panel A is the exemplary current traces recorded from hippocampal neurons before (Ctrl), during (boiled MCM+) and after (wash) bath application of the boiled MCM(+). Panel B is the bar graph illustrating the average IA current densities. Note that denature of MCM+ proteins failed to prevent MCM(+)-induced enhancement of neuronal current. * p=0.018 vs Ctrl, # p≤0.0001 vs Ctrl.
Figure 7.
Incubation of MCM(+) with glutamate decarboxylase (GAD) for 3 hr significantly reduced MCM(+)-mediated increase of IA. Panels A-C are current traces recorded from same neuron before (A), during (B) and after (C) bath perfusion of GAD-treated MCM(+)[GAD+MCM+]. The GAD-treated MCM(+) produced a slightly increase of IA, but there is no statistical significance when compared with the recorded during control (Ctrl). However, there is a statistical significance when compared with the IA recorded during bath perfusion of MCM(+), suggesting that glutamate released by MDM plays a partial role in MCM(+)-associated increase of IA. *p=0.223 vs Ctrl, # p≤0.0001 vs Ctrl .
Discussion
Several different macrophage populations exist in the CNS, including transient perivascular macrophages and resident microglia. In healthy individuals, these populations support critical immune and homeostatic functions without pathological consequences (Cotter et al., 2002; Williams and Hickey, 2002). However, under certain circumstances, a cycle of macrophage activation can cause neurotoxicity through excessive secretion of inflammatory and immunoactive substances. For example, neuropsychiatric decline in AIDS patients is correlated with increasing numbers of macrophage in the brain (Glass et al., 1995), and the neuronal damage observed in AIDS patients is closely associated with markers of macrophage activation (Adle-Biassette et al., 1999). This suggests the source of neuronal dysfunction may be the release of soluble factors from infected and/or activated macrophage. In this study, we demonstrated that soluble factor(s) released from LPS-stimulated MDM enhanced voltage-dependent, 4-AP sensitive IA in cultured rat hippocampal neurons in a concentration-dependent manner. The enhancement of neuronal IA by MCM(+) was recapitulated in a MDM-hippocampal neuronal co-culture system, suggesting that macrophage enhances neuronal transient A-type K+ current by releasing soluble factors. Although LPS stimulation is not an ideal disease model, it does act as the most reliable and widely accepted means in vitro to induce MDM activation and resultant secretion, an immunopathophysiological process involved in neurodegenerative disorders. As LPS was shown to induce outward K currents(Chung et al., 1998; Seydel et al., 2001), the presence of residual LPS in the MCM(+) was ruled out by endotoxin detection showing that the residual LPS was undetectable by an endotoxin assay kit with high sensitivity and by experimental results demonstrating that the “immediately”-collected MCM(+), which might contain residual LPS but without MDM-secreted factors, failed to produce an enhancement of neuronal IA.
The IA plays a crucial role in hippocampal neuronal dendritic membrane excitability and synaptic plasticity as there is a linear increase in its density with distance from soma to distal dendrites in the hippocampal pyramidal cells (Hoffman et al., 1997). The presence of these A-type K+ channels prevents the dendritic initiation of Na+ and Ca2+ action potentials, limits the back-propagation of action potentials into the dendrites and reduces the amplitude of excitatory synaptic events. As a result, the A-type K+ channels expressed in the dendrites powerfully dampen the excitability of the dendritic membrane (Hoffman et al., 1997). Thus, the enhancement of IA by MCM(+) or MDM(+), which was observed in our experiments, may cause a significant compromise in neuronal excitability as observed in our previous report(Wang et al., 2008), resulting in neuronal dysfunction or injury.
The correlation between increased outward K+ current and cell apoptosis has been demonstrated in multiple cells types including neurons (Yu, 2003; Burg et al., 2006). Neuronal apoptosis, resulting from the enhancement of neuronal outward K+ current, was shown by Yu and his colleagues (Yu et al., 1997) and supported by many subsequent studies (Hribar et al., 2004; Remillard and Yuan, 2004; Grishin et al., 2005; Hu et al., 2006). The apoptotic cell shrinkage is believed to be a consequence of an increased K+ and Cl- efflux and the activation of Kv channels (Yu et al., 1997; Bock et al., 2002). Thus, enhanced K+ efflux has been considered as an essential mediator for not only early apoptotic cell shrinkage but also downstream caspase activation and DNA fragmentation (Remillard and Yuan, 2004). Our results that MCM(+) and MDM(+) increased neuronal IA and induced neuronal apoptosis as detected by PI/DAPI staining and that the MCM(+)-induced neuronal apoptosis was blocked by A-type K+ channel antagonist are in an full agreement with the above mentioned studies and further support the notion that enhancement of neuronal K+ efflux induces neuronal apoptosis. Our results also suggest that under disease condition, activated brain macrophages (and microglia) release soluble factors causing neuronal apoptosis via enhancement of neuronal outward K+ current.
The Kv channel blocking agent, 4-AP, is used clinically to relieve neurological symptoms secondary to conduction block in patients with multiple sclerosis and chronic spinal cord injury (Jensen and Shi, 2003; Judge and Bever, 2006). 4-AP treatment restores conduction and increases presynaptic action potential duration and amplitude to increased transmitter release in experimentally demylinated peripheral nerves by blocking Kv channels either on demylinated axons or at synaptic endings (Hayes, 2004). 4-AP has also been used for the treatment of Alzheimer's disease, possibly by enhancing neurotransmitter release via blockade of presynaptic K+ channels (Davidson et al., 1988). The therapeutic efficacy of 4-AP suggests a role for IA in the pathogenesis of neurological disorders. We found that addition of 4-AP to the hippocampal neuronal cultures blocked MCM(+)-induced neuronal injury in vitro. As MCM(+) contains predominately pro-inflammatory cytokines and glutamate released from MDM(+), our results suggest that under disease conditions, such as HIV-1 brain infection, immune activated macrophages (brain microglia) may induce neuronal injury by activating neuronal Kv channels. This suggestion is supported by our previous studies that systemic administration of 4-AP improves learning and memory in a murine model of HIV-1 encephalitis(Keblesh et al., 2009b).
Biological significance of MCM(+)-associated increase of IA and resultant neuronal apoptosis remain to be determined. Recent genetic targeting studies indicate that Kv channel activity is of great importance in memory processes (Giese et al., 1998; Giese et al., 2001; Solntseva et al., 2003). As the number and pattern of action potentials (APs) are thought to encode information (Reike et al., 1997) and A-type K+ channel activity influences the number and pattern of APs, the MCM(+)-associated increase of IA and resultant neuronal apoptosis might alter information processing in the brain, resulting in disturbance in learning and memory as seen in AIDS patients with HAD. In fact, experiments in several different model systems have now shown decreased K+ channel current correlates with improved long-term potentiation (LTP) and memory, while increased K+ current corresponds to learning and memory deficiencies (Ghelardini et al., 1998; Alkon, 1999; Solntseva et al., 2003). At present, the effect of altered IA is best characterized by studies of Kv4 channels in the distal dendrites. In particular, Kv4 channels are thought to provide a convergence point for LTP signal transduction pathways (Olds et al., 1989; Alkon et al., 1998; Dineley et al., 2001; Birnbaum et al., 2004). Increasing or decreasing the IA inversely affects back-propagating AP amplitudes (Watanabe et al., 2002), which helps determine the depolarization sensed by N-methyl-D-aspartic acid (NMDA) receptors and may underlie learning and memory networking properties (Paulsen and Sejnowski, 2000; Johnston et al., 2003; Birnbaum et al., 2004). Importantly, increased Kv4 current lowers LTP induction probability (Watanabe et al., 2002), while decreased Kv4 current enhances LTP (Frick et al., 2004), increases excitatory post-synaptic potential (EPSP)-spike potentiation (Frick et al., 2004) and improves learning and memory (Lilliehook et al., 2003). Therefore, enhancement of IA by macrophages may contribute to the neurocognitive impairment as seen in neurological disorders including HAD.
It is well known that activated macrophages release a variety of immune and inflammatory substances including, but not limited to, cytokines, chemokines, glutamate, quinolinic acid, arachidonic acid and its metabolites, platelet activating factor, nitric oxide (Xiong et al., 2000; Kaul et al., 2001). To explore the active components causing the enhancement of IA, we focused our studies on cytokines/chemokines and glutamate, as they are reliably detected in the conditioned media (Cotter et al., 1998; Jiang et al., 2001; Williams and Hickey, 2002; Erdmann et al., 2007). Our results showed that heat (boiled MCM+) denature of the proteinous components (i.e. cytokines and chemokines) had no significant effects on the MCM(+)-mediated enhancement of neuronal IA, indicating that cytokines and chemokines released by MDM might not be the main active factors for increasing neuronal IA. However, treatment of MCM(+) with GAD significantly attenuated the effects of MCM(+) on IA, suggesting that glutamate released by LPS-stimulated MDM plays a role in enhancing neuronal IA and consequently mediating neuronal injury. Our results are in consistent with the recent findings showing that glutamate enhances neuronal IA and that Kv channels are involved in glutamate-induced neuronal apoptosis (Zhao et al., 2006; Shen et al., 2008).
It is worth pointing out that the current densities recorded in neurons co-cultured with MDM(-) for 24 h, as shown in Fig 4E, was much larger than those recorded in neurons exposed to MCM(-) for 20 - 30 min via bath perfusion (Fig 1C). The difference might be generated most likely by the experimental conditions that during human MDM - rat hippocampal neuronal co-culture human MDM might “recognize” rat hippocampal neurons as foreign cells, leading to activation of human MDM and resultant secretion of immune active substances, which produce larger outward K+ current, in addition to much longer exposure time of hippocampal neurons to MDM-secreted active substances in the co-culture system (24 h) than in a bath perfusion system (20-30 min).
In summary, our results demonstrated that immune activated macrophages or the conditioned media recovered from immune activated macrophages produced an enhancement of IA in cultured rat hippocampal neurons. The link between macrophage-mediated increase of neuronal IA and neuronal apoptotic injury was demonstrated by experimental results showing that addition of 4-AP, an IA blocker, protects hippocampal neurons from apoptotic injury induced by activated macrophage or the conditioned media recovered from immune activated macrophages. Further investigation revealed that glutamate released by LPS-stimulated MDM is at least in part involved in MCM(+)/MDM(+)-mediated enhancement of neuronal IA. These results provide not only a new insight into the mechanism for macrophage-associated neuronal injury, but also a potential target for the development of therapeutic strategies.
Acknowledgments
The authors thank Ms. Robin Taylor and Mr. Benjamin C. Reiner for their critical reading of the manuscript. The authors extend special thanks to Ms. Julie Ditter, Ms. Robin Taylor and Ms. Johna Belling for their excellent administrative support. This work was supported by NIH grant 2 R01 NS041862 to H.X.
References
- Adle-Biassette H, Chretien F, Wingertsmann L, Hery C, Ereau T, Scaravilli F, Tardieu M, Gray F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol. 1999;25:123–133. doi: 10.1046/j.1365-2990.1999.00167.x. [DOI] [PubMed] [Google Scholar]
- Alkon DL. Ionic conductance determinants of synaptic memory nets and their implications for Alzheimer's disease. J Neurosci Res. 1999;58:24–32. [PubMed] [Google Scholar]
- Alkon DL, Favit A, Nelson T. Evolution of adaptive neural networks: the role of voltage-dependent K+ channels. Otolaryngol Head Neck Surg. 1998;119:204–211. doi: 10.1016/S0194-5998(98)70055-5. [DOI] [PubMed] [Google Scholar]
- Arantes RM, Lourenssen S, Machado CR, Blennerhassett MG. Early damage of sympathetic neurons after co-culture with macrophages: a model of neuronal injury in vitro. Neuroreport. 2000;11:177–181. doi: 10.1097/00001756-200001170-00035. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev. 2004;84:803–833. doi: 10.1152/physrev.00039.2003. [DOI] [PubMed] [Google Scholar]
- Bock J, Szabo I, Jekle A, Gulbins E. Actinomycin D-induced apoptosis involves the potassium channel Kv1.3. Biochem Biophys Res Commun. 2002;295:526–531. doi: 10.1016/s0006-291x(02)00695-2. [DOI] [PubMed] [Google Scholar]
- Burg ED, Remillard CV, Yuan JX. K+ channels in apoptosis. J Membr Biol. 2006;209:3–20. doi: 10.1007/s00232-005-0838-4. [DOI] [PubMed] [Google Scholar]
- Cameron DJ, Churchill WH. Macrophage mediated cytotoxicity in man: role of hydrocortisone, trypan blue, chloroquine and prednisolone. Int J Immunopharmacol. 1981;3:77–85. doi: 10.1016/0192-0561(81)90047-3. [DOI] [PubMed] [Google Scholar]
- Chen X, Chi S, Liu M, Yang W, Wei T, Qi Z, Yang F. Inhibitory effect of ganglioside GD1b on K+ current in hippocampal neurons and its involvement in apoptosis suppression. J Lipid Res. 2005;46:2580–2585. doi: 10.1194/jlr.M500252-JLR200. [DOI] [PubMed] [Google Scholar]
- Chung S, Joe E, Soh H, Lee MY, Bang HW. Delayed rectifier potassium currents induced in activated rat microglia set the resting membrane potential. Neurosci Lett. 1998;242:73–76. doi: 10.1016/s0304-3940(98)00029-9. [DOI] [PubMed] [Google Scholar]
- Colom LV, Diaz ME, Beers DR, Neely A, Xie WJ, Appel SH. Role of potassium channels in amyloid-induced cell death. J Neurochem. 1998;70:1925–1934. doi: 10.1046/j.1471-4159.1998.70051925.x. [DOI] [PubMed] [Google Scholar]
- Cotter R, Zheng J, Gendelman HE. The role of mononuclear phagocytes in neurodegenerative disorders: Lessons from multisclerosis, Alzheimer's disease and HIV-1 associated dementia. In: Marwah J, Teitelbaum H, editors. Advances in Neurodegenerative Disorders Vol2, Alzheimer's and Aging. Scottsdale, AZ: Prominent Press; 1998. pp. 203–241. [Google Scholar]
- Cotter R, Williams C, Ryan L, Erichsen D, Lopez A, Peng H, Zheng J. Fractalkine (CX3CL1) and brain inflammation: Implications for HIV-1-associated dementia. J Neurovirol. 2002;8:585–598. doi: 10.1080/13550280290100950. [DOI] [PubMed] [Google Scholar]
- Davidson M, Zemishlany Z, Mohs RC, Horvath TB, Powchik P, Blass JP, Davis KL. 4-Aminopyridine in the treatment of Alzheimer's disease. Biol Psychiatry. 1988;23:485–490. doi: 10.1016/0006-3223(88)90020-0. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Weeber EJ, Atkins C, Adams JP, Anderson AE, Sweatt JD. Leitmotifs in the biochemistry of LTP induction: amplification, integration and coordination. J Neurochem. 2001;77:961–971. doi: 10.1046/j.1471-4159.2001.00321.x. [DOI] [PubMed] [Google Scholar]
- Erdmann N, Zhao J, Lopez AL, Herek S, Curthoys N, Hexum TD, Tsukamoto T, Ferraris D, Zheng J. Glutamate production by HIV-1 infected human macrophage is blocked by the inhibition of glutaminase. J Neurochem. 2007;102:539–549. doi: 10.1111/j.1471-4159.2007.04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavin MP, Coughlin K, Ho LT. Soluble macrophage factors trigger apoptosis in cultured hippocampal neurons. Neuroscience. 1997;80:437–448. doi: 10.1016/s0306-4522(97)00078-x. [DOI] [PubMed] [Google Scholar]
- Fordyce CB, Jagasia R, Zhu X, Schlichter LC. Microglia Kv1.3 channels contribute to their ability to kill neurons. J Neurosci. 2005;25:7139–7149. doi: 10.1523/JNEUROSCI.1251-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci. 2004;7:126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Banati RB, Wiessner C, Hossmann KA, Kreutzberg GW. Reactive microglia in cerebral ischaemia: an early mediator of tissue damage? Neuropathol Appl Neurobiol. 1995;21:277–289. doi: 10.1111/j.1365-2990.1995.tb01062.x. [DOI] [PubMed] [Google Scholar]
- Gelbard HA, James H, Sharer L, Perry SW, Saito Y, Kazee AM, Blumberg BM, Epstein LG. Identification of apoptotic neurons in post-mortem brain tissue with HIV-1 encephalitis and progressive encephalopathy. Neuropathol Appl Neurobiol. 1995;21:208–217. doi: 10.1111/j.1365-2990.1995.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Soukup VM, Schuenke KW, Keherly MJ, Holzer C, 3rd, Richey FJ, Lahart CJ. Acquired neuronal channelopathies in HIV-associated dementia. J Neuroimmunol. 2004;157:111–119. doi: 10.1016/j.jneuroim.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Oreinstein JM, Martin MA, Mitra R, Phipps T, Wahl LA, Lane HC, Fauci AS, Burke DS. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1498–1506. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelardini C, Galeotti N, Bartolini A. Influence of potassium channel modulators on cognitive processes in mice. Br J Pharmacol. 1998;123:1079–1084. doi: 10.1038/sj.bjp.0701709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Peters M, Vernon J. Modulation of excitability as a learning and memory mechanism: a molecular genetic perspective. Physiol Behav. 2001;73:803–810. doi: 10.1016/s0031-9384(01)00517-0. [DOI] [PubMed] [Google Scholar]
- Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ. Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvbeta1.1-deficient mice with impaired learning. Learn Mem. 1998;5:257–273. [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Grishin A, Ford H, Wang J, Li H, Salvador-Recatala V, Levitan ES, Zaks-Makhina E. Attenuation of apoptosis in enterocytes by blockade of potassium channels. Am J Physiol Gastrointest Liver Physiol. 2005;289:G815–821. doi: 10.1152/ajpgi.00001.2005. [DOI] [PubMed] [Google Scholar]
- Hayes KC. The use of 4-aminopyridine (fampridine) in demyelinating disorders. CNS Drug Rev. 2004;10:295–316. doi: 10.1111/j.1527-3458.2004.tb00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Hribar M, Bloc A, Medilanski J, Nusch L, Eder-Colli L. Voltage-gated K+ current: a marker for apoptosis in differentiating neuronal progenitor cells? Eur J Neurosci. 2004;20:635–648. doi: 10.1111/j.1460-9568.2004.03520.x. [DOI] [PubMed] [Google Scholar]
- Hu CL, Liu Z, Zeng XM, Liu ZQ, Chen XH, Zhang ZH, Mei YA. 4-aminopyridine, a Kv channel antagonist, prevents apoptosis of rat cerebellar granule neurons. Neuropharmacology. 2006;51:737–746. doi: 10.1016/j.neuropharm.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Hu D, Liu J, Xiong H. Enhancement of neuronal outward delayed rectifier K+ current by human monocyte-derived macrophages. Glia. 2009;57:1492–1500. doi: 10.1002/glia.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JM, Shi R. Effects of 4-aminopyridine on stretched mammalian spinal cord: the role of potassium channels in axonal conduction. J Neurophysiol. 2003;90:2334–2340. doi: 10.1152/jn.00868.2002. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Piggee C, Heyes MP, Murphy C, Quearry B, Bauer M, Zheng J, Gendelman HE, Markey SP. Glutamate is a mediator of neurotoxicity in secretions of activated HIV-1-infected macrophages. J Neuroimmunol. 2001;117:97–107. doi: 10.1016/s0165-5728(01)00315-0. [DOI] [PubMed] [Google Scholar]
- Johnston D, Christie BR, Frick A, Gray R, Hoffman DA, Schexnayder LK, Watanabe S, Yuan LL. Active dendrites, potassium channels and synaptic plasticity. Philos Trans R Soc Lond B Biol Sci. 2003;358:667–674. doi: 10.1098/rstb.2002.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SI, Bever CT., Jr Potassium channel blockers in multiple sclerosis: neuronal Kv channels and effects of symptomatic treatment. Pharmacol Ther. 2006;111:224–259. doi: 10.1016/j.pharmthera.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Keblesh J, Hu D, Xiong H. Voltage-gated potassium channels in human immunodeficiency virus type-1 (HIV-1)-associated neurocognitive disorders. J Neuroimmune Pharmacol. 2009a;4:60–70. doi: 10.1007/s11481-008-9106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keblesh JP, Dou H, Gendelman HE, Xiong H. 4-Aminopyridine improves spatial memory in a murine model of HIV-1 encephalitis. J Neuroimmune Pharmacol. 2009b;4:317–327. doi: 10.1007/s11481-009-9161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee R, Ficker E, Heinemann U. Comparison of voltage-dependent potassium currents in rat pyramidal neurons acutely isolated from hippocampal regions CA1 and CA3. J Neurophysiol. 1995;74:1982–1995. doi: 10.1152/jn.1995.74.5.1982. [DOI] [PubMed] [Google Scholar]
- Lauritzen I, Zanzouri M, Honore E, Duprat F, Ehrengruber MU, Lazdunski M, Patel AJ. K+-dependent cerebellar granule neuron apoptosis. Role of task leak K+ channels. J Biol Chem. 2003;278:32068–32076. doi: 10.1074/jbc.M302631200. [DOI] [PubMed] [Google Scholar]
- Lees GJ. The possible contribution of microglia and macrophages to delayed neuronal death after ischemia. J Neurol Sci. 1993;114:119–122. doi: 10.1016/0022-510x(93)90285-7. [DOI] [PubMed] [Google Scholar]
- Lilliehook C, Bozdagi O, Yao J, Gomez-Ramirez M, Zaidi NF, Wasco W, Gandy S, Santucci AC, Haroutunian V, Huntley GW, Buxbaum JD. Altered Abeta formation and long-term potentiation in a calsenilin knock-out. J Neurosci. 2003;23:9097–9106. doi: 10.1523/JNEUROSCI.23-27-09097.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12:893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Carpenter-Hyland EP, Hearing MC, Becker HC, Woodward JJ, Chandler LJ. Glutamate transporters regulate extrasynaptic NMDA receptor modulation of Kv2.1 potassium channels. J Neurosci. 2008;28:8801–8809. doi: 10.1523/JNEUROSCI.2405-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau H, McKinney S, Anderson DJ, Lester HA. ROMK1 (Kir1.1) causes apoptosis and chronic silencing of hippocampal neurons. J Neurophysiol. 2000;84:1062–1075. doi: 10.1152/jn.2000.84.2.1062. [DOI] [PubMed] [Google Scholar]
- Olds JL, Anderson ML, McPhie DL, Staten LD, Alkon DL. Imaging of memory-specific changes in the distribution of protein kinase C in the hippocampus. Science. 1989;245:866–869. doi: 10.1126/science.2772638. [DOI] [PubMed] [Google Scholar]
- Paulsen O, Sejnowski TJ. Natural patterns of activity and long-term synaptic plasticity. Curr Opin Neurobiol. 2000;10:172–179. doi: 10.1016/s0959-4388(00)00076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piani D, Spranger M, Frei K, Schaffner A, Fontana A. Macrophage-induced cytotoxicity of N-methyl-D-aspartate receptor positive neurons involves excitatory amino acids rather than reactive oxygen intermediates and cytokines. Eur J Immunol. 1992;22:2429–2436. doi: 10.1002/eji.1830220936. [DOI] [PubMed] [Google Scholar]
- Reike F, Warland R, de Ruyter van Steveninck R, Bialek W. Spikes: Exploring the Neural Code. Cambridge, MA: MIT Press; 1997. [Google Scholar]
- Remillard CV, Yuan JX. Activation of K+ channels: an essential pathway in programmed cell death. Am J Physiol Lung Cell Mol Physiol. 2004;286:L49–67. doi: 10.1152/ajplung.00041.2003. [DOI] [PubMed] [Google Scholar]
- Seydel U, Scheel O, Muller M, Brandenburg K, Blunck R. A K+ channel is involved in LPS signaling. J Endotoxin Res. 2001;7:243–247. [PubMed] [Google Scholar]
- Shen B, Zhou K, Yang S, Xu T, Wang Y. The Kv4.2 mediates excitatory activity-dependent regulation of neuronal excitability in rat cortical neurons. J Neurochem. 2008;105:773–783. doi: 10.1111/j.1471-4159.2007.05179.x. [DOI] [PubMed] [Google Scholar]
- Sodickson DL, Bean BP. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J Neurosci. 1996;16:6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solntseva EI, Bukanova Iu V, Skrebitskii VG. Memory and potassium channels. Usp Fiziol Nauk. 2003;34:16–25. [PubMed] [Google Scholar]
- Thery C, Chamak B, Mallat M. Cytotoxic effect of brain macrophages on developing neurons. Eur J Neurosci. 1991;3:1155–1164. doi: 10.1111/j.1460-9568.1991.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Thery C, Chamak B, Mallat M. Neurotoxicity of brain macrophages. Clin Neuropathol. 1993;12:288–290. [PubMed] [Google Scholar]
- Wang W, Hu D, Xiong H. Macrophage attenuation of neuronal excitability: implications for pathogenesis of neurodegenerative disorders. Glia. 2008;56:241–246. doi: 10.1002/glia.20609. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Hoffman DA, Migliore M, Johnston D. Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2002;99:8366–8371. doi: 10.1073/pnas.122210599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Xiao AY, Jin C, Yang A, Lu ZY, Yu SP. Effects of chloride and potassium channel blockers on apoptotic cell shrinkage and apoptosis in cortical neurons. Pflugers Arch. 2004;448:325–334. doi: 10.1007/s00424-004-1277-2. [DOI] [PubMed] [Google Scholar]
- Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- Xiong H, Zeng YC, Lewis T, Zheng J, Persidsky Y, Gendelman HE. HIV-1 infected mononuclear phagocyte secretory products affect neuronal physiology leading to cellular demise: relevance for HIV-1-associated dementia. J Neurovirol. 2000;6 1:S14–23. [PubMed] [Google Scholar]
- Yu SP. Regulation and critical role of potassium homeostasis in apoptosis. Prog Neurobiol. 2003;70:363–386. doi: 10.1016/s0301-0082(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Yu SP, Farhangrazi ZS, Ying HS, Yeh CH, Choi DW. Enhancement of outward potassium current may participate in beta- amyloid peptide-induced cortical neuronal death. Neurobiol Dis. 1998;5:81–88. doi: 10.1006/nbdi.1998.0186. [DOI] [PubMed] [Google Scholar]
- Yu SP, Yeh CH, Sensi SL, Gwag BJ, Canzoniero LM, Farhangrazi ZS, Ying HS, Tian M, Dugan LL, Choi DW. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science. 1997;278:114–117. doi: 10.1126/science.278.5335.114. [DOI] [PubMed] [Google Scholar]
- Zhao YM, Sun LN, Zhou HY, Wang XL. Voltage-dependent potassium channels are involved in glutamate-induced apoptosis of rat hippocampal neurons. Neurosci Lett. 2006;398:22–27. doi: 10.1016/j.neulet.2005.12.073. [DOI] [PubMed] [Google Scholar]