Abstract
We have recently shown that HIV-1 Pr55gag virus-like particles (HIV-VLPs), produced in a baculovirus expression system and presenting a gp120 molecule from a Ugandan HIV-1 isolate of clade A (HIV-VLPAs), induce maturation and activation of antigen-presenting cells (APCs) in fresh peripheral blood mononuclear cells (PBMCs) from seronegative as well as seropositive, with either low or high viremia, HIV-1 subjects. A Th2 polarization has been observed in both HIV seropositive groups, which is efficiently boosted by HIV-VLP induction and does not switch into a Th1 pattern. Here we show that the production of the known immune-suppressive IL-10 is induced in both HIV-seropositive groups at a significantly lower level by HIV-VLPs compared to LPS. These levels, however, appear to still negatively interfere with the innate as well as adaptive Th1-polarized response observed in HIV-seropositive groups. These results indicate that vaccines and novel adjuvants (i.e., TLR agonists, such as LPS) must be evaluated not only for their immunogenicity but also for their potential immune-suppressive effects. In this perspective, fresh ex vivo PBMCs can be of high value for screening the responses as well as eventual failures of vaccinees enrolled in clinical trials.
Virus-like particles (VLPs) represent a form of subunit vaccine based on viral capsid proteins that self-assemble into particulate structures closely resembling immature virus particles.1–4 VLPs are replication as well as infection incompetent, lacking regulatory proteins as well as infectious genetic material, and can be employed to deliver antigenic structures. In particular, VLPs efficiently also reach the MHC class I pathway in antigen-presenting cells (APCs) in the absence of infection or intracellular replication.5–7
The VLPs developed in our laboratory are based on the HIV-1 Pr55gag precursor protein (HIV-VLPs) and display an entire gp120 molecule from a Ugandan HIV-1 isolate of the A clade.8–10 These HIV-VLPAs induce HIV-1-specific CD4+ and CD8+ T cell responses as well as cross-clade neutralizing antibodies in immunized BALB/c mice.11 Moreover, the intraperitoneal and intranasal administration of HIV-VLPAs in mice induces antibody responses at systemic as well as mucosal (vaginal and intestinal) levels.12,13
We have recently shown that these HIV-VLPs are able to induce maturation and activation of monocyte-derived dendritic cells (MDDCs), which produce a pattern of cytokines indicative of both Th-1 and Th-2 pathways [including interleukin (IL)-6, IL-10] and induce primary and secondary responses in autologous human CD4+ T cells in an ex vivo immunization assay.14 Moreover, the HIV-VLP-activated MDDCs show specific transcriptional profiles of genes involved in the morphological and functional changes characterizing the MDDCs activation and maturation.15 In addition, we have shown that the morphological and gene transcriptional maturation pattern induced by HIV-VLPs in ex vivo-generated MDDCs can also be observed in CD14+ uncultured peripheral blood mononuclear cells (PBMCs) from seronegative as well as seropositive individuals with low or high viremia levels.16,17 In particular, the results show that HIV-1 seropositivity status does not impair the immune activation status; furthermore, circulating monocyte CD14+ cell populations respond to an antigenic stimulus, although a Th2 polarization is observed in HIV-seropositive individuals, regardless of the viremia levels.17
Interleukin (IL)-10 is produced by macrophages, dendritic cells (DCs), B cells, as well as various subsets of CD4+ and CD8+ T cells18 and it was first described as a product of Th2 cells that inhibited cytokine synthesis in Th1 cells.19 Many of the effects of IL-10 on T cell and NK cell function are mainly mediated via a direct effect of IL-10 on monocyte-macrophages. In particular, IL-10 inhibits MHC class II and costimulatory molecule B7-1/B7-2 expression on monocytes and macrophages and limits the production of proinflammatory cytokines and chemokines.18 Importantly, autocrine IL-10 signaling in DCs can inhibit chemokine production and prevent their trafficking to lymph nodes as shown in mycobacterial infection, leading to the failure to recruit and induce Th1 differentiation of naive T cells.20 Nevertheless, IL-10 can act directly on CD4+ T cells, inhibiting proliferation and production of IL-2, interferon (IFN)-γ, IL-4, IL-5, and tumor necrosis factor (TNF)-α.18,21,22 Moreover, IL-10 produced by DCs may directly activate IL-10-secreting Tregs that inhibit effector T cell activity and reduce parasite control.23,24 Thus, IL-10 can directly regulate innate and adaptive Th1 and Th2 responses by limiting T cell activation and differentiation in the lymph nodes as well as suppressing proinflammatory responses in tissues, leading to impaired pathogen control and/or reduced immunopathology.25
In the present study, the effect of HIV-VLPs on IL-10 production has been evaluated ex vivo on total circulating PBMCs, according to HIV-1 seropositivity status. This is to assess and consequently circumvent the immune suppressive effect of IL-10 on the Th1-type response, which is extremely relevant in an HIV-1 vaccine perspective and in particular for therapeutic strategies. Lipopolysaccharide (LPS) was used as a term of comparison given that it is a well-known inducer of IL-1026 and it is the agonist of the same TLR (TLR-4) targeted by adjuvants currently used in clinical trials (i.e., GSK's AS04).27 We show that in HIV-1-seropositive subjects, with either low or high viremia, the HIV-VLPs induce a one-log lower amount of IL-10 in PBMCs compared to LPS.
Twenty-three subjects were enrolled in the study and all human specimens were obtained and processed at the Institute of Human Virology in Baltimore under informed consent, as approved by the University of Maryland Baltimore Institutional Review Board. Of these 19 were HIV-1 seropositive, of whom 11 were under antiretroviral treatment (ART) and showed a low HIV-1 viremia (1.34–2.54 log RNA copies/ml), while 8 were enrolled at their first visit and were naive for ART and showed a high viremia (4.74–5.92 log RNA copies/ml). Four healthy seronegative subjects were enrolled as controls. Human PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation, plated in six-well plates at a concentration of 1 × 107/well, and incubated with 6 μg/ml of HIV-VLPs or 1 μg/ml of LPS. The residual endotoxin activity possibly present in the HIV-VLP preparation was inhibited by preincubation with polymixin B sulfate (Sigma) at a concentration of 10 μg/ml. The absence of interference due to the polymixin B sulfate in the activation results was verified in parallel on PBMCs treated only with polymixin.17 After 16 h cellular supernatants were collected and the level of IL-10 was assessed by ELISA (University of Maryland Cytokine Core Laboratory, Baltimore, MD).
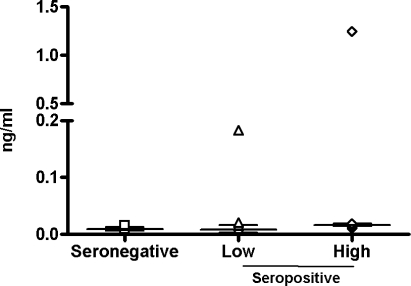
The average basal level of IL-10 does not show a significant difference between HIV-1-seronegative and seropositive individuals (8.45–15.9 pg/ml); moreover, in the latter group, the level of viremia does not make a significant difference. Only two subjects, one in the low viremia and one in the high viremia groups, show very high basal IL-10 levels (183.9 and 1249.9 pg/ml), which could be due to individual characteristics (Fig. 1).
FIG. 1.
Basal expression of IL-10 in PBMCs. The basal expression of IL-10 by PBMCs was measured by ELISA in the culture supernatant. The value for each sample in each group has been represented. The median and interquartile range of the values in each group are shown.
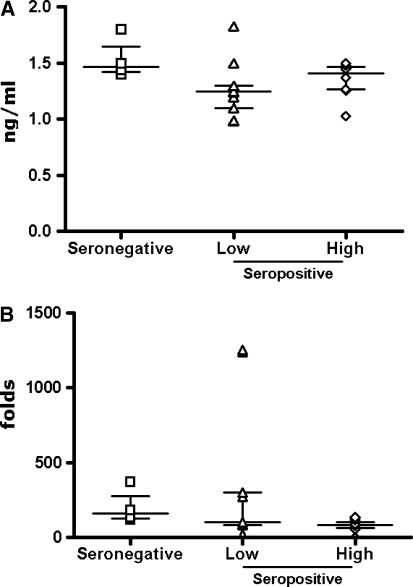
As expected, the treatment of PBMCs with LPS induces an increased production of IL-10 in seronegative (average 1534.29 pg/ml) as well as in seropositive individuals (average 1138.29 and 1356.37 pg/ml, in low and high viremia groups, respectively) (Fig. 2A), which represents a considerable fold induction over the basal levels (200 folds in seronegatives and 330 folds in low viremia and 78 folds in high viremia seropositives) (Fig. 2B).
FIG. 2.
Expression of IL-10 induced by LPS in PBMCs. PBMCs were incubated in the presence of the LPS for 16 h and the expression of IL-10 by PBMCs was measured by ELISA in the culture supernatant. For each group, the value for each sample has been represented (A), and the fold-induction over the basal level in Fig. 1 has been represented (B). The median and interquartile range of the values in each group are shown; asterisks indicate a p < 0.05.
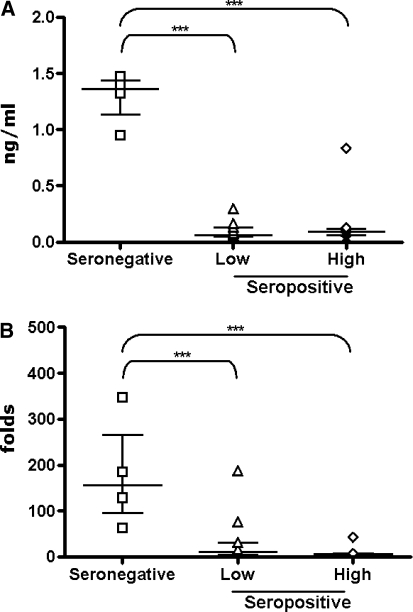
The treatment of PBMCs with HIV-VLPs induces levels of IL-10 in seronegative individuals (average of 1292.62 pg/ml) comparable to LPS induction with an average 180-fold induction over the basal levels. On the contrary, it induces a log lower level of IL-10 in seropositive individuals compared to LPS induction (average 96.04 and 171.37 pg/ml, in the low and high viremia groups, respectively) (Fig. 3A), which represents a significantly lower average fold induction over the basal levels (32.67 and 9.03 folds in the low and high viremia groups, respectively) (Fig. 3B). All intergroup comparisons were performed with the Mann–Whitney U test (for univariate nonparametric group analysis); p-values were two-tailed and considered significant if less than 0.05. Considering the observed mean values in the three cohort groups (seronegative, low-viremia and high-viremia seropositive), the size of the enrolled subjects per group has a statistical power of 100% at a two-tail t-test with a 99% confidence interval.
FIG. 3.
Expression of IL-10 induced by HIV-VLPs in PBMCs. PBMCs were incubated in the presence of the HIV-VLPs for 16 h and the expression of IL-10 by PBMCs was measured by ELISA in the culture supernatant. For each group, the value for each sample has been represented (A), and the fold-induction over the basal level in Fig. 1 has been represented (B). The median and interquartile range of the values in each group are shown; asterisks indicate a p < 0.05.
These data indicate that HIV-VLPs produced in our laboratory induce a significantly lower production of IL-10, compared to LPS, in seropositive individuals regardless of their viremia levels. However, considering the Th2 polarization observed in both HIV-seropositive groups, which is not switched into a sought Th1 pattern by HIV-VLP induction,17 these IL-10 levels could still be sufficient to negatively interfere with the innate as well as adaptive Th1-polarized response. The observed IL-10 levels, indeed, are in the range of the amount with a documented immunosuppressive effect, as shown by others.26 Moreover, our findings confirm studies by others showing that the induction in vivo of Th2-biased responses with gp120 DNA vaccine is mediated in part through the stimulation of innate IL-10, which inhibits activation of DCs that trigger the induction of Th1 cells.28
The present results are very attractive and, although obtained on a single vaccine approach, suggest that vaccines and novel adjuvants (i.e., TLR agonists, such as LPS) should be evaluated not only for their immunogenicity but also for their potential immune-suppressive effects. Therefore, additional detailed studies on HIV-seropositive subjects are needed to identify strategies for reducing the factors possibly involved in the observed Th2 polarization and also for boosting the Th1-type response (i.e., doses, route, and timing of administration of vaccines and/or adjuvants). In vivo preclinical and clinical studies will be needed to validate the present data generated in ex vivo cultured human cells.
In this perspective, the experimental approach described here and based on fresh ex vivo PBMCs can be of great value for screening individual responses as well as eventual failures of vaccinees enrolled in clinical trials.
Acknowledgments
This study was supported by grants from the Ministero Italiano Università e Ricerca (MIUR, 2004) and the Ministero Italiano della Sanità (Ricerca Corrente and Progetto Finalizzato AIDS 2006), and by grants from the Institute of Human Virology and the NIH (to G.K.L.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Gheysen D. Jacobs E. de Foresta F, et al. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- 2.Delchambre M. Gheysen D. Thines D, et al. The GAG precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 1989;8(9):2653–2660. doi: 10.1002/j.1460-2075.1989.tb08405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyanohara A. Imamura T. Araki M, et al. Expression of hepatitis B virus core antigen gene in Saccharomyces cerevisiae: Synthesis of two polypeptides translated from different initiation codons. J Virol. 1986;59:176–180. doi: 10.1128/jvi.59.1.176-180.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirnbauer R. Booy F. Cheng N. Lowy DR. Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann MF. Lutz MB. Layton GT, et al. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur J Immunol. 1996;26(11):2595–2600. doi: 10.1002/eji.1830261109. [DOI] [PubMed] [Google Scholar]
- 6.Ruedl C. Schwarz K. Jegerlehner A, et al. Virus-like particles as carriers for T-cell epitopes: Limited inhibition of T-cell priming by carrier-specific antibodies. J Virol. 2005;79(2):717–724. doi: 10.1128/JVI.79.2.717-724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruedl C. Storni T. Lechner F. Bachi T. Bachmann MF. Cross-presentation of virus-like particles by skin-derived CD8(−) dendritic cells: A dispensable role for TAP. Eur J Immunol. 2002;32(3):818–825. doi: 10.1002/1521-4141(200203)32:3<818::AID-IMMU818>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 8.Buonaguro L. Del Gaudio E. Monaco M, et al. Heteroduplex mobility assay and phylogenetic analysis of V3 region sequences of HIV 1 isolates from Gulu–Northern Uganda. J Virol. 1995;69:7971–7981. doi: 10.1128/jvi.69.12.7971-7981.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buonaguro L. Buonaguro FM. Russo F, et al. A novel gp120 sequence from an HIV-1 isolate of the A clade identified in North Uganda. AIDS Res Hum Retroviruses. 1998;14:1287–1289. doi: 10.1089/aid.1998.14.1287. [DOI] [PubMed] [Google Scholar]
- 10.Buonaguro L. Buonaguro FM. Tornesello ML, et al. High efficient production of Pr55gag virus-like particles expressing multiple HIV-1 epitopes, including a gp120 protein derived from an Ugandan HIV-1 isolate of subtype A. Antiviral Res. 2001;49:35–47. doi: 10.1016/s0166-3542(00)00136-4. [DOI] [PubMed] [Google Scholar]
- 11.Buonaguro L. Racioppi L. Tornesello ML, et al. Induction of neutralizing antibodies and CTLs in Balb/c mice immunized with virus-like particles presenting a gp120 molecule from a HIV-1 isolate of clade A (HIV-VLPAs) Antiviral Res. 2002;54:189–201. doi: 10.1016/s0166-3542(02)00004-9. [DOI] [PubMed] [Google Scholar]
- 12.Buonaguro L. Visciano ML. Tornesello ML, et al. Induction of systemic and mucosal cross-clade neutralizing antibodies in BALB/c mice immunized with human immunodeficiency virus type 1 clade A virus-like particles administered by different routes of inoculation. J Virol. 2005;79:7059–7067. doi: 10.1128/JVI.79.11.7059-7067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buonaguro L. Devito C. Tornesello ML, et al. DNA-VLP prime-boost intra-nasal immunization induces cellular and humoral anti-HIV-1 systemic and mucosal immunity with cross-clade neutralizing activity. Vaccine. 2007;25(32):5968–5977. doi: 10.1016/j.vaccine.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 14.Buonaguro L. Tornesello ML. Tagliamonte M, et al. Baculovirus-derived human immunodeficiency virus type 1 virus-like particles activate dendritic cells and induce ex vivo T-cell responses. J Virol. 2006;80:9134–9143. doi: 10.1128/JVI.00050-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aricò E. Wang E. Tornesello ML, et al. Immature monocyte derived dendritic cells gene expression profile in response to virus-like particles stimulation. J Transl Med. 2005;3:45. doi: 10.1186/1479-5876-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buonaguro L. Monaco A. Arico E, et al. Gene expression profile of peripheral blood mononuclear cells in response to HIV-VLPs stimulation. BMC Bionform. 2008;9(Suppl. 2):S5. doi: 10.1186/1471-2105-9-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buonaguro L. Tornesello ML. Gallo RC, et al. Th2 Polarization in peripheral blood mononuclear cells from human immunodeficiency virus (HIV)-infected subjects, as activated by HIV virus-like particles. J Virol. 2009;83(1):304–313. doi: 10.1128/JVI.01606-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore KW. de Waal MR. Coffman RL. O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 19.Fiorentino DF. Bond MW. Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demangel C. Bertolino P. Britton WJ. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur J Immunol. 2002;32(4):994–1002. doi: 10.1002/1521-4141(200204)32:4<994::AID-IMMU994>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Schandene L. Onso-Vega C. Willems F, et al. B7/CD28-dependent IL-5 production by human resting T cells is inhibited by IL-10. J Immunol. 1994;152(9):4368–4374. [PubMed] [Google Scholar]
- 22.Joss A. Akdis M. Faith A. Blaser K. Akdis CA. IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway. Eur J Immunol. 2000;30(6):1683–1690. doi: 10.1002/1521-4141(200006)30:6<1683::AID-IMMU1683>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 23.van der KD. Latz E. Brouwers JF, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277(50):48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 24.Jang S. Uematsu S. Akira S. Salgame P. IL-6 and IL-10 induction from dendritic cells in response to Mycobacterium tuberculosis is predominantly dependent on TLR2-mediated recognition. J Immunol. 2004;173(5):3392–3397. doi: 10.4049/jimmunol.173.5.3392. [DOI] [PubMed] [Google Scholar]
- 25.Couper KN. Blount DG. Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180(9):5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 26.den Haan JM. Kraal G. Bevan MJ. Cutting edge: Lipopolysaccharide induces IL-10-producing regulatory CD4+ T cells that suppress the CD8+ T cell response. J Immunol. 2007;178(9):5429–5433. doi: 10.4049/jimmunol.178.9.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcon N. Chomez P. Van MM. GlaxoSmithKline Adjuvant Systems in vaccines: Concepts, achievements and perspectives. Expert Rev Vaccines. 2007;6(5):723–739. doi: 10.1586/14760584.6.5.723. [DOI] [PubMed] [Google Scholar]
- 28.Daly LM. Johnson PA. Donnelly G, et al. Innate IL-10 promotes the induction of Th2 responses with plasmid DNA expressing HIV gp120. Vaccine. 2005;23(7):963–974. doi: 10.1016/j.vaccine.2004.03.072. [DOI] [PubMed] [Google Scholar]