Abstract
To assess differences in arterial wave reflection, a marker of atherosclerosis, in HIV-positive and HIV-negative Rwandan women, applanation tonometry was performed on 276 HIV+ and 67 HIV− participants. Radial artery pressure waveforms were recorded and central aortic waveforms were derived by validated transfer function. Central augmentation index (C-AI), central pulse pressure (C-PP), and peripheral augmentation index (P-AI) were measured. HIV+ participants were younger and had lower diastolic blood pressure (BP) and 41% of the HIV+ women were taking antiretroviral therapy (ART). Mean C-AI and P-AI were significantly lower in HIV-infected than in uninfected participants (20.3 ± 12.0 vs. 25.5 ± 12.1, p = 0.002 and 74.6 ± 18.8 vs. 83.7 ± 20.0, p < 0.001). After age matching, C-AI, C-PP, and P-AI were similar among the groups. On multivariate analysis, age, heart rate, weight, and mean arterial pressure were independently associated with C-AI (R2 = 0.33, p < 0.0001). Among HIV-infected women, current CD4 count did not correlate with C-AI (Rho = −0.01, p = 0.84), C-PP (Rho = 0.09, p = 0.16), or P-AI (Rho = −0.01, p = 0.83). In conclusion, HIV infection was not associated with increased arterial wave reflection in women with little exposure to antiretroviral therapy and without CV risk factors. Whether long-term ART increases measures of arterial stiffness remains unknown.
Introduction
There is growing concern that HIV infection increases cardiovascular (CV) risk.1–4 While a number of studies have shown that HIV infection is associated with more extensive subclinical atherosclerosis, these studies are confounded by the presence of conventional cardiovascular risk factors, treatment with antiretroviral therapy (ART), and variable duration of infection. Although there has been a dramatic decline in HIV-related mortality rates since the introduction of highly active antiretroviral therapy (HAART), protease inhibitors (PI) have been associated with dyslipidemia, insulin resistance, hyperglycemia, and central adiposity, which increase the risk for cardiovascular disease. Few studies have assessed markers of atherosclerosis in patients who have low CV risk. With the growing use of ART in resource-limited settings, where CV disease (CVD) and CV risk factors are less prevalent, it will be important to determine whether HIV infection, ART, or both are associated with measures of CVD.
Atherosclerotic changes within the arterial wall are known to increase arterial stiffness and alter wave reflection.5–7 Increased arterial wave reflection is associated with cardiovascular risk factors including age, smoking, hypertension, diabetes mellitus (DM), hypercholesterolemia, and estrogen status.6,8–10 Increased arterial wave reflection is associated with coronary artery disease and is an independent predictor of adverse coronary events,11–13 but has not been well studied in the setting of HIV infection. The objective of this study was to compare measures of arterial stiffness using radial artery tonometry in HIV-infected and HIV-uninfected women enrolled in the Rwanda Women's Interassociation Study and Assessment (RWISA), a cohort with minimal background prevalence of CVD or CV risk factors.
Materials and Methods
Study population
The Rwanda Women's Inter-association Study and Assessment (RWISA) is an observational prospective cohort study investigating the effectiveness and toxicity of antiretroviral therapy in HIV-infected Rwandan women, with a control group of HIV-negative women. Between May 15 and November 15, 2005, 936 women (715 HIV infected and 221 HIV uninfected) were enrolled from community-based associations, with semiannual follow-up visits. Informed consent was obtained in Kinyarwanda in accordance with protocols approved by the Rwandan Ethics Committee and the Institutional Review Board of Montefiore Medical Center, Bronx, NY. At study entry participants provided historic information including demographic parameters, medical and psychosocial history, information on experience of trauma during the 1994 Rwandan genocide, and symptoms of depression and posttraumatic stress. A physical examination was performed, and fasting blood specimens were taken for real time laboratory testing and for storing for future testing. Women were enrolled sequentially into this substudy if an RWISA visit occurred in one of the three 2-week periods in which a person trained to perform the needed measurements was present.
All the HIV+ women were naive to ART at study entry. A proportion initiated ART after enrollment as clinically indicated by WHO treatment guidelines and the Rwandan National Protocol. Nearly all ART in Rwanda is provided as a fixed-dose combination of nevirapine and lamivudine, with either zidovudine or stavudine.
Laboratory measurements
At study enrollment, laboratory measurements performed in real time were CD4 cell count, HIV serology, plasma glucose, serum levels of total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides. Low-density lipoprotein cholesterol (LDL-C) was estimated from the Friedewald equation, for specimens with triglyceride levels < 400 mg/dl, and are not provided for women with triglycerides > 400 mg/dl.
Measurement of arterial wave reflection
Repeat blood pressure (BP) measurements were completed in the seated position using a standardized protocol, and an average of two readings was calculated. Arterial stiffness was measured in the sitting position using a commercial pulse wave analysis system (Sphygmocor Atcor Medical, version 6.2) in a quiet, private room after lying supine for at least 5 min. In brief, peripheral pressure waveforms were recorded from the radial artery at the wrist, using applanation tonometry. The arm was supported to ensure optimal positioning and minimal movement. The obtained waveforms (mean of 30-s recording) were calibrated to the systolic and diastolic cuff pressures. Aortic pressure waveforms were derived from radial tonometry by using a previously validated transfer function, which relates radial to aortic pressure waveform within the system software.14 The central aortic pulse wave was used to measure the augmentation index (AI), which is the proportional increase in systolic pressure due to the reflected wave. AI was expressed as a percentage of the pulse pressure. Larger AI values indicate greater wave reflection from the periphery or earlier return of the reflected wave.15 Therefore, AI provides an indirect estimate of arterial wave reflection. The AI has been validated with simultaneously recorded direct aortic measurements and is highly reproducible in both healthy and diseased populations.16,17 In addition, stiffness was assessed by measuring the central pulse pressure (PP) and peripheral AI; the greater the waveform reflection and stiffer the artery, the higher the values for each of these indices. Only high-quality recordings, defined as an in-device quality index >80% and confirmed by visual analysis, were analyzed.
The primary outcome variables were central and peripheral AI and central PP. The primary exposure variables were HIV serostatus (positive vs. negative) and CD4 cell count. The HIV-negative group was retested 6 months later with ELISA. Secondary exposure variables included age, mean arterial pressure (MAP) as reported by the device, height, and weight, HIV disease, CD4 count, serum lipoprotein levels, and plasma glucose levels.
Staging of HIV disease was performed with World Health Organization (WHO) criteria, by self-report of clinical conditions. Hypertension was defined as either systolic BP >140 mm Hg or diastolic BP >90 mm Hg or by self-report with treatment with medications. Body mass index (kg/m2) based on measured weights and heights was classified as underweight (<18.5), normal weight (18.5–24.9), overweight (25.0–29.9), and obese (≥30.0). Alcohol use was categorized as abstainers (<1 drink per week), low-to-moderate consumption (1–3 drinks per week), and heavy consumption (≥3 drinks per week).
Statistical analysis
Values are represented as mean ± SD or medians (interquartile range). Continuous data were compared using the t-test. Fisher's exact test was used to compare dichotomous variables. Univariate associations between study variables were analyzed using Spearman's correlation coefficients. Multivariate linear regression was used to determine independent correlates of central AI. In further analyses, we age matched HIV-infected patients to within 2 years of uninfected controls. A probability of < 0.05 was considered significant. Data were analyzed with SAS software (version 9.1; Cary, NC).
Results
Hemodynamic measurements were obtained from 276 HIV-infected and 67 HIV-uninfected participants. Clinical characteristics and hemodynamic values of the included women are shown in Table 1. HIV+ women were younger and shorter and had lower diastolic BP, lower total cholesterol (C) and higher triglyceride levels, and lower HDL-C levels compared to the HIV-negative women. There were no significant differences between the groups in weight, systolic BP, heart rate, LDL-C, or blood glucose levels. The median CD4+ count in the HIV-infected women was 267 cells/mm3 (172, 388). At the time of the measurements of arterial stiffness, 112 (41%) of the HIV-infected women were receiving ART, for a median of 10 months. Of the women on ART, 95 (84.8%) were taking a nevirapine-containing fixed dose combination. Only five women were taking cardiac or antihypertensive medications: three were HIV negative and two were HIV positive. Of the seven total patients who used aspirin, one was HIV negative and six were HIV positive, of whom one was on ART.
Table 1.
Clinical Characteristics and Hemodynamic Values of HIV-Infected and Uninfected Rwandan Womena
| HIV-positive (n = 276) | HIV-negative (n = 67) | p-value | |
|---|---|---|---|
| Age, years | 35 ± 7 | 41 ± 10 | <0.0001 |
| Height, cm | 157.1 ± 6.7 | 159.7 ± 6.7 | 0.02 |
| Weight kg | 53.6 ± 9.8 | 53.9 ± 12.2 | 0.83 |
| Peripheral SBP, mm Hg | 116 ± 11 | 119 ± 14 | 0.11 |
| Peripheral DBP, mm Hg | 71 ± 8 | 74 ± 12 | 0.019 |
| Central SBP, mm Hg | 99 ± 12 | 99 ± 13 | 0.37 |
| Central DBP, mm Hg | 70 ± 9 | 72 ± 9 | 0.07 |
| Heart rate, bpm | 81 ± 12 | 81 ± 13 | 0.81 |
| Total cholesterol, mg/dl | 127.6 ± 32.3 | 145.7 ± 33.6 | <0.0001 |
| LDL, mg/dl | 68 ± 26 | 70 ± 27 | 0.68 |
| HDL, mg/dl | 44 ± 15 | 54 ± 15 | <0.0001 |
| Triglycerides, mg/dl | 101 ± 67 | 83 ± 40 | 0.009 |
| Glucose, mg/dl | 85 ± 47 | 88 ± 89 | 0.80 |
| Hypertension (%) | 0.4 | 1.5 | 0.35 |
| Smoking (%) | 2.5 | 4.6 | 0.38 |
| Alcohol consumption (%) | 19.2 | 23.3 | 0.47 |
| Central AI | 20.3 ± 12.0 | 25.5 ± 12.1 | 0.002 |
| Central PP (mm Hg) | 28.1 ± 8.0 | 27.4 ± 9.0 | 0.54 |
| Peripheral AI | 74.6 ± 18.8 | 83.7 ± 20.0 | 0.0006 |
SBP, systolic blood pressure; DPB, diastolic blood pressure; AI, augmentation index; PP, pulse pressure.
Mean central AI was significantly lower in the HIV-infected than in HIV-uninfected women (20.3 ± 12.0 vs. 25.5 ± 12.1, p = .002). Peripheral AI (74.6 ± 18.8 vs. 83.7 ± 20.0, p < 0.001) was also lower in the HIV-infected group. Central PP was similar between the groups (28.1 ± 8.0 vs. 27.4 ± 9.0, p = 0.54). In stepwise regression analysis, age (β = 0.55, p < 0.001), HR (β = −0.40, p < 0.001), weight (β = −0.18, p = 0.0023), and MAP (β = 0.21, p = 0.001) were independently associated with central AI (R2 = 0.33; p < 0.0001) (model). HIV status, cholesterol, glucose, and ART were not. Central PP was independently associated only with age (β = 0.19, p < 0.001) and HR (β = −0.21, p < 0.001) (R2 = 0.15, p < 0.0001). Peripheral AI was independently associated with age (β = 0.97, p < 0.001), HR (β = −0.36, p < 0.001), weight (β = −0.49, p > 0.001), and MAP (β = 0.29, p = 0.008) (R2 = 0.28, p < 0.0001).
Among HIV-infected women, current CD4 count was not significantly correlated with central AI (Rho = −0.01, p = 0.84), central PP (Rho = 0.09, p = 0.16), or peripheral AI (Rho = −0.01, p = 0.83). There were no significant differences between each of the three outcome measures between subjects with CD4 count <200 and those with CD4 counts ≥200 cells/mm3.
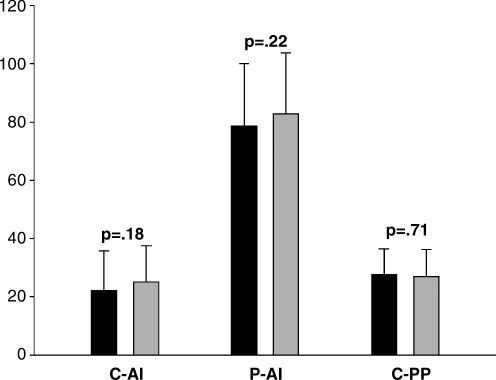
In an age-matched analysis, we compared 116 HIV-infected and 58 HIV-uninfected women (Fig. 1; Table 2). The HIV-infected women had lower diastolic BP but were otherwise similar to the HIV-negative women. Among the HIV-infected group, the median CD4 count was 275 cells/mm3 and the mean (± SD) was 293 ± 173 cells/mm3. For all primary outcome measures of arterial wave reflection (central AI, peripheral AI, and central PP), the differences between HIV-positive and HIV-negative women narrowed, and were no longer statistically significant.
FIG. 1.
Comparison between age-matched HIV+ and HIV− patients. AI, augmentation index; PP, pulse pressure; C, central; P, peripheral.
Table 2.
Clinical Characteristics and Hemodynamic Values of HIV-Infected and Uninfected Rwandan Women after Age Matchinga
| HIV-positive (n = 116) | Controls (n = 58) | p-value | |
|---|---|---|---|
| Age, years | 38 ± 8 | 39 ± 9 | 0.53 |
| Height, cm | 157.5 ± 7.0 | 160.4 ± 7.8 | 0.016 |
| Weight, kg | 52.0 ± 10.0 | 55.2 ± 11.4 | 0.06 |
| Peripheral SBP, mm Hg | 117 ± 12 | 119 ± 15 | 0.23 |
| Peripheral DBP, mm Hg | 71 ± 9 | 75 ± 12 | 0.026 |
| Central SBP, mm Hg | 98 ± 14 | 98 ± 13 | 0.92 |
| Central DBP, mm Hg | 71 ± 9 | 71 ± 9 | 0.59 |
| Heart rate, bpm | 82 ± 13 | 81 ± 13 | 0.93 |
| Total cholesterol, mg/dl | 126 ± 30 | 144 ± 32 | 0.0006 |
| LDL, mg/dl | 67 ± 24 | 70 ± 28 | 0.45 |
| HDL, mg/dl | 42 ± 14 | 54 ± 15 | <0.0001 |
| Triglycerides | 109 ± 74 | 79 ± 36 | 0.0009 |
| Glucose, mg/dl | 91 ± 70 | 89 ± 95 | 0.93 |
| Diabetes, % | 0.9 | 0 | 0.66 |
| Hypertension, % | 0.9 | 1.7 | 0.55 |
| Smoking, % | 4.4 | 1.7 | 0.34 |
| Central AI | 22.1 ± 13.4 | 24.9 ± 12.3 | 0.18 |
| Central PP, mm Hg | 27.3 ± 9.1 | 26.7 ± 9.3 | 0.71 |
| Peripheral AI | 78.5 ± 21.5 | 82.8 ± 20.8 | 0.22 |
SBP, systolic blood pressure; DPB, diastolic blood pressure; AI, augmentation index; PP, pulse pressure.
Discussion
Concern over cardiovascular disease in HIV-infected individuals has led to multiple studies examining the associations between HIV infection, ART, and atherosclerosis. In this study of Rwandan women with a low prevalence of CV risk factors and CV disease, we were unable to detect significant differences in measures of arterial wave reflection between the HIV-infected and HIV-uninfected participants after adjustment for potential confounders and matching for age. These findings suggest that HIV infection itself is not associated with altered arterial wave reflection. In observational studies HIV infection has been found to be associated with both higher cardiovascular event rates1,2 and higher measures of cardiovascular risk,18 and other studies have found higher risk due to antiretroviral therapy.1,2 The DAD project, a large collaborative study of HIV-infected cohorts, reported an adjusted 26% increase in the hazard of incident myocardial infarction (MI) with each additional year of combination antiretroviral treatment.2 Of note, DAD does not include HIV-negative controls. Subsequent data from DAD suggested that use of PI-based regimens was responsible for the increased risk of MI, while use of nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimens was not associated with MI risk.
Multiple studies have found both HIV infection and its treatment are associated with more extensive subclinical cardiovascular disease as measured by carotid intimal medial thickness, coronary calcium content, and impaired endothelial function. Although each diagnostic technique poses advantages and limitations, interpretation of prior studies is made difficult by confounding variables including traditional cardiovascular risk factors, ART, and variable duration of HIV infection.
We compared HIV-uninfected women who were similar to HIV-infected women, and found lower, not higher measures of arterial wave reflection in HIV-infected participants. The HIV-infected women were younger, had lower BMI, HDL-C, and diastolic BP, and had higher triglyceride levels than the uninfected women. In multivariate models, measures of arterial wave reflection were unrelated to HIV status or CD4 count. Similarly, after age matching there were no significant differences in any of the arterial measures. The patient population was unique in that the prevalence of cardiovascular risk factors including lipid abnormalities, tobacco smoking (4%), hypertension (1%), and diabetes (< 1%) was quite low in both HIV-infected and HIV-uninfected women. Despite differences in lipoprotein levels by HIV status, LDL levels were very low in both groups, which likely conferred cardioprotection.
Few studies have specifically measured arterial stiffness in HIV-infected individuals. Bonnett found carotid wall stiffness assessed by elastic modulus to be greater in 49 HIV-infected compared to 24 HIV-uninfected children.19 Two prior studies have used applanation tonometry to evaluate arterial wave reflection/stiffness. In 59 HIV-infected adults treated with HAART, AI was related to duration of ART, cumulative exposure to nucleoside reverse transcriptase inhibitors (NRTIs), and to PIs.20 Similarly, another study of 84 HIV-infected patients, half of whom were treated with statins, found mean arterial pressure, age, duration of HIV infection, and PI exposure to be independent predictors of aortic stiffness.21 Disparate results may be explained by differences in the participants studied. The present study was larger in size, excluded children, evaluated subjects only on short-term antiretroviral therapy, and included a control group.
Several explanations may account for the lack of an association between HIV status and higher AI. First, HIV infection may not directly predispose low-risk patients to atherosclerosis as the immunological sequelae of HIV infection including T cell dysfunction and decreased production of Th1 cytokines22 have been suggested to retard the development of vascular disease.23 The very low LDL levels in both groups likely conferred cardioprotection. Although the pathogenesis of atherosclerosis might differ in the HIV and the non-HIV settings, this seems an unlikely explanation. The inflammatory state of HIV infection is evidenced by increased levels of circulating markers including erythrocyte sedimentation rate and C-reactive protein,24 which are predictive of higher cardiovascular risk.24 Other systemic inflammatory diseases including rheumatoid arthritis, systemic lupus erythromatosis, and Behcet's disease have also been found to be associated with premature atherosclerosis and higher cardiovascular event rates.25,26 The presence of medium sized arterial vasculitis has been well described in these disorders, and has also been observed in two necropsy studies of HIV infection.27,28 Higher measures of aortic wave reflection and arterial stiffness have been found in these other disorders. Whether women are more prone than men to altered waveform reflection because of lower lean mass and smaller vessel size is unknown.
AI is often used as a measure of arterial stiffness. Increased arterial stiffness is associated with an increased risk of cardiovascular events.12,29 Recently, the use of AI as a measure of true arterial stiffness has come into question.30,31 Atherosclerosis causes arterial stiffening by amplifying waveform reflection and summation. In our study age, height, weight, HR, and BP were independently associated with central and peripheral AI and central PP. These findings are consistent with prior studies of uninfected individuals.16
This study is subject to the limitations inherent in cross-sectional analyses. CD4 cell counts, use of ART, and CVD risk factors such as lipid levels are dynamic variables that change over time. However, the present study provides baseline measurements that will be used to study longitudinal changes in arterial stiffness. Use of ART in our cohort was of brief duration, but in some participants preceded measurement of arterial stiffness in order to avoid treatment delay. The duration of HIV infection was unknown, although the low median CD4 cell count suggests a duration of at least 10 years. Information on viral load at the time of the studies was not available. Although information as to the presence of metabolic syndrome was also lacking, it is likely that its prevalence was quite low given the paucity of risk factors and relatively low BMI. The women may well have been too young to demonstrate changes in arterial stiffness.
We used radial artery tonometry to study arterial wave reflection, which is a well-validated approach to assessing subclinical vascular disease.31,32 Although AI is influenced by heart rate, it was not indexed for heart rate in the present study because mean heart rate was the same in the HIV-infected and HIV-uninfected groups.33 Arterial wave reflection was assessed by measuring AI rather than aortic pulse wave velocity. Although central AI may not be as sensitive a measure of arterial stiffness as aortic PWV in individuals over 50 years of age,34 the study subjects were young (mean age < 40 years). The exclusive use of AI is consistent with prior studies.26 AI is a composite measure that depends on wave velocity, the site of reflection, and the amplitude of the reflected wave. Therefore, AI may appear normal in the HIV-infected group in the setting of faster wave speed and reduced wave reflection, resulting from reduced impedance mismatch at the point of reflection due to peripheral vasodilatation. Although central PP is a relatively crude measure of arterial stiffness because it is strongly influenced by stroke volume, particularly in younger individuals, we included this index as well as peripheral AI because there currently exist a number of applanation tonometry devices worldwide, which provide multiple indices including central PP and peripheral AI.
Conclusions
In conclusion, HIV infection was not associated with greater arterial wave reflection in Rwandan women with little exposure to ART and without other CV risk factors.
Whereas previous studies of cardiovascular disease and HIV infection have been confounded by the presence of conventional cardiovascular risk factors and treatment with ART, this study evaluated a unique cohort of women. The finding that HIV infection itself is not associated with greater arterial waveform reflection, a functional marker of atherosclerosis would suggest that other factors including ART may be responsible for increased cardiovascular risk. Whether long-term ART increases reflective indices remains unknown. The present study provides baseline data for assessing the influence of ART by repeating these measurements. Whether arterial wave reflection in HIV-infected individuals is associated with acute or chronic inflammation and its predictive value for future CV events merit further study.
Acknowledgments
We acknowledge the contribution of the following individuals who participated in this study: Rachel Knipe (New York Presbyterian-Cornell Hospital, New York, New York), Zachary Rosner (University of Chicago Pritzker School of Medicine, Chicago, Illinois), and Jeremy Fox (University of Michigan Medical School, Ann Arbor, Michigan). This study was supported by a supplement to the Bronx/Manhattan Women's Interagency HIV Study (WIHS), and by the Brooklyn, NY and Chicago, IL WIHS, all funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, and UO1-AI-42590). This work was also supported in part by the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center funded by the National Institutes of Health (NIH AI-51519) and by the National Institute of Diabetes and Digestive and Kidney Disease (DK54615).
Disclosure Statement
No competing financial interests exist.
References
- 1.Friis-Moller N. Weber R. Reiss P, et al. Cardiovascular disease risk factors in HIV patients-association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;23(17(8)):1179–1193. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 2.Friis-Moller N. Sabin CA. Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349(21):1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 3.Mooser V. Atherosclerosis and HIV in the highly active antiretroviral therapy era: Towards an epidemic of cardiovascular disease? AIDS. 2003;17(S):65–69. doi: 10.1097/00002030-200304001-00009. [DOI] [PubMed] [Google Scholar]
- 4.Vittecoq D. Escaut L. Chironi G, et al. Coronary heart disease in HIV infected patients in the highly active antiretroviral treatment era. AIDS. 2003;17(S):70–76. doi: 10.1097/00002030-200304001-00010. [DOI] [PubMed] [Google Scholar]
- 5.Van Bortel LM. Duprez D. Starmans-Kool MJ, et al. Clinical applications of arterial stiffness, Task Force III: Recommendations for user procedures. Am J Hypertens. 2002;15(5):445–452. doi: 10.1016/s0895-7061(01)02326-3. [DOI] [PubMed] [Google Scholar]
- 6.McVeigh GE. Hamilton PK. Morgan DR. Evaluation of mechanical arterial properties: Clinical, experimental and therapeutic aspects. Clin Sci. 2002;102(1):51–67. [PubMed] [Google Scholar]
- 7.Syeda B. Gottsauner-Wolf M. Denk S. Pichler P. Khorsand A. Gloqar D. Arterial compliance: A diagnostic marker for atherosclerotic plaque burden? Am J Hypertens. 2003;16:356–362. doi: 10.1016/s0895-7061(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 8.McVeigh GE. Morgan DJ. Finkelstein SM. Lemay LA. Cohn JN. Vascular abnormalities associated with long-term cigarette smoking identified by arterial waveform analysis. Am J Med. 1997;102:227–231. doi: 10.1016/S0002-9343(96)00454-8. [DOI] [PubMed] [Google Scholar]
- 9.Resnick LM. Militianu D. Cunnings AJ, et al. Pulse waveform analysis of arterial compliance: Relation to other techniques, age, and metabolic variables. Am J Hypertens. 2000;13:1243–1249. doi: 10.1016/s0895-7061(00)01219-x. [DOI] [PubMed] [Google Scholar]
- 10.Mahmud A. Feely J. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension. 2003;41(1):183–187. doi: 10.1161/01.hyp.0000047464.66901.60. [DOI] [PubMed] [Google Scholar]
- 11.Ravikumar R. Deepa R. Shanthirani C. Mohan V. Augmentation index is associated with cardiovascular risk. J Hypertens. 2002;20:2407–2414. doi: 10.1097/00004872-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Boutouyrie P. Tropeano AI. Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: A longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 13.London GM. Cohn JN. Prognostic application of arterial stiffness: Task Forces. Am J Hypertens. 2002;15(8):754–758. doi: 10.1016/s0895-7061(02)02966-7. [DOI] [PubMed] [Google Scholar]
- 14.Chen CH. Nevo E. Fetics B, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure: Validation of generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 15.O'Rourke MF. Gallagher DE. Pulse wave analysis. J Hypertens. 1996;14:147–157. [PubMed] [Google Scholar]
- 16.Wilkinson IB. Fuchs SA. Jansen IM, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16(12 Pt 2):2079–2084. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 17.Siebenhofer A. Kemp CRW. Sutton AJ. Williams B. The reproducibility of central aortic blood pressure measurements in healthy subjects using applanation tonometry and sphygmocardiography. J Hum Hypertens. 1999;13:625–629. doi: 10.1038/sj.jhh.1000887. [DOI] [PubMed] [Google Scholar]
- 18.Quiros-Roldan E. Torti C. Tinelli C, et al. Risk factors for myocardial infarction in HIV-positive patients. Int J STD AIDS. 2005;16(1):14–18. doi: 10.1258/0956462052932665. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet D. Aggoun Y. Szezepanski I. Bellal N. Blanche S. Arterial stiffness and endothelial dysfunction in HIV-infected children. AIDS. 2004;18(7):1037–1041. doi: 10.1097/00002030-200404300-00012. [DOI] [PubMed] [Google Scholar]
- 20.Sevastianova K. Sutinen J. Westerbacka J. Ristola M. Yki-Jarvinen H. Arterial stiffness in HIV-infected patients receiving highly active antiretroviral therapy. Antivir Ther. 2005;10(8):925–935. [PubMed] [Google Scholar]
- 21.Boccara F. Simon T. Lacombe K, et al. Influence of pravastatin on carotid artery structure and function in dyslipidemic HIV-infected patients receiving antiretroviral therapy. AIDS. 2006;20(18):2395–2398. doi: 10.1097/QAD.0b013e32801120e3. [DOI] [PubMed] [Google Scholar]
- 22.Ostrowski SR. Gerstoft J. Pedersen BK. Ullum H. Impaired production of cytokines is an independent predictor of mortality in HIV-1-infected patients. AIDS. 2003;17(4):521–530. doi: 10.1097/00002030-200303070-00007. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X. Nicoletti A. Elhage R. Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102(24):2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 24.Lau B. Sharrett AR. Kingsley LA, et al. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch Intern Med. 2006;166(1):64–70. doi: 10.1001/archinte.166.1.64. [DOI] [PubMed] [Google Scholar]
- 25.Ghaffari S. Detection and management of coronary artery disease in patients with rheumatologic disorders. Rheum Dis Clin North Am. 1999;(3):657–668. doi: 10.1016/s0889-857x(05)70091-0. [DOI] [PubMed] [Google Scholar]
- 26.Van Doornum S. McColl G. Wicks IP. Tumour necrosis factor antagonists improve disease activity but not arterial stiffness in rheumatoid arthritis. Rheumatology (Oxford) 2005;44(11):1428–1432. doi: 10.1093/rheumatology/kei033. [DOI] [PubMed] [Google Scholar]
- 27.Otedo AE. Oyoo GO. Obondi JO. Otieno CF. Vasculitis in HIV: Report of eight cases. East Afr Med J. 2005;82(12):656–659. doi: 10.4314/eamj.v82i12.9373. [DOI] [PubMed] [Google Scholar]
- 28.Joshi VV. Pawel B. Conner E. Sharer L. Oleske JM. Morrison S. Arteriopathy in children with AIDS. Pediatr Pathol. 1987;7:261–275. doi: 10.1080/15513818709177129. [DOI] [PubMed] [Google Scholar]
- 29.Blacher J. Asmar R. Djane S. London GM. Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 30.Vyas M. Izzo JL., Jr Lacourciere Y, et al. Augmentation index and central aortic stiffness in middle-aged to elderly individuals. Am J Hypertens. 2007;20(6):642–647. doi: 10.1016/j.amjhyper.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Sugawara J. Komine H. Hayashi K. Magda S. Matsuda M. Relationship between augmentation index obtained from carotid and radial artery pressure waveforms. J Hypertens. 2007;25(2):375–381. doi: 10.1097/HJH.0b013e32801092ae. [DOI] [PubMed] [Google Scholar]
- 32.Yasmin Brown MJ. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. QJM. 1999;92(10):595–600. doi: 10.1093/qjmed/92.10.595. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson IB. MacCallum H. Flint L. Cockcroft JR. Newby DE. Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525:263–267. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McEniery CM. Yasmin Hall IR, et al. Normal vascular aging: Differential effects on wave reflection and aortic pulse wave velocity: The Anglo-Cardiff Collaborative Trial (ACCT) J Am Coll Cardiol. 2005;46(9):1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]