Summary
Sudden cardiac death, or cardiac arrest, is a major health problem, causing about 166,200 deaths each year among adults in the United States. It may be caused by almost all known heart diseases. Most cardiac arrests occur when the diseased heart begins to exhibit rapid and/or chaotic activity, such as ventricular tachycardia or fibrillation. Some are due to extreme slowing of the heart. All these events are called life-threatening arrhythmias. Arrhythmogenic cardiomyopathy is a frequent feature in several muscular dystrophies with a potential risk of cardiac sudden death. Among the measures able to predict the propensity to develop life-threatening arrhythmias, heart rate variability is an accepted non invasive measurement of cardiac autonomic modulation. The use of heart rate variability to measure the extent of changes in autonomic nervous system is an established risk stratification procedure in different diseases. In fact numerous studies have demonstrated the positive prognostic power of altered heart rate variability values to predict all-cause mortality, cardiac events, sudden cardiac death and heart transplantation. Usefulness of heart rate variability as a predictor of sudden cardiac death in muscular dystrophies has been reviewed.
Keywords: Heart rate variability, sudden cardiac death, muscular dystrophies
The last two decades have witnessed the recognition of a significant relationship between the autonomic nervous system and cardiovascular mortality, including sudden cardiac death (1–4). Experimental evidence of an association between vulnerability to lethal arrhythmias and signs of either increased sympathetic or reduced vagal activity has spurred efforts for the development of quantitative markers of autonomic activity.
Heart rate variability (HRV) represents one of the most promising markers. The apparently easy derivation of this measure has led to its use becoming popular. As many commercial devices now provide an automated measurement of HRV, the cardiologist has been provided with a seemingly simple tool both for research and clinical studies (5). Indices of heart rate variability provide a window onto autonomic modulation of the heart. HRV indices, determined in either the time or frequency domain, are closely related and reflect parasympathetic, mixed sympathetic, and parasympathetic and circadian rhythms. Depression of HRV has been observed in many clinical scenarios, including autonomic neuropathy, heart transplantation, congestive heart failure (CHF), myocardial infarction (MI), and other cardiac and non-cardiac diseases.
The use of HRV to measure the extent of changes in the autonomic nervous system (ANS) is an established risk stratification procedure in different diseases. Numerous studies have recently shown the positive prognostic power of altered HRV values to predict all-cause mortality (ACM), cardiac events (CE), sudden cardiac death (SCD) and heart transplantation. The analysis of beat-to-beat variations in heart rate has been used to investigate sympatho-vagal balance within the cardiovascular system. In CHF, markedly reduced HRV has been demonstrated which coincides with the severity of CHF as well as being an independent marker of sympatho-excitation. On the other hand, after acute myocardial infarction, depressed HRV predicts cardiac mortality and malignant arrhythmias independently of other conventional risk factors.
These frequency domain analyses contributed to understand the autonomic background of the RR interval fluctuations in the heart rate record (6, 7). The clinical importance of HRV became appreciated in the late 1980s, when it was confirmed that HRV was a strong and independent predictor of mortality after acute myocardial infarction (8–10). From a general point of view, HRV can be used in clinical practice to estimate i) the integrity of cardiac autonomic innervation, ii) the physiologic status of cardiac autonomic activity, and iii) the vulnerability to various cardiac arrhythmias resulting from autonomic imbalance. With the availability of new, digital, high-frequency, 24-hour multi-channel electrocardiogram (ECG) recorders, HRV has the potential to provide additional valuable insight into the physiological and pathological conditions and to enhance risk stratification.
Measurement of HRV
Time domain methods
Variations in heart rate may be evaluated by a number of methods, but perhaps the simplest to perform are the time domain measures with which, either the heart rate, at any point in time, or the intervals between successive normal complexes, are defined. In a continuous ECG record, each QRS complex is detected, and the so-called normal-to-normal (NN) intervals (i.e., all intervals between adjacent QRS complexes resulting from sinus node depolarisations) or the instantaneous heart rate is determined. Simple time domain variables that can be calculated include mean NN interval, mean heart rate, difference between the longest and shortest NN interval, difference between night and day heart rate, etc.
From a series of instantaneous heart rates or cycle intervals, particularly those recorded over longer periods, traditionally 24 hours, more complex statistical time domain measures can be calculated. These can be divided into two classes: i) those derived from direct measurements of the NN intervals or instantaneous heart rate and ii) those derived from the differences between NN intervals. These variables may be derived from analysis of the total ECG recording or may be calculated using smaller segments of the recording period. The latter method allows comparison of HRV to be made during varying activities, for example, rest, sleep, etc.
The simplest variable to calculate is the standard deviation of the NN intervals (SDNN), that is, the square root of variance. Since variance is mathematically equal to total power of spectral analysis, SDNN reflects all the cyclic components responsible for variability in the period of recording. In many studies, SDNN is calculated over a 24-hour period and thus encompasses short-term HF variations as well as the lowest-frequency components observed in a 24-hour period. As the period of monitoring decreases, SDNN estimates shorter cycle lengths. It should also be noted that the total variance of HRV increases with the length of analyzed recording (11). Thus, on arbitrarily selected ECGs, SDNN is not a well-defined statistical quantity because of its dependence on the length of the recording period. In practice, it is inappropriate to compare SDNN measures obtained from recordings of different durations. On the contrary, durations of the recordings used to determine SDNN values (and likewise other HRV measures) should be standardized. Short-term 5-minute recordings and nominal 24-hour long-term recordings appear to be appropriate options. Other commonly used statistical variables, calculated from segments of the total monitoring period, include the standard deviation of the average NN (SDANN) intervals calculated over short periods, usually 5 minutes, which is an estimate of the changes in heart rate due to cycles longer than 5 minutes, and the SDNN index, the mean of the 5-minute standard deviations of NN intervals calculated over 24 hours, which measures the variability due to cycles shorter than 5 minutes. The most commonly used measures derived from interval differences include root-mean square of difference of successive RR intervals, (RMSSD), the number of interval differences of successive NN intervals greater than 50 ms (NN50), and the proportion derived by dividing NN50 by the total number of NN intervals (pNN50). All these measurements of short-term variation estimate high-frequency variations in heart rate and thus are closely correlated. Selected frequency domain measures are outlined in Table 1 (12).
Table 1. Selected time domain measures of HRV.
| Variable | Units | Description |
| Statistical measures | ||
| SDNN | msec | Standard deviation of all NN intervals |
| SDANN | msec | Standard deviation of averages all NN intervals in all 5-minute segments of entire recording |
| RMSDD | msec | Square root of mean of sum of squares of differences between adjacent NN intervals |
| SDNN Index | msec | Mean of standard deviations of all NN intervals for all 5-minute segments of entire recording |
| SDSD | msec | Standard deviation of differences between adjacent NN intervals |
| NN50 count | msec | Numbers of pairs of adjacent NN intervals differing by more than 50 msec in entire recording; three variants are possible counting all such NN intervals in which the first or second interval is longer |
| pNN50 | % | NN50 count divided by total number of all NN intervals |
Frequency domain methods
Various spectral methods (13) for the analysis of the tachogram have been applied since the late 1960s. Power spectral density (PSD) analysis provides the basic information of how power (variance) distributes as a function of frequency. Regardless of the method used, only an estimate of the true PSD of the signal can be obtained by appropriate mathematical algorithms.
Methods for the calculation of PSD may be classified as: i) non-parametric and ii) parametric. In most instances, the two methods provide comparable results. The advantages of the non-parametric methods are: i) the simplicity of the algorithm used (fast Fourier transform – FFT – in most cases) and ii) the high processing speed, while the advantages of parametric methods are: i) smoother spectral components that can be distinguished independently of pre-selected frequency bands, ii) easy post-processing of the spectrum with an automatic calculation of low- and high-frequency power components with easy identification of the central frequency of each component, and iii) accurate estimation of PSD, even on a small number of samples in which the signal usually remains stationary.
Spectral components
Short-term recording
Three main spectral components can be distinguished in a spectrum calculated from short-term recordings of 2 to 5 minutes (14–18): VLF, LF, and HF components. The distribution of the power and the central frequency of LF and HF are not fixed but may vary in relation to changes in autonomic modulations of the heart period (18, 19). The physiological explanation of the VLF component is less well defined, and the existence of a specific physiological process attributable to these heart period changes might even be questioned. The non-harmonic component, which does not display coherent properties and is affected by algorithms of baseline or trend removal, is commonly accepted as a major constituent of VLF. Thus, VLF assessed by means of short-term recordings (≤ 5 minutes) is not a reliable measure and should be avoided when the PSD of short-term ECGs is interpreted. Measurement of VLF, LF, and HF power components is usually made in absolute values of power (milliseconds squared). LF and HF may also be measured in normalized units (18, 19) which represent the relative value of each power component in proportion to the total power minus the VLF component. The representation of LF and HF in normalized units emphasizes the controlled and balanced behaviour of the two branches of the autonomic nervous system. Moreover, normalization tends to minimize the effect of the changes in total power on the values of LF and HF components. Nevertheless, normalized units should always be quoted with absolute values of the LF and HF power in order to describe the overall distribution of power in spectral components.
Long-term recordings
Spectral analysis also may be used to analyse the sequence of NN intervals of the entire 24-hour period. The result then includes a ULF component, in addition to VLF, LF and HF components. The slope of the 24-hour spectrum can also be assessed on a log-log scale by linear fitting of the spectral values. Selected frequency domain measures (12) are outlined in Table 2.
Table 2. Selected frequency domain measures of HRV.
| Variable | Units | Description | Frequency range |
| Analysis of Short-term Recordings (5 minutes) | |||
| 5-min Total power | msec2 | Variance of NN intervals over temporal segment | ≤ 0.4 Hz |
| VLF | msec2 | Power in VLF range | ≤ 0.04 Hz |
| LF | msec2 | Power in LF range | 0.04-0.15 Hz |
| LF normalised | nu* | LF power in normalised units LF/(total power-VLF)x100 | |
| HF | msec2 | Power in HF range | 0.15-0.4 Hz |
| HF normalised | nu* | HF power in normalised units HF/(total power-VLF)x100 | |
| LF/HF | Ratio LF[msec2]/HF[msec2] | ||
normalised units
Heart rate variability in muscular dystrophies
Muscular dystrophies (MDs) are frequently not confined to the skeletal muscles but also involve other organs or tissues. In the last few years, remarkable progress has been made in understanding the close and various relationships between skeletal muscle disease and heart muscle disease. Cardiac involvement has been documented in a number of primary MDs (20) and is even the dominant feature in some of them. Myocardial disease, manifesting predominantly as cardiomyopathy and congestive heart failure, is characteristic of dystrophies and X-linked dilated cardiomyopathy, whereas conduction system abnormalities that cause heart block, arrhythmias, and sudden death are more commonly seen in limb-girdle type 1B, myotonic, and Emery-Dreifuss MDs. Many patients with MD die on account of cardiac complications such as sudden cardiac death or congestive heart failure.
Quantifying the left ventricular ejection fraction is currently the best way to risk-stratify cardiovascular patients for SCD, and identify those who are most likely to benefit from the insertion of an implantable cardiac defibrillator (ICD). The strategy of systemically placing ICDs in patients at risk of SCD is expensive and leads to substantial psychological hardship. However, non-invasive electro-cardiographic indices of depolarization and repolarization may better identify patients who are at an increased risk of SCD.
Aim of this review is to focus on the usefulness of HRV as a valid parameter to stratify MD patients at greatest risk of arrhythmic death, markedly improving patient selection for ICD therapy.
Duchenne and Becker muscular dystrophies
Duchenne and Becker muscular dystrophies (D/BMD) are typical X-linked disorders, affecting not only skeletal and smooth muscles, but also the myocardium. The lack or reduced content of dystrophin protein at myocardium level determines the development of cardiomyopathy. This becomes a serious problem in the later stages of the disease. For most patients, cardiomyopathy becomes manifest earlier than respiratory insufficiency.
According to the literature, 15% develops in patients under 14 years of age (20–25). Clinical detection of heart damage is difficult due to the general weakness of skeletal muscles and little physical activity of patients. Heart damage may occur in any stage of the disease, and there might be no correlation between this damage and the degree of skeletal muscle damage (20). Histological findings reveal the most severe damage in the postero-basal portion of the left ventricular wall. The right ventricular septum and right ventricular and atrial myocardium are much less involved (26, 27). Persistent sinus tachycardia is the most common abnormality. The pathogenesis of this tachycardia is unknown. It is not clear whether it is associated with the function of autonomic nervous system (28). It has been suggested that fibrosis and fatty infiltration of the sinus node may allow enhanced automaticity or re-entry to occur in the node. Atrial arrhythmias including fibrillation and flutter commonly occur as pre-terminal rhythm (28). Complete heart blocks have rarely been observed. Ventricular arrhythmias are most typical for D/BMD and might be the cause of sudden death. Dilated cardiomyopathy represents the final stage of myocardium involvement in both diseases.
Yotsukura et al. (29, 30) first described an impairment of the autonomic system in 55 DMD, characterized by a significant increase in sympathetic activity and a significant decrease in parasympathetic activity. These observations were confirmed in 60 DMD patients by Lanza et al. (31), who reported a marked impairment of cardiac autonomic function mainly involving the parasympathetic branch and by Katliorienė and Zabiela (32), who observed significantly lower values of HRV parameters in D/BMD patients.
The usefulness of HRV in stratifying patients at highest risk for SCD is still controversial. Ducceschi et al., in 1997 (33), evaluated the arrhythmic profile in a population of 20 BMD patients, searching for possible correlations between the severity of the arrhythmic events, the cardiac autonomic balance (assessed by heart rate variability analysis in the time domain) and the degree of left ventricular systolic impairment. They observed that BMD patients exhibited lower values of SDNN (p = 0.013), SDANN index (p = 0.008) and 24-hour mean heart rate (p = 0.002). They concluded that, in BMD, there is cardiac autonomic imbalance characterized by sympathetic predominance and an increased susceptibility to ventricular arrhythmias, even in the absence of overt cardiomyopathy.
In 2001, Vita et al. (34) reported the results observed in 20 BMD patients investigated by a battery of six cardiovascular autonomic tests (beat-to-beat variability during quiet breathing and deep breathing, heart rate responses to Valsalva manoeuvre and standing, blood pressure responses to standing and sustained handgrip) and power spectral analysis (PSA) of heart rate variability. Although 11 patients revealed abnormal findings at some cardiovascular tests, none of them had a definite autonomic damage, as indicated by two or more abnormal tests. The authors concluded that autonomic involvement does not represent a major finding in BMD.
More recently, the study of Ammendola et al. (35) on 30 BMD patients compared with 30 normal subjects, reported that the two groups differed significantly in heart rate (p < 0.03), SDNN (p < 0.05) and LF:HF (p < 0.03). Furthermore, when BMD patients were subdivided into 2 groups – at high arrhythmic risk or not – the first group had significantly lower SDNN mean values (82 ms versus 122.2 ms, p < 0.02) and significantly higher mean heart rates (99.7 ms versus 71.8 ms, p < 0.002) and LF:HF values (4.3 versus 2.2, p < 0.001) than the patients who survived. The Authors suggested that the autonomic nervous system may have an important role in BMD and that SDNN values < 100 ms may be a significant predictor of cardiac death, independently of clinical variables; they concluded that HRV is a “reliable index to assess sympatho-vagal balance, useful to stratify arrhythmic risk in patients with BMD”.
Myotonic dystrophies
Myotonic dystrophy type 1 (DM1) is the most common form of autosomal dominant MDs, affecting 1:8000 cases among Caucasians. The genetic defect associated with DM1 is an abnormal expansion of a CTG trinucleotide repeat located in the 3’ end of the DMPK gene, on chromosome 19q13.3. Normal alleles have from 5 to 34 CTG, while DM1 alleles contain from 50 to 2000 or more CTG repeats.
In DM1, the muscle involvement is characterized by myotonia and muscle weakness involving facial, axial, semi-distal and distal compartments. Usually, symptoms become evident between the 2nd and the 4th decade of life and slowly progress with time, but, in a small number of cases, DM1 occurs as a severe, congenital form, characterized by neonatal hypotonia, facial diplegia, joint contractures, psychomotor delay, respiratory failure (36).
Besides muscle, DM1 affects eyes (cataract), endocrine (diabetes, thyroid dysfunction, hypogonadism) and nervous (mental retardation) systems, gastrointestinal tract (dysphagia, pseudo-obstruction) and heart. Cardiac involvement manifests as a selective and extensive impairment of the conduction system, not usually associated with any apparent structural heart disease. Such degeneration of the conduction system has been correlated with the significant incidence of SCD observed in DM1 patients, ranging from 2% to 30% according to data in the literature. SCD has frequently been related to the development of conduction blocks, requiring pacemaker (PM) implant in 3%-22% of DM1 patients (37). Nevertheless, some studies have reported the occurrence of SCD in DM1 patients despite pacemaker implantation; these findings, together with reports of spontaneous or inducible ventricular tachycardia (VT), suggest a potential pathogenic role of ventricular arrhythmias for the occurrence of SCD in DM1 (38, 39). However, only a few studies have appeared investigating the prognostic value of HRV analysis in DM1 and, moreover, its usefulness in the risk stratification of DM1 patients has not been established.
The function of the autonomic nervous system was studied in 23 patients with myotonic dystrophy, in a defined population in northern Sweden (40) with an extremely high prevalence of this disease. Heart-rate variability (HRV) tests showed only minor signs of parasympathetic dysfunction. Blood pressure and plasma nor-adrenaline measurements, in recumbent and upright positions, showed no signs of sympathetic neuropathy. Increased plasma levels of nor-adrenaline was an unexpected finding. This study did not support the hypothesis that cardiac arrhythmias, orthostatic hypotension, gastrointestinal motility disturbances and urinary bladder dysfunction, in myotonic dystrophy, are caused by autonomic neuropathy, and according to the authors these symptoms probably result from a defective function of the target organs.
Inoue et al. (41) analysed by means of autoregressive spectral analysis, the spontaneous beat-to-beat HRV of 10 DM1 patients (4 male, 6 female, aged 37-53 years) and 10 age- and sex-matched healthy, sedentary humans (control) at rest in the supine position. None of the DM1 patients had any cardiac conduction disturbances (i.e., atrio-ventricular or intra-ventricular conduction defects) on 12-lead electrocardiogram and were able to walk and perform daily activities. In the DM1 group, the total power, the power of the low-frequency component (a marker of sympathetic and vagal modulation of heart rate) and that of the high-frequency component (a marker of vagal modulation of heart rate) were lower than those in the control group (p < 0.01, p < 0.05 and p < 0.05, respectively). The results of their study suggest that the cardiovascular autonomic nervous system contributing to the HRV may be disturbed even in DM1 patients who can walk and perform daily activities. Therefore, one must give careful consideration to the cardiovascular autonomic dysfunction, as well as the cardiac conduction disturbance in DM1 patients.
Hardin et al. (42) evaluated HRV in 289 patients with DM1, in a multicentre study. They showed than the 24-hour time domain parameters of SDNN and SDANN decreased as age and CTG repeat length increased.
Di Leo et al. (43) evaluated the autonomic nervous system in 23 patients with DM1 type 1 by a battery of six cardiovascular autonomic tests and power spectral analysis of HRV. Although 15 patients (65%) revealed abnormal or borderline results in some tests, only one patient had definite autonomic damage, as indicated by two or more abnormal tests. As a group, DM1 patients showed a significant reduction in heart rate variability during deep breathing (p < 0.0001). The results indicated that such autonomic abnormalities are not: i) part of a peripheral neuropathy; ii) related to cytosine-thymine-guanine repeat size or breathing pattern. Power spectral analysis showed a reduction in the supine low-frequency band, which is, but not exclusively, a marker of sympathetic activity. It was inversely correlated to disease duration (p < 0.04), suggesting progression as the disease advances. A low-frequency power, recorded after standing, was significantly associated (p < 0.02) with presence of heart involvement. The Authors suggest that a mixed, especially parasympathetic, autonomic dysfunction may occur in DM1 – although it is not a major finding – playing a role in the occurrence of cardiac abnormalities, or increasing the risk of sudden cardiovascular events.
In paper by Rakocević-Stojanović et al. (44) – recently appeared in Acta Myologica – the function of ANS was studied in 20 patients with DM1 and 15 healthy controls. All subjects were investigated using a battery of six cardiovascular autonomic tests and power spectral analysis of HRV. Only one patient had normal autonomic function. Two (10%) patients had mild, 10 (50%) moderate and 7 (35%) severe autonomic dysfunction; 13 (65%) patients had vagal, and 4 (20%) sympathetic, hyperactivity; 7 (35%) patients had vagal and 15 (75%) sympathetic dysfunction; 18 (90%) patients had orthostatic hypotension. The 24-hour time domain parameters of SDNN and total power were significantly lower in DM1 patients than in healthy controls (p < 0.05). However, other parameters of HRV, such as SDANN, low frequency (LF), high frequency (HF) power and the LF/HF ratio were somewhat lower in patients with DM1 than in controls, but this was not statistically significant. There was no significant relationship between autonomic dysfunction and the severity of the disease or CTG repeat length. The Authors suggest that sympathetic dysfunction and vagal predominance may both occur in patients with DM1 and that there was no correlation between HRV and age.
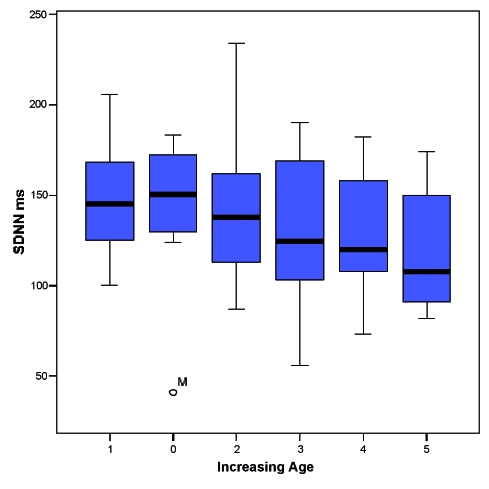
Unlike these Authors, in a study recently performed by our working team (45) on a population of 96 DM1 patients (56 male – age: 37.8 ± 15.25 years – and 40 female – age 40 ± 13.8 years), we found that, although all the time domain indexes (SDANN, SDNN, and RSMMD), and particularly SDNN were lower in DM1 patients when compared with controls (Table 3), nevertheless, the differences were not statistically different. Furthermore, only age was independently related to SDNN, with an inverse linear relationship (-5.09 ms for decade) (Fig. 1).
Table 3. HRV time domain parameters observed in DM1 patients.
| HRV parameter | DM1 patients Mean ± SD |
Controls Mean ± SD |
p value |
| SDNN | 136.4 ± 35.7 | 141.0 ± 39.0 | N.S. |
| SDANN Index | 124.5 ± 38 | 127.0 ± 35.0 | N.S. |
| RMSSD | 36.5 ± 17.0 | 27.0 ± 12.0 | N.S. |
SD = standard deviation; N.S. = Not Significant
Figure 1.
Box-plot of 6 classes of patients showing decrease of SDNN with age.
Autonomic function was also investigated in dystrophia myotonica type 2 (DM2), by Flachenecker et al. (46). Proximal myotonic myopathy is an autosomal dominant multisystem disorder with a recently defined CCTG expansion on chromosome 3 in the major subgroup (DM2). Cardiac rhythm disturbances have been described in patients with this disease, but it is not known whether DM2/proximal myotonic myopathy patients suffer from dysautonomia and whether cardiac arrhythmias relate to autonomic dysfunction. The Authors determined standard autonomic function tests (heart rate responses to Valsalva manoeuvre, deep breathing and active change of posture, and blood pressure responses to active change of posture and sustained handgrip), resting HRV in the time- and frequency-domain, and the corrected QT interval length in 16 patients with genetically defined DM2/proximal myotonic myopathy and compared to the results obtained in 16 age- and sex-matched healthy control subjects. Standard autonomic tests yielded similar results in both groups. Results of HRV measurements tended to be lower in DM2/proximal myotonic myopathy patients compared to healthy controls, but reached statistical significance only for the number of R-R intervals exceeding 50 ms (p50) and the power spectrum density in the low-frequency range (low-frequency power). Four patients (25%) suffered from mild cardiac rhythm disturbances encompassing paroxysmal tachycardia, sinoatrial block, right bundle branch block, ventricular premature beats and bradycardia. The autonomic responses of these patients were essentially similar compared to those without cardiac arrhythmias, apart from a decreased heart rate response to deep breathing in the patients with cardiac arrhythmias. The Authors concluded that no major abnormalities of cardiovascular autonomic function were found in patients with DM2/proximal myotonic myopathy, neither in the whole study group nor in the subgroup of patients with cardiac rhythm abnormalities.
Conclusions
Recent studies have demonstrated that HRV analysis, both in the time and frequency domains, may be a useful tool to assess the balance of cardiac autonomic nervous system. In particular, SDNN is considered the most important parameter to estimate the adrenergic activity. It was previously reported that BMD is associated with cardiac autonomic imbalance, characterised by sympathetic predominance, and an increased vulnerability to ventricular arrhythmias, parallel to the degree of left ventricular systolic dysfunction. Such a dysfunction has already been revealed in DMD patients, who, when compared with normal subjects, showed a higher adrenergic activity associated with a decreased vagal tone. DM1 patients, on the other hand, do not show any impairment of the cardiac autonomic system. The reason for the different behaviour observed in the two populations of patients, may reside in the fact that Duchenne and Becker patients present a systolic dysfunction whilst DM1 patients present with an early and prevalent left ventricular diastolic dysfunction (47).
References
- 1. Lown B, Verrier RL. Neural activity and ventricular fibrillation. N Engl J Med 1976;294:1165-70. [DOI] [PubMed] [Google Scholar]
- 2. Corr PB, Yamada KA, Witkowski FX. Mechanisms controlling cardiac autonomic function and their relation to arrhythmogenesis. In: Fozzard HA, Haber E, Jennings RB, et al., editors. The heart and cardiovascular system. New York, NY: Raven Press 1986. p. 1343-403. [Google Scholar]
- 3. Schwartz PJ, Priori SG. Sympathetic nervous system and cardiac arrhythmias. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology: From cell to bedside. Philadelphia: WB Saunders Co 1990. p. 330-43. [Google Scholar]
- 4. Levy MN, Schwartz PJ, editors. Vagal control of the heart: Experimental basis and clinical implications. Armonk, NY: Futura 1994. [Google Scholar]
- 5. Dreifus LS, Agarwal JB, Botvinick EH, et al. (American College of Cardiology Cardiovascular Technology Assessment Committee). Heart rate variability for risk stratification of life-threatening arrhythmias. J Am Coll Cardiol 1993;22:948-50. [DOI] [PubMed] [Google Scholar]
- 6. Pomeranz M, Macaulay RJB, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol 1985;248:H151-3. [DOI] [PubMed] [Google Scholar]
- 7. Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympathovagal interaction in man and conscious dog. Circ Res 1986;59:178-93. [DOI] [PubMed] [Google Scholar]
- 8. Kleiger RE, Miller JP, Bigger JT, et al. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987;59:256-62. [DOI] [PubMed] [Google Scholar]
- 9. Malik M, Farrell T, Cripps T, et al. Heart rate variability in relation to prognosis after myocardial infarction: selection of optimal processing techniques. Eur Heart J 1989;10:1060-74. [DOI] [PubMed] [Google Scholar]
- 10. Bigger JT, Fleiss JL, Steinman RC, et al. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992;85:164-71. [DOI] [PubMed] [Google Scholar]
- 11. Saul JP, Albrecht P, Berger RD, et al. Analysis of long-term heart rate variability: methods, 1/f scaling and implications. In: Computers in Cardiology 1987. Washington, DC: IEEE Computer Society Press 1988. p. 419-22. [PubMed] [Google Scholar]
- 12. Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology. Heart rate variability. Eur Heart J 1996;17:354-81. [PubMed] [Google Scholar]
- 13. Kay SM, Marple SL. Spectrum analysis: a modern perspective. Proc IEEE 1981;69:1380-419. [Google Scholar]
- 14. Sayers BM. Analysis of heart rate variability. Ergonomics 1973;16:17-32. [DOI] [PubMed] [Google Scholar]
- 15. Akselrod S, Gordon D, Ubel FA, et al. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat to beat cardiovascular control. Science 1981;213:220-2. [DOI] [PubMed] [Google Scholar]
- 16. Malliani A, Pagani M, Lombardi F, et al. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991;84:1482-92. [DOI] [PubMed] [Google Scholar]
- 17. Berger RD, Akselrod S, Gordon D, et al. An efficient algorithm for spectral analysis of heart rate variability. IEEE Trans Biomed Eng 1986;33:900-4. [DOI] [PubMed] [Google Scholar]
- 18. Stein PK, Bosner MS, Kleiger RE, et al. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J 1994;127:1376-81. [DOI] [PubMed] [Google Scholar]
- 19. Stein PK, Kleiger RE. Insights from the study of heart rate variability. Annu Rev Med 1999;50:249-61. [DOI] [PubMed] [Google Scholar]
- 20. Nigro G, Comi LI, Politano L, et al. Cardiomyopathies associated with muscular dystrophies. In: Engel & Franzini-Armstrong eds. Myology. New York: McGraw-Hill 2004. p. 1239-56. [Google Scholar]
- 21. Nigro G, Comi LI, Politano L, et al. Electrocardiographic evaluation of the P type stage of dystrophic cardiomyopathy. Cardiomyology 1984;3:45-52. [Google Scholar]
- 22. Nigro G, Comi LI, Politano L, et al. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Intern J Cardiol 1990;26:271-7. [DOI] [PubMed] [Google Scholar]
- 23. Nigro G, Comi LI, Politano L, et al. Evaluation of the cardiomyopathy in Becker muscular dystrophy. Muscle & Nerve 1995;18:283-91 [DOI] [PubMed] [Google Scholar]
- 24. Mansi L, Pace L, Politano L, et al. Left ventricular function and perfusion in Becker’s muscular dystrophy. J Nucl Med 1997;38:563-7. [PubMed] [Google Scholar]
- 25. Hoogerwaard EM, de Voogt WG, Wilde AA, et al. Evolution of cardiac abnormalities in Becker muscular dystrophy over a 13-year period. J Neurol 1997;244:657-63. [DOI] [PubMed] [Google Scholar]
- 26. Tsao CY, Mendell JR. The childhood muscular dystrophies: making order out of chaos. Seminars in Neurology 1999;19:9-23. [DOI] [PubMed] [Google Scholar]
- 27. Limongelli FM, Fattore L, Petretta V, et al. Atrial pacing in Duchenne muscular dystrophy. Acta Cardiomiologica 1992;11:113-8. [Google Scholar]
- 28. Zipes DP, Jalife J. Cardiac electrophysiology. From cell to bedside. 3rd edn. Philadelphia: W.B. Saunders 2000. [Google Scholar]
- 29. Yotsukura M, Sasaki K, Kachi E, et al. Circadian rhythm and variability of heart rate in Duchenne-type progressive muscular dystrophy. Am J Cardiol 1995;76:947-51. [DOI] [PubMed] [Google Scholar]
- 30. Yotsukura M, Fujii K, Katayama A, et al. Nine-year follow-up study of heart rate variability in patients with Duchenne-type progressive muscular dystrophy. Am Heart J 1998;136:289-96. [DOI] [PubMed] [Google Scholar]
- 31. Lanza GA, Dello Russo A, Giglio V, et al. Impairment of cardiac autonomic function in patients with Duchenne muscular dystrophy: relationship to myocardial and respiratory function. Am Heart J 2001;141:808-12. [DOI] [PubMed] [Google Scholar]
- 32. Katliorienė Z, Zabiela V. Arrhythmias and heart rate variability in Duchenne and Becker muscular dystrophy. Medicina 2002;38:e99-133. [PubMed] [Google Scholar]
- 33. Ducceschi V, Nigro Ge, Sarubbi B, et al. Autonomic nervous system imbalance and left ventricular systolic dysfunction as potential candidates for arrhythmogenesis in Becker muscular dystrophy. Intern J Cardiol 1997;59:275-9. [DOI] [PubMed] [Google Scholar]
- 34. Vita G, Di Leo R, De Gregorio C, et al. Cardiovascular autonomic control in Becker muscular dystrophy. J Neurol Sci 2001;186:45-9. [DOI] [PubMed] [Google Scholar]
- 35. Ammendola E, Russo V, Politano L, et al. Is heart rate variability a valid parameter to predict sudden death in patients with Becker’s muscular dystrophy? Heart 2006;92:1686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harper PS. Myotonic Dystrophy. 2nd edition. London: W.B. Saunders 1989. [Google Scholar]
- 37. Hawley RJ, Colleran JA, Fletcher R, et al. Indications for cardiac pacemaker implantation in myotonic dystrophy. Med Gen Med 1999 Sep. 7:E5. [PubMed] [Google Scholar]
- 38. Hiromasa S, Ikeda T, Kubota K, et al. Myotonic dystrophy: ambulatory electrocardiogram, electrophysiologic study, and echocardiographic evaluation. Am Heart J 1987;113:1482-8. [DOI] [PubMed] [Google Scholar]
- 39. Hiromasa S, Ikeda T, Kubota K, et al. Ventricular tachycardia and sudden death in myotonic dystrophy. Am Heart J 1988;115:914-5. [DOI] [PubMed] [Google Scholar]
- 40. Olofsson BO, Niklasson U, Forsberg H, et al. Assessment of autonomic nerve function in myotonic dystrophy. J Auton Nerv Syst 1990;29:187-92. [DOI] [PubMed] [Google Scholar]
- 41. Inoue K, Ogata H, Matsui M, et al. Assessment of autonomic function in myotonic dystrophy by spectral analysis of heart-rate variability. J Auton Nerv Syst 1995;55:131-4. [DOI] [PubMed] [Google Scholar]
- 42. Hardin BA, Lowe MR, Bhakta D, et al. Heart rate variability declines with increasing age and CTG repeat length in patients with myotonic dystrophy type 1. Ann Non invasive Electrocardiol 2003;8:227-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Di Leo R, Rodolico C, De Gregorio C, et al. Cardiovascular autonomic control in myotonic dystrophy type 1: a correlative study with clinical and genetic data. Neuromuscul Disord 2004;14:136-41. [DOI] [PubMed] [Google Scholar]
- 44. Rakocević-Stojanović V, Milovanović B, Ivić N, et al. Cardiac autonomic nervous system in patients with myotonic dystrophy type 1. Acta Myol 2007;26:112-4. [PMC free article] [PubMed] [Google Scholar]
- 45. Palladino A, Scutifero M, Ventriglia VM, et al. Is heart rate variability a useful parameter to stratify the arrhythmic risk in patients with Dystrophia Myotonica type 1? International Congress on Myotonic Dystrophy, Milan, September 2007. [Google Scholar]
- 46. Flachenecker P, Schneider C, Cursiefen S, et al. Assessment of cardiovascular autonomic function in myotonic dystrophy type 2 (DM2/PROMM). Neuromuscul Disord 2003;13:289-93. [DOI] [PubMed] [Google Scholar]
- 47. Petretta VR, Palladino A, Panico F, et al. Left ventricular diastolic function in myotonic dystrophy. Acta Myol 2004;23:44. [Google Scholar]