Summary
In the recent literature the association of facioscapulohumeraldystrophy (FSHD) with some hereditary neuromuscular diseases in the same patient has been reported. We present the first case in which the genetically confirmed familial FSHD is associated with an extramedullary thoracic tumour.
Keywords: Facioscapulohumeral dystrophy, atypical phenotype, extramedullary tumour
Introduction
The coexistence of facioscapulohumeral muscular dystrophy (FSHD) with motor neuron disease (1), Charcot-Marie-Tooth polyneuropathy 1A (CMT1A) (2, 3), myasthenia gravis (4), Leber’s hereditary optic atrophy (5), mitochondrial myopathy (6), limb girdle muscular dystrophy 2A (LGMD2A) (7), Duchenne and Becker muscular dystrophy (8, 9) has been published. We examined the patient, described herewith, in whom the familial FSHD is associated with spinal tumour.
Clinical data
The patient (the proband) aged 33 years, is an engineer. The disease began with involvement of the shoulder girdle muscles at the age of 10 years when detachment of the scapulae from the thorax was noticed. At the age of 23, he noted difficulties to work with lifted arms and problems in walking and running because of a flapping left foot.
The neurological examination showed severe weakness and slight atrophy of the orbicularis oris muscle more evident on the left side; severe atrophy and weakness of the trapezius, rhomboid, serratus anterior, latissimus dorsi, pectoralis major (both portions) and upper parts of the deltoid muscles; winging of scapulae; spindle-shaped" forearms; pronounced lumbar lordosis due to involvement of the abdominal and gluteus maximus muscles; pseudohypertrophy of the gluteus maximus muscles and slight atrophy of the posterior thigh muscles; severe atrophy and weakness of the shin muscles more evident on the left side. Pseudohypertrophy of subscapularis, supraspinatus and infraspinatus muscles was seen more clearly on the left (Fig. 1). Trophism and strength of the arm muscles were preserved, excluding the brachioradialis muscle which disappeared. Beevor’s sign was positive. No fasciculations. Coordination of movements was not disturbed. Functions of sphincters were preserved. The patient cannot abduct his arms to horizontal level nor stand up from a squatting position without assistance of arms. The patient cannot extend his toes and stand up on his heels. He has a coarse stepping gait with prominent feet drop. Deep tendon reflexes and muscle tone of the arms and legs were reduced.
Figure 1.
The patient aged 33 years. Severe atrophy and weakness of muscles fixing the scapulae, latissimus dorsi and upper part of deltoid muscles. Prominent scapular winging. Pseudohypertrophy of subscapularis, supra- and infra-spinatus muscles. The patient cannot abduct his arms to horizontal level.
However, together with signs of evident FSHD, in this patient, there are clear bilateral Babinski signs. Hyperalgesia and hyperpathia, on the feet, and a decrease in vibration sense in the toes and ankles were observed. Joint position sense, in the toes and hallux was very slightly decreased.
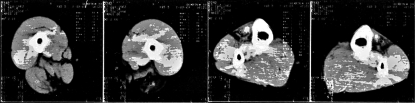
Blood and urine analyses were normal. Serum Lactodehydrogenase (LDH) and Serum Glutamic Oxaloacetic Transaminase (SGOT) values were about twice increased. Serum Creatinekinase values were 960 U/l (normal limits within190 U/L). Needle EMG showed myopathic changes. Motor nerve conduction velocities in median, ulnar, peroneal and tibial nerves were normal, on both sides, as well as the lower legs somatosensory evoked potentials (SEPs). Parameters of the blink-reflex were within normal limits. Computed tomography (CT Scan) of the legs showed a fatty replacement of some thigh and lower leg muscles (Fig. 2A, B). Muscle biopsy (supraspinatus) showed myopathic changes. Brain and spinal MRI was normal.
Figure 2A, B.
CT of leg muscles of patient aged 39 years: A). CT of mid-thighs showed fatty substitution of semi-membranosus, semi-tendinosus, biceps femoris (caput longus), sartorius and partially gracilis and adductor magnus muscles on left side and semi-membranosus muscle on right side; B). CT of mid-lower legs showed fatty replacement of tibialis anterior and extensor digitorum longus muscles on both sides.
DNA analysis revealed a p13E-11 EcoRI/BlnI DNA fragment size of 28 kb (double digestion) on chromosome 4q35 (Dr. K. Arahata).
The patient was re-examined after 6 years (April 15, 2002). His status had greatly changed: the weakness of the pelvic girdle and posterior of thigh muscles was increased; he could not stand up from a squatting position, while walking had become more difficult because the stepping gait was aggravated by ataxia. Leg muscle tone remained low but knee reflexes were deteriorated with bilateral Babinski signs and clonus of the feet, and delay of urine.
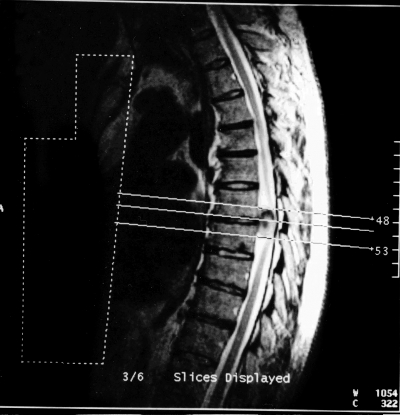
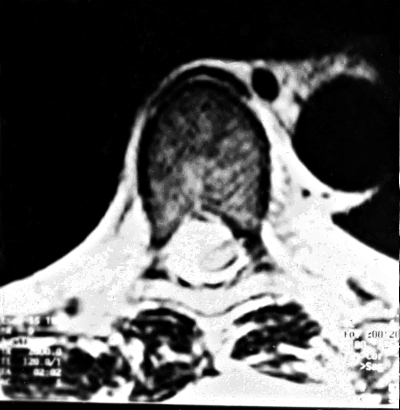
Coarse troubles of the joint position sense in the toes and ankles, less pronounced in the knee, associated with hyperalgesia on the legs were found on both sides. Sensitive ataxia was noticed. Romberg’s test was positive. On EMG study of the arm and leg muscles, myopathic changes were evident. Motor nerve conduction velocities in ulnar, peroneal and tibial nerves were normal but sensory sural nerve conduction velocities were slightly decreased (39 m/sec.). The lower leg SEPs were abnormal: cervical cord and cortical responses were practically absent on both sides and inter-peak latencies, between lumbar and cervical responses, were increased bilaterally suggesting a disorder in the posterior column. Spinal MRI showed a tumour formation (2.0 x 1.3 cm) with intradural extramedullar growth compressing the spinal cord at T6–T7 vertebral level (Fig. 3A, B). Total resection of the tumour was carried out (June 30, 2002); The histological study showed a meningioma.
Figure 3A, B.
The patient aged 39 years. MRI of thoracic column showed right intradural extramedullary tumour of spinal cord on the T6 – T7 spine level: a. longitudinal section, b. transversal section.
The patient was re-examined after surgical treatment in March 30, 2004. The pattern of muscle involvement remained the same. However, the strength of the pelvic girdle muscles increased and the patient could stand up from squatting without help of arms; leg muscle tone remained low, knee and Achilles reflexes were extremely reduced. There was no clonus of the feet nor Babinski signs. There were no urinary disturbances. Both joint position sense in the toes, hallux, ankles and knees and vibration sense were normal. Romberg’s test was negative. The patient had a coarse stepping gait without ataxia. The patient was able to climb stairs with the aid of the railing on 4 floors, to walk independently on the street and to attend to his work as an engineer.
Discussion
The coexistence of the clinical and molecular genetic typical FSHD with signs of the spinal cord affection, in the same patient, is very difficult to interpret.
Moerman et al. (1) reported on an association of atypical FSHD with symptoms of motor neuron disease, in which the diagnosis of the FSHD was established after DNA analysis, only. In their patient, aged 54 years, the onset of the first symptoms was noticed in early infancy with weakness of the lower limbs. Clinical examination showed a severe diffuse muscular weakness and atrophy, tremor of the upper limbs, spasticity of lower limbs and bilateral Babinski signs. A severe asymmetrical facial weakness was associated with bulbar symptoms (dysarthria, dysphagia and tongue paresis). EMG data supported the diagnosis of motor neuron disease and muscle biopsy showed neurogenic atrophy. Double digestion with EcoRI/BnlI of DNA of the patient showed two small alleles of 25 and 28 kb, suggesting the patient was a compound heterozygote for two low penetrance alleles.
Some authors observed the weakness and myogenic atrophy of the tongue muscle with dysphagia in patients with advanced 4q35-linked FSHD (10). However, none of these patients showed signs of pyramidal tract involvement. Palmucci et al. (2) reported on two Italian families in which two genetic diseases – typical FSHD and Charcot-Marie-Tooth polyneuropathy 1A (CMT1A) – in different members of the same family or even in the same individual were presented. In the first family, in the mother who had both mutations, the clinical expression was that of a typical FSHD only. In the case described by Buterfisch (3) – who had an unusual combination of CMT1A and FSHD mutations – both disorders were present. The disease process was devastating and resulted in severe generalized weakness and early death.
In our patient, the course of the disease (FSHD) was complicated by an extra-medullary tumour which caused the symptoms of the lateral and posterior column disorder, sensitive ataxia and sphincters disturbances, completely disappeared after resection of spinal tumour. Our report shows that patients with 4q35-linked FSHD may simultaneously present some other neurological disorders which give rise to new symptoms and signs coexisting with the clinical picture of FSHD.
References
- 1. Moerman A, Eymard B, Urtizberea A, et al. Severe facioscapulohumeral dystrophy associated with motor neuron involvement. Neuromusc Disord 2001;11:619. [Google Scholar]
- 2. Palmucci L, Mongini T, Doriguzzi C, et al. Association of FSHD and CMT1A in two Italian families. Neuromusc Disord 2001;11:619. [Google Scholar]
- 3. Buterfisch CM, Lang DF, Gutmann L. The devastating combination of Charcot-Marie-Tooth disease and facioscapulohumeral muscular dystrophy. Muscle Nerve 1998;21:788-91. [DOI] [PubMed] [Google Scholar]
- 4. McGonigal A, Thomas AM, Petty RK. Facioscapulohumeral muscular dystrophy and myasthenia gravis coexisting in the same patient: a case report. J Neurol 2002;249:219-20. [DOI] [PubMed] [Google Scholar]
- 5. Chuenkongkaew WL, Lertrit P, Limwougse C, et al. An unusual family with Leber’s hereditary optic myopathy and facioscapulohumeral muscular dystrophy. Eur J Neurol 2005;12:388-91. [DOI] [PubMed] [Google Scholar]
- 6. Filosto M, Tonin P, Scarpelli M, et al. Novel mitochondrial tRNA Leu (CUN) transition and D4Z4 partial deletion in a patient with a facioscapulohumeral phenotype. Neuromusc Disord 2008;18:204-9. [DOI] [PubMed] [Google Scholar]
- 7. Sacconi S, Salviati L, Bourget L, et al. Diagnostic challenges in facioscapulohumeral muscular dystrophy. Neurology 2006;67:1464-6. [DOI] [PubMed] [Google Scholar]
- 8. Korngut L, Siu VM, Venance SL, et al. Phenotype of combined Duchenne and facioscapulohumeral muscular dystrophy. Neuromusc Disord 2008;18:576-82. [DOI] [PubMed] [Google Scholar]
- 9. Rudnik-Schoneborn S, Weis J, Kress W, et al. Becker’s muscular dystrophy aggravating facioscapulohumeral muscular dystrophy – double trouble as an explanation for an atypical phenotype. Neuromusc Disord 2008;18:881-5. [DOI] [PubMed] [Google Scholar]
- 10. Wohlgemuth M, de Swart BJ, Kalf JG, et al. Dysphagia in facioscapulohumeral muscular dystrophy. Neurology 2006;66:1926-8. [DOI] [PubMed] [Google Scholar]