Summary
Sporadic inclusion body myositis (sIBM) is the most common myopathy presenting over the age of 40 years but its prevalence varies considerably in different populations. Genetic factors play a part in the pathogenesis of sIBM and in Caucasians susceptibility has been linked to the HLA-DR3 allele and the 8.1 MHC ancestral haplotype (AH) which is also associated with other autoimmune diseases. The variable prevalence of sIBM in different populations may be related to differences in the population frequency of this haplotype. Our recent observations indicate that the clinical phenotype at presentation is also quite variable and that the influence of the MHC is more complex than previously appreciated with HLA alleles also having modifying effects on the age-at-onset, severity and rate of progression of the disease. Recent recombinant mapping studies of polymorphisms in the Class II/III regions of the MHC by our group have further refined the susceptibility region and have identified a number of candidate genes warranting further investigation. The significance of these findings for the pathogenesis of the disease is discussed.
Keywords: S-IBM, prevalence, phenotype, MHC alleles
Introduction
Although there have so far been few epidemiological studies of sIBM the prevalence of the disease appears to vary considerably in different populations and racial groups. There is also considerable variability in the age and mode of presentation of the disease and there is increasing recognition of atypical modes of presentation which may contribute to the problem of late diagnosis and lack of response to treatment. The factors responsible for the variability in the clinical phenotype and global prevalence of the disease are poorly understood but there is increasing evidence that genetic factors are involved and that allelic variations in major histocompatibility complex (MHC) genes play an important role and may also affect the severity of the disease and rate of progression.
Prevalence of sIBM
As shown in Table 1 the reported prevalence of sIBM varies from 4.7 per million in the Netherlands to 14.9 per million in Western Australia. However, it is likely that these figures are under-estimates due to incomplete case ascertainment and delayed diagnosis of the disease. The prevalence in Western Australia was found to have increased from 9.3 per million in 2000 to 14.9 per million in 2008, probably reflecting improved case ascertainment (1). Few figures are available from other European countries, apart from a very low frequency of 1 per million in Turkey (2), and 2 per million in Sicily (G. Vita, personal communication) based on biopsy review surveys. There is a lack of published data on the prevalence of sIBM from Asian countries but the condition is considered to be rare in India (3) and Thailand (Wittoonpanich, R. personal communication) in comparison to other inflammatory myopathies. Although there have not been any formal comparative studies, it is of interest that sIBM also seems to be much rarer in non-Caucasian ethnic groups such as African-Americans and Australian aboriginals.
Table 1. Reported prevalence figures for sIBM and frequency of HLA-DR3 in different populations.
| Country | Authors | Prevalence per million | Prevalence per million(>50 yrs) | HLA-DR3 Frequency |
| Western Australia | Phillips et al. (2000) | 4.3 | 35.3 | 0.135 |
| Western Australia | Needham et al. (2008) | 14.9 | 51.2 | 0.135 |
| Netherlands | Badrising et al. (2000) | 4.9 | 16 | 0.157 |
| USA | Felice (2001) | 10.7 | 28.9 (<45) | 0.137 |
| Turkey | Serdaroglu et al. (2008) | 1.0 | 1.0 | 0.092 |
These racial and ethnic variations strongly suggest that genetic factors play an important role in determining susceptibility to the disease. Variations in the population frequencies of the susceptibility allele HLA-DRB1*0301 (DR3), which is strongly associated with sIBM in European, North American and Australian populations, and the associated 8.1 MHC ancestral haplotype, may account for the variations in disease prevalence and it is noteworthy that HLA-DR3 frequencies are much lower in countries such as Turkey and Thailand, and in African-Americans and Australian aboriginals, who have a very low incidence of sIBM (Table 1).
Further epidemiological studies are needed to determine the degree of variability in the prevalence of sIBM in other populations and racial groups and to throw further light on the contribution of the HLA genotype and other genetic and environmental factors in the disease.
Clinical phenotype
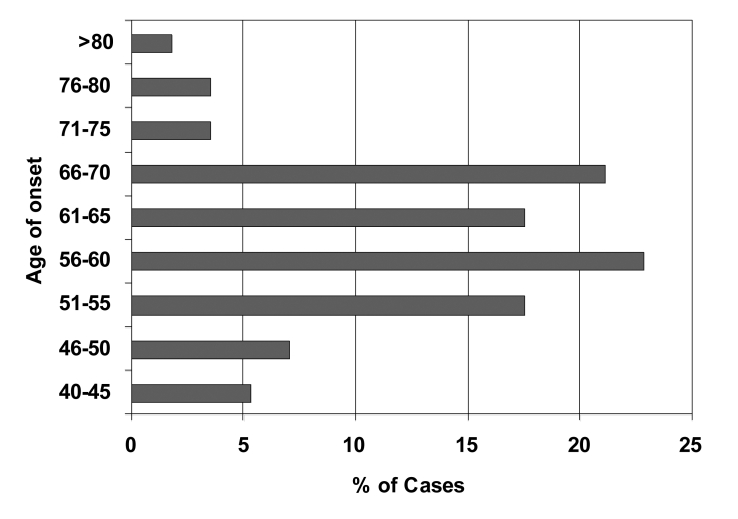
The clinical profile of sIBM is also characterised by considerable variability, firstly in the age at which symptoms first develop, and in the presenting symptoms and patterns of muscle involvement in different patients, the rate of progression of the disease and response to treatment. Although it is generally regarded as a disease of later life (4), as shown in Figure 1 the onset can be as early as the 30s in some cases. While in most cases the presenting symptoms are related to weakness of the quadriceps femoris (Figs. 2, 3) and other proximal lower limb muscles, some patients first present because of weakness of the fingers or other unusual presentations such as dysphagia, foot-drop, ‘dropped-head’ or camptocormia due to weakness of the paraspinal muscles, and scapuloperoneal or facioscapulohumeral patterns of weakness have also been reported.
Figure 1.
Distribution of age of onset of symptoms in a cohort of 56 Australian sIBM patients. (Reproduced from Needham et al., Journal of Neurology, Neurosurgery and Psychiatry, 2008;79:1056-1060 with permission from the BMJ Publishing Group).
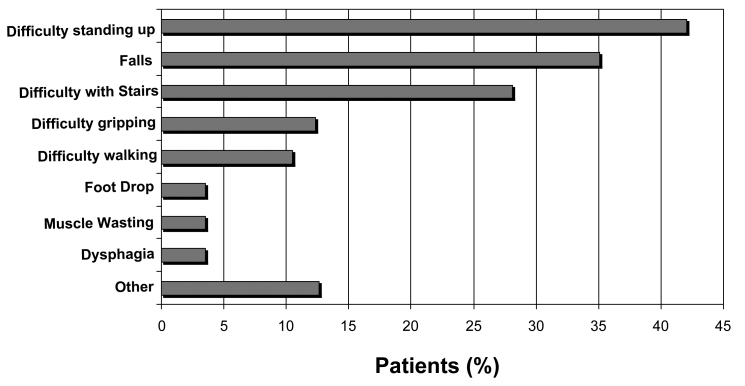
Figure 2.
Presenting symptoms in a cohort of 56 Australian sIBM patients. (Reproduced from Needham et al., Journal of Neurology, Neurosurgery and Psychiatry, 2008;79:1056-1060 with permission from the BMJ Publishing Group).
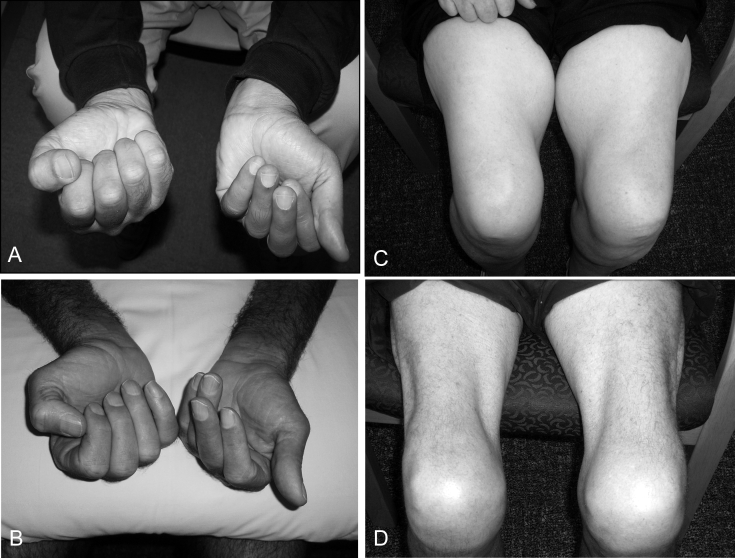
Figure 3.
A and D: Asymmetric weakness of the finger flexors in two sIBM patients with more severe weakness of the non-dominant hand in each case. C and D: Atrophy of the quadriceps femoris muscles in two patients with longstanding sIBM.
There is often still a considerable delay in the time to diagnosis of sIBM and a high rate of initial misdiagnosis. In a recent review of 57 Australian cases of sIBM (5), the average time to diagnosis was 5.2 years and the initial diagnosis was incorrect in 54% of cases, the most frequent misdiagnoses being old age, arthritis and motor neurone disease as noted in previous surveys (6). This may reflect the nonspecific nature of the early symptoms in many cases but is probably also due to failure to recognize the characteristic selective pattern of involvement of the quadriceps femoris and finger flexor muscles, which is often asymmetric and is almost invariably more severe on the non-dominant side (Fig. 3), as well as other unusual presentations. Magnetic resonance imaging is a useful diagnostic procedure prior to muscle biopsy (7).
Pathology
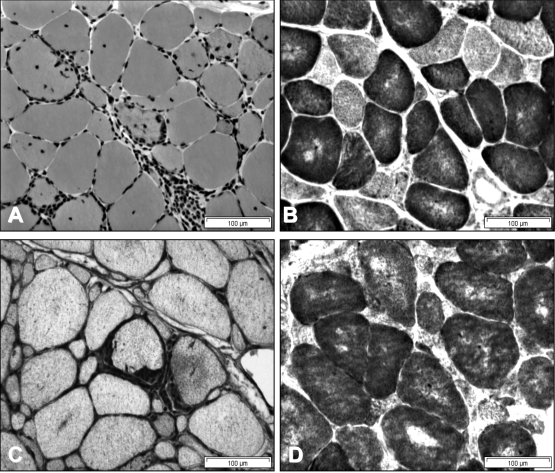
The variability in the phenotype is also reflected in the pathological changes in different muscles at different stages of the disease. It is well known that the inflammatory changes tend to be more prominent in biopsies taken in the earlier stages of the disease and may be relatively inconspicuous in longer standing cases. On the other hand, the myodegenerative features such as rimmed vacuoles and congophilic amyloid inclusions are less obvious in earlier cases and become more prominent as the disease progresses (Figs. 4, 5).
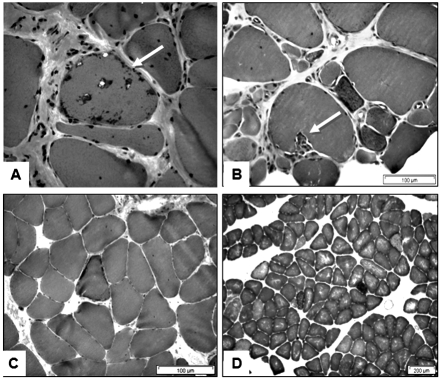
Figure 4.
Muscle biopsy findings in a 61 year old man with a 20 year history of untreated IBM. A and B: From a vastus lateralis biopsy showing chronic changes with interstitial fibrosis, myofibre atrophy and a fibre with rimmed vacuoles (arrow), and sparse inflammation (A); invasion of a non-necrotic muscle fibre by mononuclear cells (arrow) (B). C and D: From a deltoid biopsy showing a ragged-red fibre (C) and multiple blue-staining COX-negative fibres (D).
Figure 5.
Biopsies from deltoid (A and B) and biceps muscles (C and D) in a 35 year old male with a 3 year history of sIBM showing endomysial mononuclear inflammatory infiltrates and myofibre invasion (A); diffuse MHC-1 upregulation (C); and multiple blue-staining COX-negative fibres in both muscles, which are more atrophic in the biceps muscle (B and D). Fibres with rimmed vacuoles were present but were sparse in both muscles.
Cases of sIBM in which biopsies are taken from more than one muscle provide the opportunity to compare the severity of histopathological changes in different muscles at the same stage of the disease. For example, Figure 4 shows the paucity of endomysial inflammation and the more advanced pathological changes in the vastus lateralis muscle compared to the deltoid in a 61 year old man with untreated sIBM of 20 years duration. On the other hand, Figure 5 shows differential changes in the deltoid and biceps muscles in a 35 year old man with typical clinical features of sIBM and only a three year history, who responded to treatment with prednisolone and methotrexate. These observations show that the tempo of the disease varies in different muscles. They also emphasise the importance of making the diagnosis of sIBM at an early stage when the disease process may be more responsive to immune therapies. This will be even more important once more effective forms of disease-modifying therapy become available (8).
Pathogenesis
There is now substantial evidence that sIBM is a muscle-specific autoimmune disease in which T and B-cells and inflammatory mediators play key roles in inducing myofibre necrosis and degenerative changes in muscle fibres (9–12). An alternative point of view suggests that the inflammatory changes could be secondary to an intrinsic degenerative process associated with abnormal protein aggregation and deposition in myofibres (13).
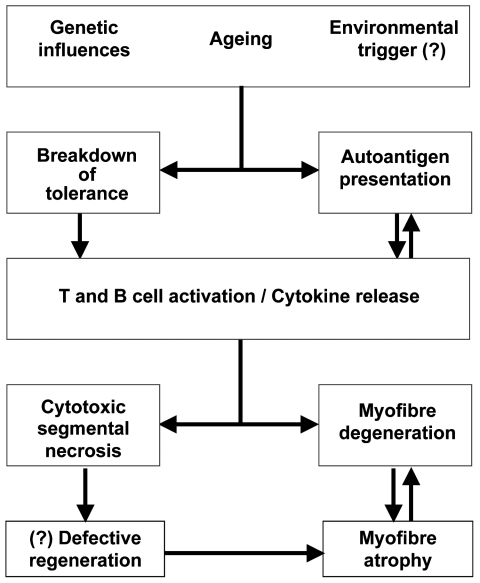
It is still unclear whether the breakdown of immune tolerance and initiation of the myositic process is a result of an aberrant response of the immune system or of abnormal MHC-I expression and presentation of immunogenic peptides by muscle fibres which are recognised as “non-self” by dendritic cells although these processes go hand in hand (Fig. 6) (11). The expression of MHC-I per se is unlikely to be of primary importance as it also occurs in other inflammatory myopathies. The occurrence of sIBM in individuals with HIV and HTLV infections (9), indicates that in some cases a viral infection may be responsible for initiating the disease as is also the case in other autoimmune diseases such as multiple sclerosis and type 1 diabetes. However, in the majority of cases of sIBM the disease begins spontaneously without any identifiable infection or environmental triggering agent.
Figure 6.
Schematic outline of the pathogenesis of sporadic inclusion body myositis.
There is increasing awareness of the role of genetic factors in the pathogenesis of the disease which may operate at a number of levels (11–14). Genetic variations could contribute to the loss of tolerance through polymorphism in MHC genes that encode Class II MHC proteins involved in antigen presentation, or alternatively in genes involved in immune regulation and T-cell activation as has been demonstrated in other autoimmune diseases (11).
Role of the MHC
The association with HLA-DR3 and the MHC 8.1 ancestral haplotype (HLA-A*01, -B*0801, -DRB1*0301, -DQB1*0201, -DQA1*05) is one of the strongest known HLA-disease associations and has been found in ~75% of sIBM cases in studies from Europe, Australia and the United States. This is known as the “autoimmune haplotype” as it is also associated with a number of other autoimmune diseases and with variations in immune function, including increased production of pro-inflammatory cytokines (15). Other HLA alleles and haplotypes have also been associated with sIBM including the 35.2 AH (HLA-B35, DR1) and the 52.1 AH (HLA-B*5201, DRB1*1502) which has been associated with the disease in Japanese (16). In addition, the HLA-DR4 and DR7 alleles (17), and HLA-DQA1 may be protective (18). Because of the strong linkage disequilibrium which exists within the MHC, alleles which are found to be statistically associated with the disease do not necessarily have a direct involvement in the pathogenesis of the disease but may simply be markers for other genes within an extended conserved haplotype. Recombinant studies in 8.1AH-associated sIBM cases lead to the definition of a 250Kb region at the junction of the central and Class II regions of the MHC which was linked to susceptibility (19, 20). This region has now been further refined and a number of candidate genes have been identified (Scott A: personal communication). Further characterisation of the disease associated gene(s) in the MHC, and determining whether they encode proteins which have an immune function or are expressed only in muscle or more ubiquitously, could lead to advances in our understanding of the pathogenesis of sIBM and to identifying new therapeutic targets.
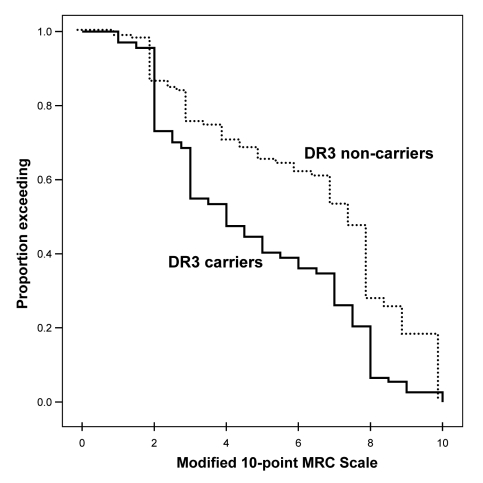
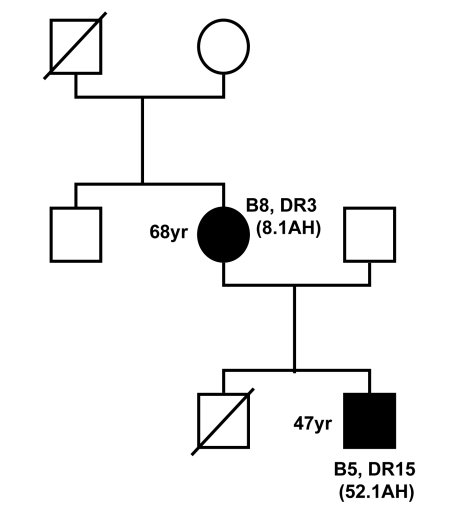
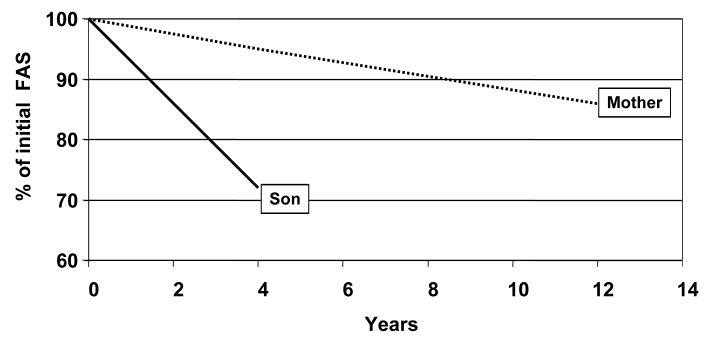
Recent observations in an Australian sIBM cohort indicate that the risk of developing the disease is also influenced by epistatic interactions between alleles at the HLA-DRB1 locus (17), and that the HLA-DRB1 genotype may also influence the disease phenotype. It was found that carriers of the HLA-DR1 allele and HLA-DR1/DR3 heterozygotes have an earlier age-at-onset of the disease, while carriers of HLA-DR3 and HLA-DR1/DR3 heterozygotes have more severe and more rapidly progressive quadriceps muscle weakness (Fig. 7) (21). Carriage of this HLA genotype therefore predicts a more severe phenotype of the disease. In addition, observations in a mother and son who both had inclusion body myositis showed that the son who carried the HLA-DRB1*1502 allele had a more rapidly progressive form of the disease than his mother who was an HLA-DRB1*0301 carrier (Figs. 8, 9) (22).
Figure 7.
Kaplan-Meier plots of quadriceps muscle strength adjusted for disease duration and treatment in DR3 carriers and non-carriers.
Figure 8.
Family tree showing a mother and son who suffered from inclusion body myositis. The mother was a carrier of HLA-B8, DR3 and the 8.1 ancestral haplotype (AH) whereas the son carried HLA-B5, DR15 and the 52.1 ancestral haplotype.
Figure 9.
Differential rate of decline in Functional Assessment Score (FAS) in the mother and son from Figure 8 showing a more rapid progression in the son.
Conclusions
Sporadic IBM therefore demonstrates considerable variability in its global prevalence, clinical phenotype and muscle pathology findings. Susceptibility to the disease is strongly linked to the HLA-DR3 locus and to the 8.1 MHC ancestral haplotype and the population frequency of this haplotype may be responsible for the differences in prevalence of the disease in different races. In addition, recent observations suggest that in addition to influencing susceptibility, the HLA genotype may have disease-modifying effects.
Acknowledgments
I express my appreciation to Dr M Needham, Dr P Zilko and Mrs S Walters for clinical contributions; to Dr V Fabian for providing access to muscle biopsies; Professor I James for statistical analyses; Dr A Scott, Dr R Allcock, Professor N Laing and Professor F Christiansen for contributions to the MHC studies and Ms J Williamson for secretarial assistance.
References
- 1. Needham M, Corbett A, Day T, et al. Prevalence of sporadic inclusion body myositis and factors contributing to delayed diagnosis. J Clin Neurosci 2008;15:1350-3. [DOI] [PubMed] [Google Scholar]
- 2. Serdaroglu P, Deymeer F, Parman Y. Prevalence of sporadic inclusion body myositis (s-IBM) in Turkey: a muscle biopsy based survey. Neuromuscul Disord 2007;17:849. [PMC free article] [PubMed] [Google Scholar]
- 3. Khadilkar SV, Patil SG, Amin SN. Study of idiopathic inflammatory myopathies with special reference to borderland between idiopathic inflammatory myopathies and muscular dystrophies. Neurol India 2008;56:356-62. [DOI] [PubMed] [Google Scholar]
- 4. Griggs RC, Askanas V, DiMauro S, et al. Inclusion body myositis and myopathies. Ann Neurol 1995;38:705-13. [DOI] [PubMed] [Google Scholar]
- 5. Needham M, James I, Corbett A, Mastaglia, FL, et al. Sporadic inclusion body myositis: phenotypic variability and influence of HLA-DR3 in a cohort of 57 Australian cases. J Neurol Neurosurg Psychiatry 2008;79:1056-60. [DOI] [PubMed] [Google Scholar]
- 6. Dabby R, Lange DJ, Trojaborg W, et al. Inclusion body myositis mimicking motor neuron disease. Arch Neurol 2001;58:1253-6. [DOI] [PubMed] [Google Scholar]
- 7. Phillips BA, Cala LA, Thickbroom GW, Mastaglia FL, et al. Patterns of muscle involvement in inclusion body myositis: clinical and magnetic resonance imaging study. Muscle Nerve 2001;24:1526-34. [DOI] [PubMed] [Google Scholar]
- 8. Dalakas MC, Rakocevic G, Schmidt J, et al. Effect of Alemtuzumab (CAMPATH 1-H) in patients with inclusion-body myositis. Brain 2009;132:1536-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dalakas MC. Sporadic inclusion body myositis-diagnosis, pathogenesis and therapeutic strategies. Nat Clin Pract Neurol 2006;2:437-47. [DOI] [PubMed] [Google Scholar]
- 10. Greenberg SA. Proposed immunologic models of the inflammatory myopathies and potential therapeutic implications. Neurology 2007;69:2008-19. [DOI] [PubMed] [Google Scholar]
- 11. Needham M, Mastaglia FL. Sporadic inclusion body myositis: a continuing puzzle. Neuromuscul Disord 2008;18:6-16. [DOI] [PubMed] [Google Scholar]
- 12. Schmidt J, Barthel K, Wrede A, et al. Interrelation of inflammation and APP in sIBM: IL-1 beta induces accumulation of beta-amyloid in skeletal muscle. Brain 2008;131:1228-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Askanas V, Engel WK. Inclusion-body myositis: muscle-fiber molecular pathology and possible pathogenic significance of its similarity to Alzheimer’s and Parkinson’s disease brains. Acta Neuropathol 2008;116:583-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Needham M, Mastaglia FL, Garlepp MJ. Genetics of inclusion-body myositis. Muscle Nerve 2007;35:549-61. [DOI] [PubMed] [Google Scholar]
- 15. Price P, Witt C, Allcock R, et al. The genetic basis for the association of the 8.1 ancestral haplotype (A1, B8, DR3) with multiple immunopathological diseases. Immunol Rev 1999;167:257-74. [DOI] [PubMed] [Google Scholar]
- 16. Scott AP, Allcock RJ, Mastaglia FL, et al. Sporadic inclusion body myositis in Japanese is associated with the MHC ancestral haplotype 52.1. Neuromuscul Disord 2006;16:311-5. [DOI] [PubMed] [Google Scholar]
- 17. Mastaglia FL, Needham M, Scott A, et al. Sporadic inclusion body myositis: HLA- DRB1 alleles interactions influence disease risk and clinical phenotype. Neuromuscul Disord 2009;19:763-5. [DOI] [PubMed] [Google Scholar]
- 18. O’Hanlon TP, Carrick DM, Arnett FC, et al. Immunogenetic risk and protective factors for the idiopathic inflammatory myopathies: distinct HLA-A, -B, -Cw, -DRB1 and -DQA1 allelic profiles and motifs define clinicopathologic groups in caucasians. Medicine (Baltimore) 2005;84:338-49. [DOI] [PubMed] [Google Scholar]
- 19. Kok CC, Croager EJ, Witt CS, Mastaglia FL, Garlepp M, et al. Mapping of a candidate region for susceptibility to inclusion body myositis in the human major histocompatibility complex. Immunogenetics 1999;49:508-16. [DOI] [PubMed] [Google Scholar]
- 20. Price P, Santoso L, Mastaglia F, et al. Two major histocompatibility complex haplotypes influence susceptibility to sporadic inclusion body myositis: critical evaluation of an association with HLA-DR3. Tissue Antigens 2004;64:575-80. [DOI] [PubMed] [Google Scholar]
- 21. Needham M, Scott A, Christiansen F, Mastaglia FL, et al. HLA alleles and MHC haplotypes in sporadic inclusion body myositis: Frequencies and phenotypic correlations. Neuromuscul Disord 2008;18:770. [Google Scholar]
- 22. Mastaglia FL, Price P, Walters S, et al. Familial inclusion body myositis in a mother and son with different ancestral MHC haplotypes. Neuromuscul Disord 2006;16:754-8. [DOI] [PubMed] [Google Scholar]