Abstract
Hyaluronan is an extracellular matrix component implicated in expansion of the extracellular space, organization of supramolecular architecture, cell motility, proliferation, tumour metastases and wound healing. Hyaluronan is highly expressed in the developing heart but it is only a minor component of the mature heart. The loss of hyaluronan synthase-2 (Has2) results in embryonic lethality with a phenotype remarkably similar to that of the versican-deficient heart defect mouse. Has2-deficient embryos lack hyaluronan-containing cardiac jelly, and at embryonic day 9.5 show arrested development, with an apparent absence of the right ventricle and underdevelopment of the conustruncus segment, and pericardial effusion consistent with heart failure. Cardiac cushions are totally absent, and endocardial cell migration over collagen gels is not detectable in Has2-deficient atrioventricular (AV) canal explants. Endothelial to mesenchymal transformation is also defective in AV explants from Has2-null embryos. The normal phenotype is restored in AV canal explants from Has2-deficient embryos by co-culture with wild type AV canal explants, with conditioned media from wild type AV explants or with exogenous hyaluronan. These results provide evidence for a direct role for hyaluronan during endocardial cushion and AV canal morphogenesis.
Keywords: Atrioventricular canal, Hyaluronan, Hyaluronan synthase-2, Morphogenesis
The heart forms early in embryogenesis as a bilayered tube. An acellular, extracellular matrix (cardiac jelly) separates the myocardial and endocardial layers. Immediately following looping of the heart tube, the cardiac jelly expands, forming the endocardial cushions. A subpopulation of the endocardial cells separates from adjacent cells, migrates into the matrix and transforms into mesenchymal cells. Subsequent cellular proliferation and remodelling within the cushion tissue form the rudimentary valve leaflets. Malformation of the endocardial cushions contributes to congenital heart defects (CHD) such as common atrioventricular (AV) canal. Atrioventricular septal defects (AVSD), atrial septal defects and ostium primum defect all contribute to common AV canal disorders. All appear to result from abnormal formation of the cushion tissue. CHD occur in approximately 1% of births, with AVSD being the second most common type of defect (1,2). These types of CHD are more common in trisomy 21 patients (3). Environmental factors such as hypoxia of the fetus and exposure to teratogens can also lead to AVSD and CHD (2,4,5). Although abnormal development of cardiac valve tissue is the most frequent type of CHD, little is known about the molecular events regulating cardiac cushion and valve formation. Thus, understanding the normal developmental process of formation of endocardial cushions and the AV canal is paramount in the development of the best treatment and prevention strategies for common AV canal disorders.
We have identified hyaluronan synthase-2 (Has2) as the principle enzyme for the production of the glycosaminoglycan hyaluronan during cardiac morphogenesis. Has2 expression is prominent in the myocardium from embryonic day (E) 8.5 through E13.5. Has2 transcripts are highly expressed in the developing endocardial cushions. Hyaluronan is abundant in the acellular cardiac jelly, organizing the extracellular matrix and maintaining integrity of the cushion tissue. The absence of Has2 and hyaluronan results in midgestation lethality from apparent cardiac failure. An in vitro assay system for AV canal morphogenesis shows that hyaluronan is necessary for migration, activation and transformation of endocardial cells into mesenchyme. These mesenchymal cells invade the collagen gel, mimicking the invasion of cells into the endocardial cushions. We show that AV canal explants from Has2−/− embryos can be restored to the normal phenotype by culturing in the presence of wild type AV canal explants, in the presence of conditioned media from wild type AV explants or with exogenous hyaluronan.
MATERIALS AND METHODS
Mice and reagents:
Animals were maintained in a pathogen-free facility meeting Institutional Animal Care and Use Committee regulations. The Has2 homozygous embryos were generated as previously described (6). Bisbenzimide and monoclonal anti-α-smooth muscle actin (clone 1A4) were purchased from Sigma Chemical Co (USA); fluorescent cyanine dye (Cy3) -conjugated goat antimouse immunoglobulin (Ig) G+IgM was purchased from Jackson Immuno-Research Laboratories Inc (USA). Hyaluronan was purchased from Calbiochem-Novabiochem Co (mean molecular weight 757,000; Catalogue No 385908, USA), reconstituted and dialyzed against AV canal explant culture medium.
In situ hybridization:
In situ hybridization was performed using a full length Has2 cDNA riboprobe (7) as described previously (8). Embryos were fixed in RNase-free 4% paraformaldehyde and embedded in paraffin, and serial sections were cut and deparaffinized. Riboprobes were labelled (35S-UTP, NEN-Dupont, USA) with T3 or T7 polymerase (Amersham-Pharmacia, USA), and sections were hybridized with the probe in 0.02% Ficoll 400, 0.02% polyvinylpyrrolidone, 0.1% bovine serum albumin, 100 μg/mL denatured salmon sperm DNA, 100 μg/mL yeast RNA and 10% dextran sulphate at 50°C for 16 h. Hybridized sections were rinsed in a formamide wash buffer at 50°C, 0.5× standard sodium citrate for 30 min at 25°C, and unhybridized probe was removed with RNase A digestion followed by 0.1× standard sodium citrate wash (×2) at 65°C for 1 h. The sections were dehydrated and autoradiographed with Kodak NTB-2 emulsion, Kodak D-19 developer and Rapid Fix (Kodak, USA).
AV canal endocardial cushion morphogenesis assay:
The AV canal (‘explant’) from E9.5 wild type and mutant embryos was placed on a type I collagen gel (rat tail collagen, Sigma) (9) and cultured in 0.1 mL of Medium 199 supplemented with 1% fetal bovine serum (Hyclone, USA), 100 U/mL penicillin and 100 μg/mL streptomycin, and 0.1% insulin, transferrin and selenium (‘culture medium’) (Gibco-BRL, USA) in four-well microculture dishes (Nalge Nunc, USA) at 37°C and 5% CO2 for 48 to 72 h. Explants were supplemented with hyaluronan the following day by the addition of 0.25 mg/mL of hyaluronan dialyzed against serumfree culture medium. As indicated, some explants were treated with hyaluronan that had been boiled for 10 min before addition to the cultures. The reduced amount of transformation in Figure 5F may be a result of eliminating minute concentrations of contaminating growth factors in the hyaluronan preparation or of creation of reactive oxygen species during boiling, which can reduce the bioactivity of hyaluronan (10,11). Co-culture experiments were conducted by placing one Has2−/− AV explant between two wild type AV explants in each well. A continuous fluid layer was maintained among all three explants with a minimum of 100 μL of culture media.
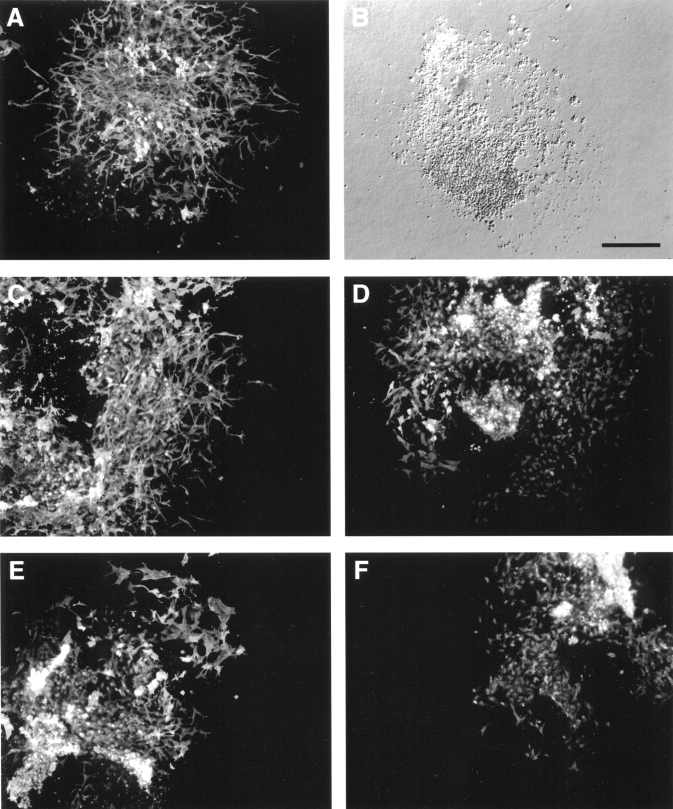
Figure 5.
Exogenous hyaluronan rescues atrioventricular (AV) canal morphogenesis in vitro in hyaluronan synthase-2−/− (Has2−/−) explants. AV canal explants were cultured as indicated for 48 h, the myocardium was removed, and remaining cells were immunostained for α-smooth muscle actin to visualize mesenchymal cells. A,B Representative AV explants derived from wild type (A) and Has2−/− embryos (B). Panel B is a differential interference contrast image: there were no mesenchymal cells in untreated Has2−/− explants. AV explant cultures from Has2−/− embryos co-cultured with wild type AV explant (C), with 100 μL conditioned media from wild type AV explants (D), with exogenous hyaluronan (0.25 mg/mL) in the media (E) and with boiled hyaluronan in the media (F) all exhibited transformation. Scale bar = 200 μm
Histological analysis:
Whole embryos were fixed in 4% paraformaldehyde and moved through a graded series of glycerol-phosphate-buffered saline (PBS) solutions to a final solution of 70% glycerol and 30% PBS for imaging. Images were acquired through a Leica M3Z inverted microscope (purchased from GTI Microsystems, USA). AV explants were processed for immunostaining by fixation in 4% paraformaldehyde for 15 min at room temperature followed by three 1× PBS washes. AV explants were blocked with PBS plus 3% bovine serum albumin and 0.05% Tween 20 for 2 h at room temperature. AV explants were incubated with the primary anti-α-smooth muscle actin at 1:500 dilution overnight at 4°C. AV explants were extensively washed with 1× PBS (×10) and then incubated with secondary antibody antimouse IgG-Cy3 at 1:2000 dilution. Following 1× PBS washes and 15 min incubation with bisbenzimide (1:500 in PBS) for nuclear staining, AV explants were lifted onto microscope slides and cover slip mounted in a SlowFade-Light Antifade kit (Molecular Probes, USA). Images were acquired using Spot Image software (Diagnostic Instruments, USA).
RESULTS
Has2 expression in the developing heart:
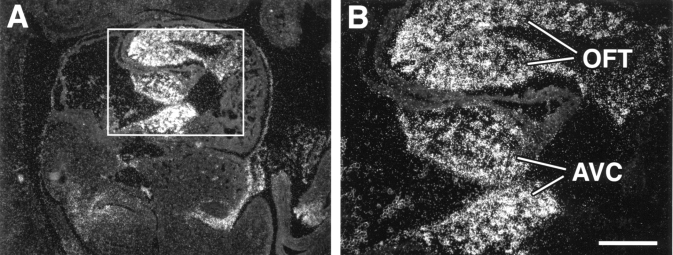
Has2 mRNA is highly expressed in the heart. Has2 transcripts are expressed predominantly in the developing endocardial cushions of the AV canal and outflow tract tissues (Figure 1A). Similar observations have been made with a green fluorescent protein reporter gene driven by putative Has2 promoter elements (unpublished data). This expression pattern (Figure 1B) at E9.5 correlates with abundant hyaluronan in the cushions (6). The high level of Has2 expression and the abundance of hyaluronan during formation of the endocardial cushions suggest that hyaluronan is critically important during heart morphogenesis.
Figure 1.
Hyaluronan synthase-2 (Has2) is highly expressed in the atrioventricular canal (AVC) and outflow tract (OFT) of the developing heart. In situ hybridization shows expression of Has2 mRNA in the endocardial cushions of the AVC and OFT tissues. A saggital section through the heart of a mouse embryo at embryonic day 10 reveals abundant Has2 mRNA in the endocardial cushions of AVC and OFT. B is the area boxed in A. Scale bar = 200 μm
Gene disruption of Has2 abrogates normal cardiac morphogenesis:
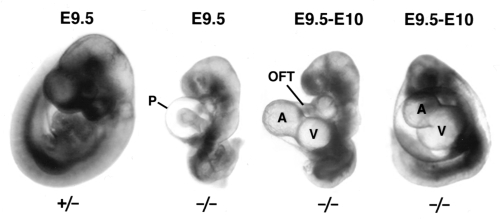
Has2 appears to be the crucial synthase for hyaluronan production during embryogenesis. The targeted inactivation of Has2 results in embryonic lethality at E9.5 with severe developmental defects including cardiovascular abnormalities (6). In contrast, single and compound gene disruptions for Has1 and Has3 cause no apparent developmental defects (unpublished observations). The Has2−/− embryos are grossly underdeveloped compared with wild type and heterozygous littermates (Figure 2). Typically, half of the observed Has2−/− mutant embryos have not turned and all lack the microvasculature network normally developed by this stage (compare far left heterozygous littermate with the three mutants, Figure 2). The Has2−/− embryos exhibit progressive pericardial edema (compare E9.5 with E10, Figure 2) indicative of insufficient heart function. The hearts lack hyaluronan, cardiac jelly and AV cushions, and have underdeveloped outflow tract and right ventricle with no trebeculations in the compacted myocardium (6). Although there are visible cardiac contractions, blood fails to be pumped through the outflow tract and the heart fails between E9.5 and E10.5. Collectively these observations indicate that hyaluronan is needed for formation of a functioning cardiovascular system.
Figure 2.
Comparison of heterozygous with hyaluronan synthase-2−/− (Has2−/−) embryos. Older (approximately embryonic day [E] 10) Has2−/− embryos have more pronounced pericardial edema and malformation of ventricular (V) and atrial (A) tissues. OFT Outflow tract; P Pericardial sac
Endothelial to mesenchymal transformation is defective in AV explants from Has2−/− embryos:
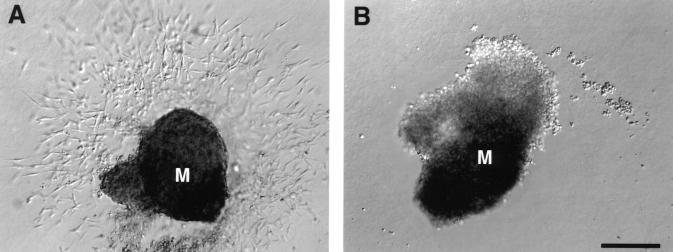
Because Has2−/− embryos have numerous developmental abnormalities, it is difficult to determine the direct consequences of hyaluronan deficiency in vivo upon AV canal morphogenesis. Furthermore, because the hearts of Has2 mutant embryos lack cardiac jelly, complete formation of the AV canal cannot be directly analyzed. Accordingly, an in vitro collagen gel invasion assay was used to directly assess deficiencies in transformation of AV canal endothelium into mesenchyme. The in vitro collagen gel invasion assay has been used to recapitulate events during AV canal morphogenesis (9). Immediately following cardiac looping, the cardiac jelly expands, forming the endocardial cushions. Subpopulations of endothelial (endocardial) cells detach themselves from adjacent cells, are activated and migrate into the matrix-rich jelly, transforming into mesenchymal cells. These mesenchymal cells organize and proliferate within the cushion tissue, forming the cardiac valve leaflets. The collagen gel (Figure 3) provides an environment for the endothelial cells from an AV canal explant to migrate and transform into mesenchymal cells expressing characteristic markers such as α-smooth muscle actin (12), fibulin-1/2 and type VI collagen (13,14). These cells invade the collagen gel, recapitulating invasion into cushion matrix in vivo (15). Markwald et al (16,17) and Brown et al (18,19) have used this system with avian AV explants to show the release of soluble signals from the myocardium, which induce endothelial to mesenchymal transformation. In the present study, this system was used to examine endothelial cell migration, transformation and invasion in wild type and Has2−/−E9.5 mouse AV explants. Figure 4 shows representative primary AV canal explants from a wild type (Figure 4A) and Has2−/− embryo (Figure 4B). The AV explants from wild type embryos exhibit extensive migration and transformation into mesenchyme. The cells have invaded the collagen gel at various depths (blurred cells out of depth of field). In contrast, the AV explants from Has2−/− embryos (Figure 4B) lack endothelial migration, transformation or invasion. Thus, even in a suitable environment such as the type I collagen gel system, AV canal explants from Has2−/− embryos have a profound defect in endothelial to mesenchymal transformation. This indicates that incomplete AV canal development in the Has2−/−embryos is a direct result of the absence of hyaluronan.
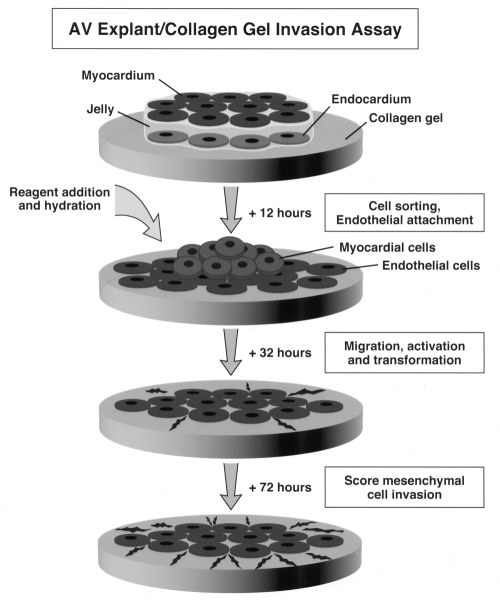
Figure 3.
Schematic representation of atrioventricular (AV) canal explant assay. The AV canal tissue is dissected from embryos at embryonic day 9.5 and placed on top of a hydrated type I collagen gel. The myocardium and endocardium are segregated, the endocardium spreads on the gel and rhythmic contractions of the myocardium are initiated. The cultures are hydrated with or without indicated reagents following firm attachment to the surface of the gel. The endocardial (endothelial) cells migrate and transform into mesenchymal cells, which then invade the collagen gel
Figure 4.
Representative atrioventricular (AV) canal explant cultures from wild type (A) and hyaluronan synthase-2−/− (Has2−/−) embryos (B) at embryonic day 9.5. The wild type AV canal explants exhibit extensive endocardial cell migration and transformation into mesenchymal cells that have invaded the collagen gel. In contrast, AV canal explants derived from Has2−/− embryos fail to exhibit migration or transformation into mesenchymal cells. The myocardium (M) in AV canal explants derived from Has2−/− embryos contracts, indicating viable cultures
Rescue of endothelial to mesenchymal transformation in Has2−/− AV explants:
The cardiac endothelial cells must be competent to respond to paracrine mediators released by the myocardium during AV canal morphogenesis. Thus, either the endocardium or the myocardium might be defective in its role, eg, either releasing or receiving inductive signals during the aborted attempt at AV canal morphogenesis in Has2−/−embryonic hearts. The ability of wild type myocardium and exogenous hyaluronan to rescue endothelial to mesenchymal transformation was examined in AV explants derived from Has2−/− embryos (Figure 5). The AV explants were examined for transformation by cell morphology and detection of α-smooth muscle actin (Figure 5) (20) and fibulin-1 and 2 staining (data not shown). The treated AV explants from Has2−/− embryos were compared with wild-type AV explants (Figure 5A) and untreated Has2−/− explants (Figure 5B) for the extent of transformation and invasion (Table 1). The wild type AV explants had abundant α-smooth muscle actin-positive mesenchymal cells that invaded the gel. Because Has2−/− AV explants neither transformed nor invaded the collagen gel (Figure 4B), no cells were immunodetected. A representative Has2−/− AV explant with the myocardium removed, leaving only a footprint of the explant (differential interference contrast image), is shown in Figure 5B. In contrast, Has2−/− AV explants co-cultured with wild type AV explants exhibited normal transformation and invasion, and expressed α-smooth muscle actin (Figure 5C). Similar results were obtained with conditioned media derived from wild type AV explants (Figure 5D), although transformation into mesenchyme was not quite as pronounced. The addition of purified hyaluronan to the culture medium also rescued endothelial to mesenchymal transformation in Has2−/− AV explants (Figure 5E). Boiling hyaluronan before treatment retained the transforming activity in the Has2−/− AV explants (Figure 5F, Table 1). It is important to note that co-culture experiments are established at the time of explantation. However, there is a 12 h delay before reagents such as conditioned medium or hyaluronan are added to Has2−/− AV explant cultures (see schematic, Figure 3). Wild type AV explants were also cultured in the presence of hyaluronidase (data not shown), and inhibition of transformation and invasion into the collagen gels was observed. These results are summarized in Table 1. These data indicate that hyaluronan is an important component of molecular induction of cardiac endothelial migration, transformation and invasion.
TABLE 1.
Qualitative summary of effects of various treatment conditions on atrioventricular (AV) explant cultures from wild type and hyaluronan synthase-2−/− (Has2−/−) embryos at embryonic day 9.5
| Source of AV explant | Culture condition | Cardiac endothelial migration | Transformation into mesenchyme |
|---|---|---|---|
| Wild type | None | ++++ | ++++ |
| Wild type* | Hyaluronidase† | +++ | − |
| Has2 mutant | None | − | − |
| Has2 mutant | Co-culture with wild type | ++++ | ++++ |
| Has2 mutant | Conditioned medium | +++ | +++ |
| Has2 mutant* | Exogenous hyaluronan | +++ | ++ |
| Has2 mutant | Boiled hyaluronan | ++ | ++ |
| Has2 mutant* | Has2 cDNA transfection | ++++ | ++++ |
| Has2 mutant* | Vector control transfection | − | − |
Results are compiled from 12 or more AV explants from at least three independent experiments.
Similar data presented by Camenisch et al (6);
Source of hyaluronidase is Streptococcus dysgalactiae (Seikagaku Inc, Japan)
DISCUSSION
CHD are the most frequent birth defects (approximately 1%) and can be as frequent as 40% in infants with defined chromosomal abnormalities such as trisomy 21 (21,22). The morphogenesis of the endocardial cushions is an important component of heart development. Genetic and environmental imbalances can contribute to the abnormal formation of cushion tissue contributing to CHD such as common AV canal. Although CHD are frequent, little information has been presented regarding the cellular and molecular events regulating cardiac cushion and valve formation until recently. We report that hyaluronan is necessary for migration of cardiac endothelial cells, transformation and subsequent invasion. All of these steps are required for cellularization and remodelling of the cushion tissue into valve leaflets.
Investigation into endocardial cushion morphogenesis has been advanced by the use of a three-dimensional in vitro collagen gel assay (15,16). Although this assay has been used predominantly with avian AV explants, we adopted it for studying mammalian AV cushion morphogenesis (6). Because AV explant cultures recapitulate the regional and temporal events during cushion morphogenesis, we exploited this system to determine whether hyaluronan has a direct role in AV cardiac endothelial to mesenchymal transformation. We observed a complete lack of endothelial to mesenchymal transformation in naive Has2−/− AV explants (Figures 4B,5B). In contrast, a continued source of hyaluronan (Figure 5C,D,E) and even low concentrations of hyaluronan (Figure 5F) (5) restored the biological response in the cardiac endothelial cells (Table 1). Endothelial to mesenchymal transformation was fully restored in the Has2−/− AV explants in cultures with a continued presence of wild type myocardium (co-culture) or with conditioned medium (Figure 5C,D). In addition, transfection of Has2−/− AV explants with Has2 cDNA restored the explants to the full wild type normal phenotype (Table 1) (6). Finally, addition of streptococcus hyaluronidase to AV explants from wild type embryos blocked endothelial transformation into mesenchyme (Table 1) (6). These findings support previous work in the in vitro collagen gel assay in which chick AV explants showed a twofold increase in cardiac endothelial transformation and invasion when cultured in the presence of exogenous hyaluronan (23). In addition, endocardial cushion development was blocked by culturing E9.5 whole rat embryos in the presence of streptomyces hyaluronidase (24). Furthermore, growth factors such as epidermal growth factor, platelet-derived growth factor and transforming growth factor-beta that are important during development can induce the biosynthesis of hyaluronan (25,26). The ubiquitous distribution of hyaluronan during embryogenesis (27) and its prominent presence in the cardiac jelly (6) indicate that hyaluronan most likely does not act unilaterally as a signalling molecule. Rather, it is likely that hyaluronan cooperates with growth factor(s) to induce the restricted activation of endothelial to mesenchymal transformation and invasion in the cardiac cushions. This mode of action is known to exist for cooperation between integrins and growth factor receptors (28). Thus, soluble inductive signals can be synergistically enhanced by changes in matrix interactions that affect cellular responses. Collectively, these observations suggest that specific growth factors act in cooperation with matrix hyaluronan through cognate receptors to stimulate full induction of AV canal morphogenesis. These findings are changing the old model that depicted hyaluronan acting solely as an inert space-filling substance to a more progressive understanding of the bioactive roles of hyaluronan (29–31). We are investigating potential growth factor candidates as well as novel hyaluronan-binding receptors in this process.
These results from the in vitro collagen gel assay show an unequivocal role for hyaluronan during AV canal morphogenesis and suggest that the observed cardiac phenotype in Has2−/− embryos is a direct result of Has2 dysfunction and the absence of hyaluronan. The rudimentary hearts of Has2−/−embryos not only lack cardiac jelly and endocardial cushions, but also exhibit compaction of the ventricular myocardium without trebeculations and diminution of the outflow tract (conus truncus) (6). Our observations provide genetic confirmation of earlier experiments in which hyaluronan in chick embryonic hearts was degraded, eliminating cardiac jelly and altering the heart morphology (32). Collectively, these data emphasize the requirement for an organized extra-cellular matrix during heart morphogenesis. The Has2−/− phenotype is very similar to that of heart defect mice that lack the hyaluronan-binding molecule versican (33). Versican mRNA is present in the atrial and ventricular endothelium as well as in the myocardium, similar to Has2. Both hyaluronan and versican are present in the cardiac jelly at E9.5 of development. Fibulin and type VI collagen are additional hyaluronan-binding molecules that are expressed in endocardial cushions (13,34). Fibulin also binds versican (35), and the alpha-1 and alpha-2 chains of type VI collagen are over-expressed in the hearts of trisomy 21 patients (3,36). These interactions suggest that a well defined and organized extra-cellular scaffold is very important for the development of the endocardial cushions. The common factor among these diverse matrix molecules is the ability to interact with hyaluronan, suggesting that hyaluronan is a key coordinator of the matrix environment in cardiac cushions. Collectively, our data suggest dual roles for matrix molecules: as structural components of cardiac jelly and as co-stimulatory agents with growth factors during heart morphogenesis. Thus, perturbations in one or more of these matrix molecules can lead to developmental abnormalities resulting in CHD.
Acknowledgments
The authors thank Marv Ruona for graphics support. We acknowledge Dr Scott Klewer and Dr Joyce Schroeder for critical discussions. We also appreciate the assistance of Carol Williams and Susan Bond for manuscript preparation. This work was supported by grants from NIH 1R01 AR-44689, T32 HL-07897, F32 HL-10299-01, the Desert Mountain Affiliate of the American Heart Association, and the Mayo Foundation for Medical Education and Research.
REFERENCES
- 1.Bouvagnet P, Sauer U, Debrus S, et al. Deciphering the molecular genetics of congenital heart disease. Herz. 1994;19:119–25. [PubMed] [Google Scholar]
- 2.Ferencz C, Boughman JA. Congenital heart disease in adolescents and adults. Teratology, genetics, and recurrence risks. Cardiol Clin. 1993;11:557–67. [PubMed] [Google Scholar]
- 3.Duff K, Williamson R, Richards SJ. Expression of genes encoding two chains of the collagen type VI molecule during human fetal heart development. Int J Cardiol. 1990;27:128–9. doi: 10.1016/0167-5273(90)90202-g. [DOI] [PubMed] [Google Scholar]
- 4.Dor Y, Camenisch TD, Itin A, et al. A novel role for VEGF in endocardial cushion formation and its potential contribution to congenital heart defects. Development. (In press) [DOI] [PubMed]
- 5.Moss JB, Xavierneto J, Shapiro MD, et al. Dynamic patterns of retinoic acid synthesis and response in the developing mammalian heart. Dev Biol. 1998;199:55–71. doi: 10.1006/dbio.1998.8911. [DOI] [PubMed] [Google Scholar]
- 6.Camenisch TD, Spicer AP, Brehm-Gibson T, et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–60. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spicer AP, Augustine ML, McDonald JA. Molecular cloning and characterization of a putative mouse hyaluronan synthase. J Biol Chem. 1996;271:23400–6. doi: 10.1074/jbc.271.38.23400. [DOI] [PubMed] [Google Scholar]
- 8.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA. 1991;88:6642–6. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Runyan RB, Markwald RR. Invasion of mesenchyme into three-dimensional collagen gels: a regional and temporal analysis of interaction in embryonic heart tissue. Dev Biol. 1983;95:108–14. doi: 10.1016/0012-1606(83)90010-6. [DOI] [PubMed] [Google Scholar]
- 10.Moseley R, Waddington RJ, Embery G. Degradation of glycosaminoglycans by reactive oxygen species derived from stimulated polymorphonuclear leukocytes. Biochim Biophys Acta. 1997;1362:221–31. doi: 10.1016/s0925-4439(97)00083-5. [DOI] [PubMed] [Google Scholar]
- 11.Agren UM, Tammi RH, Tammi MI. Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ cultures. Free Radic Biol Med. 1997;23:996–1001. doi: 10.1016/s0891-5849(97)00098-1. [DOI] [PubMed] [Google Scholar]
- 12.DeRuiter MC, Poelmann RE, VanMunsteren JC, et al. Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ Res. 1997;80:444–51. doi: 10.1161/01.res.80.4.444. [DOI] [PubMed] [Google Scholar]
- 13.Klewer SE, Krob SL, Kolker SJ, Kitten GT. Expression of type VI collagen in the developing mouse heart. Dev Dyn. 1998;211:248–55. doi: 10.1002/(SICI)1097-0177(199803)211:3<248::AID-AJA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 14.Miosge N, Sasaki T, Chu ML, Herken R, Timpl R. Ultrastructural localization of microfibrillar fibulin-1 and fibulin-2 during heart development indicates a switch in molecular associations. Cell Mol Life Sci. 1998;54:606–13. doi: 10.1007/s000180050188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernanke DH, Markwald RR. Migratory behavior of cardiac cushion tissue cells in a collagen-lattice culture system. Dev Biol. 1982;91:235–45. doi: 10.1016/0012-1606(82)90030-6. [DOI] [PubMed] [Google Scholar]
- 16.Markwald RR, Krook JM, Kitten GT, Runyan RB. Endocardial cushion tissue development: structural analyses on the attachment of extracellular matrix to migrating mesenchymal cell surfaces. Scan Electron Microsc. 1981;(Pt 2):261–74. [PubMed] [Google Scholar]
- 17.Markwald R, Eisenberg C, Eisenberg L, Trusk T, Sugi Y. Epithelial-mesenchymal transformations in early avian heart development. Acta Anat. 1996;156:173–86. doi: 10.1159/000147845. [DOI] [PubMed] [Google Scholar]
- 18.Brown CB, Boyer AS, Runyan RB, Barnett JV. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science. 1999;283:2080–2. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- 19.Brown CB, Boyer AS, Runyan RB, Barnett JV. Antibodies to the type II TGFbeta receptor block cell activation and migration during atrioventricular cushion transformation in the heart. Dev Biol. 1996;174:248–57. doi: 10.1006/dbio.1996.0070. [DOI] [PubMed] [Google Scholar]
- 20.Nakajima Y, Mironov V, Yamagishi T, Nakamura H, Markwald RR. Expression of smooth muscle alpha-actin in mesenchymal cells during formation of avian endocardial cushion tissue: a role for transforming growth factor beta3. Dev Dyn. 1997;209:296–309. doi: 10.1002/(SICI)1097-0177(199707)209:3<296::AID-AJA5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 21.Rubin JD, Ferencz C, McCarter RJ, et al. Congenital cardiovascular malformations in the Baltimore-Washington area. Md Med J. 1985;34:1079–83. [PubMed] [Google Scholar]
- 22.Marino B, Vairo U, Corno A, et al. Atrioventricular canal in Down syndrome. Am J Dis Child. 1990;144:1120–2. doi: 10.1001/archpedi.1990.02150340066025. [DOI] [PubMed] [Google Scholar]
- 23.Bernanke DH, Markwald RR. Effects of hyaluronic acid on cardiac cushion tissue cells in collagen matrix cultures. Tex Rep Biol Med. 1979;39:271–85. [PubMed] [Google Scholar]
- 24.Baldwin HS, Lloyd TR, Solursh M. Hyaluronate degradation affects ventricular function of the early postlooped embryonic rat heart in situ. Circ Res. 1994;74:244–52. doi: 10.1161/01.res.74.2.244. [DOI] [PubMed] [Google Scholar]
- 25.Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6:2397–404. [PubMed] [Google Scholar]
- 26.Toole BP. Hyaluronan and its binding proteins, the hyaladherins. Curr Opin Cell Biol. 1990;2:839–44. doi: 10.1016/0955-0674(90)90081-o. [DOI] [PubMed] [Google Scholar]
- 27.Brown JJ, Papaioannou VE. Ontogeny of hyaluronan secretion during early mouse development. Development. 1993;117:483–92. doi: 10.1242/dev.117.2.483. [DOI] [PubMed] [Google Scholar]
- 28.Aplin AE, Juliano RL. Integrin and cytoskeletal regulation of growth factor signaling to the MAP kinase pathway. J Cell Sci. 1999;112:695–706. doi: 10.1242/jcs.112.5.695. [DOI] [PubMed] [Google Scholar]
- 29.Toole BP. Hyaluronan is not just a goo! J Clin Invest. 2000;106:335–6. doi: 10.1172/JCI10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serbulea M, Kakumu S, Thant AA, et al. Hyaluronan activates mitogen-activated protein kinase via Ras-signaling pathway. Int J Oncol. 1999;14:733–8. doi: 10.3892/ijo.14.4.733. [DOI] [PubMed] [Google Scholar]
- 31.Bourguignon LYW, Zhu D, Zhu H. CD44 isoform-cytoskeleton interaction in oncogenic signaling and tumor progression. Front Biosci. 1998;3:D637–49. doi: 10.2741/a308. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura A, Manasek FJ. An experimental study of the relation of cardiac jelly to the shape of the early chick embryonic heart. J Embryol Exp Morphol. 1981;65:235–56. [PubMed] [Google Scholar]
- 33.Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202:56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- 34.Bouchey D, Argraves WS, Little CD. Fibulin-1, vitronectin, and fibronectin expression during avian cardiac valve and septa development. Anat Rec. 1996;244:540–51. doi: 10.1002/(SICI)1097-0185(199604)244:4<540::AID-AR12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 35.Aspberg A, Adam S, Kostka G, Timpl R, Heinegard D. Fibulin-1 is a ligand for the C-type lectin domains of aggrecan and versican. J Biol Chem. 1999;274:20444–9. doi: 10.1074/jbc.274.29.20444. [DOI] [PubMed] [Google Scholar]
- 36.von Kaisenberg CS, Brand-Saberi B, Christ B, et al. Collagen type VI gene expression in the skin of trisomy 21 fetuses. Obstet Gynecol. 1998;91:319–23. doi: 10.1016/s0029-7844(97)00697-2. [DOI] [PubMed] [Google Scholar]