Abstract
BACKGROUND:
Neural crest-associated congenital heart defects in humans are among the most lethal and costly to treat. In avian and mouse embryos with persistent truncus arteriosus (PTA), the most severe of the neural crest anomalies, there is poor cardiac function because of impaired excitation-contraction coupling. One possible explanation for poor excitation-contraction coupling is that peripheral junctions, composed of closely associated sarcoplasmic reticulum Ca2+ release channels (ryanodine receptors) and surface membrane L-type Ca2+ channels (dihydropyridine receptors), are not well colocalized.
OBJECTIVE:
To compare the degree of colocalization of these two Ca2+ channel proteins in isolated ventricular myocytes from normal hearts and hearts with PTA.
ANIMALS AND METHODS:
PTA was induced in the embryonic chick by laser ablation of the cardiac neural crest before migration from the neural tube. Immunofluorescent staining of dihydropyridine and ryanodine receptors along with computer-assisted image analysis was used to measure relative colocalization.
RESULTS:
Dihydropyridine and ryanodine receptor colocalization was greater by 20% in embryos with PTA. Much of the increase appeared to result from a 14% increase in the area stained for ryanodine receptors. A third observation was that a high level of colocalization was maintained even after enzymatic dissociation in which the embryonic myocytes had typically lost their elongated appearance and assumed a spherical shape.
CONCLUSIONS:
The increased colocalization of dihydropyridine and ryanodine receptors in hearts with PTA may be a compensatory response to a defect at the level of single Ca2+ channel proteins. These results indicate the high degree of stability of sarcoplasmic reticulum junctional complexes.
Keywords: Calcium release channel, Excitation-contraction coupling, Heart development, Heart defect, L-type calcium channel
Neural crest-associated congenital heart defects are among the most lethal and costly to treat. A 1992 survey involving a consortium of 60 hospitals found that three of the four most lethal heart defects are neural crest associated (Pediatric Cardiac Care Quality Assurance Consortium [PCCQAC]). Persistent truncus arteriosus (PTA), the most severe of the neural crest-associated heart anomalies, in which the outflow tract fails to septate into aortic and pulmonary arteries, is nearly 40% lethal even following corrective surgery (PCCQAC, personal communication). Moreover, it has been estimated that the neural crest-associated heart defects account for about 70% of the total cost of treating congenital heart disease (1). Our work has identified a potentially serious problem with myocardial function that may account for the high morbidity in neural crest-associated heart disease. We have determined that there are defects at every level in cardiac excitation-contraction (EC) coupling in chick embryos with PTA after complete cardiac neural crest ablation. L-type Ca2+ current is reduced at both early and late stages of development (2–4). Ca2+-induced Ca2+ release (CICR) from the sarcoplasmic reticulum (SR) is greatly inhibited (5). This is shown by reduced Ca2+ transients in isolated myocytes, trabeculae and heart tubes (5–7). There are defects at the contractile level as well (8). Similarly, impaired EC coupling has been identified in the Splotch(2H) mutant mouse, in which there is failure of neural crest migration (9). Moreover, it should be noted that human fetuses with neural crest-associated heart defects have depressed cardiac function as determined by echocardiography (10). These findings are unexpected given that a divided cardiac outflow is thought not to be essential for normal fetal circulation and in utero survival. Thus, the neural crest affects the development of myocardial function by mechanisms that are as yet unknown.
Contraction of the myocardium is initiated by depolarization of the membrane, followed by net entry of Ca2+ through voltage-gated Ca2+ channels. In the adult heart, Ca2+ entry through L-type Ca2+ channels (dihydropyridine receptors [DHPRs]) causes a further release of Ca2+ through SR Ca2+ release channels (ryanodine receptors [RyR]) by way of CICR. The subsequent rise in intracellular Ca2+ causes cyclical interactions between the contractile proteins myosin and actin, which give rise to force production and shortening of the muscle cell. Relaxation of the cell is caused primarily by a decrease in the intracellular level of Ca2+ resulting from active uptake of Ca2+ back into the SR and extrusion through Na+/Ca2+ exchange. The process that links depolarization of the membrane with the ensuing rise and fall in intracellular Ca2+ and contractility is termed EC coupling (reviewed in 11).
In mammals, it is often considered that SR content is sparse at birth and undergoes most of its development post-natally (12). This appears to be true in rabbit and rat hearts (13,14) but not in guinea pigs, which have fully developed SR at birth (15). Thus, the degree of maturity of SR at birth varies among mammalian species. In the chick, SR morphology and function are well developed by the time of hatching (16,17). CICR is present well before hatching, probably by embryonic day (E) 4 to 5 (18).
Recently, the elegant work of Sun et al (16) and Protasi et al (17) provided detailed information on the development of SR-plasma membrane junctional complexes involved in EC coupling in the chick heart. In these studies, ‘complete’ junctional complexes are described as having a junctional gap that is fully ‘zippered’ by closely spaced feet (RyRs). DHPRs are clustered in the plasma membrane close to the RyRs at junctional complexes but, as in adult hearts, lack the ordered arrays seen in skeletal muscle. The junctions are confined to the peripheral surface membrane because avian heart (and mammalian embryonic heart) lacks t-tubules, and extended junctional SR and corbular SR do not begin to appear until after hatching (19,20). Complete junctions or peripheral couplings are essentially absent at E4 but increase rapidly and are nearly at the adult level by E11. No comparable examination of the structural components of EC coupling has been published for the mammalian embryonic heart.
In the present study we used immunofluorescent staining of DHPRs and RyRs with computer-assisted image analysis to compare the degree of colocalization of these two Ca2+ channel proteins in ventricular myocytes in normal hearts and hearts with the neural crest-associated heart defect, PTA. Contrary to expectation, colocalization was seen to be greater in embryos with PTA. The increased colocalization appeared be the result of an increase in the area of RyR staining. A third observation of note is that a high level of colocalization was maintained even after enzymatic dissociation, in which the embryonic myocytes, unlike adult myocytes, have typically lost their elongated appearance and have assumed a spherical shape. This result indicates the high degree of stability of SR junctional complexes.
ANIMALS AND METHODS
Animal surgery:
Cardiac neural crest ablations were performed by the microsurgery core unit at the Medical College of Georgia, directed by Margaret L Kirby (21). Fertilized Arbor Acre chicken eggs (Seaboard Hatchery, USA) were incubated in 99% humidity at 38°C in forced draft incubators. The eggs were windowed approximately 30 h into development at Hamburger-Hamilton stage 8–9. The vitelline membrane was ruptured, and the embryos were stained with neutral red dye. Bilateral ablation of the premigratory cardiac neural crest was accomplished with a pulsed nitrogen/dye laser (Laser Sciences, Inc, USA). This surgical procedure produces PTA in over 90% of embryos (21). Sham-operated embryos underwent all preoperative procedures and served as controls. All embryos (sham and experimental) were resealed with cellophane tape and reincubated until E15.
Cell culture:
In all experiments only normal, sham-operated embryos with normal hearts and neural crest-ablated embryos with PTA were used. The presence or absence of PTA was determined by visual inspection under a dissecting microscope. At E15 embryos were killed by decapitation and the hearts were excised. The atria and large vessels were discarded and the ventricles were dissociated as previously reported (3). Briefly, the ventricles were minced and dissociated in a collagenase solution. The cells were suspended in DeHaan’s 21212 medium, plated onto glass coverslips in plastic Petri dishes, and incubated overnight in a 37°C in a 5% CO2 incubator. The overnight incubation allowed the cells to recover from the dissociation and to adhere to coverslips. Myocytes were lightly fixed and used within 24 h of dissociation for immunohistochemical analysis. It should be noted that with respect to chick ventricular myocytes, EC coupling does not appear to be affected by cell culture within a 24 h period because Ca2+ transients and the effects of ryanodine in myocytes when cultured as described above are virtually identical to similar measurements in freshly isolated trabeculae from chick ventricle (5,6,22).
Confocal microscopy and immunohistochemical analysis:
As in the studies of Sun et al (16) and Protasi et al (17), DHPR was labelled by a variety of polyclonal antibodies raised against rabbit and goat DHPR, as well as a commercial antibody (Affinity BioReagents, Inc, USA). RyR was labelled by a mouse monoclonal antibody obtained from ABR, Inc (USA), which was the same 34C antibody used by these workers (16,17), and by other commercially available RyR antibodies. (All of these antibodies yielded results similar to those shown in Figure 2.) The data for this study were obtained using a mouse monoclonal anti-DHPR antibody from Affinity BioReagents, Inc and an affinity-purified goat RyR antibody from Santa Cruz Biotechnology (USA). Myocytes were prepared and cultured overnight as described above, lightly fixed in 1% paraformaldehyde in phosphate-buffered saline for 10 min, labelled with primary and secondary (fluorescein and fluorescent cyanine dye [Cy3] -labelled) antibodies, and each label was imaged sequentially with a BioRad 1024 laser scanning confocal microscope (BioRad Corp, USA) on the stage of an Olympus IX-70 inverted microscope equipped with a 60× Olympus plan/APO oil immersion lens (numerical aperture = 1.4) (Olympus America, Inc, USA). All antibodies were diluted in 1% bovine serum albumin/phosphate-buffered saline solution at pH 7.4 and containing 0.05% sodium azide. Anti-DHPR (1:150) incubation was overnight at 4°C with subsequent incubation in anti-RyR (1:100) for 2 h at room temperature. Secondary antibodies were obtained from Jackson ImmunoResearch Laboratories, Inc (USA). Cy3-labelled goat antimouse antibody (1:10; 2 h, room temperature) was used for DHPR staining, and rabbit antigoat antibody (1:30; 2 h, room temperature) was used for RyR staining. Green and red channels on the confocal microscope were individually adjusted to eliminate bleed-through using preparations alternately stained with fluorescein and Cy3 secondary antibodies. The confocal microscope settings, after optimization to eliminate bleed-through and reduce background staining, were maintained constant for all of the images collected in this study. For controls, mouse and goat sera were substituted for primary RyR and DHPR antibodies, respectively. Controls were not labelled by the secondary antibodies (data not shown) and the staining pattern illustrated in Figure 2 was seen only when the primary antibodies were used as described above.
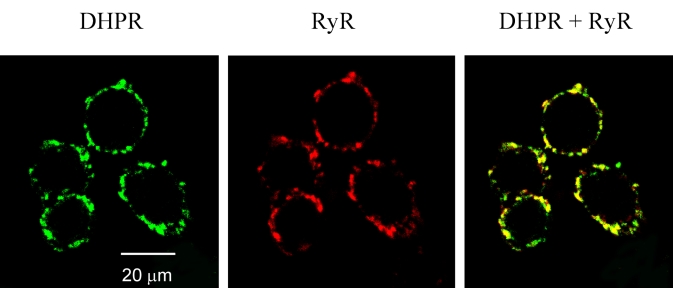
Figure 2.
Examples of several myocytes viewed sequentially for dihydropyridine receptor (DHPR) and ryanodine receptor (RyR) staining. The images were overlaid (DHPR + RyR) to indicate colocalization (yellow). Note the patchy peripheral membrane staining, indicating the location of sarcoplasmic reticulum/surface membrane junctional complexes. As with the general morphology shown in Figure 1, it was not possible to determine differences in the degree of colocalization of DHPR and RyR in normal hearts and hearts with persistent truncus arteriosus in single isolated images such as these
Image analysis:
The images, 512×512 pixels each, were analyzed using the threshold and binary functions of the Scion Image analysis software (Scion Corp, USA). With the 60× oil objective the pixels had dimensions of 0.23 μm × 0.23 μm. Only pixels above the threshold calculated by the software’s threshold algorithm were considered to be labelled. Intensity information was discarded when images were converted to binary. Unlabelled pixels were assigned a value of 0. DHPR and RyR pixels were each assigned a value of 1. The degree of colocalization for each image was determined by addition of the DHPR and RyR images, which gave colocalized pixels a value of 2. Each image was normalized to itself by dividing the number of DHPR- and RyR-labelled pixels by the total number of labelled pixels in the image.
Statistical analysis:
Data for each group were averaged and are presented as mean ± SEM. Data significance was determined by paired and unpaired Student’s t test (two-tailed). Data were considered significant at P<0.05.
RESULTS
Embryonic ventricular myocytes in culture:
For Figure 1, the transmission detector of the laser scanning confocal microscope was used to illustrate the general appearance of isolated ventricular myocytes used after approximately 24 h in culture. The myocytes were spherical with a diameter of about 20 μm. The spherical shape is typical of enzymatically dissociated embryonic myocytes that are used extensively in physiological studies in both the chick (3,23) and the mouse (9). Unlike adult cardiac myocytes, embryonic myocytes are small and quickly loose their elongated shape during the dissociation procedure. After about 48 h in culture, the myocytes begin to spread and assume a flattened shape (not shown). For this study, only spherical myocytes were used and the data were collected within 24 h of dissociation. As has been previously shown, there were no visible differences in the appearance of myocytes from sham-operated and neural crest-ablated embryos (24).
Figure 1.
Example of isolated chick ventricular myocytes at embryonic day 15 after about one day in culture. The myocytes were lightly fixed and viewed with the transmission detector of a BioRad 1024 confocal microscope. Note the typically spherical appearance of embryonic cardiac myocytes. This example is from a normal heart from a sham-operated embryo. The morphology of myocytes from sham-operated embryos and that from hearts with persistent truncus arteriosus in neural crest-ablated embryos were indistinguishable at this level
Quantitative colocalization of DHPR and RyR:
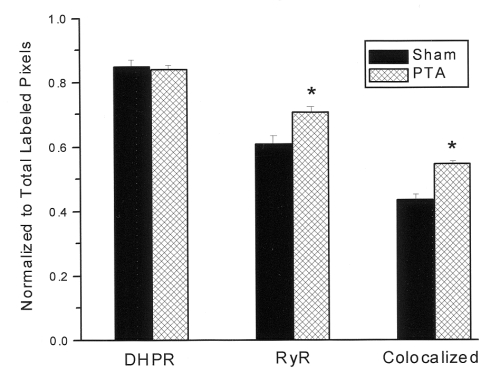
One possible explanation for defective EC coupling previously seen in hearts after neural crest ablation is a disruption of the close physical association of DHPRs and RyRs that is essential for normal and efficient EC coupling. Figure 2 shows an example of sequentially obtained confocal images of DHPR and RyR immunofluorescent costaining of a small cluster of myocytes. Virtually all of the staining was localized to the peripheral surface membrane. Colocalization appeared yellow when the DHPR and RyR images were overlaid. Note the discontinuous or patchy peripheral membrane staining pattern, which is very similar to the pattern of staining previously described by Protasi et al (17) in frozen cross-sections of intact ventricular muscle. The same pattern was observed with several other anti-DHPR polyclonal antibodies (Card C, Card 1928P and Card CT4 against rabbit) as well as another commercial antibody from Aliomone Labs (Israel). No labelling was seen in the absence of the primary antibody or when preimmune sera were substituted (not shown). Colocalization (yellow), was apparent in both sham-operated and neural crest-ablated embryos. In visual comparisons of single images such as the example in Figure 2, it was not possible to ascertain whether there was more or less colocalization of DHPR and RyR in sham-operated than in neural crest-ablated embryos. Therefore, computer-assisted image analysis was used to ascertain the relative level of colocalization of DHPRs and RyRs using multiple images from each of the hearts that were examined. In all the images used in this study, the microscope was focused in approximately the centre of the spherical myocytes. The hearts from three sham-operated and three neural crest-ablated embryos at E15 were analyzed. For each embryo, 20 to 30 512×512 pixel images containing from three to eight spherical myocytes were collected for analysis. A total of 160 images were analyzed by Scion Image analysis software as described above. The degree of labelling and colabelling was represented as a fraction of the total number of labelled pixels calculated when the DHPR and RyR stained images were overlaid. The mean results from the image analysis, shown in Figure 3, indicated a 20% increase in the level of colocalization of DHPR and RyR in the embryos with cardiac neural crest ablation (P<0.001). This result was contrary to expectation given that EC coupling was known to be substantially impaired in these embryos (6). There was also a small but significant 14% increase in the fractional area labelled by the RyR antibody (P=0.003).
Figure 3.
Quantitative measurements of relative colocalization of dihydropyridine receptors (DHPRs) and ryanodine receptors (RyRs) in normal hearts and in hearts with persistent truncus arteriosus (PTA). A total of 160 images similar to that shown in Figure 2 were analyzed to determine the relative level of colocalization of DHPRs and RyRs. *P<0.005. Bars indicate ± SEM
DISCUSSION
The results of the present study show that colocalization of DHPRs and RyRs is greater in embryos with PTA. The increased colocalization appears to result largely from an increase in the area of RyR staining. A third observation of note is that a high level of colocalization was maintained even after enzymatic dissociation and the normally elongated myocytes ‘rounded up’ and assumed a spherical shape. This latter result indicates the high degree of stability of SR junctional complexes.
Colocalization of DHPRs and RyRs:
As discussed at the beginning of this report, there is impairment at every level of the cardiac EC coupling process in embryonic chick hearts with PTA (25). Similar patterns have been observed in a mouse model of neural crest heart disease (9). Because of these observations and another report indicating that there may be a disruption of junctional complexes in diseased hearts (26), we examined the relative level of colocalization of DHPRs and RyRs in the chick heart with PTA at E15. However, contrary to expectation, we found that colocalization is actually greater in hearts with PTA. Moreover, it appears that the increased colocalization is caused by an increase in the fractional area of RyR staining because DHPR staining in embryos with PTA was not different from normal. These observations are consistent with previous reports, which indicate that the number of DHPRs detected by radiolabelled binding is normal (3) while there appears to be an increase in the number of RyRs (6) in hearts with PTA. Therefore, there is not a lack of colocalization of peripheral SR junctional complexes in embryonic myocytes from hearts with PTA to explain the well demonstrated impairment of cardiac EC coupling in these embryos. Thus, the increased colocalization of DHPRs and RyRs more likely is a compensatory response to a decreased efficiency in CICR from the SR in hearts with PTA, caused most likely by direct alteration of either L-type Ca2+ channel or SR Ca2+ release channel function. The latter possibility is supported by a recent preliminary report showing a reduction in the open channel probability for L-type Ca2+ channels (DHPRs) at E15 in the chick heart with PTA (27). A decrease in L channel open channel probability would be expected to lead to decreased CICR and account for at least part of the impaired EC coupling seen in these embryos.
Stability of SR junctional complexes:
During enzymatic dissociation, embryonic cardiac myocytes, whether mouse or avian, lose their elongated shape and become spherical. This change in shape has raised the practical question as to whether SR junctional complexes can maintain the close and precise colocalization of DHPRs and RyRs necessary for efficient EC coupling. Our results indicate that junctional complexes are present and closely resemble junctional complexes seen in fresh frozen chick ventricles that have not been subjected to enzymatic dissociation (17). Moreover, the stability of junctional complexes is maintained even in the presence of a severe congenital heart anomaly. This finding is supported by physiological studies comparing SR contributions to electrically stimulated Ca2+ transients in isolated myocytes (4,22) with Ca2+ transients elicited in freshly dissected intact cardiac trabeculae (5). The Ca2+ transients in isolated myocytes are virtually identical to those obtained in intact trabeculae whether or not SR function is blocked with ryanodine. Thus, junctional complexes are quite stable and not easily disrupted even under extreme conditions when cell shape has changed after enzymatic dissociation and cell culture.
SUMMARY AND CONCLUSIONS
It is well known that EC coupling is impaired in embryonic chick and mouse hearts with neural crest-associated heart disease (25). In addition, impaired EC coupling may explain the diminished cardiac function and increased morbidity in human fetuses with heart defects related to the neural crest (10). From the present study it is apparent that junctional complexes, the fundamental unit responsible for CICR, are not disrupted after ablation of the cardiac neural crest, and that colocalization of DHPRs and RyRs is actually increased. Thus, it appears that impaired EC coupling as a consequence of neural crest-associated heart disease is not a result of gross structural changes in the organization of peripheral junctions. It is most likely caused by functional changes at the molecular level involving surface membrane voltage-gated Ca2+ channels or SR Ca2+ release channels, or both.
Acknowledgments
This work was supported by National Institutes of Health grants HL58861 and HL36059. Polyclonal antibodies used for DHPR labelling were generous gifts from M Hosey.
REFERENCES
- 1.Clark EB. Pathogenetic mechanisms of congenital cardiovascular malformations revisited. Semin Perinatol. 1996;20:465–72. doi: 10.1016/s0146-0005(96)80062-0. [DOI] [PubMed] [Google Scholar]
- 2.Creazzo TL. Reduced L-type calcium current in the embryonic chick heart with persistent truncus arteriosus. Circ Res. 1990;66:1491–8. doi: 10.1161/01.res.66.6.1491. [DOI] [PubMed] [Google Scholar]
- 3.Aiba S, Creazzo TL. Calcium currents in hearts with persistent truncus arteriosus. Am J Physiol. 1992;262:H1182–90. doi: 10.1152/ajpheart.1992.262.4.H1182. [DOI] [PubMed] [Google Scholar]
- 4.Creazzo TL, Brotto MAP, Lutin WA, Aliff CL. Excitation-contraction coupling in cardiac dysmorphogenesis. J Physiol. 1995;487:16P. [Google Scholar]
- 5.Nosek TM, Fogaca RT, Hatcher CJ, Brotto MA, Godt RE. Effect of cardiac neural crest ablation on contractile force and calcium uptake and release in chick heart. Am J Physiol. 1997;273:H1464–71. doi: 10.1152/ajpheart.1997.273.3.H1464. [DOI] [PubMed] [Google Scholar]
- 6.Creazzo TL, Brotto MA, Burch J. Excitation-contraction coupling in the day 15 embryonic chick heart with persistent truncus arteriosus. Pediatr Res. 1997;42:731–7. doi: 10.1203/00006450-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Waldo K, Zdanowicz M, Burch J, et al. A novel role for cardiac neural crest in heart development. J Clin Invest. 1999;103:1499–507. doi: 10.1172/JCI6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godt RE, Fogaca RTH, Nosek TM. Alterations of myocardial contraction associated with a structural heart defect in embryonic chicks. Adv Exp Med Biol. 1998;453:453–9. doi: 10.1007/978-1-4684-6039-1_50. [DOI] [PubMed] [Google Scholar]
- 9.Conway SJ, Godt RE, Hatcher CJ, et al. Neural crest is involved in development of abnormal myocardial function. J Mol Cell Cardiol. 1997;29:2675–85. doi: 10.1006/jmcc.1997.0499. [DOI] [PubMed] [Google Scholar]
- 10.Lutin WA, Brumund MR, Jones C, Tharpe CE, Montegomery M, McCaffrey FM. Hemodynamic abnormalities in fetuses with congenital heart disease. Pediatr Cardiol. 1999;20:390–5. doi: 10.1007/s002469900497. [DOI] [PubMed] [Google Scholar]
- 11.Bers DM. Excitation-contraction coupling and cardiac contractile force. Boston: Kluwer Academic Publishers; 1991. [Google Scholar]
- 12.Nakanishi T, Jarmakani JM. Developmental changes in myocardial mechanical function and subcellular organelles. Am J Physiol. 1984;246:H615–25. doi: 10.1152/ajpheart.1984.246.4.H615. [DOI] [PubMed] [Google Scholar]
- 13.Seguchi M, Harding J, Jamrmakani J. Developmental change in the function of sarcoplasmic reticulum. J Mol Cell Cardiol. 1986;18:189–95. doi: 10.1016/s0022-2828(86)80471-0. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi T, Seguchi M, Takao A.Developmental changes in myocardial mechanical function and subcellular organelles Experientia 199244936–44.3058500 [Google Scholar]
- 15.Goldstein MA, Traeger L. Ultrastructural changes in postnatal development of the cardiac myocyte. In: Legato ML, editor. The Developing Heart. Boston: Nijhoff; 1985. [Google Scholar]
- 16.Sun X-H, Protasi F, Takahashi M, Takeshima H, Ferguson DG, Franzini-Armstrong C. Molecular architecture of membranes involved in excitation-contraction coupling of cardiac muscle. J Cell Biol. 1995;129:659–71. doi: 10.1083/jcb.129.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Protasi F, Sun XH, Franzini-Armstrong C. Formation and maturation of the calcium release apparatus in developing and adult avian myocardium. Dev Biol. 1996;173:265–78. doi: 10.1006/dbio.1996.0022. [DOI] [PubMed] [Google Scholar]
- 18.Dutro SM, Airey JA, Beck CF, Sutko JL, Trumble WR. Ryanodine receptor expression in embryonic avian cardiac muscle. Dev Biol. 1993;155:431–41. doi: 10.1006/dbio.1993.1041. [DOI] [PubMed] [Google Scholar]
- 19.Jewett PH, Leonard SD, Sommer JR. Chicken cardiac muscle. J Cell Biol. 1973;56:595–600. doi: 10.1083/jcb.56.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Junker J, Sommer JR, Sar M, Meissner G. Extended junctional sarcoplasmic reticulum of avian cardiac muscle contains functional ryanodine receptors. J Biol Chem. 1994;269:1627–34. [PubMed] [Google Scholar]
- 21.Kirby ML, Kumiski DH, Myers T, Cerjan C, Mishima N. Backtransplantation of chick cardiac neural crest cells cultured in LIF rescues heart development. Dev Dyn. 1993;198:296–311. doi: 10.1002/aja.1001980407. [DOI] [PubMed] [Google Scholar]
- 22.Brotto MAD, Creazzo TL. Ca2+ transients in embryonic chick heart: Contributions from Ca2+ channels and the sarcoplasmic reticulum. Am J Physiol. 1996;270:H518–25. doi: 10.1152/ajpheart.1996.270.2.H518. [DOI] [PubMed] [Google Scholar]
- 23.Fujii S, Ayer RK, Jr, DeHaan RL. Development of the fast sodium current in early embryonic chick heart cells. J Membr Biol. 1988;101:209–23. doi: 10.1007/BF01872836. [DOI] [PubMed] [Google Scholar]
- 24.Creazzo TL, Burch J, Redmond S, Kumiski D. Myocardial enlargement in defective heart development. Anat Rec. 1994;239:170–6. doi: 10.1002/ar.1092390207. [DOI] [PubMed] [Google Scholar]
- 25.Creazzo TL, Godt RE, Leatherbury L, Conway SJ, Kirby ML. Role of cardiac neural crest cells in cardiovascular development. Annu Rev Physiol. 1998;60:267–86. doi: 10.1146/annurev.physiol.60.1.267. [DOI] [PubMed] [Google Scholar]
- 26.Gomez AM, Valdivia HH, Cheng H, et al. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–5. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 27.Nichols CA, Creazzo TL. Single channel function in neural crest related heart disease. Biophys J. 2000;78:201A. [Google Scholar]