Abstract
OBJECTIVE:
To examine the spatial pattern of labelling for the gap junctional protein, connexin45, in relation to that of the other two cardiac connexins, connexin40 and connexin43, during the development of the central conduction system in mouse heart.
ANIMALS AND METHODS:
Hearts from Balb-c mice at stages from embryonic day (E) 12.5 to adult were frozen and sectioned. The sections were immunolabelled for connexins 45, 40 and 43 using fully characterized connexin-specific antibodies. Labelled sections were observed using confocal microscopy. Single, double and triple labelling were employed with sequential scanning to record images from multiple-labelled sections for the analysis of the spatial distribution of the three connexin types in relation to each other.
RESULTS:
High levels of connexin45 label were detected in specific regions within the developing mouse heart. These regions corresponded to the conus myocardium, developing interatrial septum and other developing conduction tissues of the heart. Connexin40 label was initially absent from these tissues but by E15.5 was present in the more distal regions of the conduction system. However, by E17.5, connexin45 and 40 labelling was similar to the pattern observed in the adult heart, with both connexins present in most regions of the conduction system, though they were not completely colocalized. Connexin43 label was not observed in the regions of high connexin45 labelling.
CONCLUSIONS:
These results show connexin45 to be the earliest detectable connexin in the central conduction system and to be the only connexin present throughout the whole conduction system. A distinct temporal pattern of connexin expression was also shown to occur during the development of the conduction tissues of the mouse heart.
Keywords: Conduction system, Connexin45, Development, Gap junction, Mouse
Gap junctions are clusters of transmembrane channels that link the cytoplasmic compartments of neighbouring cells to form sieve-like conduits for the direct cell to cell transfer of small signalling molecules and ions, a function necessary for tissue homeostasis, development and differentiation (1–3). In cardiac muscle, a key function of gap junctions is electrical coupling of individual myocytes to mediate the orderly spread of action potentials throughout the heart (4). Gap junction channels comprise pairs of connexons (hemichannels), one contributed by each of the apposed cells. Each connexon comprises a hexamer of six proteins. These constituent proteins, termed connexins, form a multi-gene family of conserved proteins. To date, 16 different genes have been identified in mammalian cells (5). Most tissues, including heart, express multiple connexins (6). In vitro experiments have shown that gap junctions composed of different connexins have different properties including unitary conductance, voltage sensitivity and molecular permeability (7). Hence, variations in the pattern of expression of the connexins of the heart, connexins 43, 40 and 45, are hypothesized to be key determinants of electrophysiological specialization in different regions of the heart.
We and others have shown that the tissues that comprise the impulse generation and conduction system of the heart do indeed have particular patterns of connexin expression (8–12). Connexin45 is present in all parts of the conduction system in the adult rodent heart, whereas the expression of connexin40 is not quite as extensive, being confined to a central core within the connexin45-expressing tissues. Connexin43, the most abundant connexin in the working myocardium of the heart, is barely detectable in the central parts of the conduction system (atrioventricular [AV] node, His bundle and bundle branches). Expression of connexin40 and connexin43 have both been shown to be regulated at the mRNA level during heart development (13). Connexin40 is widely expressed at embryonic day (E) 11 in ventricles and atria, but from E14 expression becomes preferentially concentrated in the ventricular conduction system, although expression remains high in the atria (14,15). A downregulation of connexin45 gene products has been shown to occur during mouse heart development (16), but little information is available on the sites of expression of this connexin. The aim of the present study was to determine the spatial pattern of connexin45 expression within the conduction system of the developing mouse heart.
ANIMALS AND METHODS
Tissue collection:
Hearts from three adult Balb-c mice were removed and the chambers injected with a 50% solution of tissue mounting medium (Cryo-M-Bed, Bright Instrument Co Ltd, United Kingdom). The hearts were then placed in a plastic mould, oriented so that frontal sections could be made with respect to the major axis of the heart, and covered with tissue mounting medium. The hearts were snap frozen by floating the moulds on isopentane cooled by liquid nitrogen. Fetal hearts (E12.5, E15.5 and E17.5) and neonatal hearts (one day) were obtained from timed pregnant Balb-c mice and prepared for frozen sectioning using similar protocols to those used for adult hearts except that the ventricular chambers were not injected with 50% tissue mounting medium before snap freezing. Animal procedures were conducted according to the Animals (Scientific Procedures) Act, 1986 (UK).
Antibodies:
The anticonnexin45 (Q14E[GP42]), anticonnexin40 (S15C[R83]) and anticonnexin43 (S10C[CK84]) antibodies, affinity purified against the peptide immunogen to which they were raised, have previously been shown to be specific for their respective connexins by Western blot and immunofluorescence of transfected cells (8,17). All three antibodies have been shown to label morphologically defined gap junctions at the electron-microscopic level. The anticonnexin45 antibody (Q14E[GP42]) was used at 1:100 dilution, the anticonnexin40 antibody (S15C[R83]) at 1:500 dilution and the anticonnexin43 antibody (S10C[CK84]) at 1:50 dilution for immunofluorescence. The direct fluorophore-conjugated (fluorescein isothiocyanate [FITC], cyanine 3 [Cy3] and cyanine 5 [Cy5]) secondary antibodies were purchased from Chemicon International (United Kingdom) except the antirabbit-FITC, which was purchased from Dako (United Kingdom) and were used at dilutions of 1:50 (antirabbit-FITC and antiguinea pig-FITC) or 1:250 (all others).
Immunofluorescent labelling and multicolour/multi-channel confocal imaging:
Frozen sections (10 μm) were cut from the hearts and mounted on poly-l-lysine-coated glass slides, which were then stored at −80°C until use. The sections were fixed by immersing the slides in methanol at −20°C for 5 min and were then washed three times with phosphate-buffered saline (PBS). Blocking was carried out for 1 h with 1% bovine serum albumin (BSA) in PBS before incubation with primary antibody (diluted in 1% BSA in PBS) for 2 h. After being washed five times with PBS over 30 min, the sections were incubated with fluorophore-conjugated secondary antibodies (diluted in 1% BSA in PBS) for 1 h. The slides were washed a further five times with PBS over 30 min and mounted with Citifluor (Agar Scientific, United Kingdom), and the coverslips were sealed with clear nail varnish. For double and triple labelling experiments, the conditions were optimized for each different combination. For double labelling, for connexin43 with connexin45 and for connexin40 with connexin45, the primary antibodies were applied as a mixture. The fluorophore-conjugated secondary antibodies were applied together in all cases (antichicken-Cy3/anti-guinea pig-FITC, antirabbit-FITC/antiguinea pig-Cy3 and antirabbit-FITC/antichicken-Cy3, respectively. For triple labelling, all three antibodies were applied together. The three primary antibodies were then immunolocalized by simultaneous incubation in antirabbit-Cy5, antiguinea pig-FITC and antichicken-Cy3. Controls for the immunofluorescent labelling experiments were omission of the primary antibody (or combinations of primary antibodies in the case of double and triple labelling); using the inappropriate secondary antibody for each individual primary antibody; and peptide inhibition, in which antibodies were incubated with 50 μg/mL of the immunogen to which they were raised at room temperature for 30 min before application on tissue sections.
Immunolabelled sections were examined by confocal laser scanning microscopy using either a Leica TCS 4D system or Leica TCS SP system (Leica microsystems (UK) Ltd, United Kingdom), equipped with argon-krypton lasers and fitted with the appropriate filter blocks for the detection of FITC, Cy3 and Cy5 fluorescence. The images recorded were projections of five consecutive single optical sections taken at 0.5 μm intervals.
RESULTS
Embryonic day 12.5:
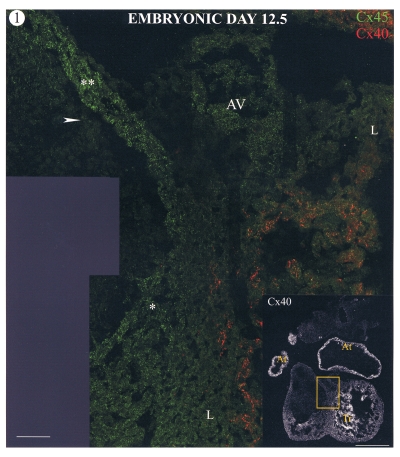
Figure 1 shows a section through an E12.5 heart labelled for connexins 45 (green) and 40 (red). The atria and trabeculated regions of the ventricles labelled positively for connexin40, which appears prominent even in low power views of whole sections. Some connexin40 labelling was also present in the compact layer of the ventricular myocardium (not shown). Low levels of connexin45 labelling (green spots) were observed throughout the heart at this stage. However, some regions of the heart, in particular the conus myocardium of the outflow tract and AV junctional myocardia, were labelled much more intensely for connexin45 than for others. These regions of high connexin45 labelling were negative for both connexin40 and connexin43. Some connexin45 label was also observed in the cushion tissue adjacent to the conus tissue.
Figure 1.
Confocal images of a section through a mouse heart at embryonic day 12.5. The low power image (insert; bar marker = 500 μm) shows connexin40 (Cx40) labelling, which is present in the atrial tissue (At) and trabeculations (Tr). The boxed area is shown at high magnification double labelled for connexin45 (Cx45) (green) and Cx40 (red). Regions of high Cx45 labelling are restricted to the conus myocardium of the outflow tract (**) and atrioventricular junctional (AV) and interventricular septal myocardium (*), which have more intense labelling than other regions (L). Cx45 label is also present in the cushion mesenchyme adjacent to the conus myocardium (arrow). Bar marker = 50 μm
Embryonic day 15:
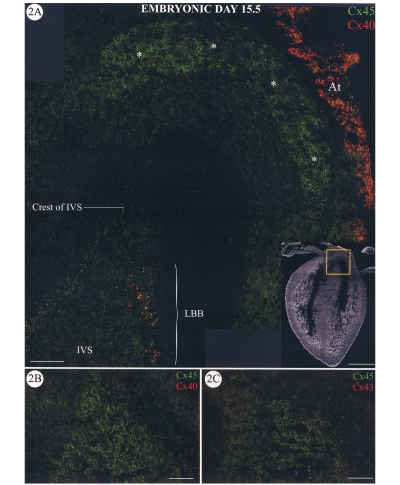
At this stage of development, connexin40 labelling was becoming more restricted; that is, there was less labelling of the compact myocardium of the ventricles. The atrial myocardium was positive for connexin40, and some connexin40 labelling was apparent in the bundle branches (Figure 2A). Regions of intense connexin45 labelling were still localized within the remnants of the conus myocardium (Figure 2A, asterisks) and less prominently in subjacent cushion mesenchyme at this stage. Connexin45 label was also present within the common AV bundle at the crest of the interventricular septum. More posterior sections through the heart double labelled for connexin45 with connexin40 (Figure 2B) or connexin43 (Figure 2C) show that connexin45 is the only connexin expressed in the AV bundle.
Figure 2.
Sections through a mouse heart at embryonic day (E) 15.5. A Confocal micrograph showing a section double labelled for connexin45 (Cx45) (green) and connexin40 (Cx40) (red). The insert shows a low power view of the section with the area shown at high power indicated by the box. Bar marker = 500 μm. The crest of the interventricular septum (IVS) labelled positively for Cx45 only (common atrioventricular bundle). Cx40 label is present in the left bundle branch (LBB) where it colocalizes, in part, with Cx45 to give yellow spots. The remnant of the conus myocardium is also labelled only for Cx45 (*). The tissue of the atrial appendage (At) is clearly positive for Cx40. More posterior sections through the E15.5 heart double labelled for Cx45 and Cx40 (B) or Cx43 (C) show that the atrioventricular bundle continues to express Cx45 only. Bar markers = 50 μm
Later stages of development and the adult heart:
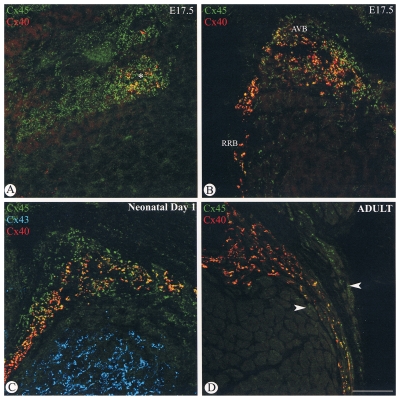
By E17.5 connexin40 labelling was observed in a subcompartment of the AV node (Figure 3A), and was present throughout the AV bundle and bundle branches (Figure 3B). However, connexin40 and connexin45 were not completely colocalized, the area of connexin45 labelling being greater than that for connexin40. The pattern of expression of connexin45 and connexin40 in the AV bundle and bundle branches at E17.5 was similar to that observed in the one-day-old neonatal heart (Figure 3C) and adult heart (figure 3D), although the degree of coexpression of connexin45 and connexin40 was greater in the adult heart. There was no detectable connexin43 labelling in either the AV node or the bundle branches at any stage during the development of the mouse heart. Connexin43 labelling was apparent in the working myocytes of the interventricular septum (Figure 3C).
Figure 3.
Confocal micrographs of sections through a mouse heart at embryonic day (E) 17.5 (A,B), one-day-old neonatal heart (C) and adult heart (D). The atrioventricular node of the E17.5 heart (A) labels positively for connexin45 (Cx45) (green), and a domain within this region is also positive for connexin40 (Cx40) (red). Colocalization of the two connexins shows up as yellow spots. Both Cx45 (green) and Cx40 (red) are localized to the atrioventricular bundle (AVB) and bundle branches (B) of the E17.5 heart. The area of Cx45 expression is larger than the area of Cx40 expression. Yellow spots represent colocalization of the labelling for both connexins. The same pattern of labelling is observed for the one-day neonatal heart (C). The Cx40 (red) label is almost all colocalized with Cx45 label (green), giving the orange-yellow spots within a region positive for Cx45 alone. Connexin43 (Cx43) (blue) is present only in the working myocytes of the interventricular septum. In the adult heart (D) the region of Cx45-only expression in the AVB/bundle branch region is not as great as in the embryonic or neonatal hearts, and the colocalization of Cx45 (green) and Cx40 (red) appears more complete, although some cells express Cx45 only (arrows). Bar marker = 50 μm. RRB Right bundle branch
DISCUSSION
The results presented here show that connexin45 is the first connexin to be expressed in the conduction tissues of the mouse heart and is the only connexin to be continuously expressed throughout the conduction system.
Connexin45 has been detected as early as E8.5, which is the stage at which the developing heart first contracts (16). The transcript was shown to be present at all stages of development but was downregulated from E12 (15). In that study, however, the authors were unable to detect connexin45 protein by immunofluorescence microscopy beyond E10.5. Using our specific and fully characterized anticonnexin45 antibody, we showed that connexin45 protein is in fact present and detectable throughout the development of the mouse heart. The expression of the protein is, however, localized to the developing conduction tissues of the heart in the latter stages of development. At E12.5 we detected connexin45 protein throughout the heart, although the amount of labelling decreased progressively during development of the heart, consistent with the reported downregulation of the transcript (16). There was still some labelling of connexin45 in ventricular myocytes at birth and beyond but the extent of labelling was insignificant compared with the intense labelling of the conduction tissues of the heart.
It is likely that the regions of the E12.5 heart intensely labelled for connexin45 (eg, conus myocardium) form part of the ring-like domains circumscribing the developing AV and outflow junctions from which tissues including the AV node and His bundle differentiate (18). Thus, the pattern of connexin45 labelling appears to be consistent with atrial and AV elements of the conduction system, which have previously been described during the development of the conduction system (reviewed in 18,19). Connexin40 expression was present in the atria, trabeculated regions and compact regions of the ventricular walls. This pattern of expression is consistent with previous reports concerning the distribution of this connexin (12). Connexin43 was absent from these connexin45-positive regions and was present at only low levels in the ventricular and atrial muscle at E12.5.
As the heart continued to develop, connexin40 labelling became more apparent in the bundle branches, but at E15.5 the developing AV bundle region still expressed only connexin45.
By E17.5 the pattern of expression of all three connexins was the same as that observed in the adult heart of this strain of mouse. The AV node consists of two domains, one expressing both connexin40 and connexin45, and the other expressing only connexin45 with the latter domain surrounding the first. This arrangement of connexin40 and connexin45 is continued into the AV bundle and bundle branches, and has been observed in the corresponding regions of the rat heart (10).
The tissues in which connexin45 is expressed include some of the most primitive elements of the chordate heart, and the fact that connexin45 is the first to be expressed may be significant phylogenetically. Expression of connexin40 in addition to connexin45 in the conduction tissues later during development introduces the possibility of redundancy into the system. The finding that mice in which the connexin40 gene was knocked out were still viable and had normal heart rates, albeit with some electrocardiographic abnormalities (20,21), shows that some degree of redundancy does exist, although both connexins are required for proper function. However, when connexin45 was knocked out, the mice died of heart failure at about E10 (22), showing that such redundancy does not apply equally to each connexin. Such a result indicates that correct heart development is dependent on the temporal sequence of connexin expression.
That the patterns of distribution of connexin45 and connexin40 are not identical suggests zonal differentiation of gap junction function in the conduction system. Where the two connexins are colocalized, there is the possibility of a wide range of molecular arrangements, including heteromeric connexons and heterotypic channels. This range of potential channels raises the possibility of complex properties and regulation in this zone. In vitro modelling of connexin coexpression to emulate the patterns observed in vivo is being developed to address these issues.
Acknowledgments
This work was supported by a project grant from the British Heart Foundation (grant number PG 93136) and the European Commission (grant number QLRT-1999-00516).
REFERENCES
- 1.Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- 2.Yamasaki H, Naus CCG. Role of connexin genes in growth control. Carcinogenesis. 1996;17:1199–213. doi: 10.1093/carcin/17.6.1199. [DOI] [PubMed] [Google Scholar]
- 3.Lo CW. The role of gap junction membrane channels in development. J Bioenerg Biomembr. 1996;28:379–85. doi: 10.1007/BF02110114. [DOI] [PubMed] [Google Scholar]
- 4.Gros DB, Jongsma HJ. Connexins in mammalian heart function. BioEssays. 1996;18:719–30. doi: 10.1002/bies.950180907. [DOI] [PubMed] [Google Scholar]
- 5.Kumar NM. Molecular biology of the interactions between connexins. In: Cardew G, editor. Gap Junction-Mediated Intercellular Signalling in Health and Disease. New York: John Wiley & Sons Ltd; 1999. pp. 6–21. [Google Scholar]
- 6.Severs NJ, Rothery S, Dupont E, et al. Immunocytochemical analysis of connexin expression in the healthy and diseased cardiovascular system. Microsc Res Tech. 2001;52:301–22. doi: 10.1002/1097-0029(20010201)52:3<301::AID-JEMT1015>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 7.Veenstra RD. Size and selectivity of gap junction channels formed from different connexins. J Bioenerg Biomembr. 1996;28:327–37. doi: 10.1007/BF02110109. [DOI] [PubMed] [Google Scholar]
- 8.Coppen SR, Dupont E, Rothery S, Severs NJ. Connexin45 expression is preferentially associated with the ventricular conduction system in mouse and rat heart. Circ Res. 1998;82:232–43. doi: 10.1161/01.res.82.2.232. [DOI] [PubMed] [Google Scholar]
- 9.Coppen SR, Kodama I, Boyett MR, et al. Connexin45, a major connexin of the rabbit sinoatrial node, is co-expressed with connexin43 in a restricted zone at the nodal-crista terminalis border. J Histochem Cytochem. 1999;47:907–18. doi: 10.1177/002215549904700708. [DOI] [PubMed] [Google Scholar]
- 10.Coppen SR, Severs NJ, Gourdie RG. Connexin45 (α6) expression delineates an extended conduction system in the embryonic and mature rodent heart. Dev Genet. 1999;24:82–90. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<82::AID-DVG9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Gourdie RG, Severs NJ, Green CR, Rothery S, Germroth P, Thompson RP. The spatial distribution and relative abundance of gap-junctional connexin40 and connexin43 correlate to functional properties of the cardiac atrioventricular conduction system. J Cell Sci. 1993;105:985–91. doi: 10.1242/jcs.105.4.985. [DOI] [PubMed] [Google Scholar]
- 12.Gros D, Jarry-Guichard T, ten Velde I, et al. Restricted distribution of connexin40, a gap junctional protein, in mammalian heart. Circ Res. 1994;74:839–51. doi: 10.1161/01.res.74.5.839. [DOI] [PubMed] [Google Scholar]
- 13.Van Kempen MJA, Vermeulen JLM, Moorman AFM, Gros D, Paul DL, Lamers WH. Developmental changes of connexin40 and connexin43 messenger RNA. Cardiovasc Res. 1996;32:886–900. doi: 10.1016/0008-6363(96)00131-9. [DOI] [PubMed] [Google Scholar]
- 14.Delorme B, Dahl E, Jarry-Guichard T, et al. Developmental regulation of connexin 40 gene expression in mouse heart correlates with the differentiation of the conduction system. Dev Dyn. 1995;204:358–71. doi: 10.1002/aja.1002040403. [DOI] [PubMed] [Google Scholar]
- 15.Gourdie RG, Litchenberg WH, Eisenberg LM. Gap Junctions and Heart Development. In: De Mello WC, Janse MJ, editors. Heart cell communication in health and disease. Boston: Kluwer Academic; 1998. pp. 19–44. [Google Scholar]
- 16.Alcolea S, Theveniau-Ruissy M, Jarry-Guichard T, et al. Downregulation of connexin 45 gene products during mouse heart development. Circ Res. 1999;84:1365–79. doi: 10.1161/01.res.84.12.1365. [DOI] [PubMed] [Google Scholar]
- 17.Ko Y-S, Coppen SR, Dupont E, Rothery S, Severs NJ.Regional differentiation of desmin, connexin43 and connexin45 expression patterens in rat aortic smooth muscle Arterioscler Thromb Vasc Biol(In press) [DOI] [PubMed] [Google Scholar]
- 18.Wessels A, Vermeulen JL, Verbeek FJ, et al. Spatial distribution of “tissue-specific” antigens in the developing human heart and skeletal muscle. III. An immunohistochemical analysis of the distribution of the neral tissue antigen G1N2 in the embryonic heart; implications for the development of the atrioventricular conduction system. Anat Rec. 1992;232:97–111. doi: 10.1002/ar.1092320111. [DOI] [PubMed] [Google Scholar]
- 19.Gourdie RG, Kubalak S, Mikawa T. Conducting the embryonic heart: orchestrating development of specialized cardiac tissues. Trends Cardiovasc Med. 1999;9:18–26. doi: 10.1016/s1050-1738(98)00035-8. [DOI] [PubMed] [Google Scholar]
- 20.Kirchhoff S, Nelles E, Hagendorff A, Kruger O, Traub O, Willecke K. Reduced cardiac conduction velocity and predisposition to arrhythmias in connexin40-deficient mice. Curr Biol. 1998;8:299–302. doi: 10.1016/s0960-9822(98)70114-9. [DOI] [PubMed] [Google Scholar]
- 21.Simon AM, Goodenough DA, Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr Biol. 1998;8:295–8. doi: 10.1016/s0960-9822(98)70113-7. [DOI] [PubMed] [Google Scholar]
- 22.Kumai M, Nishi K, Nakamura K, Takeda N, Suzuki M, Shibata Y. Loss of connexin45 causes a cushion defect in early cardiogenesis. Development. 2000;127:3501–12. doi: 10.1242/dev.127.16.3501. [DOI] [PubMed] [Google Scholar]