Abstract
BACKGROUND:
Mechanical forces related to pressure and flow are important for cell hypertrophy and proliferation.
OBJECTIVE:
To test the hypothesis that mechanosensors are present that are sensitive solely to pure atmospheric pressure in the absence of shear and tensile stresses.
METHODS AND RESULTS:
A pressure-loading apparatus was set up to examine the effects of atmospheric pressure on human aortic smooth muscle cells. Pressure application of 140 to 180 mmHg produced DNA synthesis in a pressure-dependent manner. In contrast, pressure of 120 mmHg or less produced no significant change. Pertussis toxin completely inhibited the pressure-induced increase of DNA synthesis under the high pressure of 200 mmHg. The activities of both extracellular signal-related kinase and c-Jun N-terminal kinase, but not of p38, were stimulated by a pressure of more than 160 mmHg.
CONCLUSION:
These data suggest that human aortic smooth muscle cells have a mechanosensing cellular switch for DNA synthesis that is sensitive to pure atmospheric pressure, and that the molecular switch is activated by pressure of more than 140 mmHg. The activation mechanism consists of pertussis toxin-sensitive and -insensitive pathways, and the former is activated by high pure pressure.
Keywords: c-Jun N-terminal kinase, Extracellular signal-related kinase, G-protein, Mechanical stress, p38, Pertussis toxin
Abnormal growth and proliferation of vascular smooth muscle cells have been implicated in the pathogenesis of atherosclerosis and hypertension (1). Mechanical stresses are likely to be involved in this process because they have been shown to regulate cell growth in many tissues (2). In arteries, for example, a mechanical stress related to pressure and flow is crucial in promoting blood vessel wall remodelling. Such vascular remodelling may have important clinical implications for the evolution of several vascular diseases, may alter vascular compliance in hypertension and atherosclerosis, and may cause vascular fragility and compensatory changes in atherosclerosis (3).
Various stresses have been studied, including cytokines, mitogens, ultraviolet light, oxidants, hyperosmolarity, heat stress and mechanical stress (4). The vascular endothelial and vascular smooth muscle cells covering the inner surface of blood vessels are constantly exposed to such stress. The hemodynamic forces affecting endothelial cells include shear stress caused by the frictional force of blood flow, and circumferential and pure pressure stress caused by transmural pressure. Recent studies have shown that shear stress to the vessel wall, which is one of the mechanical stresses generated by blood flow, modulates endothelial morphology and function (5). However, in an in vitro cell culture model, it has been shown that intracellular signalling produced by tensile and shear stresses may be influenced not only by pressure stress but also by morphological and cytoskeletal cell changes (6). Vascular smooth muscle cells make up the outer layer of the endothelium in blood vessels and are thus indirectly exposed to blood flow. Mechanosensing intracellular signal transduction in vascular smooth cells is defined as a cellular response to transmural pure pressure and tensile stress in the absence of shear stress. In previous studies, tensile stress was reported to accelerate DNA synthesis and activate stretch-activated cation channels in vascular smooth muscle cells (7,8). However, the molecular identities of these candidates for mechanosensitive receptors are unknown.
In the present study, we hypothesized the presence of mechanosensors and intracellular signal transduction acting under pure atmospheric pressure in the absence of tensile and shear stresses. On the basis of this hypothesis, we evaluated the DNA synthesis and intracellular signalling of human aortic smooth muscle cells (HASMC) by using an original pressure loading apparatus that was capable of directly applying various levels of pure pressure (maximum 300 mmHg) on HASMC to examine the effect of pure pressure stress on mechanosensors without inducing morphological cell changes. In this manner, we determined the threshold of pressure for activating the mechanosensing cellular switch and clarified the differential intracellular signalling mechanisms involving pertussis toxin (PTx) -sensitive and -insensitive heterotrimeric G proteins.
MATERIALS AND METHODS
Cell culture:
HASMC (Clonetics Corporation, USA) were cultured in smooth muscle cell basal medium (SmBM; Clonetics), which is modified MCDB131 containing 5% fetal bovine serum, gentamicin (50 μg/mL), amphotericin (50 μg/mL) and several growth factors: human epidermal growth factor (10 ng/mL), human fibroblast growth factor (2.0 ng/mL) and insulin (5.0 μg/mL). The cells were incubated at 37°C in a humidified, 5% CO2 atmosphere. The fourth through eighth passages of HASMC were plated on six-well plates for further investigation.
Pure pressure loading apparatus:
An original pure pressure loading apparatus was designed to expose HASMC to pure atmospheric pressure stress. The chamber allows for pumping air or nitrogen gas to raise the internal pressure (maximum 300 mmHg) and can be sealed tightly by placing several clamps at the edge. The internal pressure was monitored with an aneroid barometer during the experiments. The compression chamber was placed in an incubator and kept at 37°C, monitored by a digital thermometer mounted in the incubator. In the following series of experiments, the chamber was kept at 37°C, and PO2 and PCO2 were theoretically pre-served as constants according to Boyle-Gay-Lussac’s law. The culture medium pH was kept at a constant level (7.41±0.02) during the experiments. It was not possible to monitor the actual morphological changes of pressurized cells in this system set-up. However, the light microscopic investigations failed to find any changes in cell size or morphology after pressurization.
HASMC were cultured on six-well plates for 48 h. In three wells of each plate, the medium was changed to a starving medium (Dulbecco’s modified Eagle’s medium without serum). In the other three wells, the medium was changed to the same starving medium but containing PTx (0.1 μg/mL; Seikagaku, Japan). After 8 h of incubation, the plates were placed in the pure pressure loading apparatus in a medium containing 10 mM HEPES (N-2-hydroxyethylpiperazine-N-2-ethanesulphonic acid, pH 7.4), exposed to various levels of atmospheric pressure (0 through 240 mmHg), and then incubated in an incubator for 1 or 3 h at 37°C.
DNA synthesis:
[3H]-thymidine incorporation into DNA was studied as a marker for DNA synthesis acceleration by pure atmospheric pressure. [3H]-thymidine (2 μCi/mL, Amersham, United Kingdom) was added to the medium in all wells of the six-well plate. After pressure loading procedures, the plate was incubated in a 37°C in a CO2 incubator under normal pressure for 4 h. The cells were rinsed two times in ice-cold phosphate-buffered saline followed by precipitation three times with ice-cold 10% trichloroacetic acid, and lysed in 50 mM NaOH at 37°C by shaking for 30 min. The incorporation of [3H]-thymidine into DNA was quantified by pipetting the DNA hydrolysate into counting vials containing 4 mL of liquid scintillation cocktail (Ready Gel, Beckman, USA). The protein concentration of HASMC was normalized by Lowry’s method with bovine serum albumin as a standard (9). The counting results were expressed as disintegrations per protein type and were expressed as the percentage increase compared with controls at the basal level (0 mmHg) for 1 and 3 h. All the experiments for each pressure were performed in triplicate and were repeated two to three times.
Immunoblotting:
HASMC were incubated on 100 mm dishes until they were 80% confluent. The medium was replaced with a starving medium. After 8 h of incubation, the dishes were placed in the pure pressure loading apparatus in the medium containing 10 mM HEPES and exposed to various levels of atmospheric pressure (0 mmHg, 120 through 180 mmHg, and 240 mmHg) for 3 h at 37°C. The cells were washed twice with ice-cold phosphate-buffered saline and harvested in a buffer containing 25 mM Tris-HCl (pH 6.8), 1% Triton X-100, 150 mM NaCl and the following protease inhibitors: benzamidine (100 μM), leupeptin (2 μM), aprotinin (0.15 μM), pepstatin A (1.5 μM) and phenylmethylsulphonyl fluoride (100 μM). The protein content of each sample was measured by Lowry’s method. Samples (15 μg) were analyzed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis in a 12% gel mounted in the Mini Gel Electrophoresis System (Marysol, Japan). Protein were transferred to a nitrocellulose membrane (Hybond ECL; Amersham) by Western blotting apparatus (semidry type; Marysol) with a buffer containing 20% methanol, 48 mM Trisbase, 78 mM glycine and 0.375% sodium dodecyl sulphate for 2 h. The membranes were submerged for 1 h in 4% nonfat dry milk in TTBS (0.05% Tween-20, Tris-buffered saline, pH 7.4), followed by incubation for 2 h in TTBS containing the appropriate primary antibodies (antiactive extracellular signal-regulated kinase [ERK], antiactive c-Jun N-terminal kinase [JNK] and antiactive p38 antirabbit polyclonal antibodies, Promega, USA) in concentrations recommended by the manufacturer. The membranes were incubated in TTBS containing horseradish peroxidase-labelled donkey antirabbit immunoglobulins (1:2500, Amersham), followed by washing three times in TTBS. Protein was detected by a chemiluminescence method (ECL, Amersham). Active phosphorylated forms of ERK, JNK and p38 were scanned with a digital image analyzing system and quantified by NIH densitometer image system (sp1000, Hitachi, Japan).
Data analysis:
All the results are expressed as mean ± SEM, and significance was assessed by Student’s t test. P<0.05 was considered significant.
RESULTS
Pure pressure-dependent acceleration of DNA synthesis in HASMC:
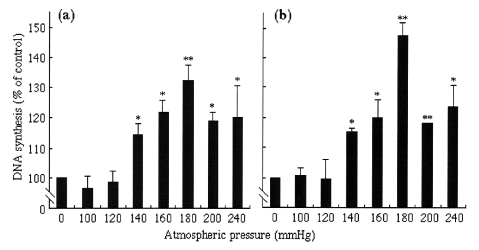
DNA synthesis was measured as [3H]-thymidine incorporation into DNA. After application of pressure for 1 h, the pure pressure of 240 mmHg induced an approximately 20% increase in [3H]-thymidine incorporation compared with the control HASMC (Figure 1). After more than 3 h, the degree of acceleration of DNA synthesis was similar.
Figure 1.
Effect of various pressures for 1 h (a) or 3 h (b) on an increase of DNA synthesis, determined by the incorporation of [3H]-thymidine into human aortic smooth muscle cells. Values are mean ± SEM (n=6 to 9). *P<0.05 versus controls; **P<0.01 versus controls
Threshold of pure atmospheric pressure in acceleration of DNA synthesis:
Various levels of atmospheric pressure (0 mmHg, 100 through 200 mmHg, and 240 mmHg) were applied to analyze the threshold of pure pressure in acceleration of DNA synthesis (Figure 1). An atmospheric pressure of less than 120 mmHg produced no significant change in [3H]-thymidine incorporation at 1 and 3 h. However, pressure of 140 mmHg produced an approximately 20% increase in [3H]-thymidine incorporation (P<0.005 versus control) at 1 and 3 h. Pressure of more than 140 mmHg also produced an increase (14±6% to 27±4%, P<0.05 versus control). At 3 h, the degree of increase was dependent on pressure levels from 140 to 180 mmHg, with 180 mmHg pressure producing an increase of 49±6% compared with control cells (P<0.01 versus control). At 200 and 240 mmHg, DNA synthesis was also significantly accelerated at both 1 and 3 h (P<0.05 versus control). However, the increase of DNA synthesis was lower than that at 180 mmHg.
PTx effect on pure pressure-dependent DNA synthesis:
The study examined whether Gi proteins are involved in pure pressure-dependent acceleration of DNA synthesis in HASMC. DNA synthesis acceleration at 200 mmHg pressure was completely inhibited by PTx (0.1 μg/mL) at 3 h (increase of DNA synthesis compared with control: 18.8±2.7 at 1 h and 18.0±1.4% at 3 h in the absence of PTx; 4.4±2.7% at 1 h and 2.3±2.9% at 3 h in the presence of PTx; Figure 2b). In contrast, an acceleration of DNA synthesis at 160 mmHg pressure was not significantly inhibited at these time points (increase of DNA synthesis versus control: 21.6±2.5% at 1 h and 27.4±5.5% at 3 h in the absence of PTx; 16.5±6.9% at 1 h and 32.5±5.5% at 3 h in the presence of PTx; Figure 2a). PTx also failed to significantly inhibit the acceleration produced by 180 mmHg pressure (increase of DNA synthesis versus control: 24.6±4.3% at 1 h and 40.1±11.4% at 3 h in the absence of PTx; 24.2±3.9% at 1 h and 49.0±6.0% at 3 h in the presence of PTx).
Figure 2.
Effect of pertussis toxin on a pure pressure-induced increase of DNA synthesis. Human aortic smooth muscle cells were incubated with (PTx) or without (NTx) pertussis toxin for 8 h. Cells were incubated under an atmospheric pressure of 160 mmHg (a) or 200 mmHg (b). Values are mean ± SEM (n=6 to 9). *P<0.05 versus in the absence of pertussis toxin; **P<0.01 versus in the absence of pertussis toxin. NS Not significant
Immunoblotting:
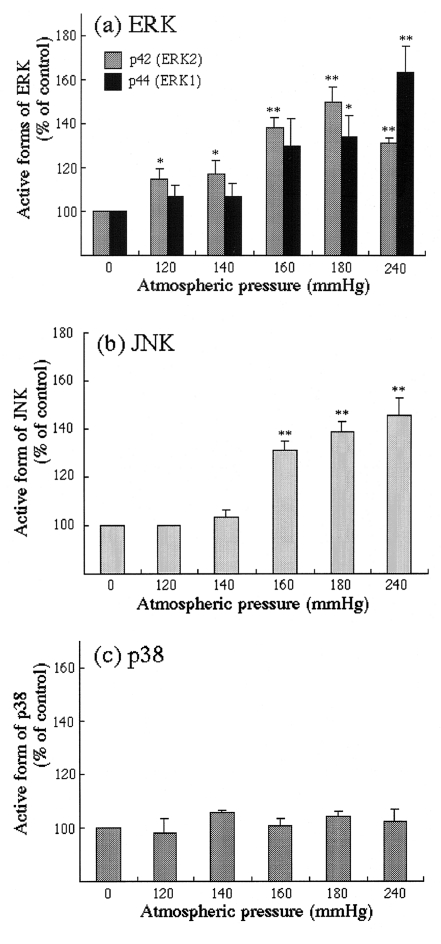
Pure atmospheric pressure stimulated ERK1/2 and JNK activities. Pressure of more than 160 mmHg induced an activation of ERK1 (p44), and ERK1 was activated to the maximal level at a pressure of 240 mmHg (increase in active form of ERK1 versus control: 63.2±12.1%). Pressures of more than 120 mmHg induced an activation of ERK2 (p42), and ERK2 was activated to a maximal level at a pressure of 180 mmHg (increase in active form of ERK2 versus control: 49.5±7.3%; Figure 3a). JNK was activated at pressures of more than 160 mmHg in a pressure-dependent manner and to a maximal level at a pressure of 240 mmHg (increase in active form of JNK versus control: 45.7±6.9%; Figure 3b). Application of pure atmospheric pressure induced no activation of p38 (Figure 3c).
Figure 3.
Densitometric analysis of (a) active extracellular signal-related kinase (ERK), (b) active c-Jun N-terminal kinase (JNK) and (c) active p38 in human aortic smooth muscle cells. Values are mean ± SEM (n=3 to 5). *P<0.05 versus controls; **P<0.01 versus controls
DISCUSSION
Mechanical forces are important modulators of cellular functions, particularly in the cardiovascular system. Mechanical stress has various components, such as wall shear stress, tensile stress and pure pressure stress. In the case of shear stress, a fluid shear stress of 1.3 to 4.1 dynes/cm2 affected endothelial cell DNA synthesis during regeneration (10). It has also been reported that shear stress stimulated ERK and JNK activity in a force-dependent manner in endothelial cells (11). Shear stress is also known to activate phospholipase C, and to generate inositol triphosphate and diacylglycerol. Inositol triphosphate releases Ca2+ from Ca2+ stores by way of the inositol triphosphate receptor, and diacylglycerol activates protein kinase C (12,13). Several protein kinase C isoenzymes have been suggested to be involved in several downstream signallings, such as ERK activation, NFκB-mediated gene transcription, erg-1 transcription and activation of c-Src families (14–16). Recently, it was reported that shear stress induces changes in the morphology and cytoskeletal organization of endothelial cells, and that these changes may be correlated to the functional change after exposure to shear stress (6). It is interesting to speculate that the focal adhesion complex plays an important role in shear stress-induced signalling, and that shear stress-induced signalling participates in intracellular cross-talk with integrin-coupled signal transductions (17,18). In these studies, however, it is difficult to separate the direct effects of pure pressure from the indirect effects caused by the morphological change of cells. The pure stress is reported to promote DNA synthesis in rat cultured vascular smooth muscle cells in an original pressure loading apparatus, as determined by immunocytochemical assay (19). This study suggests the possible presence of a mechanosensing cellular switch that is sensitive solely to pure pressure stress. However, in experiments with such an apparatus, the threshold pressure for determining the ‘on’ and ‘off’ status of the mechanosensing cellular switch has not been clarified.
In this study, we showed that pure pressure stress accelerated an increase of DNA synthesis in the absence of shear stress and tensile stress, and determined the threshold of pure pressure stress that presumably activates the mechanosensing cellular switch. Using an original pressure loading apparatus, we investigated various pressure levels from 0 to 240 mmHg. Low atmospheric pressure of less than 120 mmHg had no significant effect on DNA synthesis, while pressures of more than 140 mmHg induced an acceleration of DNA synthesis. From these results, we conclude that a mechanosensing cellular switch for DNA synthesis that is sensitive solely to pure pressure was on or off at over or under 140 mmHg, respectively. Pressures of 140 to 180 mmHg promoted an increase of [3H]-thymidine incorporation in a pressure-dependent manner at 1 and 3 h. Pressure of more than 200 mmHg also induced approximately a 20% increase of DNA synthesis. However, the degree of acceleration of DNA synthesis at a pressure of more than 200 mmHg was similar and pressure independent.
We also showed the differential pathway in HASMC in response to pure atmospheric pressures. ERK1 was activated at pressures of more than 160 mmHg and to a maximal level at 240 mmHg. ERK2 was also activated at pressures of more than 120 mmHg, to a maximal level at a pressure of 180 mmHg. JNK was activated at pressures of more than 160 mmHg in a pressure-dependent manner. In contrast, p38 was not activated at pure atmospheric pressure. The mechanosensing mechanism stimulated by pure atmospheric pressure may consist of some differential pathways involving ERK and JNK signalling, and differential levels of pressure may activate each pathway. Moreover, differential activation of ERK and JNK in HASMC by pure atmospheric pressure may result in the selective phosphorylation and activation of transcription factors leading to selective gene regulatory events. It is not clear why the degree of acceleration of DNA synthesis and activation of ERK2 were independent of pressure levels of more than 200 mmHg or why the pressure levels that accelerated DNA synthesis and activated ERK and JNK were different.
A role for heterotrimeric G protein in mechanical stress-induced signal transductions in endothelial cells has recently emerged (20,21). It is known that both heterotrimeric G proteins and small G proteins are activated by shear stress. Shear stress-induced activation of G proteins results in several flow-initiated endothelial responses that regulate vascular tone and that release such vasodilators as nitric oxide and prostaglandin I2, and such vasoconstrictors as endothelin (22–24). Therefore, we also investigated whether mechanosensing signalling pathways that are sensitive to pure pressure in HASMC were related to Gi-dependent pathways or to Gi-independent pathways. At an atmospheric pressure of 160 mmHg, PTx had no significant effect on [3H]-thymidine incorporation. However, at a high atmospheric pressure of 200 mmHg, PTx completely inhibited the increase of [3H]-thymidine incorporation. Pure pressure-induced DNA synthesis was produced through intracellular signalling pathways, including both Gi-dependent and Gi-independent pathways. Although the molecular switch was on at an atmospheric pressure of more than 140 mmHg, an atmospheric pressure below 180 mmHg may activate intracellular signalling predominantly through Gi-independent pathways, and a pressure of more than 200 mmHg may activate DNA synthesis predominantly through Gi-dependent pathways.
SUMMARY
We showed that HASMC has a mechanosensing molecular switch for DNA synthesis that is sensitive solely to pure atmospheric pressure and that the switch is on or off at over or under 140 mmHg, respectively. Moreover, mechanosensing cellular mechanisms may consist of some mechanosensors or intracellular pathways activated by different levels of pure atmospheric pressure. This study showed a change in dominance of Gi-dependent or Gi-independent intracellular signalling pathways in a pressure range from 160 to 200 mmHg. Recently, it was reported that the minimal risk for cardiovascular mortality was reached at 138.8 mmHg for a mean systolic arterial pressure in a randomized clinical study of patients with hypertension (25). Activation of mechanosensing switches in HASMC at 140 mmHg may be involved in clinical cardiovascular events.
REFERENCES
- 1.Safar ME, London G, Asmar R, Frohlich ED. Recent advances on large arteries in hypertension. Hypertension. 1998;32:156–61. doi: 10.1161/01.hyp.32.1.156. [DOI] [PubMed] [Google Scholar]
- 2.Vandenburgh HH. Mechanical forces and their second messengers in stimulating cell growth in vitro. Am J Physiol. 1992;262:R350–5. doi: 10.1152/ajpregu.1992.262.3.R350. [DOI] [PubMed] [Google Scholar]
- 3.Cowan DB, Langill BL. Cellular and molecular biology of vascular remodeling. Curr Opin Lipidol. 1996;7:94–100. doi: 10.1097/00041433-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–6. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 5.Chien S, Li S, Shyy JYJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–9. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 6.Cucina A, Sterpetti AV, Pupelis G, et al. Shear stress induces changes in the morphology and cytoskeleton organization of arterial endothelial cells. Eur J Vasc Endovasc Surg. 1995;9:86–92. doi: 10.1016/s1078-5884(05)80230-8. [DOI] [PubMed] [Google Scholar]
- 7.Weiser MC, Majack RA, Tucker A, Orton EC. Static tension is associated with increased smooth muscle cell DNA synthesis in rat pulmonary arteries. Am J Physiol. 1995;268:H1133–8. doi: 10.1152/ajpheart.1995.268.3.H1133. [DOI] [PubMed] [Google Scholar]
- 8.Setoguchi M, Ohya Y, Abe I, Fujishima M. Stretch activated whole-cell currents in smooth muscle cells from mesenteric resistance artery of guinea-pig. J Physiol. 1997;501:343–53. doi: 10.1111/j.1469-7793.1997.343bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 10.Ando J, Komatsuda T, Ishikawa C, Kamiya A. Fluid shear stress enhanced DNA synthesis in cultured endothelial cells during repair of mechanical denudation. Biorheology. 1990;27:675–84. doi: 10.3233/bir-1990-27505. [DOI] [PubMed] [Google Scholar]
- 11.Jo H, Sipos K, Go YM, Law R, Rong J, McDonald JM. Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. Gi2- and Gbeta/gamma-dependent signal pathways. J Biol Chem. 1997;272:1395–401. doi: 10.1074/jbc.272.2.1395. [DOI] [PubMed] [Google Scholar]
- 12.Helmlinger G, Berk BC, Nerem RM. Calcium responses of endothelial cell monolayers subjected to pulsatile and steady laminar flow differ. Am J Physiol. 1995;269:C367–75. doi: 10.1152/ajpcell.1995.269.2.C367. [DOI] [PubMed] [Google Scholar]
- 13.Tseng H, Peterson TE, Berk BC. Fluid shear stress stimulates mitogen-activated protein kinase in endothelial cells. Circ Res. 1995;77:869–78. doi: 10.1161/01.res.77.5.869. [DOI] [PubMed] [Google Scholar]
- 14.Mohan S, Mohan S, Sprague EA. Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. Am J Physiol. 1997;273:C572–8. doi: 10.1152/ajpcell.1997.273.2.C572. [DOI] [PubMed] [Google Scholar]
- 15.Schwachtgen JL, Houston P, Campbell C, Sukhatme V, Braddock M. Fluid shear stress activation of erg-1 transcription in cultured human endothelial and epithelial cells is mediated via the extracellular signal-related kinase 1/2 mitogen-activated protein kinase pathway. J Clin Invest. 1998;101:2540–9. doi: 10.1172/JCI1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jalali S, Li YS, Sotoudeh M, et al. Shear stress activates p60src-Ras-MAPK signaling pathways in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1998;2:227–34. doi: 10.1161/01.atv.18.2.227. [DOI] [PubMed] [Google Scholar]
- 17.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–7. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto S, Teramoto H, Coso A, et al. Integrin function: molecular hierarchies of cytoskeletal signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hishikawa K, Nakai T, Marumo T, et al. Pressure promotes DNA synthesis in rat cultured vascular smooth muscle cells. J Clin Invest. 1994;93:1975–80. doi: 10.1172/JCI117189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berthiaume F, Frangos JA. Flow-induced prostacyclin production is mediated by a pertussis toxin-sensitive G protein. FEBS Lett. 1992;308:277–9. doi: 10.1016/0014-5793(92)81292-t. [DOI] [PubMed] [Google Scholar]
- 21.Gudi SRP, Clark CB, Frangos JA. Fluid flow rapidly activates G proteins in human endothelial cells. Involvement of G proteins in mechanochemical signal transduction. Circ Res. 1996;79:834–9. doi: 10.1161/01.res.79.4.834. [DOI] [PubMed] [Google Scholar]
- 22.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured endothelial cells. Science. 1985;227:1477–9. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 23.Ranjan V, Xiao Z, Diamond SI. Constitutive NOS expression in cultured endothelial cells is elevated by fluid shear stress. Am J Physiol. 1995;269:H550–5. doi: 10.1152/ajpheart.1995.269.2.H550. [DOI] [PubMed] [Google Scholar]
- 24.Kuchan MJ, Frangos JA. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial calls. Am J Physiol. 1993;264:H150–6. doi: 10.1152/ajpheart.1993.264.1.H150. [DOI] [PubMed] [Google Scholar]
- 25.Hansson L, Zanchetti A, Carruthers SG, et al. Effect of intensive blood pressure lowering and low-dose aspirin in patients with hypertension: principal results of the hypertension optimal treatment (HOT) randomized trial. Lancet. 1998;351:1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]