Abstract
Luteinizing hormone receptor (LHR) mRNA binding protein (LRBP), identified as mevalonate kinase, has been shown to be a trans factor mediating the post-transcriptional regulation of LHR mRNA expression in ovaries. LRBP binds to the coding region of LHR mRNA and accelerates its degradation. Our previous studies in an in vitro system showed that LRBP represses the translation of LHR mRNA by forming an untranslatable ribonucleoprotein (mRNP) complex, further suggesting that the untranslatable mRNP complex is directed to the mRNA repression/decay machinery for subsequent mRNA turnover. In the present studies, we used yeast two hybrid system to screen a cDNA library which was constructed from LHR down-regulated ovaries. Two proteins were identified interacting with LRBP: ribosomal protein S20 (RP S20) and ubiquitin conjugating enzyme 2i (UBCE2i). Their interactions with LRBP were confirmed by the mating assay, co-immunoprecipitation analyses and in vitro sumoylation assays. Furthermore, we show that LRBP is a target for modification by SUMO2/3 but not by SUMO1, at K256 and/or K345. Mutation of both lysine residues is sufficient to abrogate the sumoylation of LRBP. These findings suggest that the direct interaction of LRBP with the translation machinery, through RP S20, may be responsible for the transition of LHR mRNA to an untranslatable complex, and that sumoylation of LRBP may play a role in targeting the untranslatable mRNP complex to the mRNA decay machinery in specific cytoplasmic foci.
Keywords: Luteinizing hormone receptor, LRBP, RNA decay, Sumoylation, UBCE2i
1. Introduction
Luteinizing hormone (LH) and human chorionic gonadotropin (hCG), the placental counterpart of LH, are important glycoprotein hormones regulating gonadal functions in mammals, and their actions are mediated by the same receptor designated as LHR, which is expressed primarily in the testis and ovary [1, 2]. LHR belongs to the Gs protein receptor family, and like many members of the family, LHR expression can be down-regulated by exposure to a high concentration of its ligand [3, 4]. Our laboratory has shown that LHR expression in ovarian cells is down-regulated by an endogenous preovulatory LH surge or by administration of a pharmacological dose of hCG, through a posttranscriptional mechanism [5]. Using a rodent model system, we identified an LHR mRNA binding protein (LRBP) in the down-regulated rat ovary which binds specifically to a polypyrimidine-rich sequence in the coding region of LHR mRNA [6] and accelerates its degradation [7]. The protein was purified and identified as being mevalonate kinase (MVK) [8].
Messenger RNA degradation and mRNA translation, the two important control mechanisms in regulating eukaryotic gene expression are often related events [9]. Several reports have shown that inhibition of translation or aberrant translational termination of some mRNAs destabilizes the transcripts [10, 11]. It is proposed that untranslating mRNAs assemble into translationally repressed ribonucleoprotein complexes (RNPs), which become associated with the general repression/decay machinery, at specific cytoplasmic foci referred to as P bodies, and the transcripts are then either stored or degraded [12]. Using an in vitro translation system, we demonstrated that LRBP inhibits the translation of LHR mRNA by forming a ribonucleoprotein complex [10], and mutation of the nucleotides in LHR mRNA at the LRBP binding site fully abrogates the inhibitory effect of LRBP on mRNA translation [13]. On the basis of these findings, we hypothesize that LRBP causes the formation of an untranslatable mRNP complex, which leads to the rapid decay of LHR mRNA by recruiting the general repression/decay machinery in P bodies.
To address this possibility, we used LRBP as “bait” in a yeast two-hybrid screen to identify interacting protein partners in LHR down-regulated ovaries. We identified two proteins that interact with LRBP, ribosomal protein S20 (RP S20) and ubiquitin conjugating enzyme 2i (UBCE2i). RP S20 is a component of the 40S ribosomal subunit. Based on the known function of RP S20, our results suggest that its association with LRBP may impair the translation initiation of LHR mRNA, disrupt its 5′ cap structure and render it untranslatable. UBCE2i, a SUMO conjugating enzyme is involved in multi-sumoylation of LRBP by SUMO2/3 molecules. This modification may facilitate the shuttling of mRNP complexes to specific subcellular sites, and thus gain access to the repression/decay machinery. In summary, our data suggest the possibility that the LRBP interacting proteins identified in this study might provide a mechanistic basis for the degradation of LHR mRNA.
2. Materials and Methods
2.1. Animals and treatments
All animals were housed under the care of the University of Michigan Unit of Laboratory Animal Medicine (UM-ULAM). Experimental protocols used in this study were approved by the University Committee on the Use and Care of Animals (UCUCA). Pseudopregnancy was induced in 22-day-old Sprague-Dawley female rats by a subcutaneous injection of 50 IU pregnant mare serum gonadotropin (PMSG), followed by a 25 IU hCG injection 56h later. The day of hCG injection was taken as day 0. On the fifth day of pseudopregnancy, rats were injected with 50 IU hCG to induce LH receptor down-regulation, and ovaries were collected 9h later [14].
2.2. Yeast two-hybrid screening
The bait plasmid was constructed by cloning the complete coding region of rat LRBP from pCMV4-LRBP [8] into the pGBKT7 vector, which expresses proteins in fusion with Gal4 binding domain. Since the present study focuses on the function of mevalonate kinase as an RNA binding protein, this protein is referred to as LRBP in the manuscript. The pseudopregnant rat ovary cDNA library was constructed using the Matchmaker Library Construction and Screening Kit (Clontech, Mountain View, CA). In brief, 2 μg of total RNA were extracted from pseudopregnant rat ovaries for cDNA synthesis and the first strand cDNA was amplified by Long Distance PCR. Purified cDNAs were linked to the sequence encoding Gal4 activation domain in pGADT7 vector, by homologous recombination in yeast strain AH109 [15].
pGBKT7-LRBP bait and pGADT7-cDNA library were co-transformed into yeast AH109 cells and transformants were plated on synthetic minimal medium lacking tryptrophan, leucine and histidine but supplemented with adenine. After 3 days, the growing clones were restreaked on minimal medium plates and tested by the β-galactosidase filter assay. Plasmids were isolated from positive clones, using the Zymoprep II Kit (Zymo Research, Orange, CA) and the DNA inserts of the library plasmids were sequenced at the Biomedical Sequencing Core Facility of the University of Michigan.
2.3. Protein-protein interaction analyses
The complete coding region of each LRBP interacting protein was amplified from total RNA by RT-PCR, and cloned in the proper reading frame into pGADT7 (prey) vectors, provided by the Matchmaker 3 Yeast Two-Hybrid Screening Kit (Clontech). The yeast strain AH109 transformed with a specific prey construct was mated with the Y187 strain transformed with the bait construct. The progenies were checked for growth on minimal medium plates without tryptophan, leucine and histidine, to confirm the interaction between bait and prey.
The plasmid constructs were then used to synthesize [35S] methionine labeled proteins, using the TNT Quick Coupled Transcription/Translation System (Promega, Madison, WI). Because of the location of the T7 promoter in the parent pGBKT7 and pGADT7 vectors, in vitro translation products do not contain any portion of the Gal4 protein. These products were examined by 10% SDS-PAGE as described in detail previously [10]. Any pairwise mixtures of these translation products were analyzed for in vitro association, following the instructions of Matchmaker Co-Immunoprecipitation Kit (Clontech).
2.4. Cell culture and transfection
Human embryonic kidney cells expressing the large T-antigen (293T) were maintained in Dulbecco's Modified Eagle's medium (Sigma, St. Louis, MO) containing 10 mM HEPES, 50 μg/ml gentamicin, 2 units/ml nystatin and 10% fetal bovine serum at 37 C in a humidified atmosphere containing 5% CO2. Cells were plated at 40-50% confluency in 60 mm dishes and transiently transfected with 2 μg of plasmid DNA at 12 h, using FuGENE 6 (Roche, Indianapolis, IN). Cells were then collected 48 h post-transfection, for the preparation of whole cell lysates [16] or S-100 fractions.
2.5. Preparation of cytosolic proteins (S-100 fractions)
Forty-eight hours after transfection, 293T cells were detached from culture dishes using NaCl/Pi-EDTA. The cell pellets were homogenized at 4 C in buffer A (10 mM HEPES, pH7.9; 0.5 mM MgCl2; 50 μM EDTA; 5 mM dithiothreitol; and 10% glycerol) containing 50 mM KCl and protease inhibitor mixture [6]. The homogenates were centrifuged at 105,000 × g, 4 C for 90 min, and the supernatants (S-100 fractions) were quantified using the BCA Protein Assay Kit (Pierce, Rockfond, IL).
2.6. Western blot analysis
Whole cell lysates or S-100 fractions were denatured by boiling for 5 min in the presence of loading buffer and were subjected to SDS-PAGE. After electrophoresis, the proteins were electroblotted onto nitrocellulose membranes (0.2-μm pore) and blocked with 5% milk for 1 h at room temperature. The membranes were then incubated overnight with primary antibodies at 4 C. Anti-LRBP polyclonal antibody was raised against its first 15 N-terminal amino acids – MLSEVLLVSAPGKVI [14]. Anti-myc and anti-HA antibodies were included in the Matchmaker Co-Immunoprecipitation Kit (Clontech). Anti-SUMO polyclonal antibodies were included in the Sumoylation Assay Kit (BioMol, Plymouth, Meeting, PA). The presence of immune complexes was detected using the SuperSignal West Femto Maximum Sensitivity Substrate (Pierce).
2.7. Site-directed mutagenesis
The mutants of rat LRBP in pCDNA4 vector were prepared using the QuickChange Site-directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer's protocol. The mutagenic sense (S) primers used were as follows: K256R, 5′-GTCAGAAGCAGGCTAATCAGGTTCCCTGAGATCATGGCCCCG-3′; K345R, 5′-GGCATCACCCTCCTGAGGCCAGGTCTAGAG-3′. The K256R single mutant was then employed as template for the synthesis of K256R/K345R double mutant, with the K345R mutagenic primer. Mutations were verified by DNA sequencing.
2.8. In vitro sumoylation analysis
Wild type and mutant constructs of LRBP, over-expressed in 293T cells, were assayed for sumoylation using the Sumoylation Kit (BioMol) by following the manufacturer's protocol. Briefly, aliquots of S-100 fractions were mixed with SUMO activating enzyme E1 (50 nM), SUMO conjugating enzyme E2 (500 nM), SUMO (250 nM) and Mg2+-ATP (5 mM) in the buffer supplied with the assay kit and incubated at 30 C for 60 min. The reaction was quenched by adding 20 μl of SDS-PAGE gel loading buffer and heating at 65 C for 20 min. Sumoylated proteins were then separated on 4-15% gradient gel and detected by western blot.
3. Results
3.1. Identification of proteins that interact with LRBP
We have previously shown that LRBP, binding specifically to the coding region of LHR mRNA, leads to the formation of an untranslatable mRNP complex. The mRNA component in this complex is rapidly degraded presumably by being directed to an mRNA decay pathway in the cytosol [10]. In order to identify LRBP interacting partners during this process, we employed the yeast two-hybrid system and screened a cDNA library constructed from hCG down-regulated rat ovaries.
The entire rat LRBP protein was used as the bait. A total of ∼800,000 transformants were plated and 148 clones grew out on plates with minimal medium lacking histidine. 67 of these colonies showed a strong blue color in the β-galactosidase filter assay. Library plasmids were extracted from these positive colonies, multiplied and sequenced by automated sequencing. Using GeneRunner Software 3.05, the DNA sequences obtained were translated into protein sequences, which were then subjected to BLAST analysis using the NCBI database. The results revealed that 2 clones contained in-frame cDNA encoding the sumo-conjugating enzyme UBCE2i (Table 1), 1 clone had the heat shock 70 kDa protein 5 insert, 1 clone contained the cDNA of eukaryotic initiation factor 5A (eIF5A), and 1 clone had the cDNA of ribosomal protein S20. LRBP itself was also screened out, which is consistent with the previous report that LRBP can exist as a dimer [17]. Table 1 summarizes the identified protein fragments and functional characteristics of these proteins. The other sequenced clones either represent molecules not cloned in frame with Gal4 activation domain (AD) or proteins of uncharacterized function.
Table 1. Characteristics of LRBP interacting proteins identified by the yeast two hybrid assay.
| Protein interacting with LRBP | Clone number | Insert length (bp) | Corresponding region on the protein (aa) | Function |
|---|---|---|---|---|
| UBCE2i (also named Ube2i, UBCE2A) | 1, 22 | ∼1100 | 1-158 | protein stabilization, nuclear-cytosolic transport, transcription regulation, etc. |
| Heat shock 70kDa protein 5 (HS70p5) | 13 | ∼900 | 404-650 | Protein folding and transport in ER |
| eIF5A | 35 | ∼1800 | 1-154 | RNA translation |
| RPS20 | 53 | ∼500 | 51-119 | RNA translation |
| LRBP | 17, 29 | ∼1700 | 1-395 | cholesterol synthesis enzyme, RNA binding protein |
3.2. Verification of the interactions between LRBP and prey proteins
Next we performed yeast mating assay and in vitro Co-IP to verify the observed interactions between LRBP and prey proteins. Table 1 shows that UBCE2i and eIF5A had their entire coding sequence fused to the Gal4 AD, while HS70p5 and RP S20 had only their C-terminal regions expressed in the fusion proteins. Using total RNA from rat ovaries as template, the complete coding region of each prey protein (Fig. 1A) was reverse transcribed, amplified and cloned into the pGADT7 vector. The plasmids were then transformed into AH109 yeast strain (MATa type). In addition, LRBP and lamin sequences were cloned into pGBKT7 vector and transformed into Y187 yeast strain (MATα type). AH109 strain mates with Y187 strain and their progenies inherit plasmids from both AH109 and Y187. Transformed AH109 and Y187 were mixed and cultured on minimal medium plates lacking tryptophan, leucine and histidine. Lamin functions as a negative control, because it has no known interacting protein partner, while the LRBP-LRBP interaction serves as a strong positive control. Our results showed that UBCE2i, eIF5a and RP S20, fused to Gal4 AD, interact with LRBP fused to Gal4 BD, and these proteins do not interact with lamin (Fig.1B). However, interaction between HS70p5 and LRBP was not observed in the mating assay (data not shown),
Figure 1. Examination of the interaction between LRBP-Gal4 BD (binding domain) and prey-Gal4 AD (activation domain) by yeast mating assay.
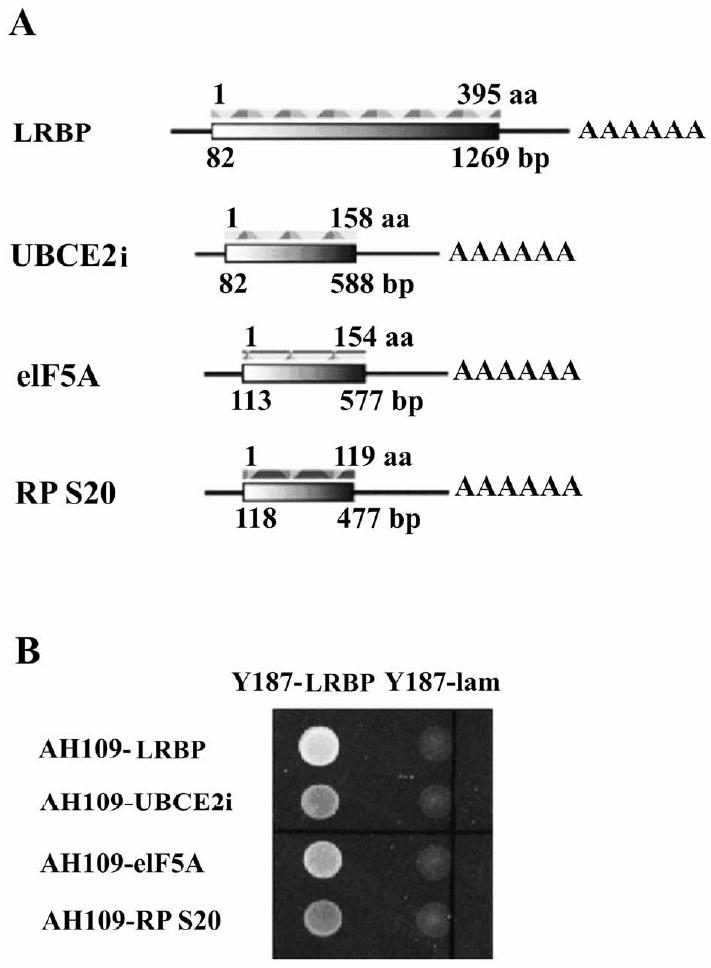
(A) Schematic representation of rat LRBP (fused in frame to the Gal4 BD sequence in plasmid pGBKT7) and prey proteins (fused in frame to the Gal4 AD sequence in plasmid pGADT7).
(B) The complete coding regions of UBCE2i, eIF5A and RPS20 were cloned and fused in frame to the Gal4 AD sequence in plasmid pGADT7 and the plasmids were transformed into AH109 yeast strain. Rat LRBP was fused to the Gal4 BD sequence in plasmid pGBKT7 and the plasmid was transformed into Y187 yeast strain. Protein-protein interactions were evaluated by mating transformed AH109 and Y187 yeasts and examining the growth on SD-WHL plates (lacking tryptophan, leucine and histidine). The presence of both plasmids in mated yeasts was verified by growth on SD-WL plates (not shown). LRBP-LRBP interaction was used as a positive control. Lamin was used as a negative control.
The protein-protein interactions were further tested by in vitro transcription-translation and co-immunoprecipitation. 35S-labeled prey proteins (UBCE2i, RP S20 and SV 40 large T) and bait proteins (LRBP and p53) were synthesized in vitro (Fig. 2A) and these proteins were immediately co-immunoprecipitated using anti-Myc monoclonal antibody (Fig. 2B and 2C). Myc-tagged 35S-labeled LRBP reacted with anti-Myc monoclonal antibody when subjected to immunoprecipitation (lane 5 in Fig. 2B and lane 2 in Fig. 2C). 35S-labeled UBCE2i or RP S20 was mixed with 35S-labeled Myc-tagged LRBP and the mixture was co-immunoprecipated using anti-Myc monoclonal antibody (lane 4 in Fig. 2B, and lane 1 in Fig. 2C). No band was observed when c-Myc immunoprecipitation was done with UBCE2i or RP S20 in the absence of Myc-tagged LRBP (lane 6 in Fig. 2B and lane 3 in Fig. 2C) indicating that neither UBCE2i nor RP S20 cross-reacted with anti-Myc antibodies. The p53-SV40 interaction served as a positive control for the Co-IP assays. Interestingly, it was observed that eIF5A, although interacting strongly with LRBP in yeast, was not co-immunoprecipitated by LRBP in vitro (data not shown). One possible explanation is that the removal of Gal4 activation domain causes a conformational change in eIF5A and prohibits the eIF5A-LRBP interaction. Another possibility is that certain co-factors present in yeast but absent in the in vitro reaction are required for the eIF5A-LRBP interaction. Taken together, our experiments verified that UBCE2i and RP S20 interact with LRBP both in yeast and in vitro.
Figure 2. Examination of the interactions between epitope-tagged LRBP and prey proteins in vitro.
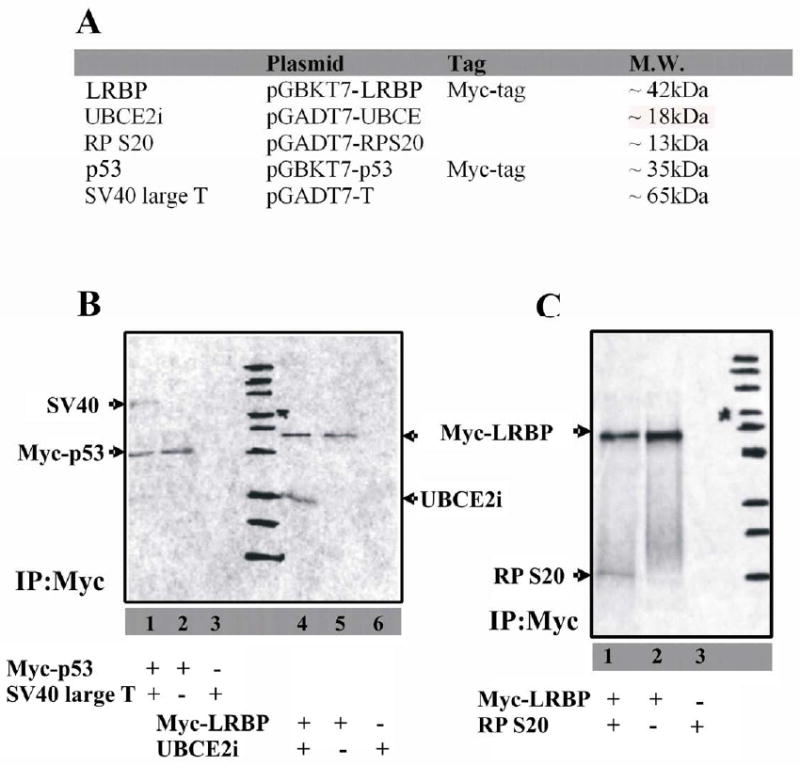
(A) List of proteins synthesized with [35S]-Methionine in vitro. Proteins expressed in pGBKT7 plasmids (LRBP and p53) are Myc tagged.
(B) Proteins were in vitro synthesized in the presence of 35S-methionine. Freshly synthesized LRBP and UBCE2i were mixed together and coimmunoprecipitated with anti-Myc antibody (lane4). The precipitated complex was analyzed by SDS-PAGE and autoradiography. Immunoprecipitation was specific (lane5, LRBP only) and no cross reaction was observed (lane6, UBCE2i only). The p53-SV40 interaction was used as a positive control (lane 1-3). Position of each protein ladder band was marked on the film.
(C) Proteins were in vitro synthesized in the presence of 35S-methionine. LRBP and RP S20 were coimmunoprecipitated with anti-Myc antibody (lane1) and analyzed by SDS-PAGE and autoradiography. Immunoprecipitation was specific (lane2, LRBP only) and no cross reaction was observed (lane3, RPS20 only). Position of each protein ladder band was marked on the film.
3.3. Analysis of the interaction between LRBP and UBCE2i
UBCE2i, the human homologue of yeast UBC9, was originally thought to be a conjugating enzyme for ubiquitination [18], leading to rapid degradation of its targets [19, 20]. Later, the enzyme was shown to be responsible for sumoylation, which does not cause protein degradation, but instead regulates subcellular localization of proteins, transcription factor activity and other cellular processes [21]. Since previous studies have shown that UBCE2i is able to interact with an mRNA binding protein AUF1, suggesting a possible role in regulating mRNA stability [22], we sought to further investigate its role in the LRBP mediated mRNA decay. The first step was to examine whether UBCE2i is associated with LRBP in mammalian cells.
293T cells were transfected to express Myc-tagged LRBP, with either HA-tagged UBCE2i or HA-tagged LRBP (lane 5 and 6 in Fig. 3A). The cell lysates were prepared 48h after transfection and were incubated with protein A agarose beads conjugated with anti-Myc antibody (lane 1-6 in Fig. 3B). Immunoprecipitated samples were examined by western blotting using an anti-HA antibody. The results showed that LRBP forms a complex with itself (lane 5 in Fig. 3B) and with UBCE2i (lane 6 in Fig. 3B). Based on the results of co-immunoprecipitation studies described, we conclude that LRBP interacts with UBCE2i under in vitro conditions.
Figure 3. Examination of the interactions between LRBP and UBCE2i in 293T cells.
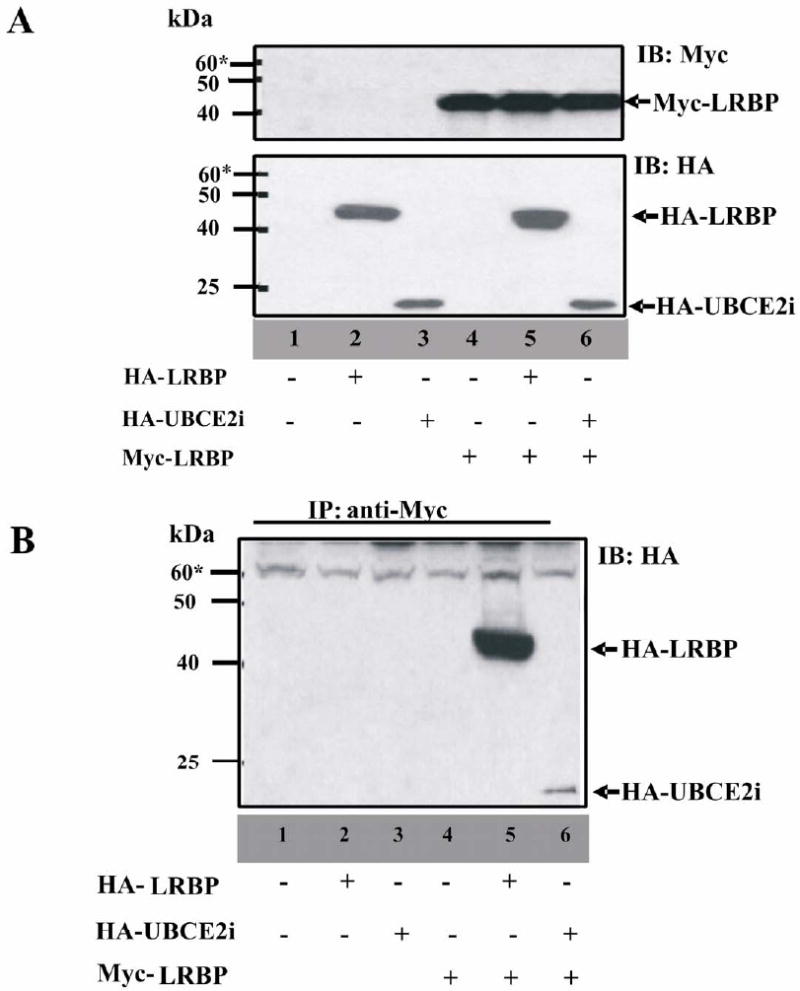
(A) 293T cells were transiently transfected with 1. pCMV-HA and pCMV-Myc; 2. pCMV-HA-LRBP and pCMV-Myc; 3. pCMV-HA-UBCE2i and pCMV-Myc; 4. pCMY-HA and pCMV-Myc-LRBP; 5. pCMV-HA-LRBP and pCMV-Myc-LRBP; 6. pCMV-HA-UBCE2i and pCMV-Myc-LRBP. 20 mg of whole cell lysate from each transfection were subjected to SDS-PAGE and examined with both Myc and HA antibodies.
(B) The lysates were immunoprecipitated with 10 ml of protein A agarose beads (Myc antibody conjugated) and the samples were subjected to SDS-PAGE and immunoblotted with HA antibody.
3.4. LRBP, a novel target of sumoylation
Since UBCE2i predominantly exerts its functions through sumoylation [20], we investigated whether LRBP is capable of SUMO modification. It has been known that most of the SUMO-modified proteins contain a SUMO consensus sequence (SUMO-CS) which is engaged in SUMO attachment. SUMOplot™ Analysis software (Abgent, San Diego, CA) was designed to predict the sites where sumoylation most likely occurs [23].
Using the software, we scrutinized the LRBP sequence and two putative sumoylation sites were identified (Fig. 4A). Both sites are on the C terminal domain of LRBP: α-8 helix and β-13 sheet respectively (Fig. 4D). These elements are highly conserved. The consensus sequence scores (CS) are 0.80 (K256) and 0.73 (K345) for human, and 0.84 (K256) and 0.73 (K345) for rat.
Figure 4. Identification of the sumoylation sites on LRBP.
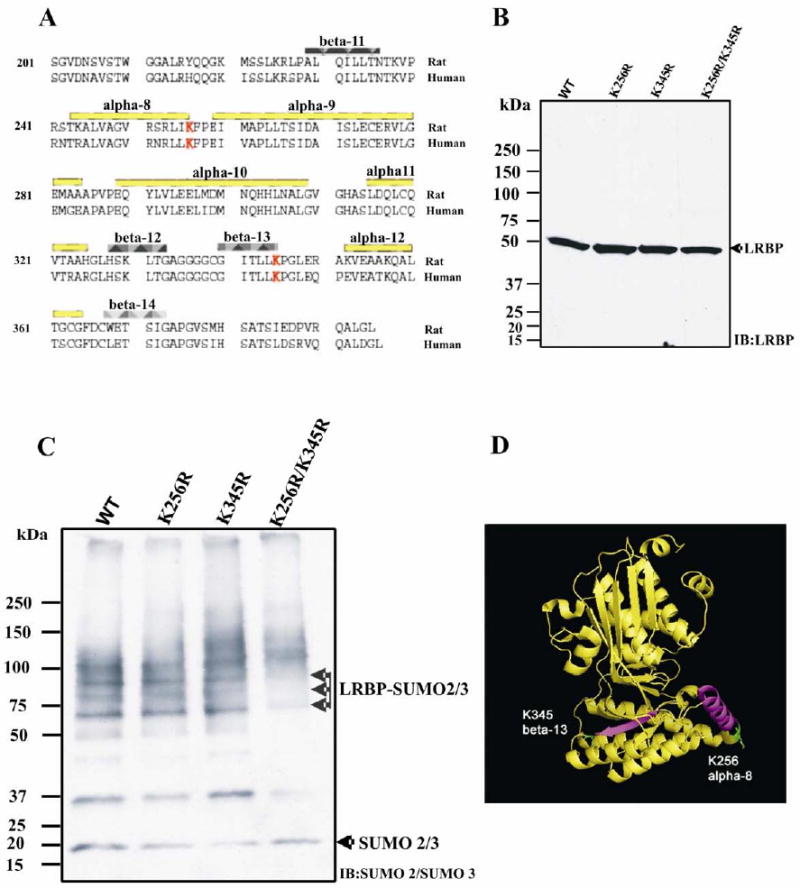
(A) Sequence alignment of C-terminal domains of rat and human LRBP (aa 225-373). The secondary structural elements are labeled in the figure, and the Sumoylation sites, predicted by SUMOplot™ software, are highlighted in red.
(B) 293T cells were transiently transfected to express LRBP wild type (WT), K256 mutant (K256R), K345 mutant (K345R), or double mutant (K256R/K345R). 10 μg of cell S-100 fraction from each transfection were subjected to SDS-PAGE and immunoblotted with anti-LRBP polyclonal antibody.
(C) 50 μg of cell S-100 fraction from each transfection were used for in vitro sumoylation assay, in the presence of Mg2+ and ATP, as described in Materials and Methods. The reaction mixtures were separated by 4-15% gradient gel and immunoblotted with anti-SUMO2/3 antibody.
(D) A ribbon diagram (generated by Pymol 1.0 software) showing N-, C-domains of rat LRBP, based on its crystal structure at the resolution of 2.4A. The Sumoylation sites (in green) are on α-8 and β-13 (in purple) respectively.
To verify that LRBP is sumoylated at K256 and/or K345, we constructed plasmids containing mutant LRBP sequences, by substituting lysine with arginine at position 256 and/or position 345. 293T cells were transfected to express wild type or mutant forms of LRBP. S-100 fractions were prepared and quantitated by BCA assay. Analysis of LRBP expression by western blot using anti-LRBP polyclonal antibody showed that the mutations had no significant influence on the expression of LRBP in 293T cells (Fig. 4B). Aliquots of S-100 fractions were then subjected to in vitro sumoylation assay, as described in the Materials and Methods. The reaction mixtures were examined by western blot using anti-SUMO2/3 antibody, which recognizes the N-terminus of SUMO2/3 molecules. Three major bands representing sumoylated proteins were observed when wild type LRBP was expressed (lane 1 in Fig. 4C). These bands migrated at 64 kDa, 75 kDa and 87 kDa, suggesting poly-sumoylation or mono-sumoylation at multiple sites. As expected, double substitution of K256 and K345 with arginine in LRBP was able to completely abrogate the sumoylation (lane 4 in Fig. 4C). However, LRBP-K256R and LRBP-K345R single mutants showed almost the same sumoylation patterns as that of wild type LRBP (lane 2 and 3 in Fig. 4C). This suggests that a single lysine residue with attachment of poly-SUMO molecules, either at position 256 or at position 345, is sufficient for the sumoylation of LRBP. When sumoylation assays were conducted in the presence of SUMO1, no sumoylated LRBP could be detected (data not shown). These results suggest that the sumoylation of LRBP occurs by the attachment of SUMO2/3, but not SUMO1. On the basis of these results, we conclude that LRBP is a novel target of sumoylation at the consensus sites - K256 and K345 by the attachment of SUMO2/3.
4. Discussion
In eukaryotes, highly regulated mRNAs are controlled not only by the rate of transcription, but also by the rate of degradation [24]. There are a number of examples where the rate of degradation is controlled by the binding of proteins to specific sequences or structures present in the cognate mRNAs [25-27]. Studies from our laboratory have shown that ligand-induced down-regulation of LRH mRNA in the ovary is mediated by its binding to a cytosolic protein LRBP. This protein recognizes an 18 mer sequence in the coding region of LHR mRNA and causes a significant decrease in LHR mRNA half life [6]. This mRNA destabilizing factor was also found to supress LHR mRNA translation, by forming an untranslatable mRNP complex [10, 13]
While the polysome bound actively translated mRNAs are distributed throughout the cytosol, the translationally inactive mRNAs often accumulate in specific cytosolic foci referred to as P bodies [28]. The complete protein composition of P bodies is not yet determined, but a conserved core of proteins functioning in translation repression and mRNA degradation has been identified in P bodies [12]. These proteins include, but are not limited to, the decapping enzyme Dcp1p/Dcp2p, the activators of decapping Dhh1p/RCK/p54, Pat1p, the Lsm1p-7p complex, and the exonuclease, Xm1p. Together, they form the general mRNA repression/decay machinery that holds or destroys the translationally inactive mRNAs [12].
We hypothesize that during hormone-induced downregulation, LHR mRNA transcripts are bound by LRBP, dissociate from the translation machinery, followed by recruitment to the repression/decay machinery. For a better understanding of how LRBP leads to LHR mRNA degradation, we performed a yeast two hybrid screen and examined potential LRBP interacting proteins. Of all the interacting proteins identified, RP S20 and UBCE2i are of particular interest with respect to mRNA degradation. These two proteins might participate in LHR mRNA degradation by translocating the untranslated LHR mRNP complex to the decay pathway. The interaction between LRBP and RP S20, a highly conserved 13 kDa ribosomal protein that belongs to the 40S ribosomal subunit [29], could impair the proper translation initiation of LHR mRNA. It is possible that when RP S20 is recruited by the cap-binding protein complex, the associated LRBP prohibits translation initiation by interfering with the cap-binding protein complex. eIF4E is most likely to be affected by this interference, since it is a well known target for regulation by various 4E-binding proteins [30]. Another possibility is that LRBP-RP S20 interaction might cause an abnormal conformational change in the 40S ribosomal subunit, making it unable to be recruited to the 5′ UTR of LHR mRNA and thus prohibiting translation. In either case, an untranslatable mRNP complex is formed and dissociated from the translation machinery.
The other LRBP interacting protein, ubiquitin conjugating enzyme E2i (UBCE2i) is a human homolog of yeast UBC9. It is highly conserved – its sequence being 100% identical with mouse UBC9 and 56% identical with yeast UBC9. As its name suggests, the protein was originally thought to be a conjugating enzyme for ubiquitination [18], but is now recognized to be responsible for a recently identified type of post-translational modification, sumoylation [31, 32]. It should be noted that UBCE2i is specific for SUMO, small ubiquitin related modifier, and is not related to the ubiquitin-proteasome degradation pathway.
Considering that the majority of UBCE2i interacting proteins are sumoylated, we analyzed the LRBP sequence using SUMOplot™ Analysis software, which predicted two putative sumoylation sites both on the C terminal domain – Lys 256 and Lys 345. By performing in vitro sumoylation assays, we consistently obtained three bands of molecular weight higher than LRBP (lane 1 in Fig. 4C). Furthermore, substitution of K256/K345 with arginines fully eliminated these bands (lane 4 in Fig. 4C). Based on these results, we conclude that LRBP undergoes sumoylation at K256 and/or K345. Appearance of three bands indicates that multiple SUMOs are attached to the same LRBP molecule simultaneously. To determine whether these SUMOs are attached at K256 or K345, we substituted each single lysine with arginine and subjected the LRBP single mutants to sumoylation assays. Surprisingly, both K256R and K345R gave similar sumoylation patterns as the wild type LRBP (lane 1, 2, 3 in Fig. 4C). It is likely that in wild type LRBP, poly-SUMO molecules attach to a single sumoylation site (K256 or K345), and use the other site as a reserve site. It is yet unclear whether poly-sumoylation of one site over the other has any functional consequences. We also observed that the LRBP is sumoylated by SUMO2/3, but not by SUMO1.
Sumoylation was thought of as primarily a nuclear phenomenon, due to the large number of substrates identified in the nucleus. Nonetheless, an expanding number of cytoplasmic proteins have been reported to exist in sumoylated form, including septins, insulin dependent glucose transporter GLUT1/4, IKB, etc. [33]. Irrespective of its localization, either in the nucleus or in the cytoplasm, sumoylation mainly affects target protein function by altering its subcellular localization or by antagonizing other modifications. It has already been reported that many mRNA binding proteins, such as hnRNP C [34], hnRNP M and SART1 [35] undergo sumoylation, which facilitates their nucleocytoplasmic transport. Sumoylation also has been shown to direct cytosolic transport of mRNA binding protein La in the sensory axons [36]. Thus, we propose that the subcellular localization of sumoylated LRBP may favor its access to the mRNA decay machinery in P bodies. It is interesting to note that another mRNA destabilizer AUF1, which binds specifically to AU-rich elements of cytokine or inflammatory mediator mRNAs, has been reported to interact with the component of translation machinery - eIF4G [37] and UBCE2i [22, 38]. It is possible that a common mechanism may exist for AUF1 and LRBP to switch their target mRNAs from translation machinery to mRNA decay machinery. In summary, our study for the first time has shown that LRBP interacts with RP S20, a component of the translation apparatus, and UBCE2i, which makes LRBP a novel member of the sumoylated protein family. These findings support the hypothesis that LRBP forms an untranslatable mRNP complex with LHR mRNA and that the untranslatable mRNP complex becomes associated with the general repression/decay machinery at specific cytoplasmic foci. Based on present in vitro data, we speculate that sumoylation of LRBP might assist in the targeting of LHR mRNA to the decay machinery. Further studies are needed to support the functional role of sumoylation of LRBP in LHR mRNA decay.
Acknowledgments
The authors express their appreciation to Helle Peegel and Dr. Pradeep Kayampilly for critical reading of the manuscript and giving valuable comments.
This work was supported by National Institutes of Health Grant R37 HD 06656
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ascoli M, Fanelli F, Segaloff D. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- 2.Menon KMJ, Munshi UM, Clouser CL, Nair AK. Regulation of luteinizing hormone/human chorionic gonadotropin receptor expression: a perspective. Biol Reprod. 2004;70:861–866. doi: 10.1095/biolreprod.103.024471. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman Y, Peegel H, Sprock M, Zhang Q, Menon KMJ. Evidence that human chorionic gonadotropin/luteinizing hormone receptor down-regulation involves decreased levels of receptor messenger ribonucleic acid. Endocrinology. 1991;128:388–393. doi: 10.1210/endo-128-1-388. [DOI] [PubMed] [Google Scholar]
- 4.LaPolt P, Jia X, Sincich C, Hsueh A. Ligand-induced down-regulation of testicular and ovarian luteinizing hormone (LH) receptors is preceded by tissue-specific inhibition of alternatively processed LH receptor transcripts. Mol Endocrinol. 1991;5:397–403. doi: 10.1210/mend-5-3-397. [DOI] [PubMed] [Google Scholar]
- 5.Lu DL, Peegel H, Mosier SM, Menon KMJ. Loss of lutropin/human choriogonadotropin receptor messenger ribonucleic acid during ligand-induced down-regulation occurs post transcriptionally. Endocrinology. 1993;132:235–240. doi: 10.1210/endo.132.1.8419125. [DOI] [PubMed] [Google Scholar]
- 6.Kash JC, Menon KMJ. Identification of a hormonally regulated luteinizing hormone/human chorionic gonadotropin receptor mRNA binding protein. Increased mRNA binding during receptor down-regulation. J Biol Chem. 1998;273:10658–10664. doi: 10.1074/jbc.273.17.10658. [DOI] [PubMed] [Google Scholar]
- 7.Nair AK, Kash JC, Peegel H, Menon KMJ. Post-transcriptional regulation of luteinizing hormone receptor mRNA in the ovary by a novel mRNA-binding protein. J Biol Chem. 2002;277:21468–21473. doi: 10.1074/jbc.M111653200. [DOI] [PubMed] [Google Scholar]
- 8.Nair AK, Menon KMJ. Isolation and characterization of a novel trans-factor for luteinizing hormone receptor mRNA from ovary. J Biol Chem. 2004;279:14937–14944. doi: 10.1074/jbc.M309484200. [DOI] [PubMed] [Google Scholar]
- 9.Coller J, Parker R. Eukaryotic mRNA decapping. Annu Rev Biochem. 2004;73:861–890. doi: 10.1146/annurev.biochem.73.011303.074032. [DOI] [PubMed] [Google Scholar]
- 10.Nair AK, Menon KMJ. Regulation of luteinizing hormone receptor expression: evidence of translational suppression in vitro by a hormonally regulated mRNA-binding protein and its endogenous association with luteinizing hormone receptor mRNA in the ovary. J Biol Chem. 2005;280:42809–42816. doi: 10.1074/jbc.M503154200. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson A, Peltz SW. Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu Rev Biochem. 1996;65:693–739. doi: 10.1146/annurev.bi.65.070196.003401. [DOI] [PubMed] [Google Scholar]
- 12.Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell. 2007;25:635–646. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Nair AK, Young MA, Menon KM. Regulation of luteinizing hormone receptor mRNA expression by mevalonate kinase-role of the catalytic center in mRNA recognition. FEBS J. 2008;275:3397–3407. doi: 10.1111/j.1742-4658.2008.06490.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Menon KMJ. Regulation of luteinizing hormone/chorionic gonadotropin receptor messenger ribonucleic acid expression in the rat ovary: relationship to cholesterol metabolism. Endocrinology. 2005;146:423–431. doi: 10.1210/en.2004-0805. [DOI] [PubMed] [Google Scholar]
- 15.Kobarg CB, Kobarg J, Crosara-Alberto DP, Theizen TH, Francini KG. MEF2C DNA-binding activity is inhibited through its interaction with the regulatory protein K1-1/57. FEBS Lett. 2005;579:2615–2622. doi: 10.1016/j.febslet.2005.03.078. [DOI] [PubMed] [Google Scholar]
- 16.Liu YV, Baek JH, Zhang H, Diez R, Cole RN, Semenza GL. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol Cell. 2007;25:207–217. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu Z, Wang M, Potter D, Miziorko H, Kim J. The structure of a binary complex between a mammalian mevalonate kinase and ATP: insights into the reaction mechanism and human inherited disease. J Biol Chem. 2002;277:18134–18142. doi: 10.1074/jbc.M200912200. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe TK, Fujiwara T, Kawai A, Shimizu F, Takami S, Hirano H, Okuno S, Ozaki K, Takeda S, Shimada Y, Nagata M, Takaichi A, Takahashi E, Nakamura Y, Shin S. Cloning, expression, and mapping of UBE2I, a novel gene encoding a human homologue of yeast ubiquitin-conjugating enzymes which are critical for regulating the cell cycle. Cytogenet Cell Genet. 1996;72:86–89. doi: 10.1159/000134169. [DOI] [PubMed] [Google Scholar]
- 19.Wang ZY, Qiu QQ, Seufert W, Taguchi T, Testa JR, Whitmore SA, Callen DF, Welsh D, Shenk T, Deuel TF. Molecular cloning of the cDNA and chromosome localization of the gene for human ubiquitin-conjugating enzyme 9. J Biol Chem. 1996;271:24811–24816. doi: 10.1074/jbc.271.40.24811. [DOI] [PubMed] [Google Scholar]
- 20.Kho CJ, Huggins GS, Endege WO, Hsieh CM, Lee ME, Haber E. Degradation of E2A proteins through a ubiquitin-conjugating enzyme, UbcE2A. J Biol Chem. 1997;272:3845–3851. doi: 10.1074/jbc.272.6.3845. [DOI] [PubMed] [Google Scholar]
- 21.Mo YY, Moschos SJ. Targeting Ubc9 for cancer therapy. Expert Opin Ther Targets. 2005;9:1203–1216. doi: 10.1517/14728222.9.6.1203. [DOI] [PubMed] [Google Scholar]
- 22.Moraes KC, Quaresma AJ, Maehnss K, Kobarg J. Identification and characterization of proteins that selectively interact with isoforms of the mRNA binding protein AUF1 (hnRNP D) Biol Chem. 2003;384:25–37. doi: 10.1515/BC.2003.004. [DOI] [PubMed] [Google Scholar]
- 23.Yurchenko V, Xue Z, Sadofsky MJ. SUMO modification of human XRCC4 regulates its localization and function in DNA double-strand break repair. Mol Cell Biol. 2006;26:1786–1794. doi: 10.1128/MCB.26.5.1786-1794.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilusz CJ, Wormington M, Peltz SW. The cap-to-tail guide to mRNA turnover. Nat Rev Mol Cell Biol. 2001;2:237–246. doi: 10.1038/35067025. [DOI] [PubMed] [Google Scholar]
- 25.Tholanikunnel B, Malbon C. A 20-nucleotide (A + U)-rich element of beta2-adrenergic receptor (beta2AR) mRNA mediates binding to beta2AR-binding protein and is obligate for agonist-induced destabilization of receptor mRNA. J Biol Chem. 1997;272:11471–11478. doi: 10.1074/jbc.272.17.11471. [DOI] [PubMed] [Google Scholar]
- 26.Tai N, Schmitz JC, Chen TM, Chu E. Characterization of a cis-acting regulatory element in the protein-coding region of human dihydrofolate reductase mRNA. Biochem J. 2004;378:999–1006. doi: 10.1042/BJ20031396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heaton J, Dlakic W, Dlakic M, Gelehrter T. Identification and cDNA cloning of a novel RNA-binding protein that interacts with the cyclic nucleotide-responsive sequence in the Type-1 plasminogen activator inhibitor mRNA. J Biol Chem. 2001;276:3341–3347. doi: 10.1074/jbc.M006538200. [DOI] [PubMed] [Google Scholar]
- 28.Balagopal V, Parker R. Polysomes, P bodies and stress granules: states and fates of eukaryotic mRNAs. Curr Opin Cell Biol. 2009;21:403–408. doi: 10.1016/j.ceb.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan YL, Wool IG. The primary structure of rat ribosomal protein S20. Biochim Biophys Acta. 1990;1049:93–95. doi: 10.1016/0167-4781(90)90088-j. [DOI] [PubMed] [Google Scholar]
- 30.von der Haar T, Gross JD, Wagner G, McCarthy JE. The mRNA cap-binding protein eIF4E in post-transcriptional gene expression. Nat Struct Mol Biol. 2004;11:503–511. doi: 10.1038/nsmb779. [DOI] [PubMed] [Google Scholar]
- 31.Netzer C, Bohlander SK, Rieger L, Müller S, Kohlhase J. Interaction of the developmental regulator SALL1 with UBE2I and SUMO-1. Biochem Biophys Res Commun. 2002;296:870–6. doi: 10.1016/s0006-291x(02)02003-x. [DOI] [PubMed] [Google Scholar]
- 32.Yan J, Yang XP, Kim YS, Joo JH, Jetten AM. RAP80 interacts with the SUMO-conjugating enzyme UBC9 and is a novel target for sumoylation. Biochem Biophys Res Commun. 2007;362:132–138. doi: 10.1016/j.bbrc.2007.07.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson VG, Rosas-Acosta G. Wrestling with SUMO in a new arena. Sci STKE. 2005;2005:pe32. doi: 10.1126/stke.2902005pe32. [DOI] [PubMed] [Google Scholar]
- 34.Vassileva MT, Matunis MJ. SUMO modification of heterogeneous nuclear ribonucleoproteins. Mol Cell Biol. 2004;24:3623–3632. doi: 10.1128/MCB.24.9.3623-3632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vertegaal AC, Ogg SC, Jaffray E, Rodriguez MS, Hay RT, Andersen JS, Mann M, Lamond AI. A proteomic study of SUMO-2 target proteins. J Biol Chem. 2004;279:33791–33798. doi: 10.1074/jbc.M404201200. [DOI] [PubMed] [Google Scholar]
- 36.van Niekerk EA, Willis DE, Chang JH, Reumann K, Heise T, Twiss JL. Sumoylation in axons triggers retrograde transport of the RNA-binding protein La. Proc Natl Acad Sci U S A. 2007;104:12913–12918. doi: 10.1073/pnas.0611562104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu JY, Bergman N, Sadri N, Schneider RJ. Assembly of AUF1 with eIF4G-poly(A) binding protein complex suggests a translation function in AU-rich mRNA decay. RNA. 2006;12:883–893. doi: 10.1261/rna.2308106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laroia G, Cuesta R, Brewer G, Schneider RJ. Control of mRNA decay by heat shock-ubiquitin-proteasome pathway. Science. 1999;284:499–502. doi: 10.1126/science.284.5413.499. [DOI] [PubMed] [Google Scholar]


