Summary
Background
Medicinal leeches (Hirudo spp.) are simultaneous hermaphrodites. Mating occurs after a stereotyped twisting and oral exploration that result in the alignment of the male and/or female gonopores of one leech with the complementary gonopores of a partner. The neural basis of this behavior is presently unknown and currently impossible to study directly because electrophysiological recording techniques disrupt the behavior.
Results
Here we report that (Arg8)-conopressin G and two other members of the oxytocin/vasopressin family of peptide hormones induce in Hirudo verbana a sequence of behaviors that closely mimic elements of spontaneous reproductive behavior. Through a series of progressively more reduced preparations, we show that one of these behaviors, a stereotyped twisting that is instrumental to aligning gonopores in preparation for copulation, is the product of a central pattern generator that consists of oscillators in ganglia M5 and M6 (the ganglia in the reproductive segments of the leech), and also in ganglion M4, which was not previously known to play a role in reproductive behavior. We find that the behavior is periodic, with a remarkably long cycle period of around five minutes, placing it among the slowest behavioral rhythms (other than diurnal and annual rhythms) yet described.
Conclusion
These results establish the leech as a new model system for studying aspects of the neuronal basis of reproductive behavior.
Highlights.
Oxytocin/vasopressin homologues induce pre-copulatory movements in a leech.
These movements are generated by a central pattern generator.
Segmental ganglia M4, M5, and M6 can each generate fictive behavior in isolation.
Introduction
Among sexually reproducing animals, finding appropriate mates is critical for species survival, and many species produce elaborate courtship behaviors that facilitate this search while reducing the probability of interspecies mating. Although the importance of hormones for reproductive behavior has long been recognized [1], the neuronal basis is less well understood. Good starts have been made using immediate early genes in birds [2], genetics in fruitflies [3] and the nematode Caenorhabditis elegans [4], and even with MRI studies of humans [5], but there is a relative dearth of detailed electrophysiological data, in part because few animals will execute courtship or mating while exposed to the invasive techniques of electrophysiology.
The medicinal leech Hirudo verbana (until recently lumped with H. medicinalis; [6]) is no exception. Although these leeches will breed in the lab, reproductive behavior is easily disrupted: even changing the light level in their environment causes them to abandon their mates. Electrophysiological recording from semi-intact leeches during mating is thus impossible. However, we here report that several members of the vasopressin/oxytocin family of peptide hormones, specifically (Arg8)-conopressin G [7], hirudotocin [8], and annetocin [9], elicit in Hirudo a sequence of behaviors that closely resemble elements of spontaneous courtship and cocoon deposition, but that are almost totally resistant to external disturbances. Using a series of progressively reduced preparations, we demonstrate that one of these behaviors, a highly stereotyped twisting that is a critical part of preparation for copulation, is driven by one or more physiologically accessible central pattern generators.
Results
Natural mating behavior
Like other leeches, Hirudo spp. are non-self-fertilizing simultaneous hermaphrodites. Fertilization is internal, and requires precise alignment of the male gonopore (located on the ventral midline of midbody segment 5) of one leech with the female gonopore (located on the ventral midline of midbody segment 6) of another. Copulation is sometimes mutual, but most often not.1 Since leeches ordinarily keep their ventral surfaces facing down, this postural alignment would not typically arise by chance. Rather, copulation is preceded by one leech scanning the body of another leech with the mouth while twisting around its longitudinal axis. (Commonly, both leeches scan each other in this way.) During this behavior, chemoreceptors on the lips [12, 13] may play a role in determining that the potential partner is, in fact, a leech, and in confirming alignment. After successful copulation, one or both leeches deposit fertilized eggs in cocoons [11] that consist primarily of material secreted by glands in the clitellum [14].
Leeches in our mating colony spent over half of their time in direct physical contact with a partner (Fig. 1A), which is more than 12 times the duration predicted by Monte-Carlo simulation of randomly placed stationary leeches in a tank of the size used for these observations. Only a small fraction of this time was spent actively exploring their partner—“tasting” their partner with their mouth wide open while twisting their bodies (Fig. 1B)—yet this was a critical prerequisite for successful copulation (Fig. 1C), because the ventral-to-ventral orientation required for aligning the gonopores can be achieved only if one of the partners twists 180° (or, less commonly, if both twist 90°) relative to the normal ventral-side-down resting state. Twist angles much greater than minimally required were commonly observed; for instance, the animals in Fig. 1B are twisted by 540° and 180°, even though the same orientation of their genital areas could have been achieved with only one animal twisted 180° and the other not twisted at all.
Figure 1.
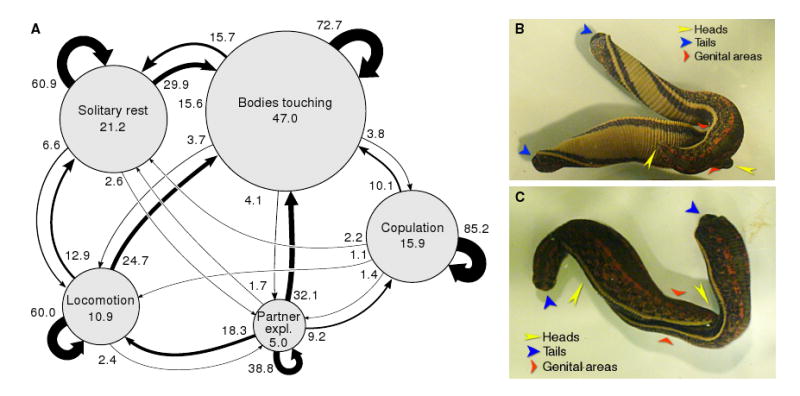
Spontaneous behavior of mating-ready leeches. A. Ethogram of the five main behavioral states of mating-ready leeches. The areas of the circles are proportional to the percentage of time spent in that state (numbers inside circles). The thickness of the arrows represents the likelihood of transitioning from one state to another (numbers by arrows: percent transitions per 5-minute observational timestep; an arrow from a circle back to itself indicates continuation of that behavior into the next 5-minute window). Data from 10 pairs of leeches that were observed for 100 minutes each. B. Typical longitudinal twisting during partner exploration. The heads, tails, and genital areas of both animals are indicated by yellow, blue, and red arrows. C. Copulation. Note the precise alignment of the genital areas and the flattening of the surrounding portion of the body.
This kind of twisting was peculiar to pairs of leeches preparing for copulation. (In particular, it differed in appearance and timing from the writhing response to noxious stimulation, which is much faster, less stereotyped, typically accompanied by whole-body shortening, and never associated with oral exploratory behavior.) Leeches from the main colony not primed for mating by the conducive conditions of the mating colony never twisted their bodies (Fig. 2A, top), even when reproductively mature. Instead, when they were placed in an observation dish, they spent some time exploring their new environment by swimming or crawling and then gradually came to rest. (The fraction of time they spent in physical contact with another leech, about 10%, was consistent with Monte-Carlo simulation results. Such contact indeed appeared to result from random encounters.)
Figure 2.
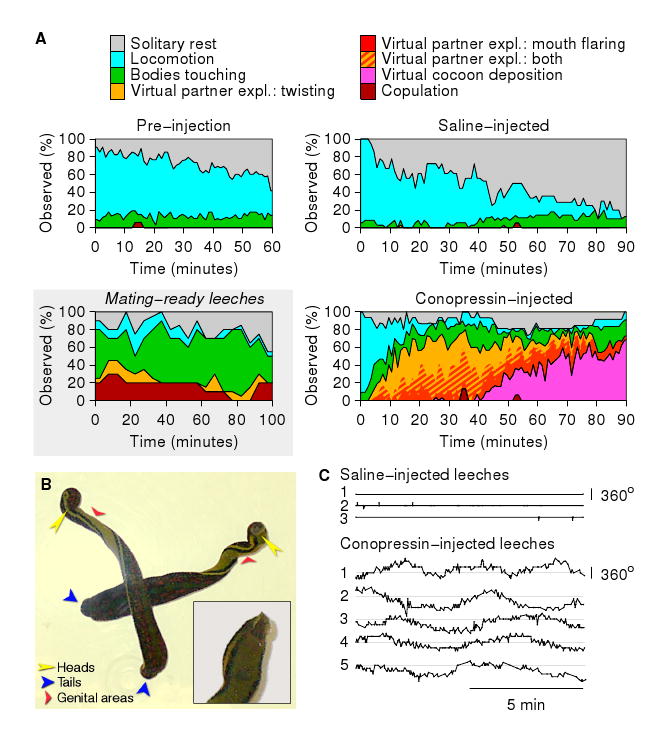
Behavior of conopressin-injected leeches. A. Fraction of time leeches spent performing each of 6 behaviors after injection with conopressin (n=16) or saline (n=18) as a function of time after injection. The “Pre-injection” panel combines data from both experimental and control groups. (Time is measured from the moment the animals were first placed in the observation tank.) Virtual partner exploration (“expl.” in legend) was seen only after injection with conopressin. We here distinguish between twisting (yellow) and mouth flaring (red). These two components of the exploration behavior commonly occurred together (hatched yellow/red). Inset: Data on mating-ready leeches replotted from Fig. 1A, for comparison. (Colors as in other panels, but yellow indicates actual, rather than virtual, partner exploration.) B. Typical longitudinal twisting of conopressin-injected leeches. Despite the fact that the posterior ends of these animals cross, their anterior ends never interacted with one another. Inset: Closeup of the anterior part of a leech with its mouth open and flared. C. Temporal pattern of longitudinal twisting: twisting angle as a function of time for 5 conopressin-injected leeches and 3 saline-injected controls.
Behavior of hormone-injected leeches
We analyzed the videotaped behaviors of 34 leeches in observation dishes containing 2–4 animals each. After one hour of habituation to this environment, 16 leeches were injected with conopressin (5–15 nanomoles/gram body mass), the other 18 with an equal volume of saline. Saline-injected animals temporarily resumed exploring their surroundings upon return to their dishes (Fig. 2A, top right). In sharp contrast, conopressin-injected leeches spent relatively little time moving around the dish (Fig. 2A, bottom right), but instead started displaying virtual partner exploration (flaring of the mouth and twisting of the body) after a median time of 9 minutes post-injection (N=16 animals). This behavior (Fig. 2B) strongly resembled spontaneous partner exploration (Fig. 1B) but was unaffected by external disturbances and did not require the presence of a mate. Other reproductive behaviors, including penile eversion [15] and (more rarely) ejaculation, were occasionally observed during this phase of the response, but occurred too infrequently to analyze in detail.
Later (at a median time of 49 minutes post-injection, N=16), twisting behavior was replaced by a periodic (period: around 0.5 s) thrusting and retraction of the anterior end that resembled the motions executed during cocoon deposition, although no cocoon material was actually secreted. At even longer latencies (data not shown), most leeches resumed twisting and continued to do so for up to 48 hours. Afterward, all leeches returned to their normal behavior. Conopressin injections did not increase mortality.
In separate experiments, we established that hirudotocin and annetocin evoked qualitatively identical behaviors. Examples of twisting induced by all three molecules may be found in Supplemental Movie 1. Quantitatively, conopressin was just as potent as hirudotocin, a peptide actually produced by Hirudo [8], and these two peptides produced stronger responses at a given dose than did annetocin, a peptide produced by earthworms [9]. For instance, the latency to the first characteristic twisting of a leech after injecting peptide (5 nanomoles/gram of body weight) was 4.1 ± 1.7 minutes for conopressin and 4.7 ± 2.7 minutes for hirudotocin (mean ± SD, N = 6 animals for each peptide; not significantly different). At lower concentrations (0.5 or 1.5 nanomoles/gram), latencies were longer, but conopressin and hirudotocin remained equipotent. For annetocin at 5 nanomoles/gram, latency to first behavior was significantly longer: 14.1 ± 7.7 minutes (N = 6, p < 0.01 compared to either hirudotocin or conopressin, t-tests); a similar latency to behavior could be obtained using conopressin at a 10-fold lower concentration: injecting conopressin at 0.5 nanomoles/gram resulted in latencies of 15.0 ± 4.1 minutes (N = 4). Of the three molecules, only conopressin is commercially available, so we performed most the following experiments with that molecule.
To further substantiate the link between the induced twisting and reproductive behavior, we cut some leeches transversely into three pieces and injected each piece with conopressin. The first piece comprised the anterior of the animal through midbody segment M3, the second comprised midbody segments M4 through M7, and the third comprised the posterior of the animal from segment M8 to the tail. Of these pieces, only the middle one twisted following conopressin injection, suggesting that the ganglia that control the male and female reproductive organs (located in segments M5 and M6) are necessary to initiate and maintain the twisting behavior. In addition, in the anterior piece the mouth periodically flared open, suggesting that this part of the behavior is controlled by the anterior brain and/or the most anterior midbody ganglia (Supplemental Movie 2, courtesy of Karrie Murphy).
Video recordings of whole animal behavior allowed us to measure the twist angle between the head and the tail of conopressin-injected animals, which revealed that the twisting was periodic (Fig. 2C), with periods of 5.6 ± 1.4 minutes (N=5 animals). Saline-injected animals never twisted, and indeed the angle between the head and the tail of a saline-injected animal always remained very close to zero.
Measurements of body wall kinematics
Sections of body wall comprising segments M4 through M7 and including a chain of ganglia from M3 to M8 were used to further quantify the kinematics of the twisting behavior. To determine in which segments the behavior was expressed, we severed all but a few selected nerves between the nerve cord and the body wall: those emanating from ganglion M5 were spared in “ci-5” preparations (“ci” for “Connections Intact”), those from ganglion M6 in “ci-6” preparations, or both those from M5 and M6 in “ci-5+6” preparations. The pieces of body wall were pinned down on Sylgard, bathed in conopressin (5 μM in saline), and videorecorded using a microscope-mounted CCD camera. We tracked the position of multiple points on the body wall (Fig. 3A) using an optic flow algorithm.
Figure 3.
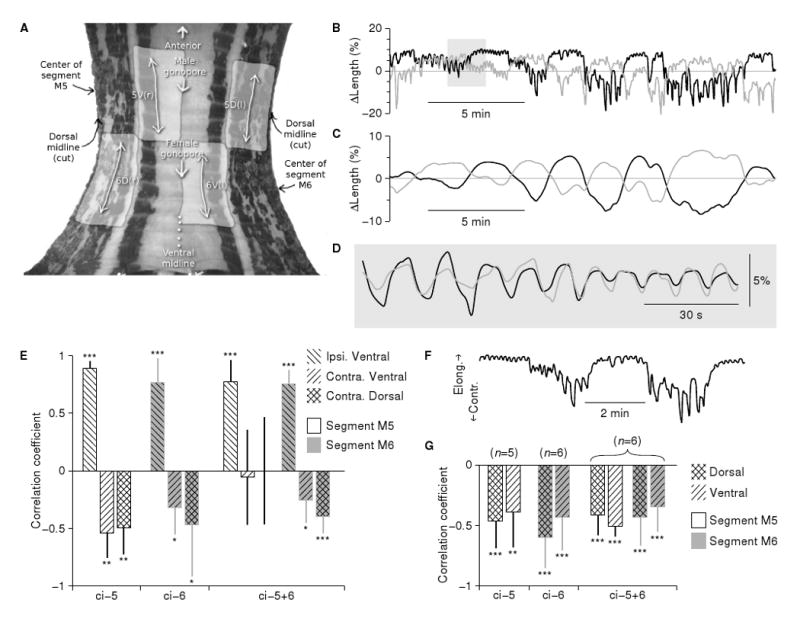
Kinematics of a semi-intact body wall during conopressin-induced twisting. A. Frame from a body wall videorecording with anatomical features indicated. Shaded regions are dorsal (D) and ventral (V) areas on the left (l) and right (r) sides of segments M5 and M6 that were used for motion analysis. (For visual clarity, areas 5D(r), 5V(l), 6D(l) and 6V(r) are not indicated on the image, although they were included in the analysis.) B. Temporal pattern of longitudinal elongation and contraction of the dorsal body wall on the left (gray line: area 6D(l)) and right (black line: area 6D(r)) around M6 in a typical “ci-6” recording. Note the alternation between contractions on the left and on the right. C. Same data after low-pass filtering. D. Expanded view of the shaded region in B after high-pass filtering. E. Temporal correlation between the lengths of various regions (patterned shading) of the body wall within a segment (M5: white bars; M6: gray bars). All are with reference to one (“ipsilateral”) dorsal region in the same sgment. Movements were analyzed on the slow time scale, i.e., the low-pass filtered as in C, and significant differences from zero are indicated: *: p<0.05; **: p<0.01; ***: p<0.001 (two tailed t-tests). F. Excerpt from the data shown in B demonstrating that the amplitude of the fast pulses was greater during the contraction phase of the slow rhythm than during its relaxation. G. Correlation between the amplitude of the fast rhythm (cf. B, D) with the simultaneously measured overall length of the same region (analyzed on the slow time scale, cf. C). Number of preparations (in parentheses) applies to E as well. An analogous presentation of the contractile rhythm in the circumferential dimension is presented in Supplemental Figure S1.
Because they were pinned down, these reduced preparations could not actually twist, but periodic motion was nevertheless well preserved. We focused on the pattern of longitudinal contraction and relaxation in both ventral and dorsal areas surrounding the male and female gonopores. Regardless of which nerves were left intact, we observed periodic motion in most preparations when the data were analyzed on a timescale of minutes (Fig. 3C), with periods of 6.0 ±1.7 minutes (N=17; not significantly different from the periods of twisting measured in intact behaving animals, Fig. 2C). The cycles of contraction and relaxation were phase-locked between different regions of the body wall, and they were left–right antisymmetric, i.e., the dorsal and ventral regions on the left contracted simultaneously when the homologous regions on the right relaxed and vice versa (Fig. 3E). Thus, the timing and symmetry of these contractions are consistent with the twisting seen in the intact animal. The circular muscles relaxed and contracted periodically too, so that circular muscles in a given area were maximally contracted when the longitudinal muscles in the same area were maximally relaxed, and vice versa (Fig. S1).
When viewed on an expanded time scale (Fig. 3D), the contraction/relaxation cycle revealed distinct contractile pulses with a variable period averaging about 10 s. The amplitude of these pulses varied systematically over time and was greatest during the contraction phase of the slow cycle (Fig. 3F–G), suggesting that the slow smooth twisting seen in intact animals is caused by biomechanical integration of many brief muscle contractions with cyclically modulated amplitudes, rather than directly by a smooth and continuous slow increase and decrease of muscle tone.
Fictive behavior in isolated chains of ganglia
The preservation of the slow rhythm of partner exploration in the body wall preparations suggested that the rhythm could be the output of a central pattern generator, because in the semi-intact body wall the sensory feedback is diminished and substantially distorted. To verify that hypothesis and to begin investigating the neural basis of this critical element of mating behavior, we further reduced the preparation by recording motor neuron output from isolated single ganglia or from short chains of ganglia after removing them from the body. To observe the pattern of motor output we recorded extracellularly from axons of the DE-3 motor neurons that innervate dorsal longitudinal muscles in each midbody segment.
Bathing chains of ganglia M4–M7 in conopressin (5 μM in saline) induced periodic bursting in DE-3 motor neurons on a 5–20-s cycle (Fig. 4A). The bursts in the left and right DE-3 neurons of a given ganglion were coordinated in-phase, and the number of spikes per burst varied systematically on a slow cycle of about 5 minutes, in such a way that the bursts on the left were strongest (had the most spikes and the fastest spike rate) when those on the right were weakest and vice versa (Fig. 4B). This pattern of motor neuron activity closely resembled the kinematics observed in body wall preparations (cf. Fig. 3B). Integrating each neuron's firing rate over time (by low-pass filtering, followed by normalization) enhanced this resemblance (Fig. 4C; cf. Fig. 3C). This further revealed that the slow left–right antisymmetric rhythm was strongly coordinated between connected ganglia. Bathing chains of ganglia in hirudotocin produced much the same results (Fig. S2 A–C). In contrast, chains bathed in saline never exhibited a left–right antisymmetric rhythm, nor rapid periodic bursting (Fig. S2 D–F).
Figure 4.
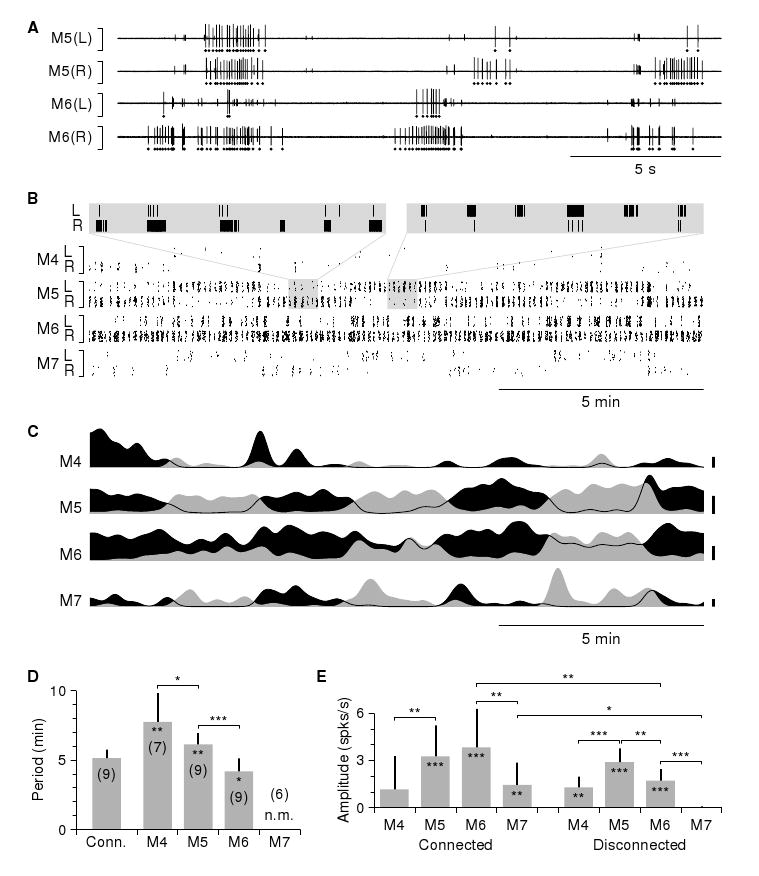
Motor neuron activity in a conopressin-bathed nerve cord. A. Simultaneously recorded sample traces of conopressin-induced motor neuron activity. The largest spikes (marked by dots below the traces) correspond to action potentials in motor neuron DE-3. B. Raster plot of a longer portion of the same recording. Each dot corresponds to an action potential in the left (L) or right (R) motor neuron DE-3 in one of the four ganglia around the genital area. Data shown were recorded simultaneously in a single trial (rather than by post-hoc alignment of multiple independent recordings); vertical placement of dots within each band was randomized to enhance visual clarity. Insets: 10x zooms showing left–right coordination of bursts when the activity was in right-dominant and left-dominant modes. C. Normalized firing rate of the left (gray) and right (black) motor neurons DE-3 in each ganglion; same recording as shown in B. Scale bars: 0.2 spikes/s for M4, M7; 2.0 spikes/s for M5, M6. D. Periods of the conopressin-induced slow left–to–right oscillations in the firing rates of motor neurons DE-3, in chains containing all of M4–M7 (“Conn.”) and in ganglia (“M4”–“M7”) disconnected from the (other) reproductive ganglion. Stars inside bars indicate significant differences in connected chains vs. disconnected chains; stars over brackets indicate significant differences between ganglia. Numbers in parentheses indicate number of preparations. Motor neurons DE-3 in isolated ganglia M7 had near-zero firing rates, so their period could not be measured in a meaningful way (indicated by “n.m.”). E. Amplitude of the oscillations described in D. Stars inside bars indicate significant deviations from zero (see Methods). Analogous data from nerve cords bathed in saline and hirudotocin are presented in Supplemental Figure S2.
To pinpoint which ganglia were responsible for generating the rhythm, we recorded from ganglion M4 either while that ganglion was connected to the reproductive ganglia M5 and M6 or when it was disconnected from both, from ganglion M5 either connected to M6 or disconnected from it, from ganglion M6 either connected to M5 or disconnected from it, and from ganglion M7 either connected to both M5 and M6 or to neither. (The preparations also varied in chain length, but we found that this had no significant impact: for instance, the periods obtained from ganglion M6 in total isolation were no different from those obtained from ganglion M6 when it was left connected to ganglia M7 through M10; data not shown.)
These recordings confirmed that conopressin induced significant periodic variation in firing rate on a time scale of minutes, most strongly in the reproductive ganglia M5 and M6. When these ganglia were left connected to each other, their firing rates oscillated with a period of 5.1 ± 0.6 minutes (N=9; Fig. 4D) as determined by fitting a sine wave to the difference between the firing rates obtained from the left and right nerves. This was not significantly different from the periods measured in intact behaving animals. Ganglia M4 and M7, when connected to M5 and M6, had their firing rates modulated at the same period, but less strongly (Fig. 4E). The modulation was always synchronized between all ganglia in a chain.
Modulation was weaker in disconnected chains, significantly so in M6 and M7; in M7, firing rates dropped to near zero and modulation was absent when it was not connected to M6. When ganglia M5 and M6 were separated from each other, M5 exhibited a longer cycle period than M6. Ganglion M4 isolated from M5–M6 had an even longer period, but—unlike M7 in isolation—it did oscillate (Fig. 4D). All other isolated non-reproductive ganglia failed to respond to conopressin (data not shown).
Spectral analysis
To more precisely characterize the described dual-timescale rhythm of fast bursts modulated on a slow cycle, we performed spectral analysis of both body wall kinematics and fictive behavior of isolated cords. The slow cycle with a period of about 5 minutes (Fig. 5A–B, left column) directly translates to a pronounced spectral peak at a frequency of 0.2/min, both in the body wall (Fig. 5A) and in the nerve cord (Fig. 5B; second column).
Figure 5.
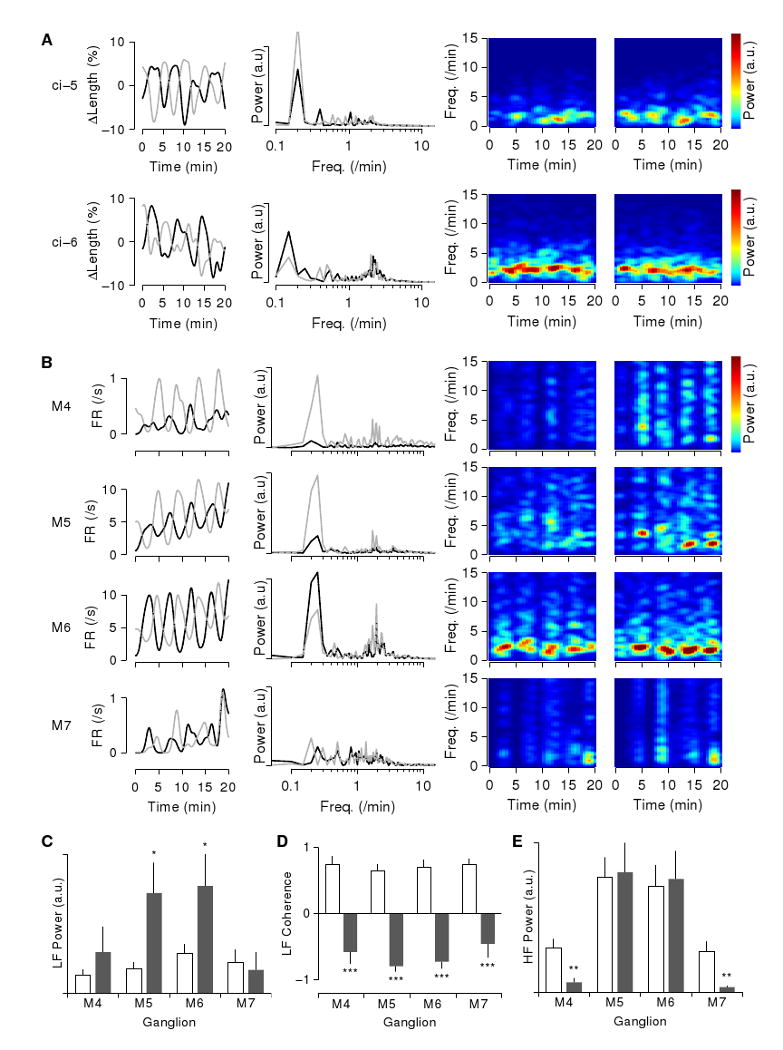
Spectral analysis of body wall movements and fictive behavior. A. Analysis of body wall movements. Left column: Contractions and relaxations in left (black) and right (gray) dorsal areas in the segment with attached ganglion (low-pass filtered). Second column: Power spectrum of the contractions (DC baseline subtracted). Third and fourth columns: spectrogram of contractions in the left and right dorsal areas (high-pass filtered with f0 = 0.5/min). The “ci-5” data (top row) and “ci-6” data (bottom row) are from separate experiments. B. Analysis of fictive behavior of a chain of ganglia M3–M8 bathed in conopressin. Left column: firing rate in left (black) and right (gray) DP1 nerves of ganglia M4 through M7 (top to bottom; low-pass filtered). Second column: Power spectrum of the firing rate (DC baseline subtracted). Third and fourth columns: spectrogram of firing rates in the left and right DP1 nerves (high-pass filtered with f0 = 0.5/min). All rows based on simultaneously recorded data, but note the different vertical scales for each row. C. Spectral power in the frequency band corresponding to the 5-minute rhythm (f = 0.15–0.25/min) in saline (white) and conopressin (gray). Data from 18, 26, 28, 16 nerves from M4, M5, M6, M7 respectively. *: p<0.05, two-tailed t-test. D. Real part of the coherence between left and right nerve activity in this band. Data from 9, 13, 14, 8 nerve pairs from M4, M5, M6, M7 respectively. ***: p<0.001. E. Spectral power in the frequency band corresponding to the bursts (f = 1–10/min). Statistics as for C; **: p<0.01. Analogous data from nerve cords bathed in saline and hirudotocin are presented in Supplemental Figure S3.
The power spectrum further reveals that the rapid bursting does not have a tightly controlled period, even within an individual preparation: the corresponding peak around 2/min is broad and consists of many subpeaks. More insight into the rapid bursts can be gleaned by plotting their power spectra as a function of time (power spectra on the right of Fig. 5A–B). Here we see a clear modulation of the spectral power on a cycle of about 5 minutes. High power density in the 2/min band alternates between left and right side of the segment.
Conopressin greatly increased the spectral power of the motor neuron activity in the reproductive ganglia M5–M6 in the frequency band 0.15–0.25/min (Fig. 5C). Interestingly, conopressin did not significantly affect the spectral power in this band of the activity of ganglia M4 and especially of M7. Nevertheless, the spectral analysis did further substantiate the observation that these ganglia participate in the rhythm: the spectral coherence (Fig. 5D) indicates that baseline activity in this frequency band is strongly in-phase between left and right nerves of a ganglion, while conopressin-induced activity is strongly in anti-phase, both for M5–M6 and for M4, M7. This could be taken as a further indication that the activity in M7 passively follows the activity in M6.
In contrast to the low frequency band, power at higher frequencies (1–10/min, corresponding to the rapid contractions shown in Fig. 3D and the bursts shown in Fig. 4A) did not increase following conopressin application (Fig. 5E). Even in control conditions, the activity pattern of the motor neurons (Fig. S2 D–F) contained considerable spectral power in this band (Fig. S3 B). Both in saline and in conopressin, coherence in this band indicates in-phase coupling between left and right nerves (data not shown). The effect of conopressin appears to be to temporally organize this activity into sharp bursts, as further evidenced by the appearance of high frequency (5–15/min) overtones in the spectrograms in conopressin (Fig. 5B, right) compared to those in saline (Fig. S3 B, right).
We subjected the fictive behavior induced by hirudotocin to analogous analysis (Fig. S3 A), once again confirming that the effect of the two hormones is indistinguishable.
Discussion
Members of the oxytocin/vasopressin family of peptide hormones induced in medicinal leeches a sequence of behaviors mimicking several of those seen during courtship and cocoon deposition. Four separate lines of evidence suggest that the observed behaviors truly are part of the reproductive repertoire (and are not just superficially similar in appearance). First, immunocytochemical staining indicates the presence of a vasopressin homolog, presumably hirudotocin, in neurons of the central nervous system of Hirudo spp. [16, 8]. Second, the peptides did not induce a single, simple behavior, but a whole sequence of behaviors—first twisting and mouth flaring as in mate exploration, then periodic thrusting and retraction of the anterior body as in cocoon deposition—and the elicited behaviors disappeared completely after 24–48 hours or less, depending on dose. Third, the behaviors were specifically generated by neurons in midbody ganglia M5 and M6, the ganglia that control the male and female genital structures. The final line of evidence stems from experiments carried out not on leeches, but on another worm: the marine polychaete Nereis succinea (unpublished observations with Jeffrey Ram). Injecting males of that species with conopressin robustly caused them to swim in tight circles and to spawn, behaviors normally induced only by exposure to a specific female pheromone [17]. As in the leech, these behaviors were much longer-lasting and more resistant to external disturbances than is spontaneous reproductive behavior. The mating behaviors of these two annelid worms do not resemble each other at all, yet in both species conopressin elicited behaviors that mimic integral parts of their reproductive repertoire. We conclude that conopressin and the two other hormones are, indeed, eliciting reproductive behavior.
Our electrophysiological data indicate that the twisting behavior seen in preparation for copulation is generated by a central pattern generator located primarily in ganglia M5 and M6, with each ganglion containing its own oscillatory circuit. Although the natural frequencies of the oscillators were found to be somewhat different (Fig. 4D), the oscillators were entrained to one another in the intact animal and then operated at a frequency between the natural frequencies of ganglia M5 and M6.
The presence of oscillations in recordings from isolated ganglia M4 indicates the presence of an autonomous oscillator in that ganglion, though it appears to be weaker than those in either M5 or M6 (in the sense that it caused the least modulation in firing rate; Fig. 4E). Notably, however, it can be activated and sustained without the help of M5 or M6, implying that receptors for conopressin are present in M4 as in M5 and M6. This is noteworthy, since only M5 and M6 were previously known to have sex-related neurons [18]. These stain positively for FMRFamide and arise late in embryonic development in response to signals from the male genitalia [19]. Interestingly, many of these neurons also contain an oxytocin-like peptide of unknown sequence [20]. The discovery of a self-sufficient oscillator in M4 indicates that this ganglion may be more directly involved in reproduction than previously appreciated. In contrast to ganglion M4, ganglion M7 (and all other ganglia) did not produce oscillatory motor output except when driven by ganglion M5 or M6.
The strong periodicity of partner exploration is remarkable in itself, because considered a priori such a behavior might be a random walk and still provide the necessary information. Furthermore, the length of the cycle (around 5 minutes) makes it one of the slowest periodic behaviors reported (other than diurnal and annual rhythms), although not quite as slow as Hirudo's heartbeat rhythm: The left and right heat tube beat every 10–30 s, but take turns contracting in a peristaltic or synchronous manner after every 20–50 heartbeats, for a full cycle period of approx. 6–20 minutes (ref. 21 and references therein). Interestingly, in the case of the heartbeat rhythm, just as for the partner exploration rhythm described here, the slow periodic behavior manifests itself in the modulation of a much faster burst rhythm, viz. the individual heart beats, and in both systems the source of the slow period remains to be discovered. In the heartbeat circuit, the heart interneurons (HN) in ganglion M5 are responsible for imposing the slow modulation on the burst rhythm, but these neurons do not themselves generate the slow oscillation; instead they rely on an external periodic hyperpolarizing current of known properties, but unknown source [22, 23].
The observation (Figures 3D and 4B) that a sequence of rapid muscle contractions underlies the long cycle of twisting raises the question why an animal would generate a very slow motion through amplitude modulation of fast pulses, rather than through a slow and continuous change in muscle tension. In the case of the heart beat rhythm, the answer is clear: contractions at a time scale of multiple minutes would not pump blood around very effectively. In the case of the partner-exploration rhythm, one possibility is that the body wall muscles can move only in discontinuous twitches similar to saccades in visual control. We do not favor this idea, because the motion in the intact animal appeared considerably smoother than in the body wall preparation. An alternative possibility is that driving the muscles in short bursts may be energetically favorable to maintaining tension continuously. Finally, it could be that using bursts is dynamically more stable: precise control over very low firing rates may be difficult to maintain.
Members of the vasopressin/oxytocin family of peptide hormones are associated with reproduction and parental behavior (as well as osmoregulation) throughout the animal kingdom, e.g., oxytocin and vasopressin in mammals [24, 25], and vasotocin in amphibians [26]. In annelids, too, hormones in this family evoke responses related to reproduction (and water balance) [27, 28], and several of them have been reported in leeches [16, 29, 8]. The association of (Arg8)-conopressin G, hirudotocin, and annetocin with reproductive behavior in the leech fits well in this general pattern: a different conopressin produces related effects on the pond snail Lymnaea stagnalis [30], and annetocin induces egg-laying behavior in earthworms [31].
Leeches are not the only animals in which neural control of complex mating behaviors takes place outside of the brain: similarly sequenced mating behavior that requires neither sensory feedback nor top-down control from the brain has been described in crickets [32]. To the best of our knowledge, however, this is the first report of reproductive behaviors elicited in isolated nerve cords by a single chemical trigger. We believe that, thanks to the accessibility and relative simplicity of its nervous system, Hirudo can now serve as a powerful new model system in which to study the cellular physiology at the interface between the phylogenetically widespread vasopressin family of hormones and the neuronal control of reproductive behavior.
Experimental Procedures
Leeches (Hirudo verbana, purchased from Leeches USA (Westbury, NY) or Carolina Biological Supply (Burlington, NC)), were housed in groups of about 50 animals in 5-gallon tanks of artificial pond water (Instant Ocean salts (Aquarium Systems, Mentor, OH) diluted with deionized water to 1/1000 of ocean strength). Leeches were kept in a cool room (15 °C; 12h/12h light/dark cycle) and fed with cow blood (Animal Technologies, Tyler, TX) at intervals of 3–6 months. Individuals that reached a weight of at least 12 grams and were judged to be ready to mate based on swelling of the clitellum were transferred to a breeding colony (29 °C; 14h/10h light/dark cycle), where they were kept in artificial pond water in groups of 10 leeches per 5-gallon tank and allowed two weeks to acclimate. Then, most of the water in the tank was removed, and the bottom of the tank was covered with moist sphagnum moss.
Behavioral observations of spontaneously mating leeches
Leeches were observed in their home tanks in the breeding colony. Detailed observations were made of two animals in each of two tanks on five consecutive days. (In each tank and on each day, we chose two leeches that were located near one another at the start of the observational period; no other criteria were applied.) Observations lasted 100 minutes on each day. During each minute, leeches were scored for any of the following behaviors: locomotion (swimming or crawling individually), bodies touching (maintaining physical contact with the other leech), partner exploration (scanning the other leech's body with open mouth), copulation (inferred from prolonged anterior ventral juxtaposition without motion, in combination with flattening of the bodies), and solitary rest (immobility while located away from the other leech).
Behavioral observations of hormone-injected leeches
For each experiment, four adult leeches of breeding size were taken from the main colony (rather than from the breeding colony), held at 20 °C for 2–4 days, and weighed just before the experiment. They were then placed together in a round plastic dish (diameter: 17 cm), and their behavior was videotaped for an hour. Following this, two of the animals received injections of either (Arg8)-conopressin G (Bachem, Torrance, CA), hirudotocin, or annetocin (the latter both synthesized to 97% purity by Biomer Technology, Pleasanton, CA), dissolved in leech saline (in mM: NaCl, 115; KCl, 4; CaCl2, 1.8; MgCl2, 2; HEPES buffer, 10). The volume injected was calculated for each leech to yield a final dosage of 0.5–15 nanomoles/gram of body mass in about 100 μL of solution. Varying the dose over this 30x range did not qualitatively alter the response. The other two animals received injections of the same volume of leech saline. Solutions were injected into the dorsal body wall 0.5–1 cm anterior to the rear sucker.
After the injections, the four leeches were returned to the observation dish, and their behavior was recorded on VHS tape for a further 90 minutes using a video camera (Hitachi HV-C20). The recordings were analyzed offline to score behaviors in each 5-minute window. Locomotion, bodies touching, copulation, and solitary rest were defined as before. In addition, we scored virtual partner exploration (mouth flaring and/or twisting as if exploring another leech, but without actual contact) and virtual cocoon deposition (periodic thrusting and retracting of the anterior end of the body). When more than one behavior was observed in a 5-minute window, each behavior was assigned a fractional score to preserve normalization.
In separate experiments, video recordings of leeches following injection of conopressin (20–50 nanomoles/gram of body mass) were analyzed frame-by-frame to determine the amount of twist, expressed in degrees of rotation between the head and the tail of the animal during virtual partner exploration. Individual animals were never used for more than one experiment.
Video analysis of body wall movements
Individual leeches weighing about 3 grams were taken from the main colony and placed in ice-cold saline. After pinning down the front and rear suckers, an incision was made along the dorsal midline and the animal was spread open. The ventral nerve cord was partially exposed and transected anterior to midbody ganglion M3 and posterior to ganglion M8. Peripheral nerves from ganglia M3 through M8 were removed, except as follows: Connections from ganglion M5 to the male reproductive ducts and to the periphery were spared in “ci-5” preparations; connections from ganglion M6 to the female gonads and reproductive ducts and to the periphery were spared in “ci-6” preparations; and connections from both M5 and M6 were spared in “ci-5+6” preparations. (The ancillary connections between M5 and M6 were cut except in “ci-5+6” preparations.) The dorsoventral flattener muscles were removed as much as was practical and the body wall was transected in segment M4 and in segment M7. The resulting patch of body wall was pinned along the anterior and posterior edges, skinside-up, onto Sylgard-184 (Dow Corning, Midland, MI) in a 35-mm Petri dish and stretched to halfway between its shortest and longest natural lengths (as judged by observing crawling). Conopressin was bath-applied at a concentration of 5 μM in saline, which reliably evoked behavior. Body wall movements were recorded for periods of 20 minutes using a digital CCD camera. Using custom-made software, fiducial marks were manually placed on several locations on each annulus in every 600th frame of the videos (corresponding to one set of markings per minute of video). Automated optic flow analysis [33] was then used to track the precise motion of each of these markers between annotated frames.
Electrophysiology
Leeches were dissected as described above, except that sections of the ventral nerve cord were entirely removed from the body and pinned down on Sylgard. Suction electrodes were attached bilaterally to the proximal region of the dorsal-posterior nerves (DP1) emanating from ganglia M4 through M7. Each DP nerve includes the axon of a single DE-3 motor neuron; DE-3 neurons excite dorsal longitudinal muscles [34, 35]. Sections of nerve cord were then transected at various points as indicated in Results, and conopressin was bath-applied as described above. Electrophysiological recordings were made using a differential AC amplifier (model 1700, A-M Systems, Sequim WA) and a DigiData 1320A data acquisition system (Axon Instruments, now Molecular Devices, Sunnyvale CA). Signals were bandpass filtered, 300 Hz–5 kHz, prior to digitization at 10 kHz. Custom software was used to extract from the recordings the times of the largest-amplitude spikes, which are the action potentials of motor neuron DE-3.
Analysis
Data were analyzed using custom Matlab (The Mathworks, Natick MA) scripts. All results are reported as mean ± sample standard deviation (SSD). Statistical significance is based on unpaired t-tests, except as noted.
Quantification of oscillations
To quantify the amplitude and period of slow oscillations in the firing pattern of motor neurons in isolated cords, we first low-pass filtered the firing rates in each neuron using a Gaussian window with a full-width-at-half-maximum of 1 minute, and then fitted a sine wave to the difference in firing rates between the left and right motor neuron DE-3 in each segment in overlapping 15-minute windows. (For the leftmost bar in Fig. 4D the firing rates in segments 4–7 were averaged together first.) Because the onset of the effect of conopressin was somewhat variable, we chose not to measure amplitude and period at a fixed time after application of the hormone; rather, for each recording we determined the time at which the oscillation had maximal amplitude and used the period from the sine-wave fit at that time. To determine whether oscillation amplitudes were significantly different from zero, we also fitted sine waves to the pre-conopressin baseline recordings (which did not exhibit oscillations) and used paired t-tests to compare the amplitude of post-conopressin oscillations with these. The resulting p-values are represented by the stars inside the bars of Fig. 4E.
Monte-Carlo simulation
To test whether mating-ready leeches actively sought each other's proximity, we simulated placing two leeches (1–1.5 cm wide by 5.5–7.5 cm long) in random locations in a two-dimensional arena (20 by 40 cm). We ran 1000 trials. In 3.8% of trials the animals touched. These results imply that with three (four) leeches in the arena, a given leech would be in contact with some other leech in 7.6% (11.4%) of trials.
Spectral analysis
Power spectra in Fig. 5A–B were calculated directly based on the Fourier transform of the firing rate or contraction as a function of time. For the purpose of plotting, spectra were convolved with a Gaussian of width 0.025 octaves in log-space. Spectrograms were estimated using a multi-taper method [36] at a resolution of Δf = 0.5/min, and used Hanning windows with a half-width of 2 minutes. Statistical analysis of spectral power and coherence was based on multi-taper estimates.
Supplementary Material
Acknowledgments
We thank Dr Baldomero Olivera for his role in earlier stages of this work, Dr Karrie Murphy and Dr June Chang Han for preliminary work (while they were undergraduates) on characterizing how conopressin affects behavior, Charlotte R. Lee for her careful studies on latency measurement, Dr Jeffrey Ram for sharing his specimens and knowledge of Nereis with us, and Joyce Murphy for maintaining our breeding colony and observing reproduction. This work was supported by grants MH43396 from NIH and IOS0825741 from NSF (both to WBK), by a fellowship from the Broad Foundations (to DAW), by Microsoft Research, and by a private gift from Richard Geckler. DAW holds a Career Award at the Scientic Interface from the Burroughs Wellcome Fund.
Footnotes
To our knowledge, mating behavior in Hirudo has not been specifically described in modern times (but see [10] for a detailed description of mating behavior in a distantly related marine leech and [11] for a textbook reference). This overview is therefore partly based on observations in our own breeding colony.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Insel TR, Young L, Wang Z. Central oxytocin and reproductive behaviours. Rev Reprod. 1997;2:28–37. doi: 10.1530/ror.0.0020028. [DOI] [PubMed] [Google Scholar]
- 2.Ball GF, Balthazar J. Ethological concepts revisited: immediate early gene induction in response to sexual stimuli in birds. Brain Behav Evol. 2001;57:252–270. doi: 10.1159/000047244. [DOI] [PubMed] [Google Scholar]
- 3.Greenspan RJ, Ferveur JF. Courtship in Drosophila. Annu Rev Genet. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. [DOI] [PubMed] [Google Scholar]
- 4.Barr MM, Garcia LR. Male mating behavior. WormBook. 2006:1–11. doi: 10.1895/wormbook.1.78.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher H, Aron A, Brown LL. Romantic love: an fMRI study of a neural mechanism for mate choice. J Comp Neurol. 2005;493:58–62. doi: 10.1002/cne.20772. [DOI] [PubMed] [Google Scholar]
- 6.Siddall ME, Trontelj P, Utevsky SY, Nkamany M, Macdonald KS. Diverse molecular data demonstrate that commercially available medicinal leeches are not Hirudo medicinalis. Proc Biol Sci. 2007;274:1481–1487. doi: 10.1098/rspb.2007.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz LJ, De Santos V, Zafaralla GC, Ramilo CA, Zeikus R, Gray WR, Olivera BM. Invertebrate vasopressin/oxytocin homologs. Characterization of peptides from Conus geographus and Conus straitus venoms. J Biol Chem. 1987;262:15821–15824. [PubMed] [Google Scholar]
- 8.Salzet M. Molecular aspect of annelid neuroendocrine system. In: Satake H, editor. Invertebrate neuropeptides and hormones: Basic knowledge and recent advances. Transworld Research Network; Trivandrum, India: 2006. [Google Scholar]
- 9.Oumi T, Ukena K, Matsushima O, Ikeda T, Fujita T, Minakata H, Nomoto K. Annetocin: an oxytocin-related peptide isolated from the earthworm, Eisenia foetida. Biochem Biophys Res Commun. 1994;198:393–399. doi: 10.1006/bbrc.1994.1055. [DOI] [PubMed] [Google Scholar]
- 10.Sawyer RT, Hammond DL. Observations on the marine leech calliobdella carolinensis (hirudinea: Piscicolidae), epizootic on the atlantic menhaden. Biol Bull. 1973;145:373–388. doi: 10.2307/1540047. [DOI] [PubMed] [Google Scholar]
- 11.Mann KH. Leeches (Hirudinea): Their structure, physiology, ecology, and embryology. Pergamon Press; New York, USA: 1962. [Google Scholar]
- 12.Elliott EJ. Chemosensory stimuli in feeding behavior of the leech Hirudo medicinalis. J Comp Physiol A. 1986;159:391–401. doi: 10.1007/BF00603984. [DOI] [PubMed] [Google Scholar]
- 13.Elliott EJ. Morphology of chemosensory organs required for feeding in the leech Hirudo medicinalis. J Morphol. 1987;192:181–187. doi: 10.1002/jmor.1051920208. [DOI] [PubMed] [Google Scholar]
- 14.Sayers CW, Coleman J, Shain DH. Cell dynamics during cocoon secretion in the aquatic leech, Theromyzon tessulatum (annelida: Clitellata: Glossiphoniidae) Tissue Cell. 2009;41:35–42. doi: 10.1016/j.tice.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Zipser B. Identifiable neurons controlling penile eversion in the leech. J Neurophysiol. 1979;42:455–464. doi: 10.1152/jn.1979.42.2.455. [DOI] [PubMed] [Google Scholar]
- 16.Nassirpour R, Norris BJ. A vasopressin-like peptide in the CNS of the medicinal leech, Hirudo medicinalis. Soc Neurosci Meeting Planner. 2001 prog no 518.8. [Google Scholar]
- 17.Ram JL, Fei X, Danaher SM, Lu S, Breithaupt T, Hardege JD. Finding females: pheromone-guided reproductive tracking behavior by male Nereis succinea in the marine environment. J Exp Bio. 2008;211:757–765. doi: 10.1242/jeb.012773. [DOI] [PubMed] [Google Scholar]
- 18.Macagno ER. Number and distribution of neurons in leech segmental ganglia. J Comp Neurol. 1980;190:283–302. doi: 10.1002/cne.901900206. [DOI] [PubMed] [Google Scholar]
- 19.Baptista CA, Gershon TR, Macagno ER. Peripheral organs control central neurogenesis in the leech. Nature. 1990;346:855–858. doi: 10.1038/346855a0. [DOI] [PubMed] [Google Scholar]
- 20.Salzet M, Wattez C, Verger-Bocquet M, Beauvillain JC, Malecha J. Oxytocin-like peptide: a novel epitope colocalized with the fmrfamide-like peptide in the supernumerary neurons of the sex segmental ganglia of leeches–morphological and biochemical characterization; putative anti-diuretic function. Brain Res. 1993;601:173–184. doi: 10.1016/0006-8993(93)91708-z. [DOI] [PubMed] [Google Scholar]
- 21.Calabrese RL, Peterson E. Neural control of heartbeat in the leech, Hirudo medicinalis. Symp Soc Exp Biol. 1983;37:195–221. [PubMed] [Google Scholar]
- 22.Gramoll S, Schmidt J, Calabrese RL. Switching in the activity state of an interneuron that controls coordination of the hearts in the medicinal leech (Hirudo medicinalis) J Exp Biol. 1994;186:157–171. doi: 10.1242/jeb.186.1.157. [DOI] [PubMed] [Google Scholar]
- 23.Lu J, Gramoll S, Schmidt J, Calabrese RL. Motor pattern switching in the heartbeat pattern generator of the medicinal leech: membrane properties and lack of synaptic interaction in switch interneurons. J Comp Physiol A. 1999;184:311–324. doi: 10.1007/s003590050329. [DOI] [PubMed] [Google Scholar]
- 24.Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 25.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 26.Moore FL, Boyd SK, Kelley DB. Historical perspective: Hormonal regulation of behaviors in amphibians. Horm Behav. 2005;48:373–383. doi: 10.1016/j.yhbeh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Fujino Y, Nagahama T, Oumi T, Ukena K, Morishita F, Furukawa Y, Matsushima O, Ando M, Takahama H, Satake H, Minakata H, Nomoto K. Possible functions of oxytocin/vasopressin-superfamily peptides in annelids with special reference to reproduction and osmoregulation. J Exp Zool. 1999;284:401–406. doi: 10.1002/(sici)1097-010x(19990901)284:4<401::aid-jez6>3.3.co;2-l. [DOI] [PubMed] [Google Scholar]
- 28.Satake H, Takuwa K, Minakata H, Matsushima O. Evidence for conservation of the vasopressin/oxytocin superfamily in annelida. J Biol Chem. 1999;274:5605–5611. doi: 10.1074/jbc.274.9.5605. [DOI] [PubMed] [Google Scholar]
- 29.Levoye A, Mouillac B, Riviere G, Vieau D, Salzet M, Breton C. Cloning, expression and pharmacological characterization of a vasopressin-related receptor in an annelid, the leech Theromyzon tessulatum. J Endocrinol. 2005;184:277–289. doi: 10.1677/joe.1.05833. [DOI] [PubMed] [Google Scholar]
- 30.Van Kesteren RE, Smit AB, De Lange RP, Kits KS, Van Golen FA, Van Der Schors RC, De With ND, Burke JF, Geraerts WP. Structural and functional evolution of the vasopressin/oxytocin superfamily: vasopressin-related conopressin is the only member present in Lymnaea and is involved in the control of sexual behavior. J Neurosci. 1995;15:5989–5998. doi: 10.1523/JNEUROSCI.15-09-05989.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oumi T, Ukena K, Matsushima O, Ikeda T, Fujita T, Minakata H, Nomoto K. Annetocin, an annelid oxytocin-related peptide, induces egg-laying behavior in the earthworm, Eisenia foetida. J Exp Zool. 1996;276:151–156. doi: 10.1002/(SICI)1097-010X(19961001)276:2<151::AID-JEZ8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 32.Sakai M, Kumashiro M. Copulation in the cricket is performed by chain reaction. Zoolog Sci. 2004;21:705–718. doi: 10.2108/zsj.21.705. [DOI] [PubMed] [Google Scholar]
- 33.Ye M, Haralick RM. Optical flow from a least-trimmed squared based adaptive approach. Proc 15th Int Conf Pattern Recognition. 2000;3:1052–1055. [Google Scholar]
- 34.Ort CA, Kristan WB, Stent GS. Neuronal control of swimming in medicinal leech. 2. Identification and connections of motor neurons. J Comp Physiol. 1974;94:121–154. [Google Scholar]
- 35.Stent GS, Kristan WB, Jr, Friesen WO, Ort CA, Poon M, Calabrese RL. Neuronal generation of the leech swimming movement. Science. 1978;200:1348–1357. doi: 10.1126/science.663615. [DOI] [PubMed] [Google Scholar]
- 36.Taylor AL, Cottrell GW, Kleinfeld D, Kristan WB. Imaging reveals synaptic targets of a swim-terminating neuron in the leech cns. J Neurosci. 2003;23:11402–11410. doi: 10.1523/JNEUROSCI.23-36-11402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


