Abstract
Numerous steroids are now believed to possess rapid membrane effects independent of the classical gene activation pathways and are potent modulators of membrane proteins, including voltage-and ligand-operated channels. The effects of steroids on the functional state of the intercellular channels clustered in gap junctions were compared by estimation of either the permeability for a fluorescent dye or the electrical conductance in cardiac myocytes of newborn rat. At 25 μM, the esters of 17β-estradiol, testosterone and two other androgen hormones rapidly abolished cell-to-cell communication, whereas none of the longer chain steroids, belonging to pregnane (17α-hydroxypregnenolone, hydrocortisone), sterol (cholesterol, 25-hydroxycholesterol), bile acid (cholic and lithocholic acids) and vitamin (D3) families, lowered the junctional permeability. Altogether, no correlation with the presence or position of double bonds nor with the trans- or cis-fusion of the A and B rings was recognized. Esterification was a prerequisite for the activity of extracellularly applied steroids but the number, nature and position of ester chain(s) had no influence. 17β-estradiol or testosterone effects were not prevented when cells were prein-cubated with blockers of the estrogen or androgen nuclear receptors (tamoxifen and cyproterone acetate, respectively). This, together with the rapid time course of the steroid effect (complete within a few minutes), in a rather high active concentration range, suggests a nongenomic mechanism of action. The reversible uncoupling effect of steroids appears to be independent of the shape of the molecules and more probably related to their size and lipo-solubility, which condition their insertion into the lipid bilayer and their subsequent disturbing effects.
Keywords: Gap junctions, Intercellular communication, Steroids
In the common theory of steroid action, steroids bind to intracellular receptors and modulate nuclear gene transcription and thus protein synthesis. These genomic steroid effects, characterized by their delayed onset of action and their dependence on transcription and protein synthesis, have been known for several decades. In contrast, very rapid actions of steroids, which are considered to be of nongenomic origin, have now been recognized more widely and characterized in detail. Specific rapid effects of steroids and related hormones such as vitamin D3 on cellular functions involve a conventional second messenger cascade that in most cases includes phospholipase C, phosphoinositide turnover, intracellular pH and intracellular calcium concentration, and modifications in the activities of protein kinases and phosphatases. Several steroids may trigger nongenomic stimulation of membrane mediators and second messengers. Binding sites in membranes have been characterized, with characteristics completely different from those of classic intracellular steroid receptors; this also includes the inability of classic steroid receptor antagonists to inhibit rapid non-genomic steroid actions. The physiological and pathophysiological relevance of these effects is still largely unclear, but their existence has been proved even under in vivo conditions. New drugs modulating nongenomic steroid actions may find applications in various areas such as the cardiovascular and central nervous systems, infertility and electrolyte homeostasis.
A central mechanism in maintaining the homeostasis of multicellular organisms is intercellular communication, allowing, for example, the transmission of intracellular signals (eg, ions or second messengers), regulation of growth, developmental and differentiation processes, synchronization and metabolic regulation. This intercellular communication occurs by means of specialized structures in the plasma membranes, termed gap junctions, that contain aggregates of oligomeric channels. Each channel is formed by the end-to-end abutment of two hemichannels or ‘connexons’ (one per membrane), which in turn comprise six protein subunits or connexins. These channels provide direct transmembrane communication pathways between cytoplasms of adjacent cells, allowing the diffusion of ions and small molecules (up to about 1 kD in size) between those cells (reviewed in, for example, 1).
This direct cell-to-cell communication has been found to be impaired by several steroids, such as certain bile acids (2), oxysterols (3,4) or sex hormones (5–8). In the present study, the effects of different steroid compounds on the degree of cell-to-cell communication were compared in cells not involved in steroidogenesis, ie, ventricular myocytes of newborn rats, by assessment of either the junctional permeability for a fluorescent dye or the junctional electrical conductance.
MATERIALS AND METHODS
Cell cultures and solutions:
Neonatal rats (about 10 one- to two-day-old pups) of either sex were killed by cervical dislocation, and their hearts were rapidly removed, minced and submitted to five sequential 10 min periods of digestion in crude trypsin (1 mg/mL; Boehringer-Mannheim, Germany) in a Ca2+- and Mg2+-free culture medium (spinner) at 37°C under gentle stirring. Supernatants from fractions 2 to 5 were centrifuged at 500 g for 5 min, resuspended in Ham’s F10 culture medium (Gibco, France) and preplated in large petri dishes for 90 min to allow nonmuscle cells to attach. The remaining unattached muscle cells were then collected and seeded in 35 mm dishes (about 55,000 cells/cm2) and cultured in Ham’s F10 medium supplemented with 10% fetal calf serum (Boehringer), 10% heat-inactivated horse serum (Gibco) and antibiotics (17 μM streptomycin sulphate and 40 U/mL penicillin G, both from Sigma [France]). The cells were maintained in a humidified incubator with 5% CO2 in air, at 37°C, for one to three days. The culture medium was replaced after 24 h with Ham’s F10 medium supplemented with the same amounts of horse serum and antibiotics.Gap junctional permeability or conductance was measured after the culture medium was replaced with Tyrode’s solution containing (in mM) NaCl 144, KCl 5.4, MgCl2 1, CaCl2 2.5, NaH2PO4 0.3, glucose 5.6 and HEPES 5 (buffered to pH 7.4 with NaOH). The dishes were then transferred onto the stage of an inverted microscope, and the cells were observed by phase-contrast microscopy. All cell-to-cell communication was measured at room temperature (20 to 22°C). The test extracellular solutions were either added to the culture dishes for the time specified in the Results section or applied very rapidly by directing a streamline flow from the opening of a stainless steel capillary (internal diameter 30 μm), positioned in the bath near the investigated cell pair.
Chemicals:
The diacetate ester of 6-carboxyfluorescein, used to introduce 6-carboxyfluorescein into the cells, was dissolved in dimethylsulphoxide (DMSO) and added to the Tyrode’s solution at the final concentration of 7 μg/mL.
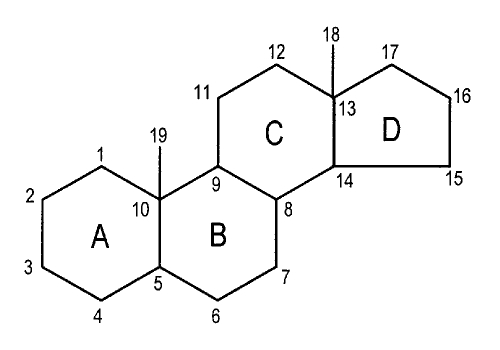
Steroids are organic compounds composed of a series of four carbon rings (A to D, Figure 1) joined together in a structural unit called cyclopentenoperhydrophenanthrene. All natural steroids have an oxygenated group present at C3 and a substituent at C17; the latter varies widely in different steroids. These compounds were dissolved in DMSO (40 mM stock solution), stored at −20°C, then diluted in the external medium to obtain the chosen concentration. The final amount of DMSO in the medium was at most 0.1% (volume/volume). At this concentration, DMSO did not affect the junctional coupling of these cells. The antiestrogen tamoxifen or the antiandrogen cyproterone acetate was added to the culture medium for 24 h before and during the assays of estradiol propionate (EP) or testosterone propionate (TP), respectively. All compounds were obtained from Sigma.
Figure 1.
Chemical structure and numbering of the steroid skeleton
Quantification of the cell-to-cell dye transfer:
The gap junctional permeability for the diffusion of a fluorescent dye was quantified by measuring the fluorescence recovery after photobleaching (gap FRAP [9]). This technique is based on the irreversible photobleaching that occurs when fluorophores are exposed to a pulse of high intensity excitation light, provided by a laser. A concentration gradient of functional (ie, unbleached) fluorophore is then superimposed on an otherwise uniform distribution of labelled molecules. The subsequent relaxation of this gradient is detected by monitoring the fluorescence signal with low intensity excitation.
Cells were loaded with dye ester (6-carboxyfluorescein diacetate) for 15 min at room temperature, in the dark. This molecule penetrates into the cells where cytoplasmic esterases release the highly fluorescent and membrane-impermeant moiety 6-carboxyfluorescein, which labels the cells. After cells were rinsed several times to stop the loading process, gap FRAP experiments were performed using the cytofluorimetric system ACAS 570 (Anchored Cell Analysis and Sorting, Meridian Instruments, USA). In selected cells in contact with other cells, the fluorescence was photobleached by strong light pulses from an argon laser tuned at 488 nm, then the redistribution of unbleached dye molecules was monitored as a function of time, as previously described (6).
The initial part of the fluorescence recovery (at least the first 4 min) follows an exponential time course (Figure 2) as expected for a diffusion process across a concentration gradient. Between interconnected cells, the gap junctional membrane constitutes the rate-limiting boundary of cell-to-cell diffusion. Under these conditions, the rate constant k of the exponential fluorescence recovery (the inverse value of the time constant), which provides a quantitative index of cell-to-cell dye transfer, can be obtained from the following equation:
where Fi, Fo and Ft are the integrated fluorescence intensities in the bleached cells before, immediately after and at time t, respectively, after photobleaching. One unbleached isolated cell was used as a control of the spontaneous decay of background fluorescence.
Figure 2.
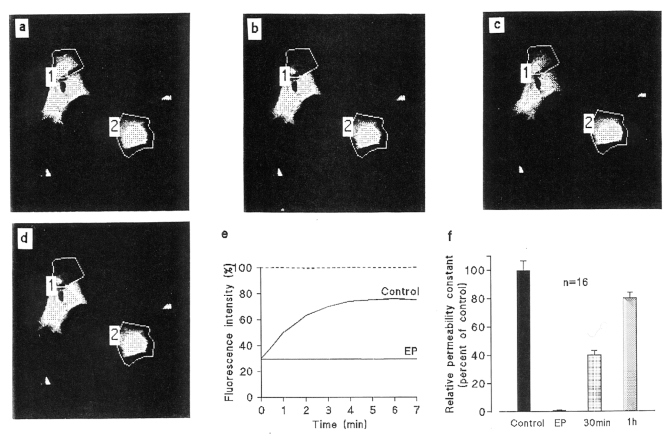
Example of interruption of cell-to-cell dye diffusion elicited by 17β-estradiol propionate (EP) in ventricular myocytes of neonatal rats in primary culture. The grey density images of fluorescence intensities were obtained by scanning a 140×140 μm field with low intensity light pulses. After a prebleach scan (a), 6-carboxyfluorescein (6-CF) was photobleached in some selected areas (polygon 1) by means of strong illumination. The fluorescence levels in the selected cells were recorded just before (a) and just after photobleaching (b), and again 7 min later in control conditions (c) then in the same cells exposed to 17β-EP (25 μM) for 15 min (d). The graphs (e) show a comparison of the time courses of the fluorescent emission of the selected cells in control conditions (Control) and after treatment with EP. The fluorescence intensity is represented as a percentage of the prebleach emission versus the time after photobleaching. In control conditions, the fluorescence emission of the bleached cell increased progressively while, in contrast, it did not significantly change cells that were exposed to the steroid. The unbleached cell in polygon 2 served as a control. (f) The relative permeability constant k was reduced to immeasurably low values after a 15 min exposure to the steroid, then progressively increased after washing, when fluorescent dye diffusion became possible from neighbouring cells through reopened junctional channels
The relative time constants k were measured at first in control conditions, then, in the same cells, after exposure to a chemical and, when necessary, a third time after another chemical was used. In preliminary experiments, k did not significantly vary when it was consecutively determined up to four times in the same cells in control conditions (n=15), showing that the cells and the intercellular communication were not altered by the photobleaching itself. Results were expressed as mean ± SEM, and the statistical significance of the differences of the means was established by Student’s t test for paired data, with a sufficiently large number of measurements in cells in contact with another cell.
Measurement of the junctional conductance:
Macroscopic junctional conductance was determined in cell pairs by using dual whole cell voltage clamp techniques as previously described (10). Low resistance (1.5 to 3 MΩ) patch pipettes were pulled by means of a horizontal puller (BB-CH PC Mecanex, Switzerland), backfilled with a filtered solution containing (in mM) KCl 140, MgATP 5, EGTA 5, glucose 10, GTP 0.1 and HEPES 10 (buffered to pH 7.2 with KOH). They were lowered onto the surface of each cell of a pair, and high resistance seals were obtained by gentle suction. The rupture of the patch membrane beneath the pipette allowed the whole cell configuration to be established. Both cells were at first clamped to the common holding potential (Vh, close to −70 mV), then a transjunctional voltage difference was established by applying a potential step to one of the cells (Figure 3). When the cytoplasms of the adjacent cells were connected by open junctional channels, a change in the holding current was recorded for the second cell, corresponding to the current that flowed through the intercellular pores. Changes in its magnitude expressed variations of the functional state of the junctional channels. Junctional conductance was calculated by dividing the current change by the applied voltage pulse.
Figure 3.
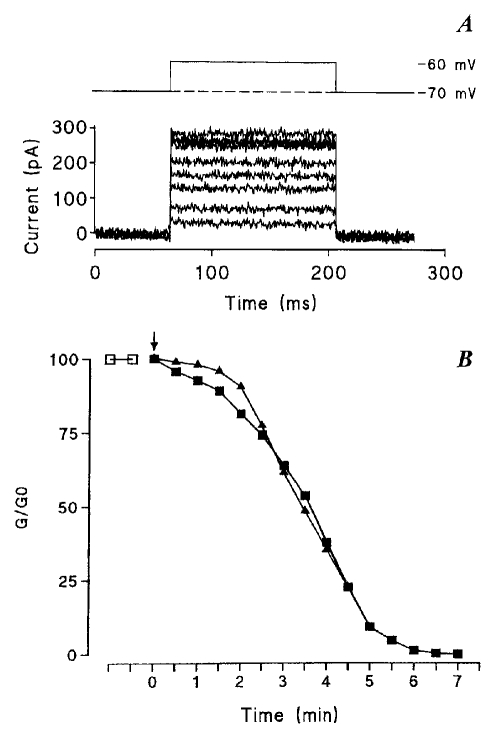
Typical examples of the decreases in junctional conductance promoted by testosterone propionate (TP) and estradiol propionate (EP) in ventricular myocytes of newborn rat. A (top) Both cells of the pair were clamped at a common holding potential (−70 mV) and a 10 mV depolarization was applied to one of them every 30 s, whereas the second cell was maintained at the holding potential. (Bottom) Because of the transjunctional driving force, a current flows through cell-to-cell channels, and its amplitude was seen to decrease gradually when the cell pair was exposed to TP (superimposed currents recorded the 5 first min after TP introduction). B Corresponding time courses of the decreases in junctional conductances (G/G0)when the cells, at first bathed in Tyrode’s solution ( ), were exposed to a stream of a steroid-containing solution (25 μM) applied close to the cell pair (filled symbols). The junctional conductance, presented in units of its original value, was found to decline progressively in the presence of both TP (▪) and EP (▴) to complete interruption of the cell-to-cell communication
), were exposed to a stream of a steroid-containing solution (25 μM) applied close to the cell pair (filled symbols). The junctional conductance, presented in units of its original value, was found to decline progressively in the presence of both TP (▪) and EP (▴) to complete interruption of the cell-to-cell communication
RESULTS
17β-EP rapidly and reversibly interrupts cell-to-cell communication:
Enzymatically dispersed cardiomyocytes, when seeded in petri dishes, progressively attach themselves to the plastic bottom, flatten, emit protrusions and pseudopodia toward neighbouring cells, and come into contact, and a cell-to-cell diffusion of the fluorescent dye can be observed. This step is interpreted as corresponding to the establishment of gap junctions and to the opening of channels connecting directly the cytoplasms of cells in contact. The cell-to-cell dye transfer is illustrated in Figure 2, upper panel (a-c) where, immediately after photobleaching of the selected cell, its fluorescence intensity was reduced to about 30% of the initial level; its light emission then rose, with a monoexponential time course. The graph (Figure 2e, control) shows a typical example of the evolution of the integrated fluorescence intensities with time after photobleaching, measured in Tyrode’s solution. The rate constant k obtained by fitting the equation was then found equal to 0.24±0.02/min (n=470); in other words, the fluorescence recovery after photobleaching followed an exponential time course with a mean time constant of 4 min. In contrast, after exposure to EP (25 μM, for 15 min), fluorescent dye was not recovered in photobleached cells in contact with other cells (Figure 2d,e; EP), and the relative permeability constant k was reduced to immeasurably low values, reflecting the suppression of the cell-to-cell diffusion of 6-carboxyfluorescein. The fluorescence decay in unbleached cells (dotted line in Figure 2e), which is the sum of background photobleaching in the successive scans and of an outflux through nonjunctional cell membranes, was very similar in control and EP-treated cultures (Figure 2a–d, polygon 2). This observation indicates that the permeability of the nonjunctional cell membrane for organic molecules was not altered. However, prolonged treatments (longer than 1 h) with high concentrations of steroid ester (100 μM) induced irreversible alterations of the cell morphology, particularly a shrinkage of the cells and the appearance of vacuoles within the cytoplasm.
The inhibition of the junctional communication was maintained as long as the cells were exposed to EP, but after its removal, cellular coupling was restored. Indeed, after a delay of approximately 5 min, the fluorescent emission was progressively redistributed, showing that the intercellular channels were gradually reopening, allowing the passage of 6-carboxyfluorescein, as shown by the rise of k levels to about 40% and 80% of their original values 30 min and 60 min, respectively, after the cells were washed (Figure 2f).
Kinetics of the interruption of gap junctional communication:
When the gap FRAP technique was used, the fluorescence levels were determined at regular time intervals and the degree of diffusional coupling was inferred from the successive light levels. In contrast, the dual voltage clamp technique allowed continuous monitoring of the junctional conductance or its measurement at short time intervals. The amplitude of the current flowing through junctional channels when a difference of potential was applied between the cells progressively decreased with time when the cell pair was exposed to a TP-containing solution (25 μM, Figure 3A), reflecting a progressive closure of junctional channels. Both hormone derivatives decreased, with similar time courses, the cell-to-cell conductance to immeasurably low values within 5 to 10 min, as illustrated in Figure 3B.
Importance of steroid esterification for the uncoupling action:
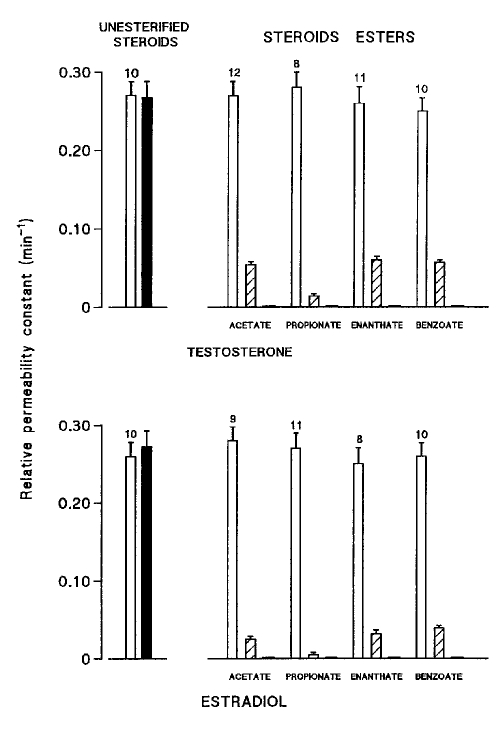
The junctional uncoupling efficiency of testosterone, of 17β-estradiol and of several of their esters, all used at the same concentration (25 μM) in the external bath, was compared (Figure 4). Short term exposures (10 to 30 min) of the cells to both nonesterified sex hormones did not alter the cell-to-cell diffusion of the fluorescent dye, whereas all the tested esters progressively reduced the rate constant k to negligibly low values. Neither the position (C3 or C17), the nature (acetate, propionate, enanthate or benzoate) nor the number (propionate or dipropionate) of the ester chains influenced the uncoupling properties of these compounds, and all of them completely abolished cell-to-cell dye transfer.
Figure 4.
Comparative effects of testosterone (top) and 17β-estradiol (bottom) and of some of their esters (all compounds used at the same concentration, 25 μM) on the relative permeability constant (k), measured in cardiac cells by means of the gap fluorescence recovery after photobleaching technique. Blank columns represent k values (means ± SEM; per min) in control conditions, and hatched and filled columns the values obtained in the same cells after exposure to the steroid compound for 10 min and 30 min, respectively, with numbers of measurements on top
Steroid derivatives act through a nongenomic pathway:
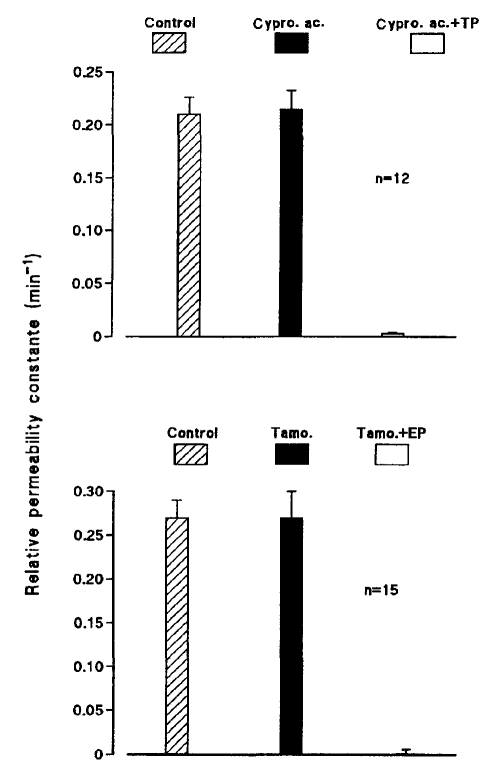
The fact that testosterone and 17β-estradiol have, at concentrations far above the known hormone concentrations, similar effects on cell-to-cell communication casts doubt on the genomic origin of the uncoupling action. Both hormones regulate biological functions by mechanisms that involve gene transcription and protein synthesis. This genomic model pre-sumes that steroid hormones cross the plasma membrane, enter the cytoplasm, and bind to and activate specific nuclear receptors. However, certain responses to steroid hormones appear to use nonclassical, nongenomic mechanisms. To verify that their uncoupling effects result from nontranscriptional actions, they were used in the presence of agents that prevent their genomic effects by binding to their nuclear receptor. Ventricular myocytes were cultured for 24 h in the presence of the antiandrogen cyproterone acetate (25 μM) or the antiestrogen tamoxifen (1 μM) before exposure to TP (Figure 5, top) or 17β-EP (Figure 5, bottom), respectively. Neither cyproterone acetate nor tamoxifen affected cell-to-cell dye diffusion, and neither prevented the interruption of the cell-to-cell communication elicited by sex hormones.
Figure 5.
The presence of antagonists of nuclear androgen or estrogen receptors did not prevent the uncoupling effects of sex steroid hormone derivatives. The relative permeability constant was estimated in cardiac cells in control conditions, in the same cells exposed for 24 h to cyproterone acetate (Cypro. ac.; 25 μM) or tamoxifen (Tamo.; 1 μM), then after they had been exposed for 15 min to testosterone propionate- (TP) or estrogen propionate-(EP) containing (25 μM) solutions
Influence of the steroid nucleus on uncoupling potential:
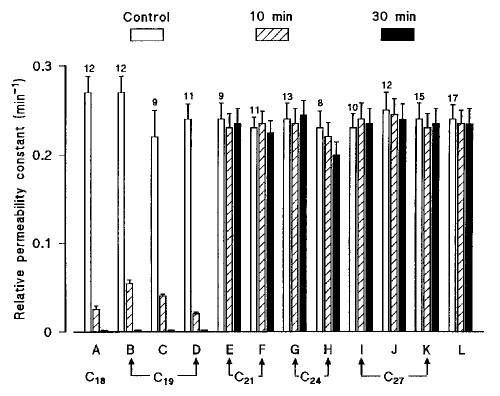
To search for a possible relation between the molecular structure of the steroid nucleus and its uncoupling potency, the effects on junctional communication of various steroids (sterols, bile acids, hormones and vitamin D3) applied at the same concentration (25 μM) in the bath fluid were examined. It can been seen from Figure 6 that, among the simplest derivatives of the perhydrocyclopentenophenanthrene nucleus, the esters of the C18 (estrane) and C19 (androstane) derivatives (A to D) totally inhibited the intercellular communication rate constants of cell-to-cell dye transfer to very low values, whereas the compounds of higher molecular weight (C21 to C27, E to L) had negligible influence. In rat Sertoli cells, steroids of molecular weight in this range, such as cyproterone acetate (C23) (6), cholesteryl acetate (C27) and ouabain (C29) (7), had no effect on the cell-to-cell dye diffusion.
Figure 6.
Comparison of the effects on the cell-to-cell diffusion of a fluorescent dye of various steroid compounds, all used at the same concentration (25 μM). The relative permeability constant was estimated in cardiac cells in control conditions, then in the same cells exposed to steroid-containing solutions for 10 min and 30 min, respectively. The effects of 12 steroid derivatives were compared: (A) 17β-estradiol acetate (1, 3, 5[10]-estratriene-3,17β-diol 17-acetate); (B) testosterone acetate (Δ4-androsten-17β-ol-3-one 17-acetate); (C) androstenediol 3 acetate (Δ5-androstene-3β,17β-diol 3-acetate); (D) dihydrotestosterone benzoate (17β-hydroxy-5α-androstan-3-one 17-benzoate); (E) 17α;-hydroxypregnenolone 3-acetate (Δ5-pregnene-3β, 17α;-diol-20-one 3 acetate); (F) hydro-cortisone 17-butyrate (Δ4-pregnene-11β, 17α, 21-triol-3, 20-dione 17-butyrate); (G) cholic acid (5β-cholan, 3α, 7α, 12α-trihydroxy 24-oic acid); (H) lithocholic acid (3α-hydroxy-5β-cholan-24-oic acid); (I) cholesteryl acetate (Δ5-cholestene-3β-ol 3-acetate); (J) cholesterol 3-sulphate (5-cholesten-3β-ol sulphate); (K) 20α-hydroxycholesterol (Δ5-cholestene-3β,20α-diol); (L) vitamin D3, or cholecalciferol. Bars indicate k values (per min), expressed as means ± SEM with numbers on top
When comparing the molecular structures with the effects on the rate constants of dye transfer, it can be noticed that the uncoupling efficiency does not depend on the kind of heterocycle because intercellular communication is interrupted as well by the C18 (estrane) and C19 (androstane) derivatives (A to D). Similarly, no correlation can be found between the effect on dye transfer and the absence (eg, D or H) or presence of double bonds on the heterocycle, whatever their position, in Δ4 (eg, D, F) or Δ5 (as C, E, J or K) or number (1 or 3). No influence of the orientation (α or β) of the ester chain, of the nature (hydroxyl or ketone) of substituted groups or, of the cis (eg, G, H) or trans (as D) fusion of the A and B rings can be detected.
DISCUSSION
Much convincing experimental evidence documenting acute and transient actions of steroid hormones in the cardiovascular system has been reported (for a recent review see, for example, 11). Steroids can rapidly activate signalling cascades within endothelial as well as cardiac or smooth muscle cells that seem to bypass the classical, genomic receptors. Activation of these signalling cascades, involving alterations in intracellular [Ca2+], modifications of activities of protein kinase and phosphatase, stimulation of NO production and participation of guanine nucleotide-binding proteins (G proteins), leads to changes in membrane potential or ion fluxes through membrane channels. In the present study, the effects on junctional communication of several steroid molecules were investigated in primary cultures of cardiac myocytes of newborn rats. A relatively rapid and reversible impairment of electrical and diffusional coupling was seen with some of these compounds at a concentration of 25 μM. This uncoupling effect may be involved in the well known cardiovascular toxicity of steroids (12).
The observation that sex steroid derivatives completely interrupt intercellular communication within 5 to 10 min, a delay too short to involve gene transcription followed by protein synthesis, did not favour a genomic origin of the junctional uncoupling. This was confirmed by the lack of efficiency of tamoxifen and cyproterone acetate to prevent the effects of 17β-estradiol and testosterone esters, respectively. The possibility of a causal involvement of calcium or hydrogen ions in the gap junction uncoupling by steroid esters was ruled out by buffering the cytosolic Ca2+ and H+ concentrations through the patch pipette solution.
Steroid binding proteins localized in the cell membrane can influence other functional proteins in a fashion similar to that of receptors for peptide hormones, which transduce the hormonal signal, by way of G-proteins, to adenylyl cyclase, phospholipase C, ion channels, etc (reviewed in 13). For example, some steroids can modulate the γ-aminobutyric acid A receptor complex, but only the steroids with a reduced A ring and a hydroxyl group at the 3α position are active at nanomolar concentrations (14). Several discrete actions of estradiol, for example, are considered to be mediated through plasma membrane receptors, not isolated up to now, which are relatively specific, so nongenomic effects of estradiol are frequently counteracted by androgens (reviewed in, for example, 15,16). In the present study, both estradiol and testosterone led, at micromolar concentrations, to complete closure of junctional channels.
In this concentration range, these lipophilic compounds had limited effects on other membrane channels. Exposure of cardiac myocytes to 10 μM estradiol, for example, rapidly and completely interrupted cell-to-cell communication whereas it only reduced the amplitudes of T- and L-type Ca2+ channels to about 90% and 82%, respectively, of their original amplitudes, whereas testosterone had no effect (17). At higher concentrations (30 μM) estradiol reduced the amplitudes of K+ and L-type Ca2+ channels to only about 60% and 50% (17), 38% and 70% (18) or 80% and 63% (19), respectively, of their original values. A similar discrepancy in sensitivity was observed with the commonly used blocker of junctional channels, heptanol. In cardiac cells, 0.5 mM of this lipophilic drug was sufficient to completely inhibit gap junctional communication (20) whereas, at this concentration, about 80% of sodium current was still present and approximately 100 mM was necessary to obtain total inhibition (21).
The junctional uncoupling potency of a range of steroids has been investigated to try to ascertain the structural requirements for this action. Among the tested compounds, derivatives from the simplest hydrocarbon nuclei, such as estradiol (C18), testosterone and other androstane or androstene (C19) derivatives, totally inhibited junctional communication, whereas compounds of higher molecular weight had no effect. Cholic acid also failed to inhibit metabolic cooperation in Chinese hamster cells, whereas lithocholic acid abolished it (2). Prolonged exposures (12 to 24 h) of the cells to an oxysterol (10 μM 25-hydroxycholesterol) reduced the cell-to-cell dye coupling of hepatocytes (4) and smooth muscle cells (3). Short term (15 min) exposures of rat cardiomyocytes to both of these compounds (25 μM) had no effect on the degree of cell-to-cell communication. No correlation with the existence or position of double bonds nor with the trans- or cis-configuration of the A and B rings was found. Altogether, the different uncoupling efficiencies of the tested steroid compounds are consistent with a mechanism involving direct incorporation of steroid compounds of appropriate shape and size into the lipidic domains that maintain the proper orientation of membrane proteins (such as connexins) in the bilayer, modifying their properties. Several other lipophilic substances (eg, aliphatic alcohols, fatty acids and aldehydes) can also interrupt gap junctional communication (reviewed in 22).
Possible modifications in the microenvironment of the membrane proteins (eg, connexins):
Many membrane proteins are considered to be embedded in a core of lipids that remain in a gel state and maintain the tertiary structure of the protein in a particular conformation (23). According to Yeagle (24), the structural specificity of the membrane sterol requirement favours interactions between the essential sterol (cholesterol) and specific binding sites of the cell membrane proteins. Steroid-induced changes in membrane biophysical properties including fluidity and bilayer width have been reported. 17β-estradiol, for example, was seen in the uncoupling concentration range to reduce the membrane fluidity in human breast cancer cells (25). Another lipophilic compound, gossypol (a polycyclic lipophilic agent naturally present in cottonseed), which similarly elicits a closure of gap junctional channels within 10 to 12 min (26), also decreases membrane fluidity (27). A decrease was also seen to occur when the gap junctional communication was interrupted by heptanol (28).
The fluidity of the acyl chains probably provides the required freedom of motion, allowing proteins within the membrane to undergo conformational changes, and rotational or translocational movements associated with their activity. A decrease of fluidity (due for example to the effects of the rigid sterol structure) is known to raise lateral pressure on proteins, affecting the functions of several membrane proteins (29). The activity of many membrane enzymes or transport systems depends on the physical state of the membrane lipids. For example, Na+/K+-ATPase activity was seen to depend on the cholesterol to phospholipid molar ratio (30). The depletion of cholesterol content decreased the fluidity of fatty acid chains of phospholipids within the membranes (31). A decrease of the fluidity of the cholesterol-rich membranous domains was observed when the gap junctional conductance was lowered by heptanol application (28). Compared with heptanol, the decay of the junctional conductance caused by TP exposure (complete in approximately 6 to 10 min at 50 μM) and its recovery after steroid washing were seen to be relatively slow. Indeed, after heptanol application, the interruption of intercellular communication had a much faster onset (within 0.5 s) and recovery (about 1 min [10]). The slower time course may indicate a protracted insertion and removal of the steroid ester into the membrane.
Intercalation of the lipophilic steroids into the membrane bilayer may cause perturbations of lipid-lipid interactions that may, in turn, alter the function of membrane proteins. Steroids may displace lipids surrounding the proteins, allowing steroid-protein interactions to occur or, alternatively, steroids may also modify protein-protein interactions (32).
Possible consequences:
Interruption of the cell-to-cell communication was observed when cells were exposed to extra-cellular high concentrations of steroid esters; however, the direct introduction of unesterified 17β-estradiol into the cytosol impaired junctional coupling at much lower concentrations. Indeed, the presence in the pipette filling solution of 250 nM unesterified estradiol was sufficient to reduce the intercellular coupling of rat cardiac myocytes to approximately 50% of its level within 25 min (8). Such a concentration is not far above the range observed during pregnancy in women, where the serum level concentration of 17β-estradiol can reach up to 105 nM (33). However, actual concentrations of this lipophilic compound may be higher in membrane than in serum and can be considerably higher in steroid drug abuse (34). It may particularly be the case in abuse of anabolic steroids, synthetic derivatives of testosterone modified to enhance the anabolic rather than the androgenic actions of the hormone, mainly by promoting protein synthesis, muscle growth and erythropoiesis. Such increasing abuse is considered to increase cardiovascular risks despite the generally observed decreases in high density lipoprotein cholesterol and apolipoprotein A-I (reviewed in, for example, 35). The deleterious effects of steroids might result directly from their destabilizing action on the membrane, resulting from their insertion into the lipid bilayer membrane, with consequent modification in the microenvironment of the membrane proteins, including membrane channels.
Acknowledgments
This study was supported in part by grants from the European Community RDT action QLG1-CT-1999-0051 and from the Fondation Langlois.
REFERENCES
- 1.Simon AM, Goodenough DA. Diverse functions of vertebrate gap junctions. Trends Cell Biol. 1998;8:477–83. doi: 10.1016/s0962-8924(98)01372-5. [DOI] [PubMed] [Google Scholar]
- 2.Noda K, Umeda M, Ono T. Effects of various chemical including bile acids and chemical carcinogens on the inhibition of metabolic cooperation. Jpn J Cancer. 1981;72:772–6. [PubMed] [Google Scholar]
- 3.Zwijsen RML, Oudenhoven IMJ, De Haan LHJ. Effects of cholesterol and oxysterols on gap junctional communication between human smooth muscle cells. Eur J Pharmacol. 1992;228:115–20. doi: 10.1016/0926-6917(92)90020-d. [DOI] [PubMed] [Google Scholar]
- 4.Guo X, Ohno Y, Miyajima A, Sunouchi A, Takanaka A. Oxysterols inhibit gap junctional communication between rat hepatocytes in primary culture. Pharmacol Toxicol. 1993;73:10–3. doi: 10.1111/j.1600-0773.1993.tb01949.x. [DOI] [PubMed] [Google Scholar]
- 5.Kihara K, Fukui I, Higashi Y, Oshima H. Inhibitory effect of testosterone on gap junctional intercellular communication of human transitional cell carcinoma cell lines. Cancer Res. 1990;50:2848–52. [PubMed] [Google Scholar]
- 6.Pluciennik F, Verrecchia F, Bastide B, Hervé JC, Joffre M, Délèze J. Reversible interruption of gap junctional communication by testosterone propionate in cultured Sertoli cells and cardiac myocytes. J Membrane Biol. 1996;149:169–77. doi: 10.1007/s002329900017. [DOI] [PubMed] [Google Scholar]
- 7.Hervé JC, Pluciennik F, Verrecchia F, et al. Influence of the molecular structure of steroids on their ability to interrupt gap junctional communication. J Membrane Biol. 1996;149:179–87. doi: 10.1007/s002329900018. [DOI] [PubMed] [Google Scholar]
- 8.Verrecchia F, Kwak BR, Hervé JC. 17β-estradiol-induced blockade of cell-to-cell communication in neonatal rat cardiomyocytes is not mediated by activation of tyrosine kinase, protein kinases A or C. In: Werner R, editor. Gap Junctions. Amsterdam: IOS Press; 1998. pp. 220–4. [Google Scholar]
- 9.Wade MH, Trosko JE, Schindler M. A fluorescence photobleaching assay of gap junction-mediated communication between human cells. Science. 1986;232:525–8. doi: 10.1126/science.3961495. [DOI] [PubMed] [Google Scholar]
- 10.Bastide B, Hervé JC, Cronier L, Délèze J. Rapid onset and calcium independence of the gap junction uncoupling induced by heptanol in cultured heart cells. Pflügers Arch. 1995;429:386–93. doi: 10.1007/BF00374154. [DOI] [PubMed] [Google Scholar]
- 11.Ruehlmann DO, Mann GE. Rapid non-genomic vasodilator actions of oestrogens and sex steroids. Curr Med Chem. 2000;7:533–41. doi: 10.2174/0929867003375038. [DOI] [PubMed] [Google Scholar]
- 12.de Voogt HJ, Smith PH, Pavone-Macaluso M, de Pauw M, Suciu S. Cardiovascular side effect of diethylstilbestrol, cyproterone acetate, medroxyprogesterone acetate and estramustine phosphate used for the treatment of advanced prostatic cancer: results from European Organization for Research on Treatment of Cancer Trials 30761 and 30762. J Urol. 1986;135:303–7. doi: 10.1016/s0022-5347(17)45620-5. [DOI] [PubMed] [Google Scholar]
- 13.Rommerts FFG. Cell surface action of steroids: a complementary mechanism for regulation of spermatogenesis? In: Nieschlag E, Habenicht UF, editors. Spermatogenesis. Fertilization. Contraception. Berlin: Springer-Verlag; 1992. pp. 1–20. [Google Scholar]
- 14.Hevers W, Lüddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- 15.Moss RL, Gu Q, Wong M. Estrogen: nontranscriptional signalling pathway. Recent Prog Horm Res. 1997;52:33–68. [PubMed] [Google Scholar]
- 16.Levin ER. Nuclear receptor versus plasma membrane oestrogen receptor. Novartis Found Symp. 2000;230:41–55. doi: 10.1002/0470870818.ch5. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima T, Iwasawa K, Oonuma H, et al. Antiarrhythmic effect and its underlying ionic mechanism of 17beta-estradiol in cardiac myocytes. Br J Pharmacol. 1999;127:429–40. doi: 10.1038/sj.bjp.0702576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger F, Borchard U, Hafner D, Putz I, Weis TM. Effects of 17beta-estradiol on action potentials and ionic currents in male rat ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:788–96. doi: 10.1007/pl00005119. [DOI] [PubMed] [Google Scholar]
- 19.Tanabe S, Hata T, Hiraoka M. Effects of estrogen on action potential and membrane currents in guinea pig ventricular myocytes. Am J Physiol. 1999;277:H826–33. doi: 10.1152/ajpheart.1999.277.2.H826. [DOI] [PubMed] [Google Scholar]
- 20.Hervé JC, Verrecchia F, Bastide B, Délèze J. Characteristics of heptanol action on cell-to-cell communication, studied by electrophysiology and intracellular calcium detection. In: Kanno Y, Kataoka K, Shiba Y, Shibata Y, Shimazu T, editors. Intercellular Communication Through Gap Junctions. Amsterdam: Elsevier Science BV; 1995. pp. 225–8. [Google Scholar]
- 21.Nelson WL, Makielski JC. Block of sodium current by heptanol in voltage-clamped canine cardiac Purkinje cells. Circ Res. 1991;68:977–83. doi: 10.1161/01.res.68.4.977. [DOI] [PubMed] [Google Scholar]
- 22.Verrecchia F, Hervé JC. Reversible inhibition of gap junctional communication elicited by several classes of lipophilic compounds in cultured rat cardiomyocytes. Can J Cardiol. 1997;13:1093–100. [PubMed] [Google Scholar]
- 23.Lee AG. Model for action of local anaesthetics. Nature. 1976;262:545–8. doi: 10.1038/262545a0. [DOI] [PubMed] [Google Scholar]
- 24.Yeagle PL. Lipid regulation of cell membrane structure and function. FASEB J. 1989;3:1839–42. [PubMed] [Google Scholar]
- 25.Clarke R, Van der Berg HW, Nelson J, Murphy RF. Pharmacological and suprapharmacological concentrations of both 17β estradiol and tamoxifen reduce the membrane fluidity of MCF-7 and MDA-MB-436 human breast cancer cells. Biochem Soc Trans. 1987;15:243–4. [Google Scholar]
- 26.Hervé JC, Pluciennik F, Bastide B, et al. Contraceptive gossypol blocks cell-to-cell communication in human and rat cells. Eur J Pharmacol. 1996;313:243–55. doi: 10.1016/0014-2999(96)00476-1. [DOI] [PubMed] [Google Scholar]
- 27.Cuellar A, Ramirez J. Further studies on the mechanism of action of gossypol on mitochondrial membrane. Int J Biochem. 1993;25:1149–55. doi: 10.1016/0020-711x(93)90593-4. [DOI] [PubMed] [Google Scholar]
- 28.Bastiaanse EML, Jongsma HJ, van der Laarse A, Takens-Kwak BR. Heptanol-induced decrease in gap junctional conductance is mediated by a decrease in the fluidity of membranous cholesterol-rich domains. J Membrane Biol. 1993;136:135–45. doi: 10.1007/BF02505758. [DOI] [PubMed] [Google Scholar]
- 29.Yeagle PL. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985;822:267–87. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- 30.Giraud F, Claret M, Bruckdorfer KR, Chailley B. The effects of membrane lipid order and cholesterol on the internal and external cationic sites of the Na+-K+ pump in erythrocytes. Biochim Biophys Acta. 1987;647:249–58. doi: 10.1016/0005-2736(81)90253-4. [DOI] [PubMed] [Google Scholar]
- 31.Verma SP, Philippot JR, Wallach DFH. Chain length dependent modification of lipid organization by low levels of 25-hydroxycholesterol and 25-hydroxycalciferol, a laser Raman study. Biochemistry. 1983;22:4587–91. doi: 10.1021/bi00288a037. [DOI] [PubMed] [Google Scholar]
- 32.Whiting KP, Restall CJ, Brain PF. Steroid hormone-induced effects on membrane fluidity and their potential roles in non-genomic mechanisms. Life Sci. 2000;67:743–57. doi: 10.1016/s0024-3205(00)00669-x. [DOI] [PubMed] [Google Scholar]
- 33.Tabei T, Ochai K, Terashima Y, Takanashi N. Serum levels of inhibitin in maternal and umbilical blood during pregnancy. Am J Obstet Gynecol. 1991;164:896–900. doi: 10.1016/0002-9378(91)90536-z. [DOI] [PubMed] [Google Scholar]
- 34.Narducci WA, Wagner JC, Hendrickson TP, Jeffrey TP. Anabolic steroids: a review of the clinical toxicology and diagnostic screening. Clin Toxicol. 1990;28:287–310. doi: 10.3109/15563659008994431. [DOI] [PubMed] [Google Scholar]
- 35.Dickerman RD, McConathy WJ, Zachariah NY. Testosterone, sex hormone-binding globulin, lipoproteins, and vascular disease risk. J Cardiovasc Risk. 1997;4:363–6. [PubMed] [Google Scholar]