Abstract
Although cardiac ischemia-reperfusion is well known as a disease of the myocytes, it is now clear that the consequences of this disease also extend to the vascular wall and especially to the endothelium. A rat model of ischemia-reperfusion in vivo was used to detect severe endothelial dysfunction characterized by a decreased nitric oxide (NO)-dependent relaxation to acetylcholine in isolated coronary arteries. Given the essential role of the endothelium and NO in the regulation of vascular tone, protection of the coronary endothelial cells is an important therapeutic target. For this purpose, a focus on the concept of endogenous protection against ischemia, ie, preconditioning, showed that endothelial dysfunction could be reversed by both the early and the delayed phase of preconditioning. With regard to the mechanisms of the coronaroprotective effects of preconditioning, it was shown that both free radicals and NO seem to have an important triggering role, leading to a delayed increase in NO production and decreased adhesion of neutrophils to endothelial cells. Identification of the precise triggers and mediators of this protection will allow the development of new therapeutic agents targeting both the myocardium and the coronary vasculature.
Keywords: Coronary endothelium, Ischemia-reperfusion, Preconditioning
Although the cardiac consequences of ischemia-reperfusion are well documented at the level of the myocardium, much less is known about the coronary vasculature. However, a number of experiments have shown that coronary endothelial cells are a major target of ischemia-reperfusion injury, especially through an altered capacity to release nitric oxide (NO), associated with an increased production of oxygen-derived free radicals. Given the central role of NO as a vasodilating agent, but also as an inhibitor of platelet aggregation and leukocyte adhesion, such a persistent impairment is likely to have important deleterious consequences for the coronary arterial wall. Thus, the coronary endothelium may be considered to be a major therapeutic target of anti-ischemic treatments.
ENDOTHELIUM AND MYOCARDIAL ISCHEMIA-REPERFUSION
Evidence that myocardial ischemia leads to coronary endothelial dysfunction was first described by Ku (1), who showed that a 90 min period of ischemia followed by reperfusion lasting 1 to 2 h was associated with decreased endothelium-dependent relaxation to thrombin in canine coronary arteries. These findings were rapidly extended to other endothelium-dependent vasodilators such as acetylcholine (2) and were confirmed in other species (3). Moreover, endothelial injury after ischemia and reperfusion is not a transient mechanism. Indeed, impaired endothelium-dependent relaxations may be prolonged at least four to 12 weeks after reperfusion in dogs (4) or rats (3). However, while acute dysfunction is the consequence of structural injury to the endothelial cells, chronic changes are rather a manifestation of dysfunctional regenerated endothelium (3), as previously shown after balloon injury (5).
The functional impairment observed after reperfusion appears to be mostly the consequence of the absence of structurally intact endothelial cells and not of a selective defect in NO synthase activity or a specific impairment of the transduction pathway linking receptors to NO synthase. This hypothesis is supported by our observations that incubation of the arterial segments with the substrate of NO synthase l-arginine did not reverse this impairment, in contrast to other diseases such as hypercholesterolemia, and is in accordance with electron microscopic studies showing structural changes in the vascular wall (3).
Several studies have investigated the mechanisms of endothelial injury after ischemia and reperfusion. First, such injury is absent or less pronounced after ischemia without reperfusion, suggesting that it is a manifestation of reperfusion injury. Moreover, the observation that the reintroduction of molecular oxygen at reperfusion is required to induce postischemic endothelial dysfunction is consistent with the view that ischemia-reperfusion injury may result from the generation of reactive oxygen species. This has now been clearly shown by the observation that endothelial dysfunction can be attenuated or prevented by scavengers of these species (6) or by treatment with superoxide dismutase and catalase in both large coronary arteries (7) and the microcirculation (8).
A potential mechanism that may explain the endothelial injury induced by free radicals is that, once produced, superoxide anions may directly inactivate NO (9,10). Because NO is a potent inhibitor of neutrophil activation and adhesion (11), the decreased NO production may lead to the development of an acute inflammatory response. Moreover, because free radicals produced during reperfusion also trigger the rapid adhesion of neutrophils to endothelial cells through the induction of adhesion molecules such as selectins or intercellular adhesion molecule-1 (ICAM-1) (12), the combined effects of decreased NO production and increased production of free radicals reinforce the adhesion of neutrophils to the endothelial cells, setting the stage for an amplification of neutrophilmediated endothelial injury.
PROTECTION OF CORONARY ENDOTHELIAL CELLS
Myocardial ischemia and reperfusion are responsible for a cascade of reactions leading to endothelial injury, characterized by a decrease in NO production. Given the important vasodilator property of NO, such an impairment may lead to increased coronary vasoconstriction and an increased risk of vasospasm. Moreover, endothelial dysfunction after reperfusion may favour platelet aggregation and thus increase the risk of thrombosis. Taken together, these observations indicate that the prevention of endothelial dysfunction or injury is an important therapeutic goal.
The most potent anti-ischemic intervention known to date is endogenous protection of ischemic myocardium, first described by Murry et al (13), and termed ‘preconditioning’. Indeed, submitting the heart to short episodes of ischemia separated by intermittent reperfusion makes it more resistant to prolonged ischemia and markedly limits infarct size. Preconditioning also confers protection against the severe ventricular rhythm disturbances that occur during subsequent ischemia and reperfusion, at least in the rat (14,15).
Although this protective phenomenon is now well described at the level of the cardiac myocytes, we were the first to describe such a protection at the level of the coronary endothelial cells. We developed a rat model of myocardial infarction to assess coronary endothelial dysfunction following ischemia and reperfusion. This model consists in submitting rats to 20 min ischemia followed by 60 min reperfusion. At the end of this infarct protocol, hearts are removed and coronary artery segments are dissected distal to the site of occlusion and mounted on small vessel wire myographs to study their reactivity in the presence of vasoactive agents. This model allows us to focus on the consequences of ischemia-reperfusion at the level of large epicardial or medium intra-myocardial coronary arteries.
With the use of this model we were able to show that ischemia-reperfusion significantly impairs endothelium-dependent relaxation of the coronary artery segments and that such an impairment can be reversed by preconditioning (16), consisting in our model of one cycle of 2 min ischemia and 5 min reperfusion followed by two cycles of 5 min ischemia and 5 min reperfusion.
Given the central role of neutrophil adhesion in endothelial injury during reperfusion, we hypothesized that the protective effect of preconditioning is due in part to a decreased production of endothelial adhesion molecules, leading to a lessened adhesion of neutrophils to the endothelium. To test this hypothesis, we subjected cultured rat aortic endothelial cells to anoxia-reoxygenation in vitro. In this context, we found that preconditioning abolished the increased expression of ICAM-1 and reduced the adhesion of neutrophils observed with anoxia-reoxygenation alone (17). Moreover, this protective effect was abolished by the free radical scavenger N2-mercapto propionylglycine (MPG), the protein kinase C inhibitor chelerythrin and the NO synthase inhibitor NG-nitro-l-arginine, suggesting a role for protein kinase C, free radicals and NO as triggers of early preconditioning in this model. Other triggers such as adenosine, KATP channels and bradykinin have also been described in the literature. Indeed, adenosine A1 and A3 receptor agonists as well as bradykinin B2 receptor activation may mimic the endothelial protection of preconditioning (18), while KATP channel blockers such as glibenclamide abolish the protection (19).
Although this early phase of preconditioning has efficient protective effects, its time course possibly limits its therapeutic application. However, although originally described as a transient phenomenon, ischemic preconditioning was subsequently found to have a biphasic effect, with an early phase of protection that develops within minutes after the initial ischemic insult and lasts 2 to 3 h (early preconditioning), and a late phase (delayed preconditioning) that appears 12 to 24 h later and lasts three to four days (20,21). In this context, we assessed whether the delayed phase of preconditioning, well documented at the level of the myocytes, could be observed at the level of the endothelium. For this purpose, we subjected rats to 20 min ischemia and 60 min reperfusion 24 h after preconditioning and again assessed endothelial dysfunction. In these experiments, we showed that delayed preconditioning protects the coronary endothelium against ischemia-reperfusion injury (6). Moreover, production of reactive oxygen species during preconditioning appears to have an important role. Indeed, the administration of MPG during preconditioning abolished its protective effect (6), suggesting that in addition to being mediators of endothelial injury during reperfusion after prolonged ischemia, free radicals produced during preconditioning protect the coronary endothelium from reperfusion injury 24 h later. With the use of the same experimental model, we also showed that delayed preconditioning can be mimicked by treatment 24 h before induction of prolonged ischemia-reperfusion with monophosphoryl lipid A, a nontoxic derivative of endotoxin (22), as observed before at the level of the myocardium (23,24).
Among the different possible triggers and mediators of delayed preconditioning, NO seems to have a dominant role. Indeed, it has been shown that brief ischemia was associated with a delayed increase in the coronary flow response to acetylcholine and bradykinin (25). Moreover, numerous studies have shown that NO plays a central part in the delayed protective effects of preconditioning in myocardial stunning (26) and infarction (27,28) through the inducible NO synthase (iNOS). Thus, we tested whether a similar involvement of iNOS can be observed at the level of the endothelial cells. For this purpose, we administered the selective iNOS inhibitor N-(3-(aminomethyl)benzyl)acetaminide (1400W) to preconditioned rats. Although 1400W indeed blocked iNOS activity in vivo under those experimental conditions, it did not affect the endothelial protection induced by delayed preconditioning (29). Thus, in contrast to myocytes, in endothelial cells iNOS is not involved as a mediator of delayed preconditioning. Moreover, in the context of monophosphoryl lipid A-induced delayed preconditioning, we also were unable to show the involvement of iNOS as a mediator of this protection (22), again in contrast to what happens at the level of the myocyte (30).
This opposite effect of iNOS inhibition may be explained in part by the distinct effect of NO on cardiomyocytes and endothelial cells. Indeed, iNOS activation by inflammatory stimuli produces high concentrations of NO (31). In cardiomyocytes, such high levels of NO probably induce a decrease in metabolic requirements and oxygen consumption, and this effect is probably responsible for the anti-ischemic role of iNOS. Contrary to cardiac myocytes, in endothelial cells an overproduction of NO may have deleterious consequences, in part by its reaction with oxygen-derived free radicals to produce highly reactive intermediates such as peroxynitrites.
While these experiments rule out the hypothesis that iNOS has a role in the endothelial effects of delayed pre-conditioning, they do not exclude a role for NO produced by endothelial NO synthase (eNOS). Indeed, endogenous stimulation of eNOS (for example by acetylcholine) may exert marked endothelial protective effects after ischemia and reperfusion (32). Moreover, our experiments on cultured endothelial cells found an increase of the nuclear factor AP-1 in the endothelial cell nucleus after preconditioning, and this may be correlated to the increased level of eNOS mRNA because AP-1 is responsible for the enhanced expression and activity of eNOS (33).
In this context, we recently evaluated the role of eNOS as a mediator of delayed preconditioning. Since there is no selective inhibitor available for this isoform, our previous results with 1400W allowed us to use the non-selective inhibitor NG-nitro-l-arginine methyl ester. Our results suggest that eNOS is a mediator of delayed preconditioning (34).
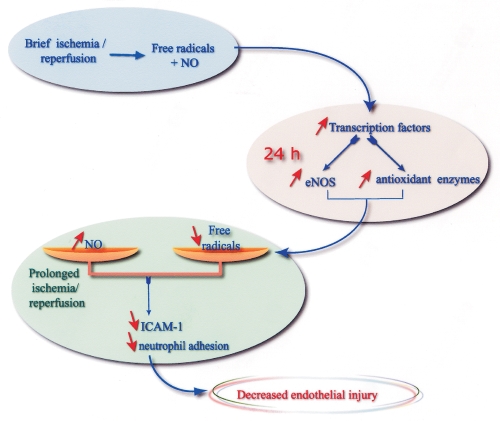
Taken together, our results suggest that the endothelial protective effects of late preconditioning involve complex interactions between NO and oxygen-derived free radicals both during brief ischemia-reperfusion (‘triggering’ mechanisms) and during reperfusion following prolonged ischemia. These potential protective pathways are summarized in Figure 1. Our hypothesis is that during preconditioning, reperfusion after brief ischemia triggers the release of both NO and free radicals (possibly at low levels), which interact to trigger delayed changes in gene expression or enzyme activity, for example, in terms of eNOS and antioxidant enzymes. The net result of these changes in activity is a change in the balance between NO and free radicals during prolonged ischemia and subsequent reperfusion. The increased NO bioavailability, together with the decreased production of free radicals, may in turn lead to a decreased adhesion of neutrophils during reperfusion, leading to endothelial protection. However, the exact respective roles of the changes in NO and oxidant stress in endothelial protection require further investigation.
Figure 1.
Potential pathways involved in the delayed endothelial protection induced by ischemic preconditioning. eNOS Endothelial nitric oxide synthase; ICAM-1 Intercellular adhesion molecule-1; NO Nitric oxide
CONCLUSION
In addition to exerting myocardial protective effects, preconditioning protects coronary endothelial cells against reperfusion injury. Identification of the mechanisms responsible for such an endogenous protective effect may lead to the development of new pharmacological interventions that can protect the endothelium during reperfusion, possibly leading to a decreased risk of vasospasm, platelet aggregation and atherosclerosis.
Acknowledgments
We thank Dr Abdesiam Chagraoui for artwork.
REFERENCES
- 1.Ku DD. Coronary vascular reactivity after acute myocardial ischemia. Science. 1982;218:576–8. doi: 10.1126/science.7123259. [DOI] [PubMed] [Google Scholar]
- 2.Van Benthuysen KM, McMurtry IF, Horwitz LD. Reperfusion after acute coronary occlusion in dogs impairs endothelium-dependent relaxation to acetylcholine and augments contractile reactivity in vitro. J Clin Invest. 1987;79:265–74. doi: 10.1172/JCI112793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaeffer N, Richard V, François A, Lallemand F, Henry JP, Thuillez C. Preconditioning prevents chronic reperfusion-induced coronary endothelial dysfunction in rats. Am J Physiol. 1996;271:H842–9. doi: 10.1152/ajpheart.1996.271.3.H842. [DOI] [PubMed] [Google Scholar]
- 4.Pearson PJ, Schaff HV, Vanhoutte PM. Long-term impairment of endothelium-dependent relaxations to aggregating platelets after reperfusion injury in canine coronary arteries. Circulation. 1990;81:1921–7. doi: 10.1161/01.cir.81.6.1921. [DOI] [PubMed] [Google Scholar]
- 5.Shimokawa H, Aarhus LL, Vanhoutte PM. Porcine coronary arteries with regenerated endothelium have a reduced endothelium-dependent responsiveness to aggregating platelets and serotonin. Circ Res. 1987;61:256–70. doi: 10.1161/01.res.61.2.256. [DOI] [PubMed] [Google Scholar]
- 6.Kaeffer N, Richard V, Thuillez C. Delayed coronary endothelial protection 24 hours after preconditioning. Role of free radicals. Circulation. 1997;96:2311–6. doi: 10.1161/01.cir.96.7.2311. [DOI] [PubMed] [Google Scholar]
- 7.Gross GJ, O’Rourke ST, Pelc LR. Myocardial and endothelial dysfunction after multiple, brief coronary occlusions: role of free radicals. Am J Physiol. 1992;263:H1703–9. doi: 10.1152/ajpheart.1992.263.6.H1703. [DOI] [PubMed] [Google Scholar]
- 8.Mehta JL, Nichols WW, Donnelly WH, et al. Protection by superoxide dismutase from myocardial dysfunction and attenuation of vasodilator reserve after coronary occlusion and reperfusion in dog. Circ Res. 1989;65:1283–95. doi: 10.1161/01.res.65.5.1283. [DOI] [PubMed] [Google Scholar]
- 9.Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250:H822–7. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- 10.Gryglewski RJ, Palmer RMJ, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived relaxing factor. Nature. 1986;320:454–60. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 11.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–5. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiagarajan RR, Winn RK, Harlan JM. The role of leukocyte and endothelial adhesion molecules in ischemia-reperfusion injury. Thromb Haemost. 1997;78:310–4. [PubMed] [Google Scholar]
- 13.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 14.Lawson CS, Hearse DJ. Ischemic preconditioning against arrhythmias: an antiarrhythmic or an anti-ischemic phenomenon? Ann NY Acad Sci. 1994;723:138–57. [PubMed] [Google Scholar]
- 15.Parratt JR, Vegh A, Kaszala K, Papp JG. Protection by preconditioning and cardiac pacing against ventricular arrhythmias resulting from ischemia and reperfusion. Ann NY Acad Sci. 1996;793:98–107. doi: 10.1111/j.1749-6632.1996.tb33508.x. [DOI] [PubMed] [Google Scholar]
- 16.Richard V, Kaeffer N, Tron C, Thuillez C. Ischemic preconditioning protects against coronary endothelial dysfunction induced by ischemia and reperfusion. Circulation. 1994;89:1254–61. doi: 10.1161/01.cir.89.3.1254. [DOI] [PubMed] [Google Scholar]
- 17.Beauchamp P, Richard V, Tamion F, et al. Protective effects of preconditioning in cultured rat endothelial cells. Effects of neutrophil adhesion and expression of ICAM-1 after anoxia and reoxygenation. Circulation. 1999;100:541–6. doi: 10.1161/01.cir.100.5.541. [DOI] [PubMed] [Google Scholar]
- 18.Giannella E, Mochmann HC, Levi R. Ischemic preconditioning prevents the impairment of hypoxic coronary vasodilatation caused by ischemia-reperfusion. Role of adenosine A1/A3 and bradykinin B2 receptor activation. Circ Res. 1997;81:415–22. doi: 10.1161/01.res.81.3.415. [DOI] [PubMed] [Google Scholar]
- 19.Bouchard JF, Lamontagne D. Mechanisms of protection afforded by preconditioning to endothelial function against ischemic injury. Am J Physiol. 1996;40:H1801–6. doi: 10.1152/ajpheart.1996.271.5.H1801. [DOI] [PubMed] [Google Scholar]
- 20.Kuzuya T, Hoshida S, Yamashita N, et al. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993;72:1293–9. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- 21.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–72. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 22.Richard V, Danielou E, Kaeffer N, Thuillez C. Delayed endothelial protective effects of monophosphoryl lipid A after myocardial ischemia and reperfusion in rats. J Mol Cell Cardiol. 1999;31:1117–23. doi: 10.1006/jmcc.1999.0943. [DOI] [PubMed] [Google Scholar]
- 23.Yao Z, Auchampach JA, Pieper GM, Gross GJ. Cardioprotective effects of monophosphoryl lipid A, a novel endotoxin analogue, in the dog. Cardiovasc Res. 1993;27:832–8. doi: 10.1093/cvr/27.5.832. [DOI] [PubMed] [Google Scholar]
- 24.Baxter GF, Goodwin RW, Wright MJ, Kerac M, Heads RJ, Yellon DM. Myocardial protection after monophosphoryl lipid A: studies of delayed anti-ischemic properties in rabbit heart. Br J Pharmacol. 1996;117:1685–92. doi: 10.1111/j.1476-5381.1996.tb15340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SJ, Ghaleh B, Kudej RK, Huang CH, Hintze TH, Vatner SF. Delayed enhanced nitric oxide-mediated coronary vasodilatation following brief ischemia and prolonged reperfusion in conscious dogs. Circ Res. 1997;81:53–9. doi: 10.1161/01.res.81.1.53. [DOI] [PubMed] [Google Scholar]
- 26.Bolli R, Manchikalapudi S, Tang XL, et al. The protective effect of late preconditioning against myocardial stunning in conscious rabbit is mediated by nitric oxide synthase. Evidence that nitric oxide acts both as a trigger and as a mediator of the late phase of ischemic preconditioning. Circ Res. 1997;81:1094–107. doi: 10.1161/01.res.81.6.1094. [DOI] [PubMed] [Google Scholar]
- 27.Imagawa J, Yellon DM, Baxter GF. Pharmacological evidence that inducible nitric oxide synthase is a mediator of delayed preconditioning. Br J Pharmacol. 1999;126:701–8. doi: 10.1038/sj.bjp.0702368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Y, Jones WK, Xuan YT, et al. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA. 1999;96:11507–12. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laude K, Richard V, Henry JP, Lallemand F, Thuillez C. Evidence against a role of inducible nitric oxide synthase in the endothelial protective effects of delayed preconditioning. Br J Pharmacol. 2000;130:1547–52. doi: 10.1038/sj.bjp.0703477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao L, Weber PA, Smith JR, Comerford ML, Elliott G. Role of inducible nitric oxide synthase in pharmacological “preconditioning” with monophosphoryl lipid A. J Mol Cell Cardiol. 1997;29:1567–76. doi: 10.1006/jmcc.1997.0390. [DOI] [PubMed] [Google Scholar]
- 31.Stoclet JC, Muller B, Andiantsitohaina R, Kleschyov A. Overproduction of nitric oxide in the pathophysiology of blood vessels. Biochemistry. 1998;63:826–2. [PubMed] [Google Scholar]
- 32.Richard V, Blanc T, Kaeffer N, Tron C, Thuillez C. Myocardial and coronary endothelial protective effects of acetylcholine after myocardial ischaemia and reperfusion in rats: role of nitric oxide. Br J Pharmacol. 1995;115:1532–8. doi: 10.1111/j.1476-5381.1995.tb16647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Ongil S, Hernandez-Perera O, Navarro-Antolin J, et al. Role of reactive oxygen species in the signalling cascade of cyclosporine A-mediated upregulation of eNOS in vascular endothelial cells. Br J Pharmacol. 1998;124:447–54. doi: 10.1038/sj.bjp.0701847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laude K, Richard V, Thuillez C.Endothelial nitric oxide synthase is a mediator of the delayed coronary endothelial protective effects of preconditioning Circulation 2000102II271(Abst) [Google Scholar]