Abstract
OBJECTIVE:
To establish whether the total antioxidant capacity of nonalcoholic extracts of three Argentine red wines (RWE) is correlated with their protection against ischemia-reperfusion injury.
ANIMALS AND METHODS:
The antioxidant properties of three RWE were determined using different free radical-generating systems. To examine the effects of these RWE during a 20 min global ischemic period followed by 30 min of reperfusion, isolated rat hearts received 50 μg/mL of RWE 1 (cabernet-sauvignon), RWE 2 (malbec) or RWE 3 (a commercial mixture of cabernet-sauvignon, malbec and merlot) 10 min before and after ischemia. Left ventricular developed pressure (LVDP), maximal velocity of rise of left ventricular pressure (+dP/dtmax) and left ventricular end-diastolic pressure (LVEDP) were used to assess contractility and diastolic function.
RESULTS:
All RWE inhibited lipid peroxidation induced by the Cl4C/NADPH system in a similar proportion (42±4%, 47±9% and 43±14% for RWE 1, RWE 2 and RWE 3, respectively). The scavenging activity of superoxide anion and 2,2-diphenyl-1-picryl-hydrazyl radical was about the same with the three RWE. In hearts without RWE treatment, LVDP and +dP/dtmax were 61±4% and 62±5%, respectively, at the end of the reperfusion period. Infusion of RWE 1 and RWE 2 significantly improved postischemic recovery (LVDP and +dP/dtmax were 102±4% and 101±4% for RWE 1 and 92±5% and 91±5% for RWE 2, respectively) and attenuated the increase of LVEDP. RWE 3 did not improve either systolic or diastolic dysfunction.
CONCLUSION:
These data show that although the three non-alcoholic RWE exhibit a similar total antioxidant capacity, only two of them protect the heart against myocardial stunning, suggesting that the protective effect is not primarily linked to the anti-oxidant properties of the extracts.
Keywords: Antioxidant capacity, Ischemia, Red wine, Reperfusion
Epidemiological studies consistently showed that light to moderate intake of alcoholic beverages is associated with a lower risk of coronary artery disease than no intake of alcoholic beverages (1–3). According to St Leger et al (4) and Renaud and de Lorgeril (5), the greatest benefit comes from the consumption of wine, especially red wine. Red wine consumption causes a decrease in platelet aggregation (6), and an increase in high density lipoprotein (7) and a decrease in low density lipoprotein peroxidation (8) independently of the alcohol content of the wine. Although other beneficial effects such as endothelium-dependent vasorelaxation in isolated arteries (9) and increase in coronary reserve in human beings (10) have been found, little information is available about the acute effects of red wine in ischemic and reperfusion injury (11–13).
Most of the studies have attributed the beneficial actions of the red wine to the antioxidant capacity of their phenolic components (12–14). The aim of the present study was to get insight into the possible correlation of the total antioxidant capacity of nonalcoholic extracts of three red wines (RWE) with their effects on myocardium subjected to ischemia and reperfusion.
ANIMALS AND METHODS
Preparation of the nonalcoholic RWE:
Red wine (200 mL) was vacuum evaporated (less than 30°C) to obtain 3.5 g of jelly-like extract. Vacuum evaporation was used to facilitate the study of the biological effects of red wine components without ethanol interference. The extract (0.5 g) was then solubilized in 100 mL of deionized double-distilled water and filtered through a 20 μm Millipore filter. Aliquots from the resulting solution were kept frozen until experiments were carried out. The wines were commercially available and obtained from local supermarkets. Three red wines from Mendoza, Argentina were used: cabernet-sauvignon (RWE 1), malbec (RWE 2) and a commercial mixture of cabernet-sauvignon, malbec and merlot grape varieties (RWE 3). The year of production was 1998 or 1999. To avoid commercial interests, the names of the producers will not be revealed.
Determination of antioxidant properties
Liver microsomal preparations:
Livers from male Wistar rats (200 to 250 g body weight) were dissected rapidly, rinsed in ice-cold saline and homogenized as described previously (15). Liver microsomes were prepared by standard differential centrifugation techniques as described by Slater and Sawyer (16). Aliquots of microsomal suspensions in 30 mM phosphate buffer (pH 7.4) containing 8.8 g/L KCl were stored at −80°C for two months.
Lipid peroxidation induced by the Fe2+/ascorbate system:
Reaction mixtures contained 2 mg of microsomal protein/mL in 0.1 M Tris-HCl, pH 7.4. Peroxidation was induced by FeSO4 (5 μM) and ascorbate (500 μM) (17). The samples were incubated in triplicate at 37°C for 20 min in the presence of different concentrations of the extracts. Products of lipid peroxidation were determined by the thiobarbituric acid method, with the absorbance measured at 532 nm (18). Appropriate controls were set up to discard any possible interference with the thiobarbituric acid assay.
Lipid peroxidation induced by the Cl4C/NADPH system:
Reaction mixtures contained 1.5 mg of microsomal protein and an NADPH-generating system (0.2 mM NADP+, 4 mM glucose-6-phosphate, 0.6 U glucose-6-phosphate dehydrogenase) in the same buffer as above (16). Peroxidation was started by the addition of Cl4C (0.02 M). After 15 min of incubation at 37°C, thiobarbituric acid-reactive substances were determined as above. Butylated hydroxytoluene (BHT) was used as a reference compound in all the lipid peroxidation assays.
Superoxide radical generation:
Superoxide anion generated by oxidation of hypoxanthine (100 μM) with xanthine oxidase grade I (0.006 U; 1 U converts 1 μmol/min of xanthine to uric acid at pH 7.5 at 25°C) in 1 mL of 10 mM KH2PO4-KOH buffer (pH 7.4) was detected by 100 μM nitroblue tetrazolium (NBT) and followed spectrophotometrically at 560 nm (19). Control experiments were performed to rule out the possibility of direct reduction of NBT or xanthine oxidase inhibition by the extracts. The influence on enzymatic activity was followed by measuring the uric acid formation from xanthine (15 min incubation at 25°C) and absorbance was measured at 295 nm. Pirogallol was used as a reference compound.
Reduction of 2,2-diphenyl-1-picrylhydrazyl radical:
For the spectrophotometric assay, 1.5 mL of a 20 mg/mL 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution in methanol was added to 0.75 mL of a solution of RWE in methanol (20). Absorbance at 517 nm was determined after 10 min and the percentage of activity was calculated. Appropriate controls were performed to discard a possible interference of the extracts with the absorbance measured at 517 nm. BHT was used as a reference compound.
Hydroxyl radical generation:
Hydroxyl radical was generated by incubating the reaction mixture containing 20 μM FeCl3, 1.4 mM H2O2, 2.8 mM deoxyribose, 2 mM EDTA and 50 μM ascorbate in 1 mL of 10 mM KH2PO4-KOH buffer, pH 7.4, for 60 min at 37°C (17). Deoxyribose degradation by hydroxyl radical was estimated by using the thiobarbituric acid method. Dimethylsulphoxide 20 mM was used as a reference compound.
In all assays cited above the extracts were tested at a final concentration of 50 μg/mL.
Isolated heart preparation:
Wistar male rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (60 mg/kg body weight). The heart was rapidly excised and perfused by the nonrecirculating Langendorff technique with Ringer’s solution containing (in mmol/L) NaCl 118, KCl 5.9, MgSO4 1.2, CaCl2 1.35, NaCO3H 20 and dextrose 11.1. The buffer was saturated with a mixture of 95% O2 and 5% CO2, pH 7.4, and was maintained at 37°C. The conductive tissue in the atrial septum was damaged with a fine needle to achieve atrioventricular block, and stimulation electrodes were sutured to the right ventricle to maintain the heart rate constant at 280 beats/min. The place of stimulation was selected to avoid damage of the suture on the left ventricular myocardium and was based on previous experience (21–23). A latex balloon tied to the end of a polyethylene tube was passed into the left ventricle through the mitral valve; the opposite end of the tube was then connected to a Statham P23XL pressure transducer (Statham Instruments, USA). The balloon was filled with water to give a left ventricular end-diastolic pressure (LVEDP) of 8 to12 mmHg, and this volume was unchanged for the remainder of the experiment. Coronary perfusion pressure was monitored at the point of cannulation of the aorta and adjusted to approximately 60 to 70 mmHg. Coronary flow, controlled with a peristaltic pump, was 11±2 mL/min. Left ventricular pressure and its first derivative (dP/dt) were recorded with a direct writing recorder. This widely used technique facilitates the assessment of contractility under controlled conditions.
Experimental protocols:
After 10 min of stabilization, the following experimental protocols were performed.
Ischemic control (n = 10):
Hearts were subjected to 20 min of normothermic global ischemia followed by 30 min of reperfusion. Global ischemia was induced by stopping the perfusate inflow line while the heart was placed in a saline bath held at 37°C.
RWE (n=18):
Hearts were treated 10 min before ischemia and the initial 10 min of reperfusion with three RWE: RWE 1 (n=8); RWE 2 (n=6) and RWE 3 (n=6) at 0.5 mg/min. Given that coronary flow is 10 mL/min, a concentration of 50 μg/mL was infused over 20 min. The total amount administered was 0.3% of the original extract obtained from 200 mL of wine. This amount results in 1 to 2 μg/mL of polyphenols, as estimated by Iijima et al (24), and is the approximate concentration reached in human blood after an intake of that volume of wine.
Systolic and diastolic function:
Myocardial contractility was assessed by the left ventricular developed pressure (LVDP), obtained by subtraction of LVEDP values from the left ventricular peak pressure and maximal velocity of rise of left ventricular pressure (+dP/dtmax). Data are expressed as the percentage of their respective preischemic values. Diastolic function was assessed by the isovolumic LVEDP.
Statistical analysis:
Data are given as mean ± SE. The Tukey-Kramer multiple comparisons test was used to analyze the antioxidant properties of the three RWE and the control.
LVDP, +dP/dtmax and LVEDP were analyzed by repeated measures of one-way analysis of variance with the Newman-Keul’s test for multiple comparisons among groups.
RESULTS
Lipid peroxidation:
Membrane lipids are particularly susceptible to oxidation. Incubation of rat liver microsomes with Fe2+/ascorbate or Cl4C/NADPH at pH 7.4 causes rapid peroxidation, detectable by the thiobarbituric acid method. Table 1 shows the thiobarbituric acid-reactive substances, expressed as nmol/mL of malonaldehyde produced in the presence of 50 μg/mL of each RWE. When the peroxidative process was induced by Fe2+/ascorbate, no change was detected with the addition of the RWE. With the Cl4C/NADPH system, the RWE produced lower malonaldehyde values than in control conditions. These data indicate that all RWE examined possess a significant capacity for attenuating membrane lipid peroxidation.
TABLE 1.
Effects of nonalcoholic red wine extracts (RWE) on lipid peroxidation of rat liver microsomes, expressed as malonaldehyde (nmol/mL) produced in the presence of 50 μg/mL of each of three extracts
| Peroxidation system | ||
|---|---|---|
| Fe2+/ascorbate | Cl4C/NADPH | |
| Control | 32.93±1.27 | 5.17±0.58 |
| RWE 1 | 31.38±0.75 | 3.00±0.35* |
| RWE 2 | 32.30±0.78 | 2.87±0.54* |
| RWE 3 | 31.22±0.82 | 2.97±0.55* |
| BHT | 0.73±0.22** | 0.33±0.15** |
Data are mean ± SE (n=4). BHT Butylated hydroxytoluene (reference compound).
P<0.05,
P<0.001 with respect to control
Scavenger activity:
Table 2 shows scavenging activities of RWE expressed as change of absorbance produced by NBT at 560 nm. The three RWE did not produce inhibition of xanthine oxidase activity and provoked a similar inhibition (between 50% and 60%) of superoxide anion generation and approximately 75% of reduction of DPPH radical production.
TABLE 2.
Scavenging activities of nonalcoholic red wine extracts (RWE) expressed as the change in absorbance produced by nitro blue tetrazolium at 560 nm (superoxide anion) or 517 nm (2,2-diphenyl-1-picrylhydrazyl [DPPH] radical)
| Superoxide anion | DPPH radical | |
|---|---|---|
| Control | 0.139±0.006 | 0.269±0.008 |
| RWE 1 | 0.055±0.007* | 0.051±0.003* |
| RWE 2 | 0.053±0.004* | 0.070±0.006* |
| RWE 3 | 0.051±0.003* | 0.059±0.006* |
| Pirogallol | 0.011±0.001* | nd |
| BHT | nd | 0.016±0.001* |
Data are mean ± SE (n=3). BHT Butylated hydroxytoluene (reference compound); nd Not determined.
P<0.001 with respect to control
In another assay, the RWE did not scavenge the hydroxyl radical produced in the Fe2+-EDTA + H2O2 system. The results of deoxyribose degradation expressed as absorbance units of RWE 1, RWE 2 and RWE 3 were 0.6989±0.039, 0.6977±0.038 and 0.6796±0.053, respectively. These values were not statistically different from that obtained in the control sample (0.6626±0.055 absorbance units).
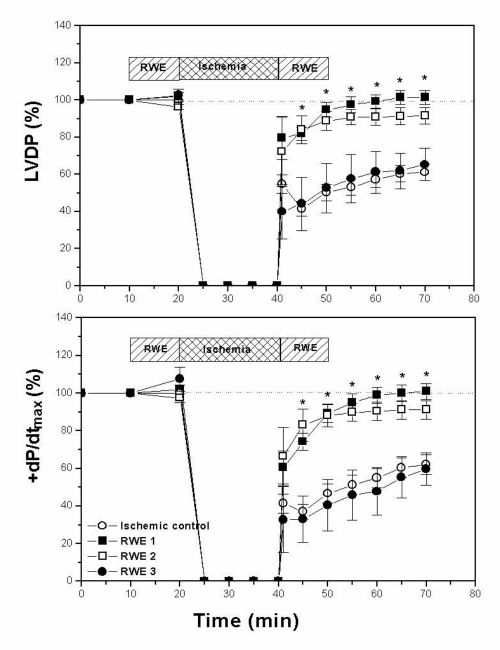
Figure 1 shows the effects of RWE 1, RWE 2 and RWE 3 on contractility during the 20 min global ischemia and 30 min reperfusion periods. In control (untreated) hearts there were decreases at the end of the reperfusion period in LVDP and +dP/dtmax to 61±4% and 62±5%, respectively, of baseline values. RWE 1 infusion 10 min before and after the ischemic period improved postischemic recovery to preischemic values. At the end of the reperfusion period, both parameters (LVDP and +dP/dtmax) reached values that were 102±4% and 101±4%, respectively, of the preischemic value. Similarly, RWE 2 protected the heart against ischemia and reperfusion injury, as shown by the improvement in postischemic recovery; after 30 min of reperfusion, LVDP and +dP/dtmax values were 92±5% and 91±5%, respectively. Treatment with RWE 3 did not improve postischemic recovery. At the end of reperfusion, LVDP and +dP/dtmax values were 65±9% and 60±9%, respectively, not statistically different from those obtained in hearts without any treatment.
Figure 1.
Changes in left ventricular developed pressure (LVDP) and maximal velocity of rise of left ventricular pressure (+dP/dtmax) of hearts exposed to 20 min of global ischemia followed by 30 min of reperfusion, expressed as a percentage of the preischemic values. At the end of reperfusion period both parameters recovered completely in hearts treated with nonalcoholic red wine extract (RWE) 1, improved significantly with RWE 2 and did not change with RWE 3 with respect to ischemic control hearts.*P<0.05 with respect to ischemic control
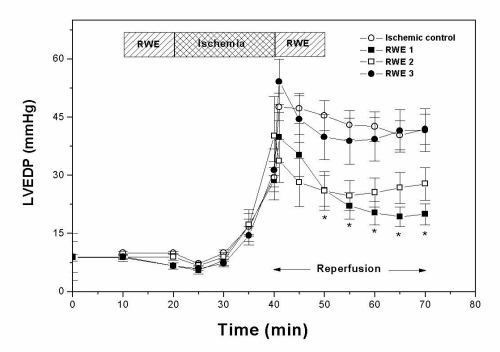
Diastolic function, assessed by measuring the isovolumic LVEDP, is shown in Figure 2. This parameter increased in control hearts from 10.0±0.4 mmHg before ischemia to 30±4 mmHg at the end of the ischemic period and 42±4 mmHg after 30 min of reperfusion. The impairment of systolic and diastolic function after the 20 min ischemic period is in agreement with previous results from this and other laboratories (22,25). RWE 1 and RWE 2, but not RWE 3, decreased the increase in diastolic stiffness obtained during reperfusion. At the end of reperfusion, LVEDP was 20±3, 28±4 and 42±5 mmHg when the hearts were treated with RWE 1, RWE 2 and RWE 3, respectively. The three RWE did not modify contracture obtained in ischemic control hearts.
Figure 2.
Effects of nonalcoholic red wine extract (RWE) on left ventricular end-diastolic pressure (LVEDP). RWE1 and RWE2, but not RWE3, decreased the increment in diastolic stiffness induced by ischemia-reperfusion. *P<0.05 with respect to ischemic control
In previous pilot experiments, the improvement of systolic and diastolic dysfunction induced by RWE was shown to be concentration dependent (unpublished data). The concentration that induced the highest protection was selected for this study.
DISCUSSION
The results presented herein show that the cardioprotection from ischemia-reperfusion elicited by RWE is not primarily associated with their total antioxidant capacity. Three RWE with similar total antioxidant capacity did not show the same protective effect on this aspect of ischemia-reperfusion injury.
Experiments were performed using a short ischemic period because it results in less impairment in function and no significant irreversible damage compared with longer periods (23). With this protocol, RWE 1 and RWE 2, but not RWE 3, improved significantly the recovery of the depressed function after ischemia-reperfusion. If we accept that there are no irreversible changes after an ischemic period of 20 min (23,26), we can conclude that two of the three RWE are protective against myocardial stunning.
Whatever factors are involved in contractile dysfunction after such a brief ischemic episode were significantly attenuated by RWE 1 and RWE 2, but not by RWE 3. The RWE that was not protective had sufficient concentrations of cabernet-sauvignon and malbec to induce protection if the grape variety were the cause of the protection. This finding suggests that it is not the kind of grape used in the wine that determines the protection.
The alterations following ischemia and reperfusion, characteristic of stunned heart, have been attributed to free radical generation (27) and Ca2+ overload (26). It has been previously shown that administration of scavengers (28) and interventions that diminish the intracellular Ca2+ concentration (29) attenuate contractile dysfunction.
The majority of the beneficial actions of red wine have been attributed to its antioxidant properties. These are mechanisms that need some time and are perhaps different from the acute effects described here. In the present study the three RWE similarly inhibited microsomal peroxidation, but only when the peroxidative process was induced by Cl4C/NADPH. Also, the extracts proved to have a similar capacity to scavenge the superoxide anion and the DPPH radical. Although the three RWEs exhibited similar antioxidant properties, only two of the three protected against ischemia-reperfusion impairment of contractility. However, we can not rule out that the antioxidant properties assessed in in vitro systems reflect their actions under in vivo conditions. It is recognized that the main problem is that the ‘antioxidant’ function is more complex than simple free radical scavenging and that dietary supplementation with a particular anti-oxidant is likely to perturb the natural balance of other antioxidants (30).
Previous studies have shown that the polyphenolic fractions of RWE exhibit a physiological profile similar to (24) or different from (31) the original extract. In ischemia and reperfusion, recent studies have shown that resveratrol, one constituent of red wine, is cardioprotective and has an anti-arrhythmic effect (11–13).
Another mechanism involved in protection may be related to nitric oxide production. Recent studies have proposed the participation of nitric oxide in the vasodilator effect of red wines (9,32,33). Nitric oxide increases cGMP and decreases cAMP concentrations, nucleotides that may act synergistically in reducing Ca2+ influx (34) and myocardial stunning (35).
In summary, this study shows that a nonalcoholic RWE may provide acute benefits against ischemia-reperfusion injury, which are not necessarily associated with the total anti-oxidant capacity of the wine.
REFERENCES
- 1.Klatsky AL, Armstrong MA, Friedman GD. Risk of cardiovascular mortality in alcohol drinkers, exdrinkers and non-drinkers. Am J Cardiol. 1990;66:1237–42. doi: 10.1016/0002-9149(90)91107-h. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg DM, Hahn SE, Parkes JG. Beyond alcohol: beverage consumption and cardiovascular mortality. Clin Chim Acta. 1995;237:155–87. doi: 10.1016/0009-8981(95)06069-p. [DOI] [PubMed] [Google Scholar]
- 3.Klatsky AL. Moderate drinking and reduced risk of heart disease. Alcohol Res Health. 1999;23:15–23. [PMC free article] [PubMed] [Google Scholar]
- 4.St Leger AS, Cochrane AL, Moore F. Factors associated with cardiac mortality in developed countries with particular reference to the consumption of wine. Lancet. 1979;i:1017–20. doi: 10.1016/s0140-6736(79)92765-x. [DOI] [PubMed] [Google Scholar]
- 5.Renaud S, de Lorgeril M. Wine, alcohol, platelets and the French paradox for coronary heart disease. Lancet. 1992;339:1523–6. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 6.Denrow H, Slane P, Folts J. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation. 1995;91:1182–8. doi: 10.1161/01.cir.91.4.1182. [DOI] [PubMed] [Google Scholar]
- 7.Gaziano JM, Buring JE, Breslow JL, et al. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions and decreased risk of myocardial infarction. N Engl J Med. 1993;329:1829–34. doi: 10.1056/NEJM199312163292501. [DOI] [PubMed] [Google Scholar]
- 8.Frankel EN, Kanner J, German JB. Inhibition of human low-density lipoprotein by phenolic substances in red wine. Lancet. 1993;341:454–7. doi: 10.1016/0140-6736(93)90206-v. [DOI] [PubMed] [Google Scholar]
- 9.Flesch M, Schwarz A, Böhm M. Effects of red and white wine on endothelium-dependent vasorelaxation of rat aorta and human coronary arteries. Am J Physiol. 1998;275:H1183–90. doi: 10.1152/ajpheart.1998.275.4.H1183. [DOI] [PubMed] [Google Scholar]
- 10.Shimada K, Watanabe H, Hosoda K, Takeuchi K, Yoshikawa J. Effect of red wine on coronary flow-velocity reserve. Lancet. 1999;354:1002. doi: 10.1016/S0140-6736(99)03478-9. [DOI] [PubMed] [Google Scholar]
- 11.Sato M, Ray PS, Maulik G, et al. Myocardial protection with red wine extract. J Cardiovasc Pharmacol. 2000;35:263–8. doi: 10.1097/00005344-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Ray PS, Maulik G, Cordis GA, Bertelli AA, Bertelli A, Das DK. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia-reperfusion injury. Free Radic Biol Med. 1999;27:160–9. doi: 10.1016/s0891-5849(99)00063-5. [DOI] [PubMed] [Google Scholar]
- 13.Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res. 2000;47:549–55. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- 14.Hertog MGL, Feskens EJM, Krombout D. Antioxidant flavonols and coronary heart disease risk. Lancet. 1997;349:699. doi: 10.1016/S0140-6736(05)60135-3. [DOI] [PubMed] [Google Scholar]
- 15.Schinella G, Marín MC, Tacconi de Analiz MJ, Mordujovich de Buschiazzo P, Tournier H. Antioxidant defence system and lipid peroxidation in lactation rats: effect of dietary vitamin E during gestation and lactation. Nutr Res. 1999;19:795–803. [Google Scholar]
- 16.Slater TF, Sawyer BC. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reaction in rat liver fractions in vitro. Biochem J. 1971;123:805–14. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halliwell B. How to characterize a biological antioxidant. Free Radic Res Commun. 1990;9:1–32. doi: 10.3109/10715769009148569. [DOI] [PubMed] [Google Scholar]
- 18.Pompella A, Maellaro E, Casini A, Ferrali M, Ciccoli L, Comporti M. Measurement of lipid peroxidation in vivo: a comparison of different procedures. Lipids. 1987;22:206–11. doi: 10.1007/BF02537304. [DOI] [PubMed] [Google Scholar]
- 19.Sanz MJ, Ferrandiz M, Cejudi M, et al. Influence of series of natural flavonoids on free radical generating systems and oxidative stress. Xenobiotica. 1994;24:689–99. doi: 10.3109/00498259409043270. [DOI] [PubMed] [Google Scholar]
- 20.Cavin A, Hostettmann K, Dyatwyko W, Potteral O. Antioxidant and lipophilic constituents of Tinospora crispa. Planta Med. 1998;64:393–6. doi: 10.1055/s-2006-957466. [DOI] [PubMed] [Google Scholar]
- 21.Mosca SM, Carriquiriborde M, Cingolani HE. Biphasic changes in relaxation following reperfusion after myocardial ischemia. Mol Cell Biochem. 1996;160/161:123–8. doi: 10.1007/BF00240041. [DOI] [PubMed] [Google Scholar]
- 22.Mosca SM, Gelpi RJ, Milei J, Fernández Alonso G, Cingolani HE. Is stunning prevented by ischemic preconditioning? Mol Cell Biochem. 1998;186:123–9. [PubMed] [Google Scholar]
- 23.Mosca SM, Salas MA, Portiansky EL, Cingolani HE, Moreyra AE. Early ischemic preconditioning protects against infarct and myocardial stunning in isolated rat heart. Exp Clin Cardiol. 1999;4:43–8. [Google Scholar]
- 24.Iijima K, Yoshizumi M, Hashimoto M, et al. Red wine polyphenols inhibit proliferation of vascular smooth muscle cells and downregulate expression of cyclin A gene. Circulation. 2000;101:805–11. doi: 10.1161/01.cir.101.7.805. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee A, Locke-Winter C, Rogers KB, et al. Preconditioning against myocardial dysfunction after ischemia and reperfusion by an α1-adrenergic mechanism. Circ Res. 1993;73:656–70. doi: 10.1161/01.res.73.4.656. [DOI] [PubMed] [Google Scholar]
- 26.Kusuoka H, Porterfield JK, Weissman HF, Weisfeldt ML, Marbán E. Pathophysiology and pathogenesis of stunned myocardium: depressed Ca2+ activation of contraction as a consequence of reperfusion-induced cellular calcium overload in ferret hearts. J Clin Invest. 1987;79:950–61. doi: 10.1172/JCI112906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao WD, Liu Y, Marbán E. Selective effects of oxygen free radicals on excitation-contraction coupling in ventricular muscle. Circulation. 1996;94:2597–604. doi: 10.1161/01.cir.94.10.2597. [DOI] [PubMed] [Google Scholar]
- 28.Koerner JE, Anderson BA, Dage RC. Protection against postischemic myocardial dysfunction in anesthetized rabbits with scavengers of oxygen-derived free radicals: superoxide dismutase plus catalase, N-2 mercaptopropionyl glycine and captopril. J Cardiovasc Pharmacol. 1991;17:185–91. doi: 10.1097/00005344-199102000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Moreyra AE, Carriquiriborde M, Mosca SM. Protective effect of nifedipine on myocardial stunning in isolated rabbit hearts: role of high energy phosphates stores. Arch Physiol Biochem. 1996;104:265–71. doi: 10.1076/apab.104.3.265.12907. [DOI] [PubMed] [Google Scholar]
- 30.Hensley K, Robinson KA, Gabbita P, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–62. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 31.Andriambeloson E, Magnier C, Haan-Archipoff G, et al. Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. J Nutr. 1998;128:2324–33. doi: 10.1093/jn/128.12.2324. [DOI] [PubMed] [Google Scholar]
- 32.Fitzpatrick DF, Hirschfield SL, Coffey RG. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am J Physiol. 1993;265:H774–8. doi: 10.1152/ajpheart.1993.265.2.H774. [DOI] [PubMed] [Google Scholar]
- 33.Stoclet J-C, Andriambeloson E, Andriantsitohaina R. Endothelial NO release caused by red wine polyphenols with specific structures. Exp Clin Cardiol. 2000;5:24–7. [PubMed] [Google Scholar]
- 34.Sperelakis N. Regulation of calcium slow channels of heart by cyclic nucleotides and effects of ischaemia. Adv Pharmacol. 1994;31:1–24. doi: 10.1016/s1054-3589(08)60605-5. [DOI] [PubMed] [Google Scholar]
- 35.Rakhit RD, Edwards RJ, Mockridge JW, et al. Nitric-oxide-induced cardioprotection in cultured rat ventricular myocytes. Am J Physiol. 2000;278:H1211–7. doi: 10.1152/ajpheart.2000.278.4.H1211. [DOI] [PubMed] [Google Scholar]