Abstract
Despite an enormous amount of research carried out in the past 10 to 20 years, the role of the renin-angiotensin system in the development of heart failure is still not very well understood. This review looks at preclinical data on the role of angiotensin II as a circulating and local hormone, and the effects of stimulation of the respective receptors in heart tissue. Recent large scale clinical trials have begun to furnish evidence of the effects of blocking the renin-angiotensin system in patients with heart failure using angiotensin-converting enzyme inhibitors or, more recently, angiotensin II receptor blockers that act directly at the receptor level, independent of pathways for angiotensin II generation. Results so far indicate that there are benefits from optimizing the blockade, but open questions remain, such as the role of endothelin and bradykinins, and the extent of crosstalk between the different systems.
Keywords: Angiotensin II, Angiotensin II receptor blocker, Angiotensin-converting enzyme inhibitors, Apoptosis, Atherosclerosis, Chymase, Heart failure, Hypertension, Valsartan
The condition of heart failure (HF) has been known from early human history, but its importance as a killer in early times was probably minor and has grown with the increase in the average human life span and prosperity. Today, in the prosperous world, the overall prevalence of HF is greater than 100/1000 people over 65 years of age. HF is responsible for around 2% of total healthcare costs and the numbers are rising. Hospital admission rates in the United States, United Kingdom and Scandinavia have doubled in the past 10 to 15 years (1). This steady increase is unique for a major cardiovascular disease (2,3).
The main risk factors for HF are well known: smoking, hypertension, atherosclerosis and diabetes. About 80% of all HF events occur in persons in the upper quintile of multivariate risk (4). The Studies of Left Ventricular Dysfunction (SOLVD) reported that 75% of the cases of chronic HF in male white patients could be attributed to coronary artery disease (5). Genes also seem to play a part: African-Americans have over twice the mortality rate of whites (6).
Hypertension has long been associated with HF. In the Framingham heart study, hypertension and coronary artery disease accounted for 90% of cases of HF (7). The correlation between high blood pressure and cardiovascular disease is valid regardless of age, ethnicity and sex (8). Treatment of high blood pressure has been described as one of the major medical highlights of the past half century (9), and though the historical focus has usually been on diastolic blood pressure, recent epidemiological work has shown that both systolic and diastolic blood pressure are important determinants of cardiovascular risk (10).
ROLE OF THE RENIN-ANGIOTENSIN SYSTEM
A common denominator in hypertension, atherosclerosis and HF is the renin-angiotensin system (RAS). Components of the RAS have a multitude of activities, both local and global, and though interfering with the RAS is among the most widespread strategies to lower blood pressure, many beneficial effects from treatments that interfere with the RAS appear to be independent of the resulting changes in blood pressure. It has long been known that all blood pressure-reducing agents are able to prevent heart disease, but, at least in monotherapy, only antihypertensive drugs that act on the RAS are of notable benefit to patients once HF occurs (11).
The vasoactive peptide angiotensin II (Ang II) is the central molecule of the RAS, with a multitude of actions (Table 1) (12). Ang II mediates increases in blood pressure and stimulation of cell growth, cell regeneration and cholesterol uptake into blood vessels (13–15). Both the antihypertensive and the protective effects of RAS modulators are related to their influence on Ang II actions.
TABLE 1.
Effects of angiotensin II related to the development of heart failure
| Vascular |
|
| Kidney and adrenal gland |
|
| Brain |
|
| Heart |
|
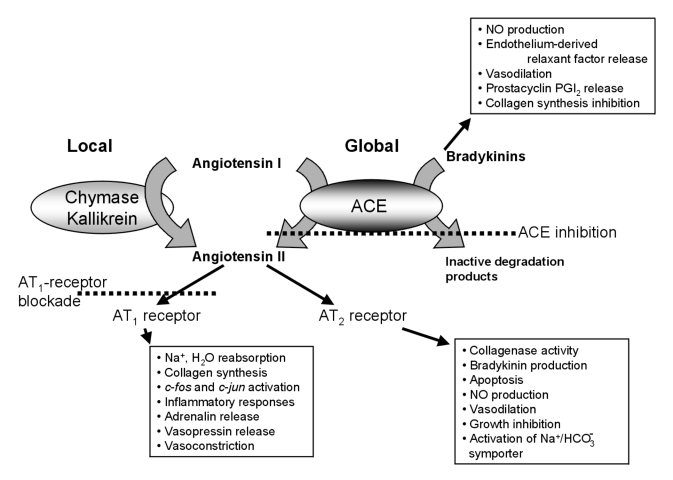
The role of Ang II in HF, as in hypertension, is complex, and it is a safe assumption that new interactions and interdependencies will continue to be described for several years yet. Two distinctions are important to keep in mind when assessing the effect of Ang II in a given setting (Figure 1):
Ang II can act as a circulating hormone or as a local hormone. Circulating Ang II is synthesized from the precursor angiotensin I by the angiotensin-converting enzyme (ACE). Vascular ACE is upregulated in HF (16,17), but the pathways for Ang II formation may differ depending on whether production is global or local. Interfering with one aspect of Ang II synthesis may not affect other pathways and actions.
The actions of Ang II are mediated by two receptors, type 1 (AT1) and type 2 (AT2), which have often opposing effects (reviewed, for example, by de Gasparo et al [18] and Unger et al [19,20]). Thus, the effects of Ang II in a given tissue depend on the distribution pattern of the two receptors in that tissue.
Figure 1.
Schematic representation of the synthesis pathways and some of the actions of angiotensin II. ACE Angiotensin-converting enzyme; AT1 Angiotensin II type 1; AT2 Angiotensin II type 2; PGI2 Prostacyclin
The situation is complicated by the fact that Ang II is involved not only in the processes that lead to hypertension and atherosclerosis but also in the responses to these phenomena. A recent study of men with hypercholesterolemia showed that Ang II receptor density in platelets increases, and thus the biological effects of Ang II are enhanced, in hypercholesterolemia (21). Cholesterol-lowering treatment reduced Ang II receptor density and reversed the increased blood pressure response to Ang II infusion. Similar feedback loops exist in the buildup to HF: Ang II as a circulating hormone can induce hypertension (through stimulation of aldosterone and arginine vasopressin release and an increase in vascular resistance). The development of left ventricular hypertrophy in response to Ang II-induced hypertension is associated with a local activation of the RAS (22). Such feedback loops aggravate the effects of cardiac hypertrophy.
ACE INHIBITION IN HF
Probably the most successful treatment for HF during the past 20 years has been ACE inhibition. Since the first trial, the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS), in 1987 (23), the efficacy of ACE inhibitors has been proved in a number of clinical trials. A recent meta-analysis of five large trials (Survival and Ventricular Enlargement study [SAVE], Acute Infarction Ramipril Efficacy study [AIRE], Trandolapril Cardiac Evaluation [TRACE], SOLVD treatment and SOLVD prevention), in which a total of 12,763 patients were studied, reported an overall 28% reduction in death, myocardial infarction and hospital admission for HF in patients with left ventricular dysfunction after myocardial infarction treated with ACE inhibitors. Benefits increased with the degree of left ventricular dysfunction (24). On the basis of these studies, ACE inhibitors are recommended as first choice treatment of HF (24,25).
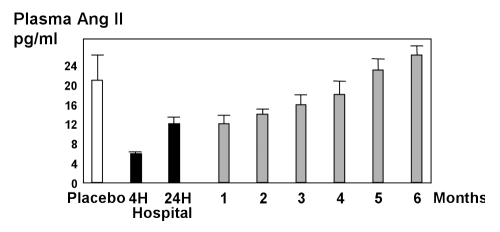
Despite these clear effects on mortality, long term survival rates with ACE inhibitor treatment of HF remain low. In the major clinical trials with ACE inhibitors, four-year mortality was almost 40% (5,26). Ang II concentrations in the ventricles are reduced less than those in the atrium by ACE inhibitors, and during chronic therapy the reduction in Ang II concentrations tends to become less pronounced or even to disappear completely (Figure 2) (27–29).
Figure 2.
Plasma angiotensin II (Ang II) concentrations in patients during long term angiotensin-converting enzyme (ACE) inhibitor therapy. Adapted from reference 27. H Hours
One of the possible reasons for this only partial efficacy is that ACE inhibitors act on the major synthesis pathway for Ang II as a circulating hormone but have no effect on the alternative routes to local Ang II formation. This leaves a major source of local Ang II unaffected by the treatment, particularly in the heart itself.
WHAT ACE INHIBITORS IGNORE: LOCAL PATHWAYS FOR ANG II FORMATION
Local Ang II production in myocardial tissues is associated with synthesis pathways involving serine proteases, the most common of which is chymase. Several investigators have reported chymase activity in the heart and coronary arteries (29–31). Daemen and Urata (32) found chymase in the normal adult human myocardium and showed that production is upregulated in the healing tissue after myocardial infarction. The chymase pathway is also important in other organs: in the kidney, findings from in vitro systems indicate that at least 40% of Ang I is converted to Ang II by pathways other than ACE, presumably a chymase (33). Preliminary data indicate that the non-ACE pathway may be substantially larger in disease states such as diabetes mellitus (33).
These alternative pathways for Ang II formation are not identical among species. Chymase-like activity is widely distributed in multiple tissues in baboons after Ang I treatment (34), but the pathway is not present in animals such as rodents or rabbits (35). A third class of Ang II-forming enzymes is the kallikrein type, which forms bradykinin as well as Ang II, depending on pH conditions, and is seen, for example, in the human leg (36).
Very recently Tipnis et al (37) described a human homologue of ACE that is insensitive to normal ACE inhibitor treatments. This enzyme is expressed predominantly in heart, kidney and testis, and thus may well contribute to local Ang II formation under ACE inhibitor therapy.
Molecular studies have shown the role of locally acting Ang II in processes leading to HF. The development of left ventricular hypertrophy is associated with the activation of several genes, including a set of fetal genes that are normally inactive in the adult heart (38–40). Left ventricular hypertrophy is associated with a 14-fold increase in the risk of HF in those aged 65 years or under (41). Other changes are collagen deposition, loss of cardiac myocytes and the proliferation of fibroblasts. All these changes increase the risk for HF and all are regulated by Ang II (42–46). In addition, the risk of vessel thrombosis is greatly increased by two Ang II-mediated effects: the increased aggregation of platelets (47,48) and the stimulated expression of plasminogen activator inhibitor 1 in endothelial cells (49).
The role of local Ang II in the buildup to HF associated with cardiac hypertrophy has been elegantly shown in vivo by Paradis et al (50) in transgenic mice harbouring cardiac myocytes that overexpress Ang II. Local production of Ang II had no effect on systolic blood pressure or heart rate, but the animals displayed significant cardiac hypertrophy and remodelling, with increased interstitial collagen formation and expression of ventricular atrial natriuretic factor.
THE CASE FOR ANG II RECEPTOR BLOCKADE
If ACE inhibition is insufficient to reduce Ang II concentrations in the heart, the alternative approach is Ang II receptor blockade. Blocking the action of Ang II at the receptor level not only offers an additional, pathway-independent reduction in Ang II-mediated effects but also can specifically target the receptor responsible for the deleterious actions. Of the two Ang II receptors, AT1 and AT2, most deleterious Ang II effects are mediated by the AT1 receptor. These include such actions as induction of aldosterone release, and the stimulation of collagen production and cell proliferation. In the heart, AT1 receptor activation is involved in the development of myocardial fibrosis (51) and the induction of ventricular arrhythmias after reperfusion (52). Ang II receptor expression has been shown to be upregulated in cardiomyocytes after myocardial infarction or in congestive HF (53–55). Through the AT1 receptor, Ang II also induces ‘late’ markers for cardiac hypertrophy, skeletal alpha-actin and atrial natriuretic factor expression (40,56).
Angiotensin receptor blockers (ARBs) such as valsartan, losartan, candesartan, irbesartan, telmisartan and others, which have been the subject of great interest in the past decade, are gaining ground in the treatment of hypertension. ARBs are at least as effective as ACE inhibitors at reducing hypertension but have a lower incidence rate of adverse effects (57). There is also scope for greater end-organ protection: ARB treatments seem to carry a lower risk for kidney complications than ACE inhibitors because glomerular blood flow is unchanged (58–60). At the molecular level, the action of ARBs is independent of Ang II-generating pathways.
Another possible advantage relevant to HF treatment is that the selective affinity for the AT1 receptor leaves the AT2 receptor unblocked. Stimulation of AT2 is associated with several beneficial effects such as local nitric oxide and bradykinin production (20,61–64), and with reducing cell proliferation and inducing apoptosis (65,66). AT2 receptor stimulation also seems to affect the downregulation of AT1 receptor expression by crosstalk between these receptors through some as yet unknown mechanism (67). It has been suggested that the selective blockade of AT1 increases local concentrations of Ang II and stimulation of the AT2 receptor, a benefit specific to selective ARBs (68–70).
Selective AT1 receptor blockade has been shown to inhibit several of the Ang II-associated phenomena seen in HF. In myocyte cultures, induction of immediate-early genes, late genes and growth factor genes by Ang II is fully inhibited by AT1 receptor blockade but not by treatment with an AT2 receptor antagonist (56). Cerbai et al (71) reported that an eight-week treatment of old (18 months) spontaneously hypertensive rats with ARBs prevented the development of myocyte hypertrophy and associated electrophysiological alterations. There are also positive effects on arrhythmias: the number of ventricular premature beats after reperfusion in mice is reduced by AT1 receptor blockade (52).
ARBs IN THERAPY: ALONE OR IN COMBINATION?
The current consensus on HF therapy is that a single medication is less effective than a combination of several drugs. In the case of ARBs, there is a strong rationale for complementing the global action of ACE inhibitors with the local ACE-independent effects, especially AT2 stimulation, from selective ARBs such as valsartan. For valsartan, there is a substantial body of preclinical work from different areas showing that adding valsartan to ACE inhibitor treatment leads to additional effects not seen in monotherapy.
For example, in a pig model of HF, concomitant ACE inhibition and highly selective AT1 receptor blockade (benazeprilat and valsartan) led to greater reduction in vascular resistance than ACE inhibition alone (72,73). The same investigators also reported, in a similar model, that the combination of benazeprilat and valsartan normalizes myocyte action potential duration; again, these effects were not achieved by monotherapy (74).
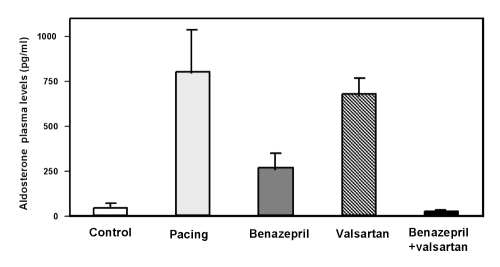
There are also promising results in humans from clinical pilot studies. Stergiou et al (75) treated 20 patients (who after six weeks of ACE inhibitor monotherapy still had uncontrolled ambulatory diastolic blood pressure) with benazepril (20 mg once daily) and valsartan (80 mg once daily) and showed a significant antihypertensive effect with a benefit over placebo for average 24 h ambulatory blood pressure (P<0.01). Pulse rate was unaffected. In a six-week study more specifically related to HF, Baruch et al (76) in 1999 studied 83 patients with HF in New York Heart Association (NYHA) functional class II to IV. The investigators added valsartan (80 or 160 mg bid) or placebo to patients’ usual ACE inhibitor regimen. The addition of valsartan had acute and long term additional effects on pulmonary capillary artery wedge pressure and diastolic pulmonary artery pressure, and led to an additional reduction in aldosterone and plasma noradrenaline concentrations (Figure 3).
Figure 3.
Effect of combination treatment with benazepril and valsartan on aldosterone concentrations in pigs with pacing-induced heart failure. Adapted from reference 72
VALSARTAN IN HEART FAILURE TRIAL
Valsartan in Heart Failure Trial (Val-HeFT) was recently completed, and the results from analyses are starting to emerge. This trial, initiated in 1997 and designed specifically to detect mortality and the combined endpoint of morbidity and mortality, enrolled 5010 patients from 300 centres in 14 European countries, South Africa and the United States. Val-HeFT is the biggest study undertaken on the use of an ARB (valsartan) in addition to standard treatment (including ACE inhibitors) for patients with HF (77,78).
In Val-HeFT, all patients enrolled have chronic stable HF (NYHA class II to IV) with an ejection fraction less than 40% and a left ventricular diameter (end-diastolic) greater than 2.9 cm/m2. All subjects were receiving standard HF therapy before and during the trial: approximately 93% of the population followed an ACE inhibitor regimen while about 35% were treated with beta-blockers. Valsartan was given in addition to the current treatment in a forced titration scheme with doses of 40, 80 and 160 mg bid.
The hypothesis behind Val-HeFT was that addition of the highly effective ARB valsartan to usual therapy for HF would result in more specific and complete blockade of the RAS, additional benefits to the proven efficacy of ACE inhibitors and a further reduction in morbidity and mortality. The study was designed with a 90% power to detect a 20% difference between the two treatments for the endpoint ‘time to death’. Three secondary endpoints will provide insight into effects on cardiac structure and function, disease progression and quality of life.
The reports from Val-HeFT so far (79) have been favourable: valsartan did have a statistically significant benefit, reducing morbidity and mortality in the total patient population by 13.3% (P=0.009). The risk reduction for hospitalizations was 27.5% (P=0.00001). In the subgroup of patients not on ACE inhibitor therapy, the reduction in morbidity and mortality from valsartan was 44.5% (P=0.0002).
These data show that there are significant benefits to be had from adding valsartan to existing treatments for HF.
A second ongoing smaller study of related design is a substudy of the Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity (CHARM), where 2300 patients are being given the ARB candesartan in combination with ACE inhibitor treatment (80). CHARM is planned to end in late 2002.
LIFE AFTER Val-HeFT: OPEN QUESTIONS
Val-HeFT will continue to generate data for quite some time. In addition to the presented data on morbidity and mortality, analyses comparing ACE inhibitor monotherapy with combination treatment are expected to provide information about other factors in HF, such as the role of bradykinins. Bradykinins can be beneficial because they mediate vasodilation through increased production of nitric oxide and have antigrowth properties (81). Some of the benefits from ACE inhibitor treatments in HF have been attributed to the increased bradykinin concentrations (82–87), but local bradykinin synthesis can also be stimulated by Ang II through activation of the unblocked AT2 receptor (63,69,88). Similar crosstalk seems to be involved in the regulation of endothelins, which also seem to have a role in cardiac remodelling (44). The potential of endothelin receptor blockers such as bosentan in treatments is being evaluated (89).
The large scope for crosstalk between all these systems is a strong argument for combination therapies in the treatment of HF. Val-HeFT showed the combination of valsartan and ACE inhibitor to be effective in reducing local Ang II concentrations further than monotherapy, which is a significant step toward both greater understanding of the pathogenesis and better survival after the advent of HF.
Acknowledgments
R Webb is an employee of Novartis Pharmaceuticals Inc, Summit, NJ; M de Gasparo has acted as a consultant for Novartis Pharma AG, Basel, Switzerland.
REFERENCES
- 1.Davis RC, Hobbs FD, Lip GY. ABC of heart failure. History and epidemiology. BMJ. 2000;320:39–42. doi: 10.1136/bmj.320.7226.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonneux L, Barendregt JJ, Meeter K, Bonsel GJ, van der Maas PJ. Estimating clinical morbidity due to ischemic heart disease and congestive heart failure: the future rise of heart failure. Am J Public Health. 1994;84:20–8. doi: 10.2105/ajph.84.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mair FS, Crowley TS, Bundred PE. Prevalence, aetiology and management of heart failure in general practice. Br J Gen Pract. 1996;46:77–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Kannel WB. Need and prospects for prevention of cardiac failure. Eur J Clin Pharmacol. 1996;49:S3–9. [PubMed] [Google Scholar]
- 5.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 6.Burt VL, Culter JA, Higgins M, et al. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Data from the health examination surveys, 1960 to 1991. Hypertension. 1995;26:60–9. doi: 10.1161/01.hyp.26.1.60. [DOI] [PubMed] [Google Scholar]
- 7.Lip GY, Gibbs CR, Beevers DG. ABC of heart failure: aetiology. BMJ. 2000;320:104–7. doi: 10.1136/bmj.320.7227.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153:598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
- 9.Luft FC. STOPPING at the CAPPP of good HOPE. Nephrol Dial Transplant. 2000;15:451–2. doi: 10.1093/ndt/15.4.451. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell CJ, Kannel WB. Cardiovascular risks of hypertension: lessons from observational studies. J Hypertens Suppl. 1998;16:S3–7. [PubMed] [Google Scholar]
- 11.MRF Intervention Trial Research Group Multiple risk factor intervention trial. Risk factor changes and mortality results. JAMA. 1982;248:1465–77. [PubMed] [Google Scholar]
- 12.Lucius R, Gallinat S, Busche S, Rosenstiel P, Unger T. Beyond blood pressure: new roles for angiotensin II. Cell Mol Life Sci. 1999;56:1008–19. doi: 10.1007/s000180050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest. 1995;95:651–7. doi: 10.1172/JCI117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoll M, Meffert S, Stroth U, Unger T. Growth or antigrowth: angiotensin and the endothelium. J Hypertens. 1995;13:1529–34. [PubMed] [Google Scholar]
- 15.Sugano M, Makino N, Yanaga T. The effects of renin-angiotensin system inhibition on aortic cholesterol content in cholesterol-fed rabbits. Atherosclerosis. 1996;127:123–9. doi: 10.1016/s0021-9150(96)05942-4. [DOI] [PubMed] [Google Scholar]
- 16.Falkenhahn M, Franke F, Bohle RM, et al. Cellular distribution of angiotensin-converting enzyme after myocardial infarction. Hypertension. 1995;25:219–26. doi: 10.1161/01.hyp.25.2.219. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari R, Bachetti T, Agnoletti L, Comini L, Curello S. Endothelial function and dysfunction in heart failure. Eur Heart J. 1998;19:G41–7. [PubMed] [Google Scholar]
- 18.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–72. [PubMed] [Google Scholar]
- 19.Unger T, Chung O, Csikos T, et al. Angiotensin receptors. J Hypertens Suppl. 1996;14:S95–103. [PubMed] [Google Scholar]
- 20.Unger T. The angiotensin type 2 receptor: variations on an enigmatic theme. J Hypertens. 1999;17:1775–86. doi: 10.1097/00004872-199917121-00001. [DOI] [PubMed] [Google Scholar]
- 21.Nickenig G, Baumer AT, Temur Y, Kebben D, Jockenhovel F, Bohm M. Statin-sensitive dysregulated AT1 receptor function and density in hypercholesterolemic men. Circulation. 1999;100:2131–4. doi: 10.1161/01.cir.100.21.2131. [DOI] [PubMed] [Google Scholar]
- 22.Ogiku N, Ishida R, Saeki K, Sugiura M. Induction of cardiac angiotensinogen mRNA and angiotensin converting enzyme (ACE) activity in isoproterenol–induced heart injury. Hypertens Res. 1996;19:179–87. doi: 10.1291/hypres.19.179. [DOI] [PubMed] [Google Scholar]
- 23.Swedberg K, for the CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study. N Engl J Med. 1987;316:1429–35. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 24.Flather MD, Yusuf S, Kober L, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet. 2000;355:1575–81. doi: 10.1016/s0140-6736(00)02212-1. [DOI] [PubMed] [Google Scholar]
- 25.Eccles M, Freemantle N, Mason J. North of England evidence based development project: guideline for angiotensin converting enzyme inhibitors in primary care management of adults with symptomatic heart failure. BMJ. 1998;316:1369–75. doi: 10.1136/bmj.316.7141.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–10. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 27.Biollaz J, Brunner HR, Gavras I, Waeber B, Gavras H. Antihypertensive therapy with MK 421: angiotensin II-renin relationships to evaluate efficacy of converting enzyme blockade. J Cardiovasc Pharmacol. 1982;4:966–72. [PubMed] [Google Scholar]
- 28.Johnston CI. Angiotensin converting enzyme inhibition. In: Robertson JIS, Nichols MG, editors. The Renin Angiotensin System. New York: Gower Medical Publishing; 1993. pp. 1–87. [Google Scholar]
- 29.Urata H, Nishimura H, Ganten D. Mechanisms of angiotensin II formation in humans. Eur Heart J. 1995;16:79–85. doi: 10.1093/eurheartj/16.suppl_n.79. [DOI] [PubMed] [Google Scholar]
- 30.Padmanabhan N, Jardine AG, McGrath JC, Connell JM. Angiotensin-converting enzyme-independent contraction to angiotensin I in human resistance arteries. Circulation. 1999;99:2914–20. doi: 10.1161/01.cir.99.22.2914. [DOI] [PubMed] [Google Scholar]
- 31.Wolny A, Clozel JP, Rein J, et al. Functional and biochemical analysis of angiotensin II-forming pathways in the human heart. Circ Res. 1997;80:219–27. doi: 10.1161/01.res.80.2.219. [DOI] [PubMed] [Google Scholar]
- 32.Daemen MJ, Urata H. Healing human myocardial infarction associated with increased chymase immunoreactivity. Heart Vessels. 1997;(Suppl 12):113–5. [PubMed] [Google Scholar]
- 33.Hollenberg NK, Fisher ND, Price DA. Pathways for angiotensin II generation in intact human tissue: evidence from comparative pharmacological interruption of the renin system. Hypertension. 1998;32:387–92. doi: 10.1161/01.hyp.32.3.387. [DOI] [PubMed] [Google Scholar]
- 34.Hoit BD, Shao Y, Kinoshita A, Gabel M, Husain A, Walsh RA. Effects of angiotensin II generated by an angiotensin converting enzyme-independent pathway on left ventricular performance in the conscious baboon. J Clin Invest. 1995;95:1519–27. doi: 10.1172/JCI117824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue K, Nishimura H, Kubota J, Kawamura K. Alternative angiotensin II formation in rat arteries occurs only at very high concentrations of angiotensin I. Hypertension. 1999;34:525–30. doi: 10.1161/01.hyp.34.3.525. [DOI] [PubMed] [Google Scholar]
- 36.Arakawa K. Serine protease angiotensin II systems. J Hypertens Suppl. 1996;14:S3–7. [PubMed] [Google Scholar]
- 37.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin converting enzyme: cloning and functional expression as a captopril insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–43. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 38.Chien KR, Zhu H, Knowlton KU, et al. Transcriptional regulation during cardiac growth and development. Annu Rev Physiol. 1993;55:77–95. doi: 10.1146/annurev.ph.55.030193.000453. [DOI] [PubMed] [Google Scholar]
- 39.Hefti MA, Harder BA, Eppenberger HM, Schaub MC. Signaling pathways in cardiac myocyte hypertrophy. J Mol Cell Cardiol. 1997;29:2873–92. doi: 10.1006/jmcc.1997.0523. [DOI] [PubMed] [Google Scholar]
- 40.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- 41.Kannel WB, Ho K, Thom T. Changing epidemiological features of cardiac failure. Br Heart J. 1994;72:S3–9. doi: 10.1136/hrt.72.2_suppl.s3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Funck RC, Wilke A, Rupp H, Brilla CG. Regulation and role of myocardial collagen matrix remodeling in hypertensive heart disease. Adv Exp Med Biol. 1997;432:35–44. doi: 10.1007/978-1-4615-5385-4_4. [DOI] [PubMed] [Google Scholar]
- 43.Anversa P, Kajstura J, Olivetti G. Myocyte death in heart failure. Curr Opin Cardiol. 1996;11:245–51. doi: 10.1097/00001573-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Colucci WS. Molecular and cellular mechanisms of myocardial failure. Am J Cardiol. 1997;80:15L–25L. doi: 10.1016/s0002-9149(97)00845-x. [DOI] [PubMed] [Google Scholar]
- 45.Bishop JE. Regulation of cardiovascular collagen deposition by mechanical forces. Mol Med Today. 1998;4:69–75. doi: 10.1016/S1357-4310(97)01193-3. [DOI] [PubMed] [Google Scholar]
- 46.Unger T. Neurohormonal modulation in cardiovascular disease. Am Heart J. 2000;139:S2–8. doi: 10.1067/mhj.2000.102901. [DOI] [PubMed] [Google Scholar]
- 47.Koppel H, Eber B, Gasser R, Klein W. [Recent molecular and pharmacological aspects of ACE inhibitors] Wien Med Wochenschr. 1995;145:633–7. [PubMed] [Google Scholar]
- 48.Touyz RM, Schiffrin EL. Effects of angiotensin II and endothelin-1 on platelet aggregation and cytosolic pH and free Ca2+ concentrations in essential hypertension. Hypertension. 1993;22:853–62. doi: 10.1161/01.hyp.22.6.853. [DOI] [PubMed] [Google Scholar]
- 49.Sironi L, Calvio AM, Arnaboldi L, et al. Effect of valsartan on angiotensin II-induced plasminogen activator inhibitor-1 biosynthesis in arterial smooth muscle cells. Hypertension. 2001;37:961–6. doi: 10.1161/01.hyp.37.3.961. [DOI] [PubMed] [Google Scholar]
- 50.Paradis P, Dali-Youcef N, Paradis FW, Thibault G, Nemer M. Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc Natl Acad Sci USA. 2000;97:931–6. doi: 10.1073/pnas.97.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baba HA, Iwai T, Bauer M, Irlbeck M, Schmid KW, Zimmer HG. Differential effects of angiotensin II receptor blockade on pressure-induced left ventricular hypertrophy and fibrosis in rats. J Mol Cell Cardiol. 1999;31:445–55. doi: 10.1006/jmcc.1998.0879. [DOI] [PubMed] [Google Scholar]
- 52.Harada K, Komuro I, Hayashi D, Sugaya T, Murakami K, Yazaki Y. Angiotensin II type 1a receptor is involved in the occurrence of reperfusion arrhythmias. Circulation. 1998;97:315–7. doi: 10.1161/01.cir.97.4.315. [DOI] [PubMed] [Google Scholar]
- 53.Busche S, Gallinat S, Bohle RM, et al. Expression of angiotensin AT(1) and AT(2) receptors in adult rat cardiomyocytes after myocardial infarction. A single-cell reverse transcriptase-polymerase chain reaction study. Am J Pathol. 2000;157:605–11. doi: 10.1016/S0002-9440(10)64571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogg H, de Gasparo M, Graedel E, et al. Angiotensin II-receptor subtypes in human atria and evidence for alterations in patients with cardiac dysfunction. Eur Heart J. 1996;17:1112–20. doi: 10.1093/oxfordjournals.eurheartj.a015008. [DOI] [PubMed] [Google Scholar]
- 55.Tsutsumi Y, Matsubara H, Ohkubo N, et al. Angiotensin II type 2 receptor is upregulated in human heart with interstitial fibrosis, and cardiac fibroblasts are the major cell type for its expression. Circ Res. 1998;83:1035–46. doi: 10.1161/01.res.83.10.1035. [DOI] [PubMed] [Google Scholar]
- 56.Sadoshima J, Izumo S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–23. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 57.Oparil S, Dyke S, Harris F, et al. The efficacy and safety of valsartan compared with placebo in the treatment of patients with essential hypertension. Clin Ther. 1996;18:797–810. doi: 10.1016/s0149-2918(96)80040-3. [DOI] [PubMed] [Google Scholar]
- 58.Plum J, Bunten B, Nemeth R, Grabensee B. Effects of the angiotensin II antagonist valsartan on blood pressure, proteinuria, and renal hemodynamics in patients with chronic renal failure and hypertension. J Am Soc Nephrol. 1998;9:2223–34. doi: 10.1681/ASN.V9122223. [DOI] [PubMed] [Google Scholar]
- 59.Muirhead N, Feagan BF, Mahon J. The effects of valsartan and captopril on reducing microalbuminuria in patients with type 2 diabetes mellitus: a placebo-controlled trial. Curr Ther Res. 2000;60:650–60. [Google Scholar]
- 60.Fricker AF, Nussberger J, Meilenbrock S, Brunner HR, Burnier M. Effect of indomethacin on the renal response to angiotensin II receptor blockade in healthy subjects. Kidney Int. 1998;54:2089–97. doi: 10.1046/j.1523-1755.1998.00220.x. [DOI] [PubMed] [Google Scholar]
- 61.Gohlke P, Pees C, Unger T. AT2 receptor stimulation increases aortic cyclic GMP in SHRSP by a kinin-dependent mechanism. Hypertension. 1998;31:349–55. doi: 10.1161/01.hyp.31.1.349. [DOI] [PubMed] [Google Scholar]
- 62.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Natl Acad Sci USA. 1999;96:6506–10. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsutsumi Y, Matsubara H, Masaki H, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999;104:925–35. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siragy HM, de Gasparo M, Carey RM. Angiotensin type 2 receptor mediates valsartan-induced hypotension in conscious rats. Hypertension. 2000;35:1074–7. doi: 10.1161/01.hyp.35.5.1074. [DOI] [PubMed] [Google Scholar]
- 65.Meffert S, Stoll M, Steckelings UM, Bottari SP, Unger T. The angiotensin II AT2 receptor inhibits proliferation and promotes differentiation in PC12W cells. Mol Cell Endocrinol. 1996;122:59–67. doi: 10.1016/0303-7207(96)03873-7. [DOI] [PubMed] [Google Scholar]
- 66.Dimmeler S, Rippmann V, Weiland U, Haendeler J, Zeiher AM. Angiotensin II induces apoptosis of human endothelial cells. Protective effect of nitric oxide. Circ Res. 1997;81:970–6. doi: 10.1161/01.res.81.6.970. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka M, Tsuchida S, Imai T, et al. Vascular response to angiotensin II is exaggerated through an upregulation of AT1 receptor in AT2 knockout mice. Biochem Biophys Res Commun. 1999;258:194–8. doi: 10.1006/bbrc.1999.0500. [DOI] [PubMed] [Google Scholar]
- 68.Matsubara H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ Res. 1998;83:1182–91. doi: 10.1161/01.res.83.12.1182. [DOI] [PubMed] [Google Scholar]
- 69.de Gasparo M, Siragy HM. The AT2 receptor: fact, fancy and fantasy. Regul Pept. 1999;81:11–24. doi: 10.1016/s0167-0115(99)00023-3. [DOI] [PubMed] [Google Scholar]
- 70.Siragy HM, Carey RM. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension. 1999;33:1237–42. doi: 10.1161/01.hyp.33.5.1237. [DOI] [PubMed] [Google Scholar]
- 71.Cerbai E, Crucitti A, Sartiani L, et al. Long-term treatment of spontaneously hypertensive rats with losartan and electrophysiological remodeling of cardiac myocytes. Cardiovasc Res. 2000;45:388–96. doi: 10.1016/s0008-6363(99)00344-2. [DOI] [PubMed] [Google Scholar]
- 72.Spinale FG, de Gasparo M, Whitebread S, et al. Modulation of the renin-angiotensin pathway through enzyme inhibition and specific receptor blockade in pacing-induced heart failure: I. Effects on left ventricular performance and neurohormonal systems. Circulation. 1997;96:2385–96. doi: 10.1161/01.cir.96.7.2385. [DOI] [PubMed] [Google Scholar]
- 73.Spinale FG, Mukherjee R, Iannini JP, et al. Modulation of the renin-angiotensin pathway through enzyme inhibition and specific receptor blockade in pacing-induced heart failure: II. Effects on myocyte contractile processes. Circulation. 1997;96:2397–406. doi: 10.1161/01.cir.96.7.2397. [DOI] [PubMed] [Google Scholar]
- 74.Spinale FG, Iannini JP, Mukherjee R, Melton DM, de Gasparo M. Angiotensin AT1 receptor inhibition, angiotensin-converting enzyme inhibition, and combination therapy with developing heart failure: cellular mechanisms of action. J Card Fail. 1998;4:325–32. doi: 10.1016/s1071-9164(98)90238-x. [DOI] [PubMed] [Google Scholar]
- 75.Stergiou GS, Skeva II, Baibas NM, et al. Additive hypotensive effect of angiotensin-converting enzyme inhibition and angiotensin-receptor antagonism in essential hypertension. J Cardiovasc Pharmacol. 2000;35:937–41. doi: 10.1097/00005344-200006000-00017. [DOI] [PubMed] [Google Scholar]
- 76.Baruch L, Anand I, Cohen I, Ziesche S, Judd D, Cohn JN. Augmented short- and long-term hemodynamic and hormonal effects of an angiotensin receptor blocker added to angiotensin converting enzyme inhibitor therapy in patients with heart failure. Vasodilator Heart Failure Trial (V-HeFT) Study Group. Circulation. 1999;99:2658–64. doi: 10.1161/01.cir.99.20.2658. [DOI] [PubMed] [Google Scholar]
- 77.Cohn JN. Improving outcomes in congestive heart failure: Val-HeFT. Valsartan in Heart Failure Trial. Cardiology. 1999;91:19–22. doi: 10.1159/000047284. [DOI] [PubMed] [Google Scholar]
- 78.Cohn JN, Tognoni G, Glazer RD, Spormann D, Hester A. Rationale and design of the Valsartan Heart Failure Trial: a large multinational trial to assess the effects of valsartan, an angiotensin-receptor blocker, on morbidity and mortality in chronic congestive heart failure. J Card Fail. 1999;5:155–60. doi: 10.1016/s1071-9164(99)90038-6. [DOI] [PubMed] [Google Scholar]
- 79.Cohn JN. Val-HeFT (Valsartan Heart Failure Trial) Clin Cardiol. 2001;24:86. [Google Scholar]
- 80.Swedberg K, Pfeffer M, Granger C, et al. Candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM): rationale and design. Charm-Programme Investigators. J Card Fail. 1999;5:276–82. doi: 10.1016/s1071-9164(99)90013-1. [DOI] [PubMed] [Google Scholar]
- 81.Weber KT, Sun Y, Guarda E. Structural remodeling in hypertensive heart disease and the role of hormones. Hypertension. 1994;23:869–77. doi: 10.1161/01.hyp.23.6.869. [DOI] [PubMed] [Google Scholar]
- 82.Zhu YC, Stauss HM, Bao G, et al. Role of bradykinin in the antihypertensive and cardioprotective actions of converting enzyme inhibitors. Can J Physiol Pharmacol. 1995;73:827–31. doi: 10.1139/y95-112. [DOI] [PubMed] [Google Scholar]
- 83.Linz W, Wiemer G, Gohlke P, Unger T, Scholkens BA. Contribution of kinins to the cardiovascular actions of angiotensin-converting enzyme inhibitors. Pharmacol Rev. 1995;47:25–49. [PubMed] [Google Scholar]
- 84.Tschope C, Gohlke P, Zhu YZ, Linz W, Scholkens B, Unger T. Antihypertensive and cardioprotective effects after angiotensin-converting enzyme inhibition: role of kinins. J Card Fail. 1997;3:133–48. doi: 10.1016/s1071-9164(97)90047-6. [DOI] [PubMed] [Google Scholar]
- 85.Sander GE, McKinnie JJ, Greenberg SS, Giles TD. Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists in the treatment of heart failure caused by left ventricular systolic dysfunction. Prog Cardiovasc Dis. 1999;41:265–300. doi: 10.1053/pcad.1999.0410265. [DOI] [PubMed] [Google Scholar]
- 86.Zhang X, Recchia FA, Bernstein R, Xu X, Nasjletti A, Hintze TH. Kinin-mediated coronary nitric oxide production contributes to the therapeutic action of angiotensin-converting enzyme and neutral endopeptidase inhibitors and amlodipine in the treatment in heart failure. J Pharmacol Exp Ther. 1999;288:742–51. [PubMed] [Google Scholar]
- 87.Hornig B, Arakawa N, Drexler H. Effect of ACE inhibition on endothelial dysfunction in patients with chronic heart failure. Eur Heart J. 1998;19:G48–53. [PubMed] [Google Scholar]
- 88.Chung O, Unger T. Angiotensin II receptor blockade and end-organ protection. Am J Hypertens. 1999;12:150S–6S. doi: 10.1016/s0895-7061(99)00218-6. [DOI] [PubMed] [Google Scholar]
- 89.Roux S, Breu V, Ertel SI, Clozel M. Endothelin antagonism with bosentan: a review of potential applications. J Mol Med. 1999;77:364–76. doi: 10.1007/s001090050363. [DOI] [PubMed] [Google Scholar]