Abstract
OBJECTIVES:
To determine whether the kappa opioid receptor agonist U-50,488H, a benzacetamide derivative of the cyclo-hexane-1,2-diamine analgesics, may be a useful molecular probe to define the structural requirements of this class of drugs for cardiac sodium channel blockade.
ANIMALS AND METHODS:
The electrophysiological effects of U-50,488H were compared with those of lidocaine, a clinically used class Ib antiarrhythmic agent, in rat heart sodium currents expressed in Xenopus laevis oocytes by using two-electrode voltage clamp.
RESULTS:
Both U-50,488H and lidocaine produced a concentration-dependent tonic block of sodium current, but U-50,488H was approximately fourfold more potent than lidocaine. Both drugs produced a hyperpolarizing shift in the voltage dependence of sodium channel inactivation and both delayed recovery from inactivation. Both drugs exhibited use-dependent block, but U-50,488H showed a 1.8-fold increase in potency compared with lidocaine at a high frequency of stimulation (30 Hz).
CONCLUSIONS:
The more potent tonic and use-dependent block of cardiac sodium channels by U-50,488H suggests that structural features of this molecule may provide it with a greater ability to block the channel. An understanding of these structural features may provide information needed in the development of novel arylacetamide-based antiarrhythmic drugs and insight into possible mechanisms describing channel block, resulting in a highly efficacious antiarrhythmic action in the heart.
Keywords: Antiarrhythmic agents; Membrane currents; Sodium channel; U-50,488H
Voltage-gated sodium channels are responsible for the initial rapid membrane depolarization phase of the action potential and the genesis of conducting action potentials in electrically excitable cells. The diverse functions associated with sodium channels in various tissues provide evidence for distinct channel subtypes. Cloning and sequencing studies have shown that at least eight distinct but homologous mammalian sodium channels exist (1). The alpha-subunit is the primary, pore-forming subunit of the sodium channel (2–4). Sodium channels can transition between at least three distinct states: resting (closed), open (active) and inactive (non-conducting) (5). The transitions between these states are voltage and time dependent. Antiarrhythmic, anticonvulsant and local anesthetic agents have been shown to block the propagation of action potentials by interacting differently with each of the states of the channel (6). Hondeghem and Katzung (7) proposed a model, the modulated receptor hypothesis (MRH), to describe the interaction of antiarrhythmic drugs with cardiac sodium channels concurrent with the model proposed by Hille (8) for the state-dependent interaction of local anesthetics with neuronal sodium channels. Both models suggest that clinically important local anesthetic and antiarrhythmic drugs have a low affinity for the resting state of the sodium channel. Selective, high affinity binding of the drug for the inactive state of the channel imparts specificity of channel block. Thus, in cardiac tissue the action of antiarrhythmic drugs such as lidocaine is to prevent abnormal electrical impulse propagation and conduction, thus suppressing nonpacemaker-generated electrical activity that results from damage to cardiac myocytes. Effective class I antiarrhythmic drugs therefore exhibit a greater efficacy in situations associated with an excessive firing of action potentials (use-dependent block) or prolonged tissue depolarization, as is associated with disease conditions. These drugs should not affect normal cellular excitability. Thus, effective arrhythmia suppression depends on the properties of the drug molecule that convey high affinity binding to the receptor on the alpha-subunit of the channel pore when the channel is in the open or inactivated state. Such high affinity binding results in a slowed recovery of the drug-bound channel from inactivation as the cell membrane repolarizes (9).
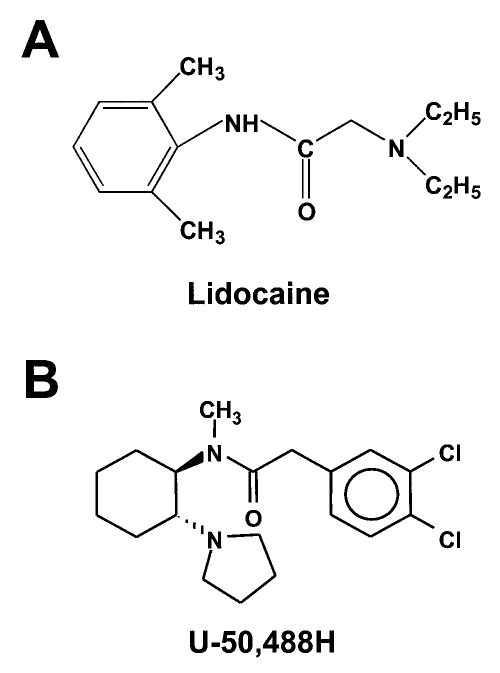
Many chemically diverse compounds exist that block cardiac sodium channels. However, blockade of the cardiac sodium channel in itself does not indicate that the compound exhibits antiarrhythmic efficacy. In addition, it has been difficult to identify the structural features of the molecule that impart antiarrhythmic efficacy because of the large number of structurally diverse compounds exhibiting sodium channel blockade. The use of molecular models for drug-channel interactions derives from biophysical, biochemical and pharmacological information that simultaneously encourages the design of novel, more potent, tissue selective drug development. Structure-activity relation studies of compounds that are effective sodium channel-blocking antiarrhythmics suggest that at least three structural features are necessary for a compound to exhibit effective class I antiarrhythmic activity (10–13). These features are a terminal aliphatic, ionizable amine group and a terminal lipophilic, usually aromatic, group interconnected by a flexible, hydrogen-bond accepting, carbonyl-containing group (Figure 1). An important category of clinically used drugs that exhibit these features and are effective sodium channel blocking antiarrhythmics are the ‘amides’, which include disopyramide and lidocaine.
Figure 1.
A Molecular structure of lidocaine (2-(diethylamino)-N-(2,6-dimethylphenyl)acetamide monohydrochloride). B General molecular structure of the arylacetamide compound U-50,488H (trans-(±)-3,4-dichloro-N-methyl-N-[7-(1-pyrrolidinyl)cyclohexyl]benzeneacetamide methane sulphonate)
Lidocaine, chemically designated as 2-(diethylamino)-N-(2,6-dimethylphenyl)acetamide monohydrochloride, is an antiarrhythmic drug used in the clinical setting to suppress ventricular arrhythmias (Figure 1A). It exhibits both tonic and use-dependent blockade of cardiac sodium current and is classified as a Ib antiarrhythmic agent according to Vaughan-Williams (14). Lidocaine is known to be relatively more selective for cardiac sodium currents than for neuronal currents (15), which may result from intrinsic differences in the affinity of lidocaine for the alpha-subunit of the channel (16,17). The mechanism by which antiarrhythmic drugs such as lidocaine and local anesthetic and anticonvulsant drugs mediate blockade of sodium channels is thought to involve their interaction with the pore of the channel, which includes residues in the S6 transmembrane segment of domain IV (18). Molecular modelling of lidocaine suggests that the orientation of the aromatic moiety and the structure of the aryl-amine connection are important determinants of channel blockade (19). Other studies suggest correlations between the potency of channel blockade and the molecular weight and lipid solubility of the amide compound (10,20). Lidocaine contains all the necessary structural features of an effective antiarrhythmic drug. Lidocaine contains a tertiary amine nitrogen atom (with an ionization constant (pKa) of 7.7), a 6 to 9 Å interconnecting aliphatic amide (-CONH-) linker and a hydrophobic benzene ring (20). The pKa of the tertiary amide nitrogen atom allows lidocaine to be either charged at low pH or neutral at physiological pH (7.4). These physicochemical properties of lidocaine make it especially suited as an antiarrhythmic drug under conditions associated with myocardial ischemia (14).
U-50,488H, trans-(±)-3,4-dichloro-N-methyl-N-[7-(1-pyrrolidinyl)cyclohexyl]-benzeneacetamide methane sulphonate, is a kappa opioid receptor agonist that is the first benzeneacetamide derivative of a trans-cyclohexane-1,2-diamine class of analgesics (21). It is unique in its chemical structure in that it contains the addition of a pyrrolidine substituent at C-2 in the cyclohexane ring (Figure 1B). In vivo, U-50,488H is an effective antiarrhythmic drug that produces marked tonic and frequency-dependent blockade of myocardial sodium current, actions independent of kappa opioid receptors (22). Structurally, U-50,488H contains a dichloro-substituted benzene ring, an aliphatic amide linker and a pyrrolidine substituted cyclohexane ring (23). Thus U-50,488H contains the three structural features that have been suggested to be necessary for a compound to mediate effective class I antiarrhythmic drug activity.
In this study we compared the efficacy of sodium channel blockade by U-50,488H with that by lidocaine to define whether this class of benzacetamide derivatives of cyclo-hexane-1,2-diamine may be a useful molecular probe with which to define the structural requirements of this class of drugs for cardiac sodium channel blockade. We examined the electrophysiological effect of U-50,488H on rat heart (rH1) sodium currents expressed in Xenopus laevis oocytes and compared the potency of sodium channel blockade of this compound with that of lidocaine, a clinically used anti-arrhythmic agent.
ANIMALS AND METHODS
Transcription of RNA and expression in X laevis oocyte:
The plasmid pSkM2 contains a coding region for the rH1 sodium channel alpha-subunit (24). Plasmid DNA was linearized by digestion with Ase I, and RNA transcripts were synthesized using the message machine SP6 RNA polymerase transcription kit (Ambion, USA). Stage V oocytes were obtained from adult female X laevis frogs, defolliculated with collagenase (2 mg/mL) and injected with 50 nL of in vitro transcribed RNA at a concentration empirically determined to obtain current amplitudes between 1 and 5 μA, as previously described (25). The oocytes were incubated for 48 h at 20°C in ND-96 (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2 and 5 mM HEPES, pH 7.5, with 0.1 mg/mL gentamycin, 0.5 mM theophylline and 0.55 mg/mL pyruvate).
All experiments were performed according to guidelines established by the Institutional Animal Care and Use Committee of the University of California, Irvine. This investigation conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Solutions and drugs:
All oocyte experiments were performed at room temperature (20 to 22°C) in ND96 bath solution. U-50,488H (trans-(±)-3,4-dichloro-N-methyl-N- [7-(1-pyrrolidinyl)-cyclohexyl]benzeneacetamide methane sulphonate) and lidocaine (2-(diethylamino)-N-(2,6-dimethylphenyl)acetamide monohydrochloride) (Sigma Chemical Co, USA) were solubilized in distilled water as a 10 mM stock solution before dilution to the final concentrations in the ND96 bath solution. U-50,488H and lidocaine were used at concentrations ranging from 1 to 3000 μM. A low volume (0.5 ml) plexiglass recording bath allowed for efficient exchange (15 to 30 s) between control and drug solutions from gravity-flow reservoirs. A suction device ensured continuous perfusion at a flow rate of 1 to 2 mL/min and maintained a constant fluid level.
Data recording and analysis:
Recording electrodes were prepared from borosilicate glass using a two-stage P-87 puller (Sutter Instrument Co, USA). Microelectrodes were filled with filtered 3 M KCl/0.5% low melting point agarose and had resistances between 1.0 and 4.0 M Ω. A grounded copper shield was inserted between the recording electrodes to minimize electrode coupling. Currents were recorded by a virtual ground circuit, and the data were filtered at 3 kHz online and digitized at a sampling frequency of 12.5 kHz. Currents were recorded and analyzed using pCLAMP 6.0.4 software (Axon Instruments, USA). Capacitance transients and leak currents were corrected by P/4 subtraction, with the depolarizations for subtraction applied after each protocol. Nonlinear curve fitting was performed using SigmaPlot (v5.0, Jandel Scientific, USA). Data are shown as the mean ± SD for n experiments. Statistical analyses were performed using SigmaStat statistical software (Jandel Scientific), with P<0.05 considered significant.
Concentration-response curves for the effect of lidocaine and U-50,488H on the rH1 sodium channel isoform were determined by measuring peak inward current for cells depolarized from −120 to −10 mV in the absence and presence of either drug (1 to 3000 μM). All sodium currents were allowed to recover from slow inactivation for 10 min before any electrophysiological protocol was started. Drugs were then perfused into the bath for 5 min before current was recorded. The resulting fractional block of sodium current by each drug at each concentration was plotted against the log concentration of drug and fitted with a Hill equation:
In this equation, INa is the fractional block of sodium current, [A] is the concentration of drug, EC50 is the concentration of drug at half-maximal block and n is the Hill coefficient describing the stoichiometry of drug binding to the sodium channel.
The voltage dependence of steady-state inactivation was determined using 500 ms conditioning prepulses from a holding potential of −120 to +15 mV in 5 mV increments, followed by a test pulse to −5 mV for 22.5 ms. The peak current amplitude evoked during the test depolarization was normalized to the maximum current amplitude and plotted as a function of the conditioning prepulse potential in the absence and presence of either U-50,488H (300 μM) or lidocaine (1000 μM). The data were fitted with a two-state Boltzmann equation of the form
in which Imax is the maximal current evoked, V is the potential of the voltage pulse, V½ is the voltage at which 50% of the current is inactivated (the midpoint of the inactivation curve), and k is the slope factor.
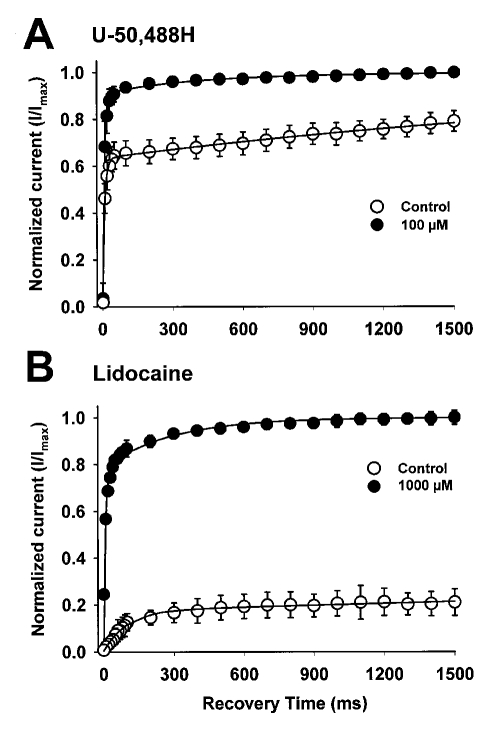
To measure recovery from inactivation, oocytes were held at −120 mV. A 500 ms prepulse to −10 mV was followed by a variable recovery period from 10 to 1500 ms. Each recovery period was followed by a 22.5 ms test pulse to −10 mV to measure the amount of current that had been recovered. Peak current amplitudes evoked during the test pulse were normalized to the current amplitude of a 22.5 ms pulse to −10 mV elicited immediately before the recovery protocol in the absence and presence of either U-50,488H (100 μM) or lidocaine (1000 μM). The peak current was plotted as a function of the duration of the recovery interval. The data were individually fitted to a double exponential equation of the form
where A1 and A2 represent the proportions of current recovering with time constants τ1 and τ2, and t is the recovery time interval.
The frequency-dependent effects of U-50,488H and lidocaine were examined from a holding potential of −120 mV with 35 depolarizations ranging from −100 to −10 mV for 24.8 ms each. Trains of pulses were delivered at frequencies of 1 and 30 Hz in the absence and presence of U-50,488H (10 and 100 μM) or lidocaine (100 and 1000 μM). The peak current amplitude during each pulse was normalized to the maximal peak current amplitude (pulse number 1), and plotted as a function of pulse number.
RESULTS
U-50,488H blocked rH1 sodium channels with greater potency than lidocaine:
U-50,488H is a potent kappa opioid receptor agonist. However, it has been shown that U-50,488H reduces the incidence of cardiac arrhythmias by blockade of cardiac sodium channels (22,26). Based on these findings, it seemed likely that U-50,488H could directly block sodium channel function independent of opioid receptor activity. To determine whether this was the case, the effects of U-50,488H were examined on the electrophysiological properties of rH1 sodium channels expressed in X laevis oocytes that are devoid of endogenous opioid receptors. Oocytes were held at −120 mV and currents were evoked by depolarizations to −10 mV every 6 s. This infrequent pulsing protocol should reveal drug interactions with the resting or open state of the sodium channel and minimize the effects of frequency-dependent block, thereby providing a reasonable estimate of the extent to which U-50,488H produces tonic block of sodium current.
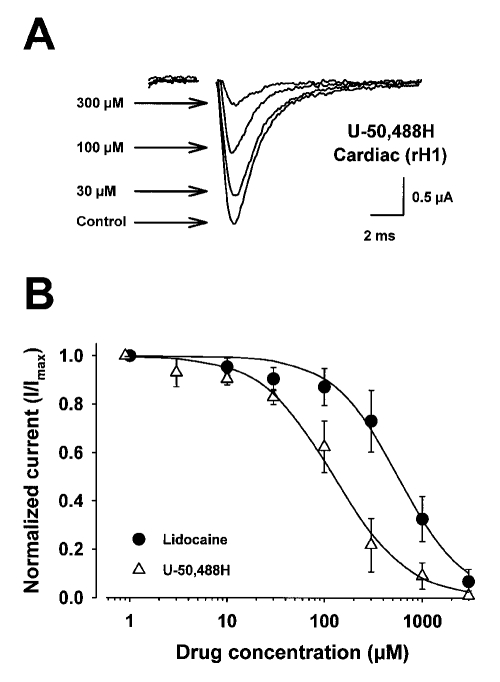
Figure 2 shows the concentration-response curves for U-50,488H and lidocaine on rH1 sodium currents. Figure 2A shows representative current traces for the blocking effects of U-50,488H on rH1 sodium currents 5 min after perfusion at each concentration. In Figure 2B are the concentration-response curves for blockade by lidocaine and U-50,488H. The smooth lines represent the best fits of the data from the Hill equation. Lidocaine blocked the heart current with an EC50 of 563±22 μM while current block by U-50,488H resulted in an EC50 of 133±20 μM. The stoichiometries of U-50,488H and lidocaine binding (nH values of 1.2±0.14 and 1.4 ± 0.11, respectively) to the sodium channels were not significantly different from each other and were not significantly different from unity, suggesting that only one drug molecule is necessary to block the channel. When EC50 values were compared, U-50,488H block of the heart sodium channel was 4.2-fold greater than that by lidocaine, suggesting that U-50,488H, the first synthetic arylacetamide kappa opioid receptor agonist developed, has a greater apparent affinity for blockade of the cardiac sodium channel than lidocaine.
Figure 2.
Concentration-response curves for the effect of lidocaine and U-50,488H on rat heart (rH1) sodium currents expressed in Xenopus laevis oocytes. Currents were recorded by two-electrode whole-cell voltage clamp. Oocytes were injected with 50 ng of in vitro transcribed RNA encoding the rH1 sodium channel alpha-subunit. After two days of incubation at 20°C in ND-96 plus supplements, current-voltage curves were evoked by depolarizing the cell to various potentials from a fixed prepulse potential of −90 mV. The data were filtered at 3 kHz. Peak sodium currents, evoked every 6 s, were measured at test potentials that elicited maximum inward current (−10 mV). Peak sodium currents were measured again after 5 min perfusion of the cell at a flow rate of 1 to 2 mL/min of ND96 containing increasing concentrations of either U-50,488H or lido-caine. The data are shown as peak current normalized to the maximum current in the absence of drug (I/Imax). The curves described by the solid lines were fitted by the Hill equation where INa = [1 + ([A]/EC50)n]−1. INa describes the fraction of maximal current remaining after block by either drug, [A] is the concentration of drug, EC50 is the concentration of drug at half-maximal block, and n is the Hill coefficient describing the stoichiometry of the drug-channel interaction. Concentration-response curves are mean ± SD for five oocytes. The parameters of the fits are shown in Table 2
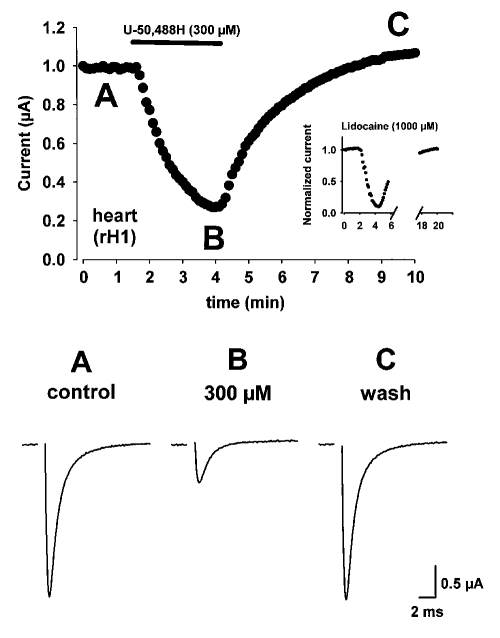
Figure 3 shows an example of the time course of rH1 sodium current block in the absence and presence of U-50,488H. Oocytes were held at −120 mV and currents were evoked by depolarizations to −10 mV every 6 s in the absence (3A, control) and presence (3B, 300 μM) of U-50,488H. U-50,488H was perfused into the bath for 2.5 min as indicated in Figure 3 by the solid bar in the upper panel. Maximal rH1 sodium channel block resulted within 2 min after the beginning of perfusion of 300 μM U-50,488H. Approximately 2.5 min after the beginning of perfusion of U-50,488H, bath flow was changed to drug-free ND96 solution and currents were evoked until a complete time course of block was determined. rH1 sodium channel amplitude was similar to control values within 5 min after the conclusion of perfusion of U-50,488H (C, wash) indicating that this arylacetamide produces rapid and reversible blockade of sodium channels. When the time-course for 1000 μM lidocaine block of the rH1 sodium current was examined in a similar manner (see inset of upper panel in Figure 3) it was observed that under identical experimental conditions lidocaine produced a similar rapid onset of channel block to U-50,488H. However, more than twice the time was required (more than 10 min) for channel amplitudes to reach control (predrug) values after lidocaine (1000 μM) perfusion. Thus, while U-50,488H produces a rapid onset blockade of rH1 channels (in a manner similar to lidocaine) it exhibits the distinct ability to be rapidly washed from the bath (and presumably from the channel) in a manner unlike lidocaine. These data suggest that U-50,488H is a potent sodium channel blocker and may interact differently with the cardiac isoform of the sodium channel. The interaction of U-50,488H with the rH1 sodium channel may result from a different affinity of the molecule for the channel or perhaps may be the result of its interaction at a different site on the channel protein.
Figure 3.
Time course for rat heart (rH1) sodium channel block by U-50,488H. Oocytes were held at −120 mV and currents were evoked by depolarizations to −10 mV every 6 s in the absence (A) and presence (B) of U-50,488H. At the time indicated by the bar, 300 μM U-50,488H was added to the solution perfusing the cell. Maximal rH1 sodium current amplitude is shown plotted against time. Current traces are shown under control conditions (A, control), at the time maximal block was attained with U-50,488H (B, 300 μM), and 5 min after removal of U-50,488H from the bath solution and recovery of pre-drug current amplitude (C, wash). The inset in the upper panel depicts the time course for 1000 μM lidocaine block of the rH1 sodium current under identical experimental conditions. Note the prolonged time scale required for current recovery from block
While it is difficult to determine from these studies whether the differences in potency of channel block observed between lidocaine and U-50,488H result from different binding affinities or interactions at different site(s) on the channel protein, an examination of the physicochemical properties of each drug may provide some structural information regarding attributes of each molecule that may indicate why U-50,488H exhibits greater potency than lidocaine. Figure 1A shows the chemical structure of lidocaine while 1B depicts the structural components of the arylacetamide, U-50,488H. Table 1 describes some of the physicochemical properties of lidocaine and U-50,488H. The parameters in this table suggest that in addition to a larger molecular weight, U-50,488H has a higher pKa and greater hydrophobicity value (log P) than lidocaine. The Henderson-Hasselbach equation (pH = pKa + log[uncharged drug/ charged drug]) determines that approximately 99% of U-50,488H molecules are charged while only 63% of lidocaine molecules are charged for their given pKa values and pH of the perfusing solution (pH 7.5). An examination of the log P values suggests that U-50,488H is 51% more hydrophobic than lidocaine. Thus, while a greater fraction of U-50,488H molecules are charged at physiological pH and because it has a greater molecular weight and hydrophobicity than lidocaine, these features of the arylacetamide molecule may optimize this structure for interaction with the sodium channel and result in an increase in potency of block compared with lidocaine.
TABLE 1.
Some physicochemical parameters of lidocaine and U-50,488H
| Drug | MW | pKa | log P |
|---|---|---|---|
| Lidocaine | 235 | 7.7 | 2.28 |
| U-50,488H | 350 | 9.4 | 4.7 |
U-50,488H produced a hyperpolarizing shift in the voltage dependence of inactivation:
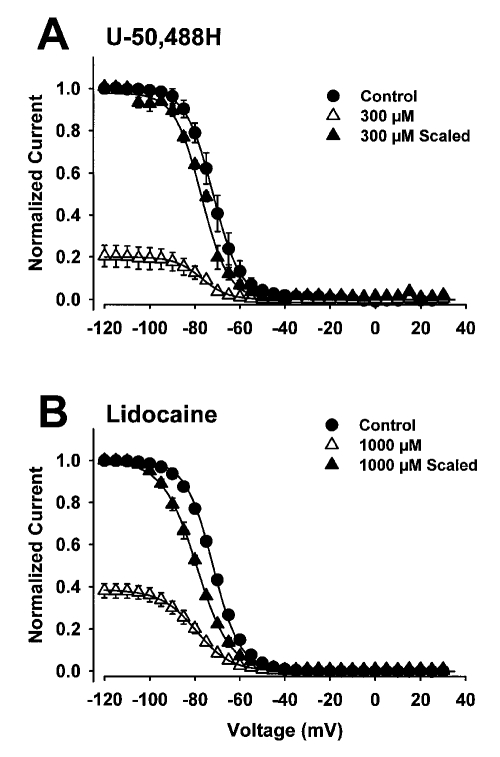
Lidocaine and other anti-arrhythmic drugs are known to bind preferentially to the inactive state of the sodium channel. This preferential binding generally results in a shift of the steady-state voltage dependence of the inactivation curve in the negative direction, reflecting a shift in equilibrium from the resting to the inactive state (15). To determine whether this was also the case for U-50,488H, the effects of the drug on the voltage dependence of steady-state inactivation were characterized and compared with the effects of lidocaine. Inactivation was examined using a two-pulse protocol with a 500 ms inactivation prepulse to ensure that all of the channels were inactivated. A concentration of U-50,488H was used to obtain marked current block. Figure 4 shows the effects of 300 μM U-50,488H (4A) and 1000 μM lidocaine (4B) on heart channels. The smooth curves represent the best fits to the data from a two-state Boltzmann function.
Figure 4.
Effects of U-50,488H (300 μM) and lidocaine (1000 μM) on the voltage dependence of steady-state inactivation of rat heart sodium channels. Inactivation was examined using a two-pulse protocol in which oocytes were held at −120 mV and depolarized from −90 to +15 mV for 500 ms, followed by a test pulse to −5 mV for 22.5 ms to determine channel availability. The data points in both panels were determined from 5 oocytes and error bars represent SD. All curves were fitted with a two-state Boltzmann function. Inactivation curves are shown in the absence (control) and presence of either 300 μM U-50,488H or 1000 μM lidocaine for the heart channel. The data obtained in the presence of either drug are also scaled to the maximum current observed without drug to show the shifts in voltage-dependence
When sodium channels were exposed to 300 μM U-50,488H, a 4 mV hyperpolarizing shift in the voltage dependence of inactivation curve was observed (Figure 4A). In control conditions, V½ occurred at −73±2.0 mV (n=5), which was shifted to −77±1.3 mV (n=5) by U-50,488H. The drug did not increase the slope factor (from 6.0±0.5 in control conditions to 6.3±1.2 in the presence of drug). Lidocaine produced a larger hyperpolarizing shift in the voltage dependence of inactivation curve for heart sodium channels (Figure 4B). Lidocaine shifted V½ by −10 mV (from −70±1.3 mV in control conditions to −80±3.0 mV in the presence of drug, n=6) but significantly changed the slope factor (6.1±0.2 in control conditions compared with 7.9 ± 0.5 in the presence of drug). These results confirm that U-50,488H blocks the inactive state of the sodium channel but that the degree of block is less than that of lidocaine.
U-50,488H affects sodium channel recovery from inactivation:
The differential binding of an antiarrhythmic drug to the different states of the sodium channel is likely to affect the kinetics of channel recovery from inactivation. To characterize the effects of U-50,488H on recovery from inactivation, a double-pulse protocol was used as outlined in the Methods. The kinetics for recovery from inactivation are plotted in Figure 5 in the absence of drug and in the presence of 100 μM U-50,488H (5A) and 1000 μM lidocaine (5B). The smooth curves represent exponential fits to the data using two time constants. The parameters of the fits are shown in Table 2. With no drug present, recovery from inactivation occurred with two components (τ1 and τ2). In the presence of U-50,488H or lidocaine, recovery from inactivation was slowed. Both of the time constants for recovery of the heart channel were increased, and the percentage of current recovering with the slow time constant increased significantly. These results indicate that U-50,488H delays recovery from inactivation for cardiac sodium channels, presumably by binding to the inactive state of the channel.
Figure 5.
Effects of 100 μM U-50,488H (A) and 1000 μM lidocaine (B) on recovery from inactivation of rat heart sodium channels. Oocytes were held at −120 mV and depolarized with an inactivating pulse to −10 mV for 500 ms. This was followed by a variable recovery interval ranging from 10 to 1500 ms at −120 mV. The recovery interval was followed by a 20 ms test depolarization to −10 mV. The peak current amplitude elicited during the test pulse was normalized to the peak current amplitude during a 22 ms pulse elicited from a holding potential of −120 mV for the rat heart channel immediately before the recovery protocol. The mean ± SD for the normalized current is shown as a function of the recovery time for at least four oocytes either in the absence of drug or in the presence of 100 μM U-50,488H (A) or 1000 μM lidocaine (B). The smooth curves represent fits of the data to a double exponential equation
TABLE 2.
Effects of U-50,488H and lidocaine on sodium channel recovery from inactivation in rat heart
| τ1 (ms) | % | τ2 (ms) | % | |
|---|---|---|---|---|
| Control | 8±0.7 | 91±2 | 405±99 | 9±2 |
| U-50,488H 100 μM | 14±0.8* | 64±4* | ∼3000† | 36±4* |
| Lidocaine 1000 μM‡ | 96±9* | 19±2* | ∼23,000† | 81±2* |
Data are mean ± SD for four oocytes.
P<0.05 compared with control;
Precise values for τ2 could not be determined because the longest recovery interval that was used was 1500 ms;
Lidocaine control data are not shown in the table, but are not statistically different from U-50,488H control data. Time constants τ1 and τ2 were 9±0.4 and 395±29 ms, respectively
U-50,488H produced use-dependent block of rH1 sodium channels:
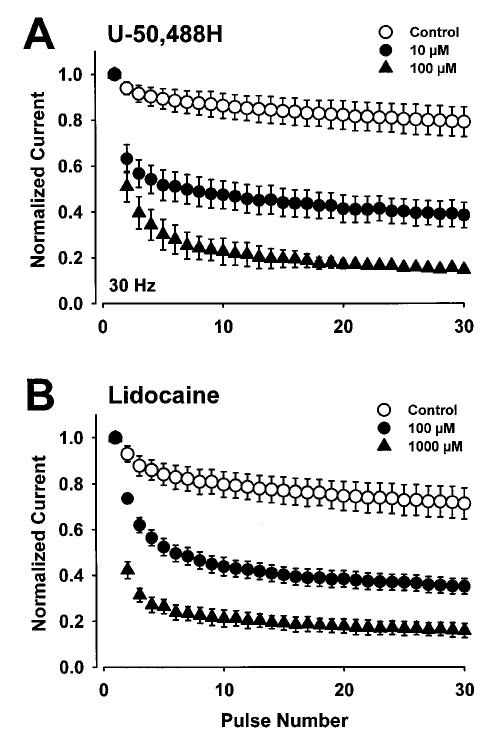
Differential block of sodium channels in closed, open or inactive states by antiarrhythmic drugs can result in use-dependent block, which is an important characteristic for efficacy during pathophysiological processes characterized by rapidly firing action potentials (such as during cardiac arrhythmias). Therefore, we examined use-dependent block of heart sodium channels by U-50,488H and lidocaine. Steady-state tonic block of sodium current was assessed using single depolarizing pulses delivered every 6 s in the absence and presence of drug. Two concentrations of U-50,488H and lidocaine were used to obtain minimal current block and marked current block. Following steady-state drug block (tonic block), trains of depolarizing pulses were delivered to determine the extent of phasic block at different frequencies. The results are shown in Figure 6 for a pulse frequency of 30 Hz. The percentage of block during the 30th pulse is shown at frequencies of 1, 5 and 30 Hz in Table 3.
Figure 6.
Effects of U-50,488H (A) and lidocaine (B) on use-dependent block of rat heart sodium channels in the absence (control) or presence of low or high concentrations of U-50,488H or lidocaine. A series of 20 ms depolarizing pulses to −10 mV was applied from a holding potential of −120 mV for rat heart channels at 30 Hz. The peak currents were normalized to the current during the first depolarization and plotted as a function of pulse number. The peak currents measured in the presence of drug were normalized to the current during the first depolarization in the presence of drug. Those currents measured approximately 10% of the current amplitudes in the absence of low concentrations of U-50,488H and lidocaine and approximately 40% in the absence of high concentrations of these drugs. This normalization emphasizes the amount of use-dependent block that developed independently of the tonic block by either drug
TABLE 3.
Frequency-dependent blocking actions of U-50,488H and lidocaine on rat heart sodium current evoked at 1 and 30 Hz showing percentage reduction of the peak sodium current measured during pulse number 30 of a series of depolarizing pulses delivered to the oocyte at 1 or 30 Hz
| 1 Hz | 30 Hz | |
|---|---|---|
| U-50,488H | ||
| Control | 5.8±0.7 | 19±6.0 |
| 10 μM | 11±4.4 | 54±11* |
| 100 μM | 27±10* | 83±3.4* |
| Lidocaine | ||
| Control | 4.0±1.0 | 23±8.0 |
| 100 μM | 7.4±1.0* | 64±3.0* |
| 1000 μM | 11±1.0* | 85±3.0* |
Data are expressed as means ± SD for five oocytes.
P<0.05 compared with control
Both the low (10 μM) and high (100 μM) concentrations of U-50,488H blocked heart channels in a use-dependent manner, with significant decreases in peak current during the final pulse at both frequencies (Table 3). These concentrations of U-50,488H produced a similar proportion of frequency-dependent block as the concentrations of lidocaine that were used; however, 10-fold lower concentrations of U-50,488H were used. These studies suggest that U-50,488H blocks heart sodium channels in a use-dependent manner.
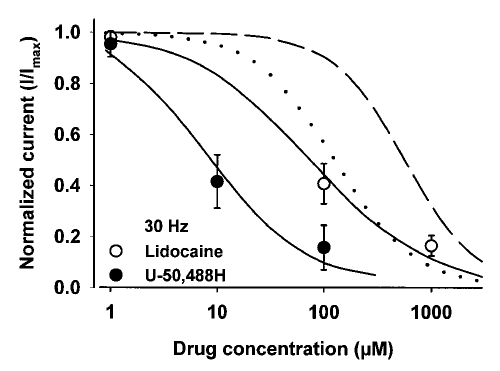
Both U-50,488H and lidocaine produced potent use-dependent blockade of heart sodium channels (Figure 6). The potency of each compound at producing use-dependent blockade was compared by plotting the concentration-response curves for U-50,488H and lidocaine at 30 Hz (Figure 7). Three concentrations of each drug were used to investigate use-dependent block. Following steady-state tonic block, 30 Hz trains of depolarizing pulses were delivered to assess the extent of phasic block at each drug concentration. The results are shown in Figure 7 for U-50,488H (1, 10 and 100 μM) and lidocaine (1, 100 and 1000 μM) at 30 Hz. The order of potency was the same as that found for tonic block. The calculated EC50 values for use-dependent block were 9.4±1.4 μM for U-50,488H and 74±19 μM for lidocaine. Neither drug changed the stoichiometry of drug binding (nH) to the channel. Thus the EC50 value was reduced by about 14-fold for U-50,488H and about eightfold for lidocaine with respect to values determined for tonic (0.16 Hz or one pulse every 6 s) block.
Figure 7.
Concentration-response curves for the use-dependent block by U-50,488H and lidocaine on rat heart (rH1) sodium currents expressed in Xenopus laevis oocytes. Currents were recorded by two-electrode whole-cell voltage clamp. Oocytes were injected with in vitro transcribed RNA encoding the rH1 sodium channel alpha-subunit. The amount of use-dependent block is determined by the residual current that exists at the end of a 30 Hz series of 20 ms depolarizing pulses to −10 mV that were applied from a holding potential of −120 mV to rH1 channels compared with the current in the absence of drug. The data are shown as residual current normalized to the maximum current in the absence of drug (I/Imax). The smooth curves described by the solid lines were fitted by the Hill equation where INa = [1 + ([A]/EC50)n]−1. INa describes the fraction of maximal current remaining after block by either drug, [A] is the concentration of drug, EC50 is the concentration of drug at half-maximal block, and n is the Hill coefficient describing the stoichiometry of the drug-channel interaction. Concentration-response curves are mean ± SD for five oocytes. For comparison, the concentration-response curves describing tonic block (from Figure 2) of U-50,488H (smooth dotted curve) and lidocaine (short dashed curve) on rH1 sodium currents are shown when peak sodium currents were evoked every 6 s and measured at test potentials that elicited maximum inward current (−10 mV)
DISCUSSION
The pharmacological actions of U-50,488H on the rH1 sodium channel were compared with those of lidocaine, a class Ib antiarrhythmic drug. These results complement previous studies (26,27) and suggest that the structural features of this arylacetamide drug provide it with potent tonic and use-dependent block of recombinant cardiac (rH1) sodium channels expressed in oocytes. Thus, there is much potential for the use of arylacetamides and their derivatives as a safer therapeutic treatment of ventricular arrhythmias. While the features of the molecule that define the blocking activity of U-50,488H are not known, molecular modelling of pyrrolidinylmethyl phenol fragments (11) suggests that the free pyrrolidinyl ring orientation contains the proposed receptor binding amine and that the mutual orientation of the two functional ring moieties is important to the active shape of the molecule. These features may be optimized within the molecular structure of U-50,488H and related arylacetamide compounds compared with lidocaine, which lacks these additional moieties. This indicates that U-50,488H may be useful as a novel pharmacophore with which to explore functional groups that are important for cardiac ion channel blockade.
Although lidocaine and U-50,488H share some common chemical features, they vary considerably in their molecular weight, pKa and lipophilicity (log P) (Table 1). U-50,488H is larger and more lipophilic than lidocaine. Molecular weight has been shown to be an important property for local anesthetic and antiarrhythmic drug potency (10). Drugs that have a larger molecular weight tend to exhibit a greater potency for blockade of sodium channels (28). When the tonic component of U-50,488H block of sodium channels was compared with that of lidocaine using an infrequent pulsing protocol, U-50,488H (which has an approximately 1.5-fold greater molecular weight than lidocaine) exhibited a 4.2-fold increase in potency for cardiac sodium channel blockade. As has been suggested with many other sodium channel blocking drugs, tonic block may reflect interactions of the drug with the resting state of the channel (29). Therefore, studies were conducted to examine the effects of U-50,488H on the open and inactive states of the sodium channel because differential block of either of these states is a more important criterion for clinical drug efficacy in cardiac disease states.
The state-dependent sodium channel blocking actions of a drug depend on several physicochemical properties of the molecule. In addition to molecular weight, the degree of ionization of a compound (pKa) has been shown to be relevant to the potency of sodium channel blockade by a drug (10). It is thought to be necessary for antiarrhythmic and local anesthetic drugs to cross the cell membrane so that they can bind to the sodium channel from the inside of the cell. Drug molecules are partitioned into either charged (cationic) or uncharged (lipophilic) species based on the pKa of the most easily hydrolyzed moiety (8). The charged drug species can block the channel by passing through the hydrophilic channel, while the uncharged drug species is not restricted to the channel and can pass through the membrane to the binding site on the alpha-subunit protein. For most antiarrhythmic and local anesthetic drugs the neutral (uncharged) species is more lipophilic, and hence is more membrane permeant. The enhanced membrane permeability associated with uncharged drugs suggests that chemical compounds with low pKa values may be more potent channel blockers. However, this is not the case for U-50,488H compared with lidocaine. U-50,488H has a pKa of 9.6 resulting in 99% of drug molecules that are charged at physiological pH, while only 63% of lidocaine molecules are similarly charged. Thus, the enhanced potency of U-50,488H may result from a restriction and stabilization of the orientation of the pyrrolidine nitrogen atom to a position outside of the conformational plane described by the benzamide and cyclohexane rings. This preferred conformation by the charged U-50,488H molecule may result in a tighter binding to the antiarrhythmic drug-binding site. A similar mechanism has been suggested for class I antiarrhythmic agents containing a bis(1-pyrrolidinylmethyl)phenol structure (11).
The previously determined octanol:water partition coefficient (log P) is used as a measure of the hydrophobicity of a drug. The hydrophobicity of U-50,488H is approximately twofold greater than that of lidocaine. In general it is theorized that the hydrophobicity of a molecule increases with its molecular weight, ie, with the addition of aryl or alkyl substituents to the core nucleus of the drug molecule (28). Thus, potency usually increases with an increase in drug hydrophobicity. This is true for U-50,488H compared with lidocaine. The addition of moieties such as pyrrolidine and dichlorobenzene greatly enhance hydrophobicity of this arylacetamide compared with lidocaine and may make an important contribution to channel blockade.
Thus, the more potent tonic component of cardiac sodium channel block by U-50,488H suggests that features of the molecule such as the larger molecular weight and enhanced hydrophobicity may provide it with a greater ability to cross lipid membranes and therefore successfully block the sodium channel in myocytes compared with lidocaine. In oocytes, U-50,488H shifted the voltage dependence of inactivation for the cardiac sodium channel in the hyperpolarizing direction, although the shift for U-50,488H was only 4 mV compared with 10 mV for lidocaine (Figure 4). According to the MRH, such a shift can be attributed to a higher affinity of the drug for the inactive state of the channel, as has been postulated to account for the actions of lidocaine (8,15). The smaller inactivation shift observed with U-50,488H is not consistent with the higher potency of this drug on the cardiac channel.
The reduced interaction of U-50,488H with the inactive state of the channel relative to lidocaine suggests that the charged nature of the drug at physiological pH may reduce accessibility to the binding site on the cardiac channel. The passage of charged molecules across the membrane may be confined to the hydrophilic sodium channel pore. Consistent with this prediction, U-50,488H shows a rapid, potent and reversible sodium channel block (Figure 3) unlike lidocaine, which required a significantly greater time to be washed from the bath and therefore its binding site on the cardiac channel. This finding is in accordance with the physicochemical differences observed between U-50,488H and lidocaine, and the biophysical properties of channel block described by the MRH.
The MRH also predicts that recovery of the drug-bound channel from inactivation should be slowed and biphasic, with a fast component reflecting recovery of unbound channels and a slow component reflecting recovery of channels bound to drug (8). The time course of sodium channel recovery from inactivation in the presence of U-50,488H is consistent with this prediction as has been observed for many sodium channel blocking drugs (18,30). All these results are consistent with the hypothesis that U-50,488H interacts with the inactive state of the sodium channel.
The frequency-dependent block produced by U-50,488H is consistent with its physicochemical properties. The decrease in sodium current at high rates of stimulation results from an accumulation of drug-associated channels because sodium channels spend more time in the open state as the interpulse (or diastolic) interval shortens (31). When a train of short (24.8 ms) depolarizing pulses is applied to evoke current, blockade by U-50,488H is rapid and suggests that the drug also interacts with the open (active) state of the sodium channel. The frequency-dependent block of sodium currents by U-50,488H can be interpreted in terms of the MRH as resulting from differences in the binding and unbinding ratio of the agent to the binding site, with the resulting block ratio being dependent on higher binding affinity to the open state than to the resting state of the channel (7,8). The use-dependent behaviour observed for U-50,488H was comparable with that of lidocaine, except that use-dependent blockade by U-50,488H resulted at concentrations 14-fold greater than for lidocaine (Figure 7). Lipophilicity does not seem likely to account for the high use-dependent behaviour of U-50,488H because lipophilic drugs tend to equilibrate rapidly with the membrane phase of the cell and result in a rapid recovery from block (32). The potent use-dependent properties of U-50,488H may be due to the concomitant increase in molecular weight and the high pKa value of its ionizable amine moiety (Table 1). Drug molecules with pKa values similar to that of U-50,488H have a large fraction of the drug in the charged form and exhibit marked use-dependent activity (13,20,33). The affinity of U-50,488H for the inactive state of the sodium channel receptor may also contribute to the observed potent use-dependent block by slowing the unbinding of drug-associated channels (34).
Our findings with U-50,488H are consistent with observations of Wang et al (35), who characterized the binding properties of a series of quaternary ammonium sodium channel blockers and suggested that there are two hydrophobic binding domains associated with the sodium channel and that each binding domain can adequately accommodate up to a 12-hydrocarbon chain (35,36). Similarly, studies by De Luca et al (13) showed substitutions at the amino terminal of mexiletine, a class Ib antiarrhythmic drug, that increase molecular weight, and that lipophilicity of the molecule can enhance high affinity hydrophobic (or τ) interactions with the sodium channel binding domains and increase drug potency. Similar structural features are present in U-50,488H, but not lidocaine. These structural modifications corroborate the evidence for a specific binding site on the sodium channel for antiarrhythmic drugs. Studies by Ragsdale et al (18,37) identified a putative antiarrhythmic, local anesthetic and anticonvulsant drug binding site on the S6 transmembrane-spanning region of domain IV of the channel. Within this region exist two important amino acid residues that line the pore of the sodium channel. These residues, phenylalanine 1764 and tyrosine 1771, are instrumental in blockade of the open and inactive states of the sodium channel by drugs (37,38). Unlike lidocaine, the cyclohexyl aromatic ring that adjoins the N-methyl amino group, the pyrrolidinyl moiety and the terminal lipophilic dichlorobenzene group of U-50,488H may provide it with structural features that promote strong interactions with the aromatic substituents (phenylalanine and tyrosine) of these domain IV, S6 amino acid residues. The resulting interaction of these two aromatic-aromatic (π-π) groups would favour a strong drug-receptor association that may enhance the potency of the arylacetamide compared with lidocaine. Lidocaine would be capable of forming only a single π-π interaction and a single cation-π interaction. The latter interaction would occur between the protonated amino terminal moiety of lidocaine and the aromatic ring of the amino acid residue on the pore (13,38,39). This is likely to account for the reduced potency observed with lidocaine in the present study.
Thus, U-50,488H, a benzacetamide derivative of cyclo-hexane-1,2-diamine, contains at least three structural features associated with compounds that exhibit effective cardiac sodium channel blocking activity and antiarrhythmic action in vivo. Additional pharmacological studies are required to investigate whether this compound can specifically block the cardiac isoform of the sodium channel. Studies with related analogues are also necessary to provide crucial structural information needed in the development of novel arylacetamide-based antiarrhythmic drugs that show therapeutic potential against pathological conditions associated with ventricular arrhythmias.
Acknowledgments
pSkM2 plasmid was generously provided by Dr Roland Kallen (University of Pennsylvania). AL Goldin is an Established Investigator of the American Heart Association. At the time this work was conducted MK Pugsley was a Medical Research Council of Canada Post-Doctoral Research Fellow.
REFERENCES
- 1.Goldin AL. Voltage-gated sodium channels. In: North RA, editor. Ligand- and Voltage-Gated Ion Channels. Boca Raton: CRC Press; 1995. pp. 73–112. [Google Scholar]
- 2.Noda M, Ikeda T, Suzuki H, et al. Expression of functional Na+ channels from cloned cDNA. Nature. 1986;322:826–8. doi: 10.1038/322826a0. [DOI] [PubMed] [Google Scholar]
- 3.Isom LL, DeJongh KS, Patton DE, et al. Primary structure and functional expression of the β1 subunit of the rat brain sodium channel. Science. 1992;256:839–42. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- 4.Goldin AL. Molecular analysis of Na+ channel inactivation. In: Perracjia C, editor. Hanbook of Membrane Channels, vol 1. San Diego: Academic Press; 1994. pp. 121–35. [Google Scholar]
- 5.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–44. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strichartz G, Rando T, Wang GK. An integrated view of the molecular toxinology of sodium channel gating in excitable cells. Annu Rev Neurosci. 1987;10:237–67. doi: 10.1146/annurev.ne.10.030187.001321. [DOI] [PubMed] [Google Scholar]
- 7.Hondeghem LM, Katzung BG. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977;472:373–98. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- 8.Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catterall WA. Common modes of drug action on Na+ channels: local anesthetics, antiarrhythmics and anticonvulsants. Trends Pharmacol Sci. 1987;8:57–65. [Google Scholar]
- 10.Courtney KR. Interval-dependent effects of small antiarrhythmic drugs on excitability of guinea-pig myocardium. J Mol Cell Cardiol. 1980;12:1273–86. doi: 10.1016/0022-2828(80)90071-1. [DOI] [PubMed] [Google Scholar]
- 11.Glowka ML, Dargie RL, Codding PW. Spatial requirements of the Na channel binding site for class I antiarrhythmics as derived from the crystal structures of 4-substituted 2,6-bis(1-pyrrolidinylmethyl) phenols. J Med Chem. 1991;34:2679–84. doi: 10.1021/jm00113a003. [DOI] [PubMed] [Google Scholar]
- 12.Zamponi GW, French RJ. Open-channel block by internally applied amines inhibits activation gate closure in batrachotoxin-activated sodium channels. Biophys J. 1994;67:1040–51. doi: 10.1016/S0006-3495(94)80569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Luca A, Natuzzi F, Desaphy J-F, et al. Molecular determinants of mexiletine structure for potent and use-dependent block of skeletal muscle sodium channels. Mol Pharmacol. 2000;57:268–77. [PubMed] [Google Scholar]
- 14.Vaughan-Williams EM. A classification of antiarrhythmic actions reassessed after a decade of new drugs. J Clin Pharmacol. 1984;24:129–47. doi: 10.1002/j.1552-4604.1984.tb01822.x. [DOI] [PubMed] [Google Scholar]
- 15.Bean BP, Cohen CJ, Tsien RW. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983;81:613–42. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nuss HB, Tomaselli GF, Marban E. Cardiac sodium channels (hH1) are intrinsically more sensitive to block by lidocaine than are skeletal muscle (μ1) channels. J Gen Physiol. 1995;106:1193–209. doi: 10.1085/jgp.106.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang DW, Nie L, George AL, Jr, Bennett PB. Distinct local anesthetic affinities in Na+ channel subtypes. Biophys J. 1996;70:1700–8. doi: 10.1016/S0006-3495(96)79732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–8. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- 19.Zamponi GW, Sui X, Codding PW, French RJ. Dual procainamide action on batrachotoxin-activated cardiac sodium channels. Open channel block and prevention of inactivation. Biophys J. 1993;65:2324–34. doi: 10.1016/S0006-3495(93)81291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehring GR, Myoer JW, Hondeghem LM. Quantitative structure activity studies of antiarrhythmic properties in a series of lidocaine and procainamide derivatives. J Pharmacol Exp Ther. 1988;244:479–92. [PubMed] [Google Scholar]
- 21.Clark CR, Halfpenny PR, Hill RG, et al. Highly selective κ opioid analgesics. Synthesis and structure-agonist relationships of novel N-[(2-aminocyclohexyl)aryl]acetamide and N-[C2-amino cyclohexyl)aryloxy]acetamide derivatives. J Med Chem. 1988;31:831–6. doi: 10.1021/jm00399a025. [DOI] [PubMed] [Google Scholar]
- 22.Pugsley MK, Saint DA, Walker MJA. An electrophysiological basis for the antiarrhythmic actions of the κ-opioid receptor agonist U50,488H. Eur J Pharmacol. 1994;261:303–9. doi: 10.1016/0014-2999(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 23.Szmuszkovicz J, Von Voigtlander PF. Benzeneacetamide amines: structurally novel non-mμ opioids. J Med Chem. 1982;25:1125–26. doi: 10.1021/jm00352a005. [DOI] [PubMed] [Google Scholar]
- 24.Kallen RG, Sheng Z-H, Yang J, Chen L, Rogart RB, Barchi RL. Primary structure and expression of a sodium channel characteristic of denervated and immature rat skeletal muscle. Neuron. 1990;4:233–42. doi: 10.1016/0896-6273(90)90098-z. [DOI] [PubMed] [Google Scholar]
- 25.Goldin AL, Sumikawa K. Preparation of RNA for injection into Xenopus oocytes. Methods Enzymol. 1992;207:279–97. doi: 10.1016/0076-6879(92)07018-j. [DOI] [PubMed] [Google Scholar]
- 26.Pugsley MK, Penz WP, Walker MJA, Wong TM. Cardiovascular actions of the κ-agonist, U50,488H, in the absence and presence of opioid receptor blockade. Br J Pharmacol. 1992;105:521–6. doi: 10.1111/j.1476-5381.1992.tb09012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pugsley MK, Penz WP, Walker MJA. Cardiovascular actions of U50,488H and related kappa agonists. Cardiovasc Drug Rev. 1993;11:151–64. [Google Scholar]
- 28.Bokesch PM, Post C, Strichartz G. Structure-activity relationship of lidocaine homologs producing tonic and frequency-dependent impulse blockade in nerve. J Pharmacol Exp Ther. 1986;237:773–81. [PubMed] [Google Scholar]
- 29.Valenzuela C, Snyders DJ, Bennett PB, Tamargo J, Hondeghem LM. Stereoselective block of cardiac sodium channels by bupivacaine in guinea pig ventricular myocytes. Circulation. 1995;92:3014–24. doi: 10.1161/01.cir.92.10.3014. [DOI] [PubMed] [Google Scholar]
- 30.Pugsley MK, Goldin AL. Effects of bisaramil, a novel class I antiarrhythmic agent, on heart, skeletal muscle and brain Na channels. Eur J Pharmacol. 1998;342:93–104. doi: 10.1016/s0014-2999(97)01420-9. [DOI] [PubMed] [Google Scholar]
- 31.Courtney KR. Sodium channel blockers: The size/solubility hypothesis revisited. Mol Pharmacol. 1990;37:855–9. [PubMed] [Google Scholar]
- 32.Liu L, Wendt DJ, Grant AO. Relationship between structure and sodium channel blockade by lidocaine and its amino-alkyl derivatives. J Cardiovasc Pharmacol. 1994;24:803–12. doi: 10.1097/00005344-199424050-00016. [DOI] [PubMed] [Google Scholar]
- 33.Yeh JZ, Ten Eick RE. Molecular and structural basis of resting and use-dependent block of sodium current defined using disopyramide analogs. Biophys J. 1987;51:123–35. doi: 10.1016/S0006-3495(87)83317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan C, Mok WM, Wang GK. Use-dependent inhibition of Na+ currents by benzocaine homologs. Biophys J. 1996;70:194–201. doi: 10.1016/S0006-3495(96)79563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang GK, Simon R, Bell D, Mok WM, Wang SY. Structural determinants of quaternary ammonium blockers for batrachotoxin-modified Na+ channels. Mol Pharmacol. 1993;44:667–76. [PubMed] [Google Scholar]
- 36.Wang GK, Quan C, Vladimirov M, Mok WM, Thalhammer JG. Quaternary ammonium derivative of lidocaine as a long-acting local anesthetic. Anesthesiology. 1995;83:1293–301. doi: 10.1097/00000542-199512000-00020. [DOI] [PubMed] [Google Scholar]
- 37.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci USA. 1996;93:9270–5. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li HL, Gaule A, Meadows L, Ragsdale DS. A molecular basis for the different local anesthetic affinities of resting versus open and inactivated states of sodium channels. Mol Pharmacol. 1999;55:134–41. doi: 10.1124/mol.55.1.134. [DOI] [PubMed] [Google Scholar]
- 39.Dougherty DA. Cation-π interaction in chemistry and biology. A new view of benzene Phy, Tyr, and Trp. Science. 1996;271:163–8. doi: 10.1126/science.271.5246.163. [DOI] [PubMed] [Google Scholar]