Abstract
Cardiac sarcoma is a very rare neoplasm and is difficult to diagnose. The case of a 51-year-old man with a left atrial tumour, locally recurrent three months after its surgical removal, is presented. Computed tomography showed metastatic spread to the lung parenchyma. On revised histology, the mass extirpated was a sarcoma. Because of the metastatic spread, further therapy was symptomatic only; the patient died 15 months after the first manifestation of his problems. Immunohistochemical staining confirmed cardiac rhabdomyosarcoma with metastatic spread to the lungs. Difficulty in diagnosing and treating cardiac tumours is discussed.
Keywords: Cardiac rhabdomyosarcoma, Echocardiography, Immunohistochemical staining
The incidence of primary cardiac tumours is as low as 0.0017% to 0.28%: they are markedly rarer than secondary cardiac tumours. Twenty-five per cent of all these neoplasms are malignant and show typical histology and invasiveness. Most of them are sarcomas occurring in humans irrespective of sex, mainly during the third and fourth decades of life. They are most frequently found in the right and left atrium, and less frequently in the right or left ventricle and in the area of the interventricular septum. On histology using immunohistochemical methods, the most frequent to be diagnosed are angiosarcomas, rhabdomyosarcomas, fibrosarcomas and osteosarcomas (1).
Clinical signs of sarcomas depend on location and propagation of the tumour. Classical symptoms are heart failure inexplicable by other causes, chest pain, pericardial effusion sometimes causing cardiac tamponade, arrhythmias or conduction impairments, obstruction of the inferior vena cava or sudden death. Tumours developing in the myocardium are clinically silent. When sarcomas invade the cardiac cavities and pericardium, the presence of hemorrhagic pericardial effusion is revealed; 75% of the patients develop distant metastases – most frequently in the lung parenchyma, thoracic lymph nodes, mediastinum and backbone (1,2).
None of the clinical symptoms is typical of cardiac tumours, but, on the other hand, the patient rarely remains asymptomatic. The main examination methods are transthoracic and transesophageal echocardiography, possibly complemented by computed tomography (CT) and magnetic resonance imaging. Cardiac catheterization is not needed before cardiac surgery if good visualization of the tumour and cardiac chambers is achieved (3,4).
The prognosis is generally poor; the survival time is reported to be from several weeks to two years (2,5).
CASE PRESENTATION
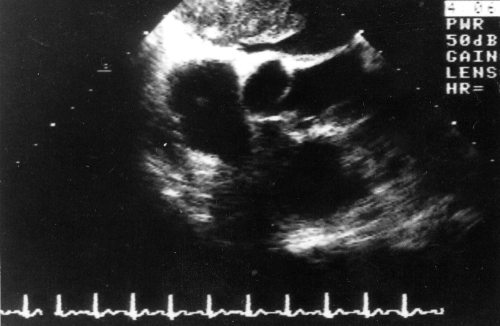
A 51-year-old man was hospitalized in the internal medicine ward of the Motol University Hospital, Prague, Czech Republic, with a suspicious mass-like lesion in the left atrium. He had been treated for bleeding from a gastric ulcer when he was 27 and for lung tuberculosis when he was 35 years old, which had not been followed up when he presented with his cardiac problems because he had completely recovered. He was a nonsmoker, he drank alcohol only casually, and no cancer had occurred in his family. Before his last admission, he was hospitalized twice in the local internal medicine ward for lung edema. Because transthoracic echocardiography was suggestive of a tumour mass in the left atrium, he was referred to the university hospital for trans-esophageal echocardiography. A pedunculated 35×60 mm mass was found in the left atrium, obturating the orifice of the mitral valve, with a slight mitral insufficiency (Figure 1).
Figure 1.
Transesophageal echocardiography, transversal view: the left atrium is occupied by a nonhomogeneous mass arising from the interatrial septum
Objectively, the patient did not show any particular features: normosthenic, height 164 cm, body weight 66 kg, eupnoic at rest, with regular heart beat and compensated circulation. Chest x-ray showed thickening of the lung parenchyma in the right pericardial region that was not focal in nature, the old specific process appeared in the right apical region, and the heart was not dilated. An electrocardiogram did not show any focal changes. The erythrocyte sedimentation rate (ESR) was 40/62 mm, and blood count, coagulation and basic biochemical parameters were within normal values. The finding of the left atrial mass-like lesion was an indication for cardiac surgery. The left atrium was entirely occupied by a tumour about the size of a 10×6×4 cm mandarin orange, white-grayish, solid, resilient and compact in consistency, not crumbling, firmly adhering to the interatrial septum, and with multiple radiating lines invading the right pulmonary veins. Another, smaller 1×0.8×0.5 cm node was removed from the posterior left atrial wall above the mitral valve. The tumour was completely extirpated and the artificial defect caused in the interatrial septum by its enucleation was resolved by pericardial patch angioplasty of the right pulmonary veins. The postoperative course was uneventful, and the patient was temporarily stimulated for bradycardia and was discharged from the hospital after institution of anticoagulant therapy with warfarin (for three months because of the extensive surgery). On histology, the mass extirpated was described as a fibromyxoma with central necrosis.
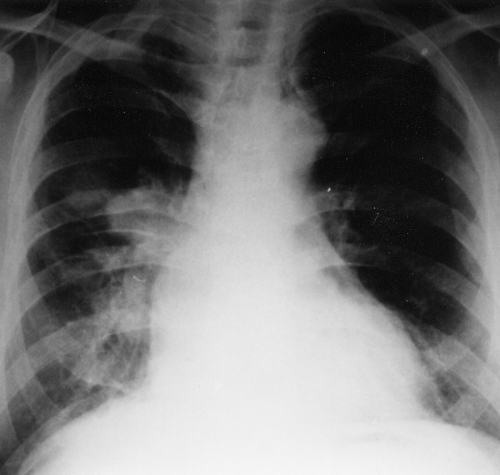
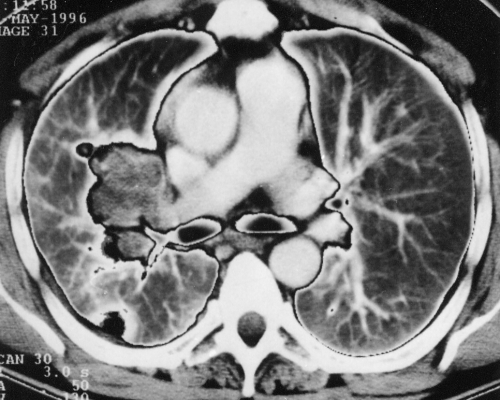
Three months after this cardiac surgery, the patient was hospitalized for repeated events of unconsciousness lasting for several seconds and not preceded by any prodroms; he always regained consciousness spontaneously. His somatic parameters were within normal values, no loss in body weight was recorded, no neurological deficiency was detected, and his heart beat was regular with no arrhythmia. Chest x-ray (Figure 2) showed a spherical enlargement of the right hilum with another adjacent 3×2 cm oval mass. The lateral view displayed projection of both masses on the hilum structures. Transesophageal echocardiography showed an oval nonhomogeneous 32×50 mm mass arising from the posterior upper part of the interatrial septum and the posterior wall of the left atrium that was slightly mobile, reaching up to the entry of the right pulmonary veins, with the possibility of invading even the right superior pulmonary vein and causing obstruction. The right inferior pulmonary vein was not visualized. Some parts of the mass were hyperechogenic and others were hypoechogenic. The mitral orifice was not obturated. The left pulmonary veins were normal, and mitral regurgitation was only slight. Chest CT confirmed a neoplastic process in the left atrium, extending to the right superior pulmonary vein and causing enlargement of the superior pole of the right hilum. At the same time, CT revealed peripheral foci, suspected as metastases, in the apical segment of the right inferior lobe and the laterobasal segment of the left inferior lobe of the lungs (Figure 3). Neither the hilum nor the mediastinal lymph nodes were infiltrated. Lung scan confirmed defective perfusion in the whole right superior lobe of the lungs; pulmonary angiography also showed the arterial branch of the right superior lobe disappearing at a distance of 4 cm. Both the left pulmonary veins and the right inferior pulmonary veins were evident. The right superior pulmonary vein was missing. Catheterization showed only a slight increase in pulmonary arteriolar resistance (degree 1 on a scale of 4) and normal left ventricular ejection fraction. Bronchoscopy including cytology was negative; ultrasound scan, abdominal CT and skeleton scintigraphy did not reveal any pathological changes. The ESR was 12/39, and blood count and biochemistry including creatine kinase, C-reactive protein and tumour markers (human chorionic gonadotropin, B2micro, carcinoembryonic antigen) were negative. The histogram showed normal intervals. CT of the brain revealed seven 5 to 10 mm hypodense circular foci.
Figure 2.
Posteroanterior chest x-ray showing a spherical mass reaching to the left hilum
Figure 3.
Chest computed tomogram showing metastasis on the right periphery and enlargement of the superior pole of the hilum caused by the invasive growth of the tumour from the left atrium to the right superior pulmonary vein
Because a malignant intracardial tumour with metastatic spread was clinically suspected, the analysis of the initial perioperative histological specimen, which could also be suggestive of focally increased cellularity including atypical mitoses and nuclear polymorphism and, consequently, of possible fibrosarcoma or angiosarcoma (given the closeness of the mass and the vascular wall), needed to be revised. The patient received permanent VVI stimulation and antiedemic therapy because of the brain metastases described. No other oncological therapy was instituted because it would have been only palliative without any substantial effect. The patient was followed up for his oncological diagnosis and died with a clinical picture of heart failure six months later, ie, 15 months after symptoms first appeared.
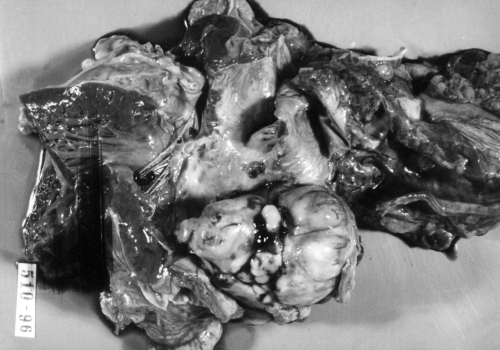
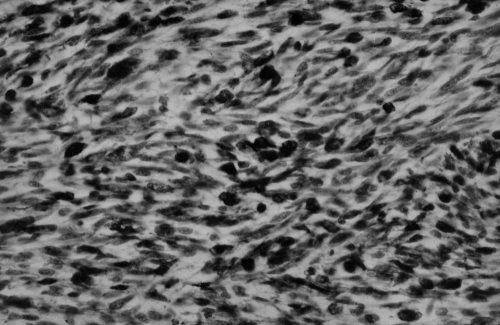
Autopsy revealed a tumour occupying almost the entire left atrium (Figure 4), adhering to the interatrial septum and the posterior wall of the left atrium, and invading the right pulmonary veins since growing through the atrium wall to reach the hilum and the adjacent right pulmonary parenchyma, where an expansively growing focus about 3 cm in diameter was found. The sentinel hilum lymph nodes were free of metastases; the right atrium also remained uninvaded. Metastases were also found in the lymph nodes of the anterior perimeter of the superior vena cava and on the posterior wall of the right atrium between the superior vena cava and the inferior vena cava, where a mass 3 cm in diameter protruded into the right atrium and diaphragm. Metastatic foci 0.5 to 1 cm in diameter were dispersed in the parenchyma of the right lung. No metastases were seen in the brain. Macroscopically, the tumour had a rough flat surface, and was light yellow on section, infiltrated with pseudocystic foci, elastic and soft. Histologically, this was an expansively growing tumour with fascicled or whirled spindle and cubic cells with atypical nuclei showing multiple mitoses, as well as atypical ones. Immunohistochemical staining showed positivity of the tumour cells to desmin and muscle-specific actin by the antibody muscle-specific actin 35; that is, it was suggestive of rhabdomyosarcoma (Figure 5). Alpha-actin, antigen CD 34, factor VIII, S-100 protein and neuron-specific enolase were negative.
Figure 4.
Spherical mass occupying the left atrium at postmortem examination
Figure 5.
Immunohistochemical detection of actin in tumorous cells. (Original magnification ×250)
DISCUSSION
The present case points out several problems associated with the care of patients with a malignant cardiac tumour. A diagnosis of left atrial sarcoma was established only when the tumour recurred with metastatic spread to the lung parenchyma. When a malignant origin of the tumour was clinically suspected, the initial histological finding was revised and immunohistochemical analysis of the autopsy specimen led to the correct diagnosis of rhabdomyosarcoma. The results of the analyses were confirmed at postmortem examination, except that suspicious metastases or embolization was not found in the brain.
Before surgery, differentiation between benign and malignant tumours is difficult. Indicative of a malignant cardiac tumour are the presence of metastases, propagation of the tumour to the mediastinum, fast growth of the tumour, hemorrhagic pericardial effusion, chest pain, localization to the right chambers of the heart, presence of the tumour both in the wall and in the chambers of the heart, and propagation of the tumour to the pulmonary veins. Benign cardiac tumours may be accompanied by peripheral embolization mimicking the metastatic process associated with malignant tumours. Histological analysis of peripheral metastases is recommended in such cases (5,6). Relapsing cardiac tumour as seen in our patient is also suggestive of malignant origin of the neoplasm (7).
Accurate histological examination is essential. The present case also illustrates the difficulty in establishing this rare diagnosis. Histological findings can also be predictive of the biological behaviour of the tumour. The prognosis is negatively influenced by a higher number of mitoses, the presence of necroses and expression of antigen Ki-67 (8).
When the correct diagnosis was reached in our patient, no surgery, radiotherapy or chemotherapy could be envisaged because of the size of the cardiac tumour and its metastatic spread. Generally speaking, treatment of malignant cardiac tumours is very precarious. Radical surgical removal of the tumour is often impossible. Furthermore, metastatic spread can be found. Low perioperative mortality is reported for small groups of surgically treated patients with cardiac sarcoma; nevertheless, survival more than one year after surgery is rather rare. However, surgery may lead to improvement of clinical signs due to the obstruction of the veins or valvular areas. While lymphoma is frequently reactive to chemotherapy and irradiation, these procedures mostly fail in other histological types of sarcoma (1,5). Consequently, only palliative treatment can be proposed to the patients. Because most patients show metastases at the time of diagnosis, even heart transplantation may not lead to the patient’s recovery (2).
REFERENCES
- 1.Collucci WS, Shoen FJ, Braunwald E. Primary tumors of the heart. In: Braunwald E, editor. Heart Disease. 5th edn. Philadelphia: WB Saunders Company; 1997. pp. 1464–77. [Google Scholar]
- 2.Simon BC, Funck R, Drude L, Bohle RM, Reichart B, Maisch B. Malignes Angiosarkom im rechtem Vorhof waerend der Schwangerschaft. Herz. 1994;19:166–70. [PubMed] [Google Scholar]
- 3.Chadimová M, Šochman J, Ouhrabková R. Myxom srdeční –morfologie, klinické pozorování, diagnostika. Cor Vasa. 1993;1:41–7. [PubMed] [Google Scholar]
- 4.Morelli S, Testa G, Voci P. Large left atrial thrombus mimicking atrial myxoma. Eur Heart J. 1996;17:1290. doi: 10.1093/oxfordjournals.eurheartj.a015049. [DOI] [PubMed] [Google Scholar]
- 5.Burke AP, Cowan D, Virmani R. Primary sarcomas of the heart. Cancer. 1992;69:387–95. doi: 10.1002/1097-0142(19920115)69:2<387::aid-cncr2820690219>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 6.Burke AP, Virmani R. Cardiac myxoma. Am J Clin Pathol. 1993;100:671–80. doi: 10.1093/ajcp/100.6.671. [DOI] [PubMed] [Google Scholar]
- 7.Reyen K. Cardiac myxomas. N Engl J Med. 1995;333:1610–5. doi: 10.1056/NEJM199512143332407. [DOI] [PubMed] [Google Scholar]
- 8.Harris GJ, Tio FO, Grover FJ. Primary left atrial myxosarcoma. Ann Thorac Surg. 1993;56:564–6. doi: 10.1016/0003-4975(93)90902-t. [DOI] [PubMed] [Google Scholar]