Abstract
Clinical studies converge on the observation that circulating cytokines are elevated in most cancer patients by anti– vascular endothelial growth factor (VEGF) therapy. However, the source of these molecules and their relevance in tumor escape remain unknown. We examined the gene expression profiles of cancer cells and tumor-associated macrophages in tumor biopsies before and 12 days after monotherapy with the anti-VEGF antibody bevacizumab in patients with rectal carcinoma. Bevacizumab up-regulated stromal cell–derived factor 1α (SDF1α), its receptor CXCR4, and CXCL6, and downregulated PlGF, Ang1, and Ang2 in cancer cells. In addition, bevacizumab decreased Ang1 and induced neuropilin 1 (NRP1) expression in tumor-associated macrophages. Higher SDF1α plasma levels during bevacizumab treatment significantly associated with distant metastasis at three years. These data show that VEGF blockade up-regulates inflammatory pathways and NRP1, which should be evaluated as potential targets for improving anti-VEGF therapy.
Introduction
Antiangiogenic therapy with bevacizumab—an anti–vascular endothelial growth factor (VEGF) antibody (Genentech)—with standard chemotherapy has proven efficacious in multiple advanced cancers (1). However, overall survival benefit is modest, and even for responding patients, the benefit is often short lived. Moreover, adjuvant bevacizumab with chemotherapy did not result in an overall statistically significant prolongation in disease-free survival (DFS) at 3 years in colorectal cancer (2). The angiogenic pathway targeted might be insufficient because an advanced tumor may use multiple pathways for blood vessel formation (3). Alternatively, the tumor might switch from the targeted pathway to another pathway that supports its acquisition of new vessels. Increases in key mediators of angiogenesis in circulation have been reported in multiple studies of anti-VEGF agents in patients with cancer, but it is unclear if they originate from the tumor or directly affect the tumor (1). Plasma stromal cell–derived factor 1α (SDF1α) and basic fibroblast growth factor (bFGF) seem to associate with resistance to antiangiogenic therapy in patients with glioblastoma, whereas SDF1α and interleukin (IL)-6 associate with progression of hepatocellular carcinoma after antiangiogenic therapy (4, 5). These findings are in line with preclinical data suggesting a potential role for bFGF and other cytokines beyond VEGF in tumor angiogenesis (6, 7). However, the pathways that are activated after VEGF blockade in human tumors remain unknown.
Identifying pathways of resistance could help optimize the sequencing of different antiangiogenic agents already approved and/or currently under development. Gene expression profiling studies are frequently used to identify new candidate genes for diagnostic, prognostic, and therapeutic purposes. However, a common problem in the analysis of changes in tumor tissue is their spatial and temporal heterogeneity. Laser capture microdissection (LCM) can isolate highly pure cell populations from a heterogeneous tissue section, permitting gene expression profiling of specific cell populations. Therefore, we used LCM to explore changes in tissue biomarkers in response to bevacizumab in rectal carcinoma patients enrolled in a phase II study of bevacizumab monotherapy followed by bevacizumab with chemoradiation (8). The unique design of this trial permitted us to compare the expression of 20 angiogenic cytokines and their receptors in cancer cells and tumor-associated macrophages (TAM) in tumor biopsies before and after bevacizumab monotherapy.
Materials and Methods
Biopsies
Rectal tumor biopsies were obtained with informed consent— after approval by the National Cancer Institute-Cancer Therapy Evaluation Program and Institutional Review Boards of Massachusetts General Hospital and Duke University—before, and then 12 d after the first bevacizumab infusion, using flexible sigmoidoscopy to visualize the tumor. Tissues were fixed in 4% paraformaldehyde for paraffin embedding or snap frozen in liquid nitrogen and stored at −80°C.
LCM
For LCM, frozen tumor sections were air dried and fixed in 70% ethanol. Slides were incubated with anti–human CD68/macrosialin rat antibody (1:10 dilution in blocking serum supplemented with SuperaseIn RNase inhibitor; 0.4 units/mL; Serotec), then with biotinylated rabbit anti-rat IgG (20 mg/mL), and finally, Vectastain ABC Elite kit and 3,3′-diaminobenzidine chromogenic substrate were used for development.
Laser capture was done using Veritas Microdissection System (Arcturus, Molecular Devices). Microdissections were performed under ×40 objectives using CapSure LCM macro caps. Laser settings ranged from 50 to 80 mW (power), 1,500 to 2,000 ms (duration), and 15 to 20 µm (diameter). We performed ~500 laser pulses per specimen. To avoid contamination by adjacent cells, paper Prepar strips (Arcturus) were used before placing LCM caps to remove tissues that were not well fixed on the sections. The efficiency of LCM was evaluated by examining the cap after capture, and by examining the tissue before and after lifting off the cap. The analysis was done only when frozen biopsy tissues contained clear areas of carcinoma— evaluated by an experienced pathologist. LCM was performed on 10 sections from each patient. Due to the technical difficulty in isolating endothelial cells without contamination from pericytes, we did not pursue studies in these tumor-associated host cell types. Also, given the limited amount of RNA collected, we pursued transcriptional studies using quantitative PCR (qPCR). Based on these results, we validated the qPCR analysis for the candidate genes with non–amplified RNA.
To determine the potential cellular contamination of the laser-captured macrophages by surrounding tumor cells, we measured CD68 expression by reverse transcription-PCR. In cDNA from captured TAMs, CD68 levels were 96-fold higher compared with that in cancer cells, which remained at background levels after 40 cycles of amplification.
RNA extraction
Total RNA was extracted using the PicoPure RNA isolation kit (Arcturus) using the protocol of the manufacturer.
Real-time PCR assay
RNA from each sample was used for linear mRNA amplification to generate the amplified RNA as a template for real-time PCR assay. Two rounds of linear mRNA amplification were performed using the RiboAmp RNA Amplification kit (Arcturus). First-strand cDNA synthesis was done using 100 pg of RNA and the oligo-dT primers. One microgram of amplified RNA templates from each sample was used to synthesize cDNAs using TaqMan Reverse Transcription kit (Applied Biosystems). Quantitative RT-PCR (ABI Prism 7300, Applied Biosystems) was used to determine the mRNA levels. The thermal cycling conditions consisted of 45 cycles of amplification (annealing/extension: 68°C). The primers were designed using Primer3 software (Applied Biosystems; Supplementary Table S1). Quality control studies included assessment of the tRNA integrity by using the Agilent 2100 Bioanalyzer, elimination of genomic DNA contamination (verified by DNA-targeted qPCR on DNase-treated RNA), evaluation of the efficiency, dissociation curve, and sensitivity of the PCR reaction, and verification of the amplicon size on a 4% Nusieve 3:1 agarose gel (Bio Whittaker).
Immunohistochemistry
Five-micrometer-thick paraffin and frozen sections were cut and immunostained overnight with antibodies against SDF1α, CXCR4, and neuropilin 1 (NRP1; Supplementary Note).
Analysis of circulating biomarkers
Peripheral blood biomarker measurements in these patients have been previously reported (8). Here, we evaluated the correlation between their levels after treatment and DFS using Cox proportional hazards model with rank-transformed covariates.
LCM data analysis
We chose relative quantification to determine the amplification of genes. The change in amplification of genes was normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control gene. qPCR data were analyzed by the Relative Quantification Study in Sequence Detection Software v1.2 (Applied Biosystems). Changes ( fold) in gene expression level were calculated by the 2DDCt method. In brief, we used the following formula:
in which the variables are as follows: x0, number of PCR cycles at baseline; x1, number of PCR cycles at 12 d after bevacizumab treatment; GAPDH0 , number of PCR cycles for GAPDHat baseline; and GAPDH1 , number of PCR cycles for GAPDHat 12 d after bevacizumab treatment. When the limit of 50 PCR cycles was reached for x0, we placed “>” in front of y, because the fold difference is greater than y (Table 1 and Table 2). When the limit of 50 PCR cycles was reached for x1, then we put “<” in front of y, because the fold difference is lower than y. In several instances ( for example, the expression of CXCR4 in cancer cells in patient no. 2; Table 1), the limit of 50 cycles was reached both at baseline and posttreatment (i.e., CXCR4 was undetectable at both time points). Changes in the gene expression were analyzed for the patient cohort using paired exact Wilcoxon test (taking into consideration the number of patients showing increases/decreases and the extent of change).
Table 1.
Changes in gene expression in cancer cells after bevacizumab monotherapy measured using qPCR*
| Gene | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | Patient 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SDF1α | 34 | 3.7 | 2 | 9.8 | 4.9 | 0.57 | 6.5 | 4 | 2.5 | 2.8 | 5.7 | 79 |
| CXCR4 | 28 | 7 | 2 | 14 | 15 | 7 | 2.8 | >360 | 91 | >890 | 4 | 64 |
| CXCL6 | 30 | 6.5 | 0.31 | 28 | 2.5 | 5.3 | 3.2 | 0.062 | 45 | 0.41 | >49,000 | 5.3 |
| ANG1 | <0.0002 | 0.2 | 0.81 | <0.0014 | <0.0001 | 0.082 | 0.25 | 2 | 0.0073 | <0.0001 | <0.0001 | <0.051 |
| ANG2 | 0.088 | <0.0003 | <0.0002 | 0.00074 | 0.41 | <0.0002 | 0.11 | <0.0059 | 8.6 < | <0.0001 | 0.062 | 0.33 |
| PlGF | 0.41 | 0.33 | 0.009 | <0.0063 | <0.0001 | 0.31 | 0.31 | <0.0001 | 0.0068 | 0.31 | 0.2 | <0.0078 |
| NRP1 | 2.8 | <0.036 | 0.012 | 39 | 4.6 | <0.0068 | 4 | 2.5 | 510 | 2.8 | 150 | 9.2 |
| CXCL5 | 4 | 49 | 0.00013 | 340 | 170 | 0.87 | 0.036 | 0.22 | 39 | 0.62 | 60 | 9.8 |
| IL-8 | 0.00023 | 1.6 | 0.054 | 1.5 | 18 | 2 | 2.6 | 0.81 | 180 | 9.8 | 32 | 450 |
| bFGF | 0.31 | 0.23 | 0.19 | 0.41 | 18 | 0.2 | 0.57 | 1.7 | 84 | 1.2 | 100 | 16 |
| TGFB1 | 0.41 | 1.7 | 0.013 | 0.088 | 17 | 49 | <0.0001 | <0.0001 | 5.7 | 0.019 | 1.9 | 2 |
| TNFAIP2 | 28 | >300 | 0.095 | 16 | 0.14 | 2.8 | 0.66 | 1.1 | 26 | 0.29 | >420 | 1,800 |
| MIF | 0.5 | 1 | 0.66 | 0.029 | 11 | 0.29 | 2.6 | 0.41 | 1.1 | 0.76 | 0.12 | 0.81 |
| NRP2 | 0.93 | 2.1 | 0.54 | 1.2 | 11 | 0.0048 | 1.1 | 0.57 | 0.57 | 0.71 | 0.018 | 0.33 |
| VEGF | 6.1 | 0.1 | 14 | 0.058 | 12 | 0.81 | 0.0063 | 69 | 0.54 | 2 | 49 | 0.35 |
| VEGFR1 | <0.0024 | <0.0063 | 0.99 | <0.0001 | 15 | <0.0018 | >340 | >320 | 7.5 | >550 | >52 | 0.00091 |
| VEGFR2 | 180 | 56 | 7 | 39 | 91 | 1.4 | 0.18 | 0.051 | 79 | 0.16 | 0.054 | 0.38 |
| VEGF-C | <0.0024 | <0.0063 | 1 | <0.0001 | 15 | <0.0018 | 0.088 | >320 | 5.7 | >510 | >52 | 0.00091 |
| VEGFR3 | 45 | 6.5 | 1.2 | 0.16 | <0.0003 | 0.57 | 2.2 | 0.44 | 52 < | <0.0001 | >180 | <0.0073 |
Values are fold changes after bevacizumab treatment which are normalized to housekeeping gene (GAPDH) expression. Data are shown in fold difference at day 12 after bevacizumab treatment compared with baseline (red, up-regulated >2-fold; white, changed <2-fold; green, down-regulated >2-fold), and normalized to the expression of a housekeeping gene (GAPDH).
Table 2.
Changes in gene expression in TAMs after bevacizumab monotherapy measured using qPCR
| Gene | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 |
|---|---|---|---|---|---|---|---|---|
| SDF1α | 0.87 | 45 | 1.4 | 0.095 | 1.4 | 0.87 | 2 | 0.0062 |
| CXCR4 | 0.35 | 16 | 0.13 | 1.7 | >56 | 0.35 | 1.4 | 0.045 |
| NRP1 | 1.3 | 2.6 | 2.1 | 1.1 | 120 | 590 | 2.1 | 4.9 |
| ANG1 | 0.28 | 0.095 | 0.22 | 0.29 | 0.15 | 0.35 | 0.38 | 0.11 |
| ANG2 | 0.037 | 0.12 | 1.3 | 0.5 | 0.22 | 8 | 0.22 | 0.095 |
| DLL4 | 0.81 | 1.1 | 49 | 0.077 | 0.067 | 0.067 | 1.3 | 0.33 |
| PlGF | 0.25 | 9,400 | 1 | 0.0008 | —* | 28 | 13 | 0.37 |
| CXCL5 | 0.077 | 2.7 | 0.095 | 84 | 1.9 | 0.35 | 12 | 0.57 |
| CXCL6 | 0.0003 | 0.011 | 4 | 52 | 1.7 | 150 | 4.9 | 0.54 |
| GM-CSF | 0.57 | 1.1 | 1.6 | 3.5 | 1.1 | 0.0055 | 4.3 | 0.19 |
| IL-8 | 1.1 | 2.6 | 0.38 | 1.1 | 370 | 1.1 | 7.5 | 0.02 |
| TGFβ1 | 0.0024 | 4 | 1 | 0.62 | 10 | 49 | 0.2 | 0.0049 |
| MIF | 0.082 | 2.1 | 680 | 0.47 | 0.054 | 0.0068 | 1.5 | 0.57 |
| NRP2 | 0.22 | 1.1 | 1.6 | 8.6 | 0.31 | 21 | 13 | 0.37 |
| VEGF | 2.6 | 42 | —* | 1.1 | 200 | 0.29 | 15 | 0.009 |
NOTE: Data are shown in fold difference at day 12 after bevacizumab treatment compared with baseline (red, up-regulated >2-fold; white, changed <2-fold; green, down-regulated >2-fold), and normalized to the expression of a housekeeping gene (GAPDH).
Gene expression undetectable at both time points.
Results and Discussion
VEGF blockade by bevacizumab treatment significantly induces expression of SDF1α and CXCL6 in rectal cancer cells
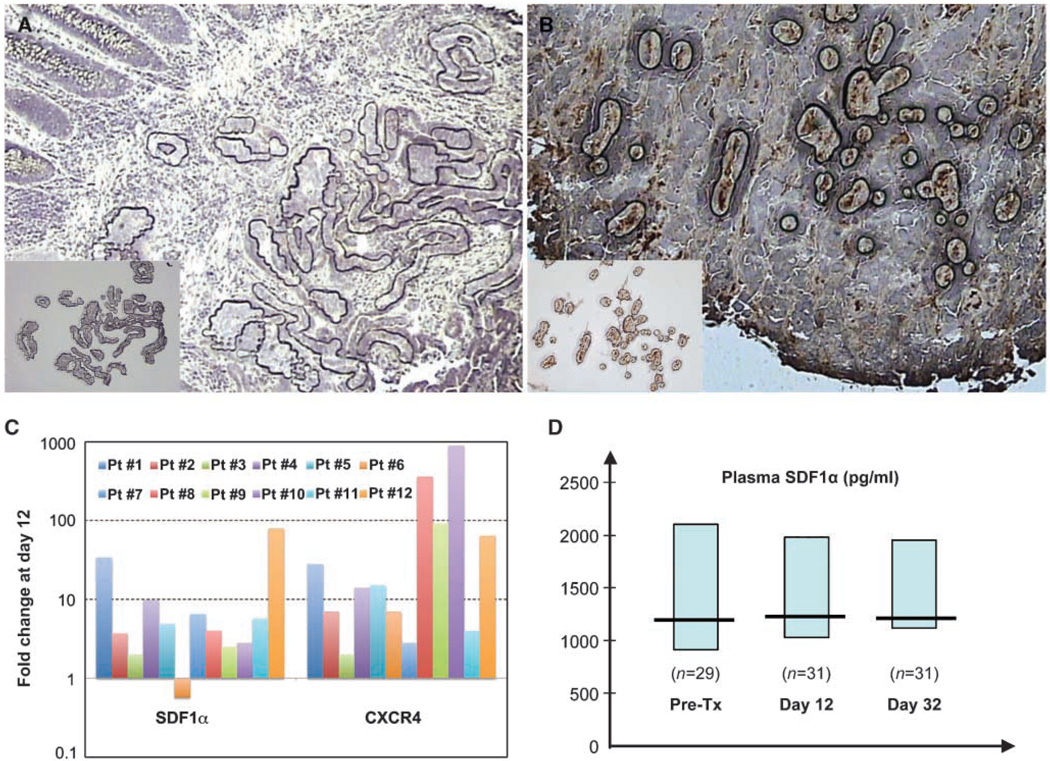
For LCM, we identified cancer cells afterH&Estaining, and TAMs after CD68 immunostaining (Fig. 1A and B). We detected analyzable cancer tissue in 12 serial biopsy pairs. TAMs were identified and collected in sufficient numbers from 8 of these 12 serial biopsy pairs.
Figure 1.
Changes in expression in cancer cells and TAMs from rectal carcinoma and in circulating plasma SDF1α levels after bevacizumab treatment. A, representative image of tumor tissue after selected tumor cells were burned by the capture laser and before RNA extraction (inset). B, selection of TAMs for LCM guided by CD68 immunostaining. Images of TAMs, burned by the capture laser of the cap containing TAMs (inset ; magnification, ×20). C, comparison of relative SDF1α and CXCR4 RNA levels in tumor cells captured by LCM from 12 pairs of serial biopsy samples collected before and 12 d after bevacizumab monotherapy. Columns, mean fold change in gene expression compared with pretreatment value. GAPDH was used as RNA control in the PCR. D, kinetics of plasma SDF1α after treatment with bevacizumab alone (day 12) and after bevacizumab with chemoradiation (day 32). Note the relatively high circulating levels of SDF1α (median of >1 ng/mL, shown with 95% confidence intervals) and the lack of consistent change in the overall population. Pre-Tx, pretreatment.
Because circulating levels of VEGF and PlGF are significantly elevated after bevacizumab treatment (8), we first examined the transcription of these factors in the tumor. Bevacizumab did not consistently upregulate VEGF mRNA expression in the cancer cells or TAMs captured, but surprisingly led to a lower PlGF mRNA expression in cancer cells (P < 0.001; Table 1 and Table 2). Of note, 6 of 12 patients had substantial increases and 4 of 12 patients had decreases in VEGF mRNA expression. Next, we measured the changes in expression of a set of proangiogenic transcription factors, cytokines, and their receptors, which might facilitate escape from VEGF blockade.
VEGF blockade up-regulated the expression of SDF1α (P < 0.01) as well as that of granulocyte chemotactic protein-2 (GCP-2/CXCL6, P < 0.05; Table 1; Fig. 1C). Expression of SDF1α protein in cancer cells was confirmed by immunohistochemistry in the biopsy tissues before and after bevacizumab treatment, as well as in lung metastases (Supplementary Figs. S1 and S2). The SDF1α pathway is known to modulate angiogenesis independent of VEGF pathway (9), as well as metastasis in gastrointestinal tumors (10). Similarly, GCP-2/CXCL6 is expressed in gastrointestinal tumors and promotes angiogenesis (11).
In addition, bevacizumab significantly decreased the expression of Ang1 and Ang2—key factors in angiogenesis and vascular normalization (12)—in cancer cells and lowered Ang1 in TAMs at day 12 (P < 0.05; Table 1 and Table 2). We had previously found by immunohistochemistry that VEGF blockade reduces the expression of Ang2 in endothelial cells in rectal tumors (13).Other factors analyzed in cancer cells and TAMs (e.g., bFGF, transforming growth factor-β, IL-8, granulocyte macrophage colony-stimulating factor, etc., Table 1 and Table 2) showed no significant changes at day 12. Of interest, we found no change in HIF1α transcription in cancer cells. Future studies should establish the changes in HIF1α protein level and stability after VEGF blockade. Thus, we identified molecules that are upregulated in tumors but not in plasma. We propose that the latter are potential targets to delay the escape from anti-VEGF therapy.
VEGF blockade by bevacizumab treatment induces significant changes in gene expression of cellular receptors in cancer cells and TAMs
We next examined the gene expression changes of VEGF receptors in cancer cells. Transcripts of NRP1 and VEGFR2 but not VEGFR1 were detectable in cancer cells. This suggests the possibility that bevacizumab might directly affect the rectal cancer cells. NRP1 binds VEGF and modulates VEGFR2 signaling, and may also independently modulate cell migration and survival. NRP1 but not VEGFR2 protein expression was detectable by immunohistochemistry in cancer cells (Supplementary Figs. S1 and S2). VEGFR2 and NRP1 transcription was not significantly changed after VEGF blockade in this patient cohort; however, NRP1 increased in 9 of 12 patients (Table 1). In addition, we evaluated NRP1 expression in TAMs and found a significant increase at 12 days after bevacizumab (Table 2). Although the function of NRP1 in TAMs is currently unclear, NRP1 is upregulated in macrophages of the alternatively activated M2 phenotype (14). In the tumor microenvironment, these TAMs may adopt a trophic role that promotes angiogenesis, matrix proteolysis, and tumor progression (15).
Finally, VEGF blockade by bevacizumab up-regulated the expression of the SDF1α receptor CXCR4 in rectal cancer cells (P < 0.01; Table 1). Expression of CXCR4 protein in cancer cells was confirmed by immunohistochemistry in the biopsy tissues (Supplementary Fig. S1).
Collectively, these LCM data show that gene expression changes in rectal carcinomas following bevacizumab treatment are cell specific and different from changes in circulating cytokines, which likely emanate primarily from normal tissues (1).
Higher circulating SDF1α levels after VEGF blockade by bevacizumab are seen in patients with locally advanced rectal cancer who progress more rapidly to distant metastatic disease
With a median follow-up of 36 months (4–73 months), none of the 32 patients enrolled in this study experienced local recurrence after neoadjuvant treatment, surgery, and adjuvant chemotherapy (8). However, 6 of 32 patients did suffer distant failures (lung and/or liver metastasis) postsurgery. Because SDF1α was detectable in the plasma in concentrations of >1 ng/mL (Fig. 1D), and is known to promote cancer cell migration, proliferation, and survival (16), we explored the possible association between DFS and plasma SDF1α before, during, and after treatment. The levels of circulating plasma SDF1α during treatment (at days 12 and 32) were associated with a more rapid disease progression to metastasis (P < 0.05; Table 3), and similar trends were seen for plasma SDF1α at 3 days after bevacizumab alone, but not for plasma SDF1α measured at pretreatment or after neoadjuvant treatment (presurgery; Table 3). Of note, none of the other plasma circulating biomarkers—previously reported to correlate with the extent of primary tumor regression in these patients (i.e., PlGF, VEGF, IL-6, and CEA; ref. 8)—nor the pretreatment tumor and nodal stage were associated with distant failure postsurgery at 3 years (Table 3).
Table 3.
Correlation between DFS and circulating markers measured pretreatment, after bevacizumab alone, and after combination therapy as well as between DFS and pretreatment T and N staging
| Marker | Pretreatment | Day 3 | Day 12 | Day 32 | Presurgery |
|---|---|---|---|---|---|
| Pre-Tx | BV Monotherapy | Combination | Post-Tx | ||
| Plasma PlGF | 1.12; 0.82–1.52 (n = 31) | 1.18; 0.85–1.63 (n = 31) | 1.31; 0.93–1.84 (n = 29) | 0.94; 0.69–1.29 (n = 30) | 0.95; 0.72– 1.26 (n = 22) |
| P* | 0.48 | 0.32 | 0.12 | 0.71 | 0.73 |
| Plasma VEGF | 1.15; 0.81–1.63 (n = 31) | 1.07; 0.78–1.47 (n = 31) | 1.11; 0.80–1.55 (n = 29) | 0.94; 0.72–1.23 (n = 30) | 0.87; 0.64–1.18 (n = 22) |
| P | 0.43 | 0.68 | 0.54 | 0.64 | 0.38 |
| Plasma sVEGFR1 | 1.04; 0.77–1.42 (n = 31) | 1.10; 0.81–1.47 (n = 31) | 1.04; 0.79–1.38 (n = 29) | 1.13; 0.85–1.50 (n = 30) | 1.05; 0.77–1.42 (n = 22) |
| P | 0.79 | 0.55 | 0.76 | 0.40 | 0.76 |
| Plasma SDF1α | 1.26; 0.82–1.94 (n = 29) | 1.48; 0.97–1.97 (n = 30) | 2.73; 1.21–6.18 (n = 31) | 2.32; 1.18–4.59 (n = 31) | 1.45; 0.94–2.24 (n = 27) |
| P | 0.30 | 0.067 | 0.016 | 0.015 | 0.092 |
| Plasma IL-6 | 0.87; 0.51–1.50 (n = 26) | 0.73; 0.48–1.12 (n = 27) | 0.93; 0.52–1.65 (n = 26) | 1.31; 0.90–1.89 (n = 29) | 1.18; 0.82–1.69 (n = 24) |
| P | 0.62 | 0.15 | 0.80 | 0.16 | 0.38 |
| Plasma IL-8 | 0.69; 0.35–1.37 (n = 25) | 0.88; 0.62–1.23 (n = 27) | 0.95; 0.57–1.58 (n = 26) | 0.81; 0.59–1.13 (n = 29) | 0.60; 0.38–0.96 (n = 24) |
| P | 0.30 | 0.45 | 0.84 | 0.22 | 0.032 |
| Serum CEA | 1.05; 0.76–1.43 (n = 25) | 1.03; 0.74–1.43 (n = 25) | 0.98; 0.69–1.41 (n = 25) | 1.01; 0.68–1.50 (n = 23) | 1.12; 0.76–1.65 (n = 22) |
| P | 0.78 | 0.85 | 0.93 | 0.97 | 0.57 |
| T stage (3 or 4) | 1.15; 0.75–1.74 (n = 32) | N/A | N/A | N/A | N/A |
| P | 0.52 | ||||
| N stage (0 or >0) | 1.08; 0.82–1.43 (n = 32) | N/A | N/A | N/A | N/A |
| P | 0.56 | ||||
NOTE: Data are shown as hazard ratios (with 95% confidence intervals), derived from Cox proportional hazards model with rank-transformed covariates.
Abbreviations: BV, bevacizumab; Post-Tx, posttreatment combination therapy; NA, not applicable.
P values are from the Wald test.
Implications
The SDF1α-CXCR4 pathway may be a relevant escape/resistance mechanism when anti-VEGF agents are used in monotherapy (e.g., in adjuvant therapy or advanced disease; refs. 2, 17). In this context, we have found that significant increases in plasma SDF1α after antiangiogenic treatment in patients with glioblastoma and hepatocellular carcinoma was associated with poor outcomes (4, 5). In this relatively small group of patients, we found that bevacizumab consistently increased SDF1α as well as CXCR4 expression in rectal cancer cells, despite the known heterogeneity of tumors. Moreover, high levels of circulating SDF1α during neoadjuvant treatment associated with rapid distant disease progression. Interestingly, SDF1α and CXCR4 expression was detectable in metastatic cancer cells in the surgical specimens from liver and lung lesions of patients. Similarly, CXCL6 has been reported to be up-regulated in colon cancer, and plays a key role in the induction and maintenance of gut inflammation, enhancing the development and growth of colitis-associated colorectal cancer (18). Finally, the increase in NRP1 during bevacizumab treatment might be relevant because NRP1 promotes tumor growth and angiogenesis in human colorectal carcinomas (19), and NRP1 and VEGF blockade has been shown to additively inhibit tumor growth (20).
Although antiangiogenesis is a promising and actively employed cancer therapeutic, mechanistic insight into the molecular mechanisms underlying tumor resistance and escape from anti-VEGF therapy is sorely lacking. The identification of SDF1α-CXCR4, CXCL6, and NRP1 as potential pathways of escape from anti-VEGF therapy in patients with rectal carcinoma provides the necessary and critical insight for guiding further therapy.
Supplementary Material
Acknowledgments
Grant support: NIHgrants R21-CA99237, P01-CA80124, R01-CA115767, R01-CA85140, and R01-CA126642; Federal Share/National Cancer Institute Proton Beam Program Income grants; and by the National Foundation for Cancer Research.
We thank Sylvie Roberge, Christina Koppel, and Carolyn Smith for their technical support and Madeline Carroll for clinical trial oversight.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
B.G. Czito: commercial research grant, Genentech. C.G. Willett: speaker honorarium, Genentech. R.K. Jain: commercial research grant, AstraZeneca and Dyax; consultant/advisory board, AstraZeneca, Dyax, Millennium, and SynDevRx; speaker honorarium, Pfizer. The other authors disclosed no potential conflicts of interest.
References
- 1.Jain RK, Duda DG, Willett CG, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6:327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolmark N, Yothers G, O’Connell MG, et al. A phase III trial comparing mFOLFOX6 to mFOLFOX6 plus bevacizumab in stage II or III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2009;27 suppl doi: 10.1200/JCO.2010.30.0855. abstr LBA4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 4.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu AX, Sahani DV, Duda DG, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshiji H, Harris SR, Thorgeirsson UP. Vascular endothelial growth factor is essential for initial but not continued in vivo growth of human breast carcinoma cells. Cancer Res. 1997;57:3924–3928. [PubMed] [Google Scholar]
- 7.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol. 2009;27:3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guleng B, Tateishi K, Ohta M, et al. Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factor-independent manner. Cancer Res. 2005;65:5864–5871. doi: 10.1158/0008-5472.CAN-04-3833. [DOI] [PubMed] [Google Scholar]
- 10.Kollmar O, Rupertus K, Scheuer C, et al. Stromal cell-derived factor-1 promotes cell migration and tumor growth of colorectal metastasis. Neoplasia. 2007;9:862–870. doi: 10.1593/neo.07559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gijsbers K, Gouwy M, Struyf S, et al. GCP-2/CXCL6 synergizes with other endothelial cell-derived chemo-kines in neutrophil mobilization and is associated with angiogenesis in gastrointestinal tumors. Exp Cell Res. 2005;303:331–342. doi: 10.1016/j.yexcr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 13.Willett CG, Boucher Y, Duda DG, et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23:8136–8139. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 14.Gustafsson C, Mjosberg J, Matussek A, et al. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS ONE. 2008;3:e2078. doi: 10.1371/journal.pone.0002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 16.Brand S, Dambacher J, Beigel F, et al. CXCR4 and CXCL12 are inversely expressed in colorectal cancer cells and modulate cancer cell migration, invasion and MMP-9 activation. Exp Cell Res. 2005;310:117–130. doi: 10.1016/j.yexcr.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Grothey A. Future directions in vascular endothelial growth factor-targeted therapy for metastatic colorectal cancer. Semin Oncol. 2006;33:S41–S49. doi: 10.1053/j.seminoncol.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Waldner MJ, Neurath MF. Cytokines in colitis associated cancer: potential drug targets? Inflamm Allergy Drug Targets. 2008;7:187–194. doi: 10.2174/187152808785748137. [DOI] [PubMed] [Google Scholar]
- 19.Parikh AA, Fan F, Liu WB, et al. Neuropilin-1 in human colon cancer: expression, regulation, and role in induction of angiogenesis. Am J Pathol. 2004;164:2139–2151. doi: 10.1016/S0002-9440(10)63772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan Q, Chanthery Y, Wu Y, et al. Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J Biol Chem. 2007;282:24049–24056. doi: 10.1074/jbc.M703554200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.