SUMMARY
Wnt/β-catenin and NF-κB signaling mechanisms provide central controls in development and disease, but how these pathways intersect is unclear. Using hair follicle induction as a model system, we show that patterning of dermal Wnt/β-catenin signaling requires epithelial β-catenin activity. We find that Wnt/β-catenin signaling is absolutely required for NF-κB activation, and that Edar is a direct Wnt target gene. Wnt/β-catenin signaling is initially activated independently of Eda/Edar/NF-κB activity in primary hair follicle primordia. However, Eda/Edar/NF-κB signaling is required to refine the pattern of Wnt/β-catenin activity, and to maintain this activity at later stages of placode development. We show that maintenance of localized expression of Wnt10b and Wnt10a requires NF-κB signaling, providing a molecular explanation for the latter observation, and identify Wnt10b as a direct NF-κB target. These data reveal a complex interplay and inter-dependence of Wnt/β-catenin and Eda/Edar/NF-κB signaling pathways in initiation and maintenance of primary hair follicle placodes.
INTRODUCTION
Hair follicle development requires reciprocal communication between surface epithelial cells and the underlying mesenchyme that is mediated by secreted signaling molecules (Schmidt-Ullrich and Paus, 2005). A signal from the dermis is thought to initiate formation of a regular array of epithelial thickenings, known as hair follicle placodes (Hardy, 1992). Whether this initiating dermal signal is broadly expressed or patterned is unknown. Signaling from the placodes promotes clustering of underlying dermal fibroblasts, forming dermal condensates that are the precursors of hair follicle dermal papillae (Schmidt-Ullrich and Paus, 2005). Further signaling interactions between the hair placode and the nascent dermal papilla lead to placode down-growth and hair follicle morphogenesis. Mouse hair follicle development occurs in several waves, with primary (guard) hair follicle placodes appearing at approximately E14.5, and secondary (awl and zigzag hair) placodes forming between E16.5 and birth (Schmidt-Ullrich and Paus, 2005).
Among known signaling mechanisms involved in hair follicle development, the Wnt/β-catenin and Eda/Edar pathways appear to play the earliest roles (Fuchs, 2007; Schmidt-Ullrich and Paus, 2005). Expression of several Wnt ligands and Wnt reporter transgenes is specifically elevated in developing hair follicles (DasGupta and Fuchs, 1999; Maretto et al., 2003; Reddy et al., 2001), and forced activation of β-catenin signaling promotes hair follicle fate in both embryonic and postnatal skin (Gat et al., 1998; Narhi et al., 2008; Zhang et al., 2008). Conversely, ectopic expression of the secreted Wnt inhibitor Dkk1 in embryonic mouse epidermis prevents the initiation of hair follicle development and blocks patterned expression of all molecular placode markers, including Wnt ligands, suggesting the importance of an earlier acting, broadly expressed Wnt signal (Andl et al., 2002). Inefficient depletion of β-catenin from embryonic epidermis also blocks early stages of hair follicle development (Huelsken et al., 2001), although the precise stage of arrest remains unclear.
Binding of the A1 isoform of the Tumor Necrosis Factor α family member Ectodysplasin to its receptor EDAR induces nuclear translocation of the transcription factor NF-κB, and NF-κB pathway activation in developing hair follicle placodes (Kumar et al., 2001; Schmidt-Ullrich et al., 2006; Yan et al., 2000). Loss of function mutations in these genes or suppression of NF-κB activity by ubiquitous expression of the transdominant super-repressor IκBαΔN block very early stages in the formation of primary and zigzag hair follicles, but do not affect awl or vibrissa follicle development (Schmidt-Ullrich and Paus, 2005; Schmidt-Ullrich et al., 2006). Transient primary pre-placode structures are detected in the absence of Eda/Edar/NF-κB signaling (henceforth referred to as Edar signaling), but these fail to express Shh or cyclin D1 and are not maintained (Schmidt-Ullrich et al., 2006).
Formation of a regular, patterned array of primary hair follicles is thought to occur via a reaction-diffusion mechanism based on competition between placode promoting and placode-inhibitory morphogens (Jiang et al., 2004). Secreted Wnt inhibitors such as Dkk4 may contribute to array establishment by blocking the actions of placodal Wnts in adjacent interfollicular epidermis (Bazzi et al., 2007; Sick et al., 2006), while the Edar-BMP mutual activation-inhibition system is suggested to stabilize a labile pre-pattern established by early Wnt/β-catenin signaling (Mou et al., 2006). However, it is unclear how the Wnt/β-catenin and Edar signaling pathways intersect at the molecular level, and to what extent these pathways are inter-dependent. It is also not clear whether Wnt/β-catenin signaling operates solely within the ectoderm in its interactions with Edar pathway components or whether Wnt indirectly controls such interactions via the dermis (Andl et al., 2002).
To address these questions we analyzed the effects of specific genetic manipulations of the Wnt/β-catenin pathway on the pattern of Wnt signaling activity, and on Edar signaling and function. Conversely, we determined the effects of loss of Edar signaling on Wnt pathway activity. The results of these experiments demonstrated an unexpected requirement for epithelial β-catenin in establishing patterned dermal Wnt activity, and revealed a complex interplay and interdependence between the Wnt and Edar signaling pathways in primary hair follicle placode formation.
RESULTS
Wnt/β-catenin pathway activation is first observed broadly in the dermis
To detect Wnt/β-catenin signaling pathway activity in embryonic skin we utilized three independent Wnt reporter lines: conductin+/lacZ (cond-lacZ) mice in which β-galactosidase reporter gene expression is regulated by the endogenous promoter of the conductin/axin2 gene, a direct target of canonical Wnt/β-catenin signaling (Jho et al., 2002; Yu et al., 2005); and TOPGAL and BAT-gal mice that carry transgenes containing 3 or 7 copies of a consensus LEF1/TCF DNA binding sequence, respectively, placed upstream of different minimal promoters and lacZ (DasGupta and Fuchs, 1999; Maretto et al., 2003). Wnt reporter activity is blocked by ectodermal Dkk1 expression, indicating that it is specific (Chu et al., 2004; Liu et al., 2007).
Cond-lacZ activity, assayed by X-gal staining or immunoflurescence for β-galactosidase, was present uniformly in the upper dermis at E12.5 (Figure 1A; Supplementary Figure S1A). Several Wnt genes that could potentially activate β-catenin signaling in the upper dermis are expressed in the surface epithelium or dermis at E12.5 – E13.5 (Supplementary Figure S1B and data not shown), and the Fzd10 Wnt receptor gene shows prominent expression in both surface epithelial and immediately underlying dermal cells (Supplementary Figure S1C).
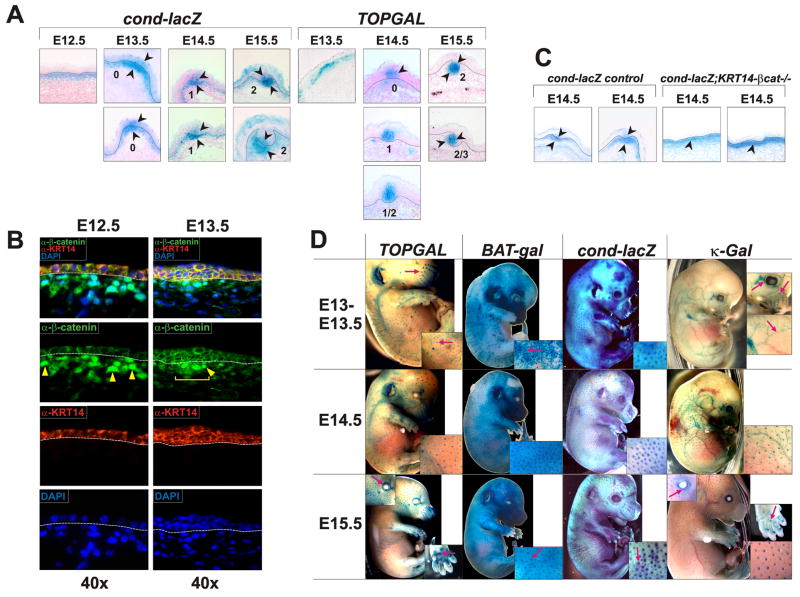
Figure 1. Wnt/β-catenin and NF-κB signaling in embryonic skin.
(A) Technovit sections of X-Gal stained cond-lacZ and TOPGAL Wnt reporter embryos at E12.5 – E15.5. Arrowheads indicate X-Gal staining. Dashed lines indicate the dermal-epidermal boundary. Numbers specify developmental stages of placodes. (B) Immunofluorescence detection of β-catenin (green). α-KRT14 antibody (red) was used to mark the epidermis. Yellow arrowheads indicate nuclear β-catenin staining in the dermis. A yellow bracket indicates localized dermal nuclear β-catenin at E13.5. Dashed lines indicate the dermal-epidermal boundary. (C) Technovit sections of E14.5 X-Gal stained cond-lacZ and cond-lacZ;KRT14-βcat−/− embryos. Arrowheads indicate X-Gal staining. Dashed lines indicate the dermal-epidermal boundary. (D) Whole mount X-Gal staining of Wnt reporter (TOPGAL, BAT-gal, cond-lacZ) and NF-κB reporter (κ-Gal) embryos at the time points indicated. Red arrows in insets indicate hair placodes and vibrissae, blood vessels, eyelids, and developing sweat glands in footpads.
At E13.5 Cond-lacZ activity remained in the upper dermis, and was focally elevated in the dermis and epithelium at sites of future pelage hair placode formation (Figure 1A, Stage 0). At E14.5 cond-lacZ activity persisted in the dermal condensate, and, within the epithelium, became elevated and restricted to cells in the center of the placode (Figure 1A). TOPGAL expression was also focally observed in the upper dermis at E13.5, but faded from this site at E14.5, appearing instead in developing Stage 0 and Stage 1 epithelial placodes. At E15.5 both cond-lacZ and TOPGAL were expressed in the center of the epithelial placode, and in the dermal condensate (Figure 1A, Stage 2). Lack of dermal X-Gal staining in Stage 1 hair follicles in TOPGAL embryos could be due to differences in promoter sensitivity to Wnt signaling between TOPGAL mice and cond-lacZ mice. LacZ expression in cond-lacZ mice reflects activity of an endogenous Wnt-responsive promoter and so may provide a more accurate readout of β-catenin signaling. Consistent with this, nuclear localized β-catenin, another indicator of β-catenin pathway activity (Clevers, 2006), is prominent in the upper dermis at E12.5 before becoming mostly restricted to dermal condensates at E13.5 (Figure 1B). Epithelial pre-placodes displayed elevated β-catenin protein at this stage, but clear nuclear localization was not evident in the epithelium, possibly due to the prominent membrane staining in epithelial cells.
β-catenin mRNA is transcribed ubiquitously at low levels, including in the dermis, but shows specific transcriptional upregulation in certain tissues (Huelsken et al., 2001). In contrast to Wnt reporter gene activity and nuclear localized β-catenin in dermal cells, specific transcriptional up-regulation of β-catenin, assayed by in situ hybridization or by X-gal staining of β-catlacZ/+ (βcatlacZ) knock-in embryos (Huelsken et al., 2001), was confined to skin epithelial cells. Within the epithelium, β-catenin mRNA was expressed uniformly at E12.5, was locally upregulated in developing placodes at E13.5 (Supplementary Figure S1D, stage 0), and remained elevated in specific subsets of hair follicle epithelial but not dermal cells throughout embryonic development and in adult life (Huelsken et al., 2001; Supplementary Figure S1D; and data not shown). Thus transcriptional upregulation of β-catenin mRNA may contribute to signaling in hair follicle epithelial, but not dermal cells.
Patterning of Wnt/β-catenin activity in the dermis requires epithelial β-catenin
The most sensitive Wnt reporter examined here, cond-lacZ, shows patterned activity similar to that of nuclear localized β-catenin, and is observed earlier than other described pre-placode markers, suggesting that its expression reflects early patterning events. As we could not temporally separate the appearance of patterned cond-lacZ activity in the dermis and epidermis at E13.5, patterned epithelial signaling could occur in response to patterned dermal signaling, or vice versa; alternatively these patterning events could be due to independent competitive interactions in dermis and epidermis. To begin to distinguish between these mechanisms we generated cond-lacZ embryos in which the β-catenin gene Ctnnb1 is efficiently deleted from embryonic surface ectoderm by E11.5 using an early-acting KRT14-Cre line (Liu et al., 2007) (Supplementary Figure S3). As expected, cond-lacZ expression was absent from the epithelium of KRT14-Cre;Ctnnb1fl/fl;cond-lacZ (KRT14-β-cat−/−;cond-lacZ) embryos at E13.5 and E14.5. Patterned dermal cond-lacZ expression was not observed in these mutants; instead, cond-lacZ staining was observed uniformly in the upper dermis, both at E13.5 and E14.5 (Figure 1C and data not shown). Thus, patterning of β-catenin signaling in the dermis requires epithelial β-catenin.
Focal Wnt/β-catenin activity and β-catenin mRNA up-regulation are observed prior to the onset of NF-κB signaling
To determine the relative timing of Wnt/β-catenin and Edar activation at E13.5 – E15.5, the developmental stages at which primary (guard) hair follicles, start to develop, we compared β-galactosidase expression in TOPGAL, BAT-gal, and cond-lacZ Wnt reporter embryos and the NF-κB reporter line (Igκ)3xcona-lacZ (κ-Gal) (Schmidt-Ullrich et al., 1996) (Figure 1D). At E13.5, Wnt reporter activity appeared in a pattern corresponding to primary pelage hair follicle development (Figure 1D). By contrast, NF-κB reporter gene activity was not observed in the skin until E14.5 (Schmidt-Ullrich et al., 2006; Figure 1D). While differing reporter gene sensitivities may influence these data, they suggest that localized Wnt/β-catenin pathway activation occurs approximately 24 hours before the onset of NF-κB signaling.
At E14.5, NF-κB reporter activity was confined to epithelial placodes while cond-lacZ and BAT-gal activity was observed in dermal condensates as well as placodes (Figure 1A; Schmidt-Ullrich et al., 2006). At E14.5 and E15.5 similar numbers of X-gal stained spots were observed in TOPGAL and κ-Gal embryos, corresponding to guard hair placodes. In cond-lacZ and BAT-gal embryos additional spots were visible. These may correspond to the locations of secondary hair follicle pre-placodes, reflecting greater sensitivity of the cond-lacZ and BAT-gal compared with TOPGAL reporter genes; alternatively they may represent transient placodal structures that are not maintained.
Similar to expression of Wnt reporter transgenes, elevated focal β-catenin transcriptional activity was observed one day before the onset of NF-κB reporter gene expression (Schmidt-Ullrich et al., 2006; Supplementary Figure S1D). At subsequent stages, elevated β-catenin transcriptional activity and NF-κB reporter gene expression coincided, and were confined to hair follicle epithelial cells, first in the proliferating hair germ, and at later stages in the follicle pre-cortex and cortex (Schmidt-Ullrich et al., 2006; Supplementary Figure S1D, and Figure 2D).
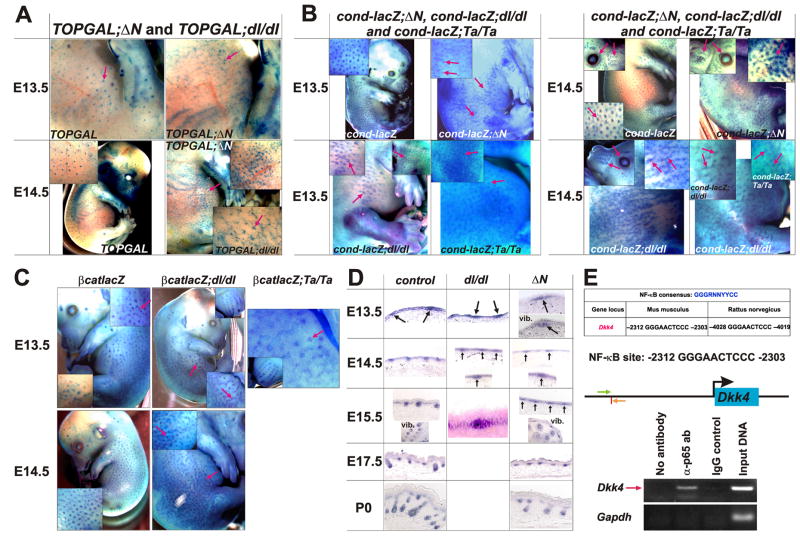
Figure 2. Wnt activity and β-catenin mRNA up-regulation in the absence of Edar signaling.
(A, B) X-gal stained E13.5 and E14.5 embryos of the following genotypes: control TOPGAL, TOPGAL;ΔN, and TOPGAL;dl/dl (A); and control cond-lacZ, cond-lacZ; ΔN, cond-lacZ;dl/dl and cond-lacZ;Ta/Ta (B). Arrows indicate hair placodes displaying Wnt reporter gene expression. Arrows in insets indicate hair placodes, eyelids and vibrissae. (C) X-gal stained E13.5 and E14.5 βcatlacZ, βcatlacZ;dl/dl and βcatlacZ;Ta/Ta embryos. (D) In situ hybridization for β-catenin mRNA using sagittal skin sections of control, dl/dl and ΔN embryos at the time points indicated. Arrows indicate developing placodes. (E) Dkk4 is an NF-κB target gene. Upper panel: The upstream promoter of Dkk4 contains a consensus NF-κB DNA binding site, located at -2303 - -2312 in mouse Dkk4 (vertical red line). Green and orange arrows indicate the positions of ChIP primers in the mouse Dkk4 promoter. Lower panel: ChIP using wild type E14.5 epidermal extracts, anti-p65 antibody (α-p65 ab) or IgG control, and Dkk4 or control Gapdh primers.
Focal Wnt/β-catenin signaling occurs in the absence of Eda-A1/Edar/NF-κB signaling
The overlapping patterns of Wnt and NF-κB reporter gene expression and β-catenin transcriptional elevation in hair follicle epithelial cells are consistent with direct cross-talk between these signaling pathways. However, the earlier appearance of patterned Wnt reporter activity suggests that Wnt/β-catenin signaling may be activated independently of NF-κB. To test this, we crossed TOPGAL, cond-lacZ and βcatlacZ mice with Eda−/− (tabby) (Ta/Ta) (Mikkola et al., 1999) and Edar−/− (downless) (dl/dl) (B6C3FE-a/a-Edardl-J, Jackson Laboratories #000210) (Headon and Overbeek, 1999) mice, or mice with suppressed NF-κB activity (cIκBαΔN) (ΔN) (Schmidt-Ullrich et al., 2001) (Figure 2A, B). Localized Wnt reporter gene activity was detected in dl/dl, Ta/Ta and ΔN embryos between E13.5 and E14.5. However, consistent with prior data (Mou et al., 2006), the borders of X-gal positive placodes were often not well defined, resulting in string-like structures that may result from placode fusion (insets in Figure 2A, B). β-catenin expression at E14.5 showed similar ill-defined borders and string-like structures in dl and Ta mutants (Figure 2C, D). Interestingly, at E13.5 irregular X-gal positive foci and string-like structures were observed in some control embryos as well as in dl and Ta mutants, suggesting that these structures represent an early stage in placode formation. These data indicate that initial localized upregulation of both β-catenin transcription and Wnt/β-catenin signaling activity is independent of NF-κB signaling. However, subsequent refinement of the pattern of Wnt/β-catenin activity into placodes with well-defined borders requires activation of the Edar pathway.
The secreted Wnt inhibitor and direct Wnt target gene Dkk4 has been suggested to engage in negative feedback signaling to regulate placode size and spacing (Bazzi et al., 2007; Sick et al., 2006). To ask whether Eda-A1/Edar/NF-κB signaling might co-regulate Dkk4, we carried out chromatin immunoprecipitation (ChIP) assays with extracts of E14.5 embryonic epidermis, using primers that amplify a region of the Dkk4 promoter containing a perfect consensus NF-κB binding site that is conserved between mouse and rat (Figure 2E). These experiments demonstrated that NF-κB complexes bind directly to the Dkk4 promoter (Figure 2E). In line with this, Dkk4 expression was strongly reduced, although not absent, in ΔN and dl/dl embryonic skin compared with littermate controls (Supplementary Figure S2A), and was enhanced in KRT14-Eda-A1 embryos that constitutively express EDA-A1 in the epidermis (Mustonen et al., 2003) compared with littermate controls (Supplementary Figure S2B), consistent with independently obtained data from another group (Fliniaux et al., 2008). Thus Dkk4 expression is regulated by NF-κB as well as by Wnt signaling, providing a possible mechanism for the failure of refinement of Wnt active patches in NF-κB pathway mutant ectoderm.
β-catenin is required within the skin epithelium for activation of Eda-A1/Edar/NF-κB signaling
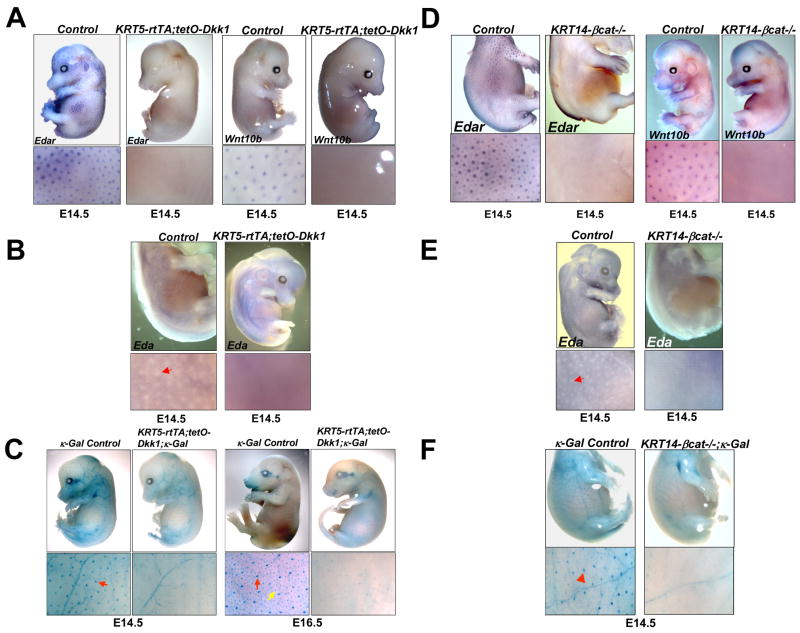
Forced constitutive expression of the secreted Wnt/β-catenin pathway inhibitor DKK1 in embryonic mouse surface ectoderm blocks patterned expression of Edar (Andl et al., 2002), suggesting that NF-κB signaling may require Wnt/β-catenin signaling in order to be activated. To test this, we generated KRT5-rtTA;tetO-Dkk1 embryos in which epidermal Dkk1 expression can be induced by placing the pregnant mothers on oral doxycycline, efficiently blocking Wnt/β-catenin signaling in epithelial and immediately underlying dermal cells by E11.5 (Chu et al., 2004). Localized upregulation of Edar and an additional placode marker, Wnt10b, was absent as expected in induced E14.5 KRT5-rtTA;tetO-Dkk1 embryos (Figure 3A). Expression of Eda-A1 is downregulated at sites of primary placode formation in E14.5 wild type embryos (Laurikkala et al., 2002). We found that ectopic Dkk1 blocked this patterned downregulation (Figure 3B). To determine whether inhibition of Wnt/β-catenin signaling affected epithelial NF-κB signaling, we X-gal stained KRT5-rtTA;tetO-Dkk1; κ-Gal embryos and their littermates that had been treated with doxycycline from E0.5. NF-κB reporter gene expression was maintained in the blood vessels of induced KRT5-rtTA;tetO-Dkk1; κ-Gal embryos at E14.5 and E16.5, but its activity was completely absent in the skin epithelium (Figure 3C). Thus activation of NF-κB signaling in the embryonic ectoderm is entirely dependent on Wnt/β-catenin signaling pathway activity.
Figure 3. Wnt/β-catenin pathway activity is required for Eda/Edar/NF-κB signaling.
(A, B) Whole mount in situ hybridization of E14.5 littermate control and KRT5-rtTA;tetO-Dkk1 embryos doxycycline treated from E0.5, using Edar and Wnt10b (A) and Eda (B) probes. (C) Whole mount X-gal staining of E14.5 and E16.5 κ-Gal control and KRT5-rtTA;tetO-Dkk1; κ-Gal embryos doxycycline treated from E0.5. Red arrows indicate primary hair placodes; yellow arrow indicates a secondary hair placode. (D, E) E14.5 KRT14-Cre;Ctnnb1fl/fl (KRT14-βcat−/−) and control littermate embryos hybridized with the probes indicated. (F) Whole mount X-gal stained E14.5 KRT14-Cre;Ctnnb1fl/fl; κGal (KRT14-βcat−/−; κ-Gal) and control littermate κ-Gal NF-κB reporter embryos. Red arrowheads indicate hair placodes.
In contrast with the effects of Dkk1, a diffusible molecule, prior data suggested that patterned expression of Edar is maintained following late depletion of β-catenin in the surface ectoderm (Huelsken et al., 2001). These data raise the possibility that Dkk1 might affect Edar signaling indirectly, by inhibiting dermal β-catenin signaling and subsequent production of secreted dermal factor(s) expressed in response to β-catenin activation. To re-examine this question, we utilized KRT14-β-cat−/− embryos generated using our early-acting KRT14-Cre line (Liu et al., 2007) (Supplementary Figure S3). In contrast with previously reported data (Huelsken et al., 2001), patterned expression of Edar was completely absent from the surface ectoderm of KRT14-β-cat−/− embryos at E14.5 (Figure 3D). Similarly, patterned upregulation of the Wnt10b placode marker, and patterned downregulation of Eda, were not observed in KRT14-β-cat−/− embryos (Figure 3D, E), and NF-κB reporter expression was completely absent from the skin of KRT14-β-cat−/−; κ-Gal embryos at E14.5 and later stages (Figure 3F and data not shown). While these data do not rule out functions for β-catenin signaling in the dermis, or indirect effects of epithelial β-catenin signaling in activating the Edar pathway, they demonstrate unequivocally that β-catenin is required within ectodermal cells for activation of Eda-A1/Edar/NF-κB signaling.
Edar is a potential direct target of β-catenin transcriptional complexes
As our results indicate that epithelial Wnt/β-catenin is required for activation of Edar signaling, we asked whether the broad expression of Edar that is observed in the surface ectoderm prior to placode induction (Headon and Overbeek, 1999; Schmidt-Ullrich et al., 2006) is β-catenin-dependent. Quantitative RT-PCR assays revealed a more than 5-fold reduction in Edar transcript levels in both Dkk1-expressing and β-catenin deleted skin at E13.5, one day before patterned Edar upregulation is observed in controls (Figure 4A, B). Thus, initial uniform expression of Edar depends on β-catenin signaling within the surface ectoderm. In line with previous data identifying Eda as a direct Wnt/β-catenin target (Durmowicz et al., 2002; Laurikkala et al., 2001), Eda transcripts were also reduced in Dkk1-expressing and β-catenin deleted skin at E13.5 (Figure 4A, B).
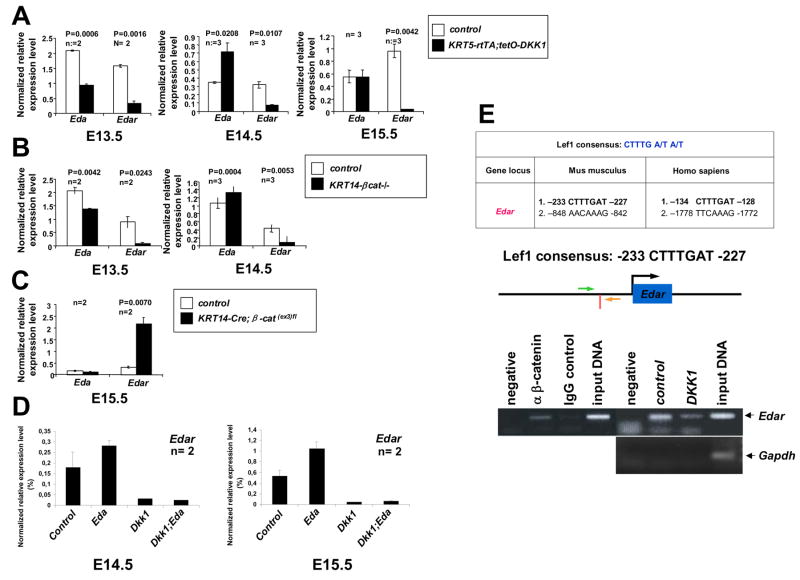
Figure 4. Edar is a direct β-catenin target.
(A–C) Epidermal Eda and Edar mRNA expression assayed by real time RT-PCR in E13.5–E15.5 littermate controls and embryos with forced epidermal Dkk1 expression (KRT5-rtTA;tetO-Dkk1 (A)), epidermal β-catenin deficiency (KRT14-βcat−/−, (B)), or carrying an epidermal β-catenin gain of function mutation (KRT14-Cre; β-cat(ex3)fl, (C)). Results are mean ± SEM. Two or three embryos were analyzed for each genotype. (D) Edar mRNA expression assayed by real time RT-PCR in epidermis from doxycycline treated control, KRT14-Eda-A1 (Eda), KRT5-rtTA;tetO-Dkk1 (Dkk1) and KRT5-rtTA;tetO-Dkk1;KRT14-Eda-A1 (Dkk1;Eda) embryos at E14.5 and E15.5. Two embryos were analyzed for each genotype. (E) Upper panel: two conserved LEF/TCF binding sites in murine and human Edar promoters. Middle panel: vertical red bar indicates the position of the proximal LEF/TCF site in murine Edar; arrows indicate the locations of primers. Lower panels:- left: ChIP using wild-type E14.5 dorsal epidermal extracts, primers for Edar promoter, and anti-β-catenin antibody (α β-catenin) or IgG control; right: ChIP using dorsal epidermal extracts from doxycycline treated E14.5 control or KRT5-rtTA;tetO-Dkk1 (DKK1) embryos, anti-β-catenin antibody, and primers for Edar promoter (upper right) or Gapdh negative control (lower right).
As expected from in situ hybridization data (Figure 3A, D), Edar expression was strongly reduced in Dkk1-expressing and β-catenin deleted skin compared with control skin at E14.5 and E15.5 (Figure 4A, B). Consistent with down-regulation of Eda at sites of placode formation in control embryos, Eda levels were increased or similar to controls in Dkk1-expressing and β-catenin deleted skin at these stages (Figure 4A, B). Thus while initial uniform Eda expression is regulated at least in part by β-catenin signaling, other factors likely control its expression at later time points. A similar temporal shift in the regulatory relationship between Wnt/β-catenin and Eda has been described in chick feather bud development (Houghton et al., 2005). In line with this conclusion, mutation of ectodermal β-catenin to a constitutively active form (Zhang et al., 2008) caused significantly increased Edar expression but did not affect levels of Eda at E15.5 (Figure 4C). Interestingly, qPCR analyses showed that forced expression of Eda-A1 in KRT14-Eda-A1 transgenic skin increased levels of Edar transcripts at E14.5 and E15.5, and that this increase was completely blocked by ectopic Dkk1 (Figure 4D).
Two potential LEF/TCF binding sites were identified in the Edar promoter region, with the site most proximal to the transcription start site being best conserved between humans and mice (Figure 4E), suggesting Edar as a direct Wnt target. Chromatin IP (ChIP) using primers to amplify this region of the mouse Edar promoter and antibody to β-catenin revealed binding of β-catenin complexes to this site in vivo in wild-type E14.5 epidermal extracts, and reduced binding in Dkk1 transgenic epidermis (Figure 4E). These data identify Edar as a potential direct LEF/TCF/β-catenin target gene in embryonic skin epithelial cells.
Forced expression of Eda-A1 or activated Edar fails to rescue primary hair follicle formation or NF-κB activity in embryos with impaired ectodermal Wnt/β-catenin signaling
Epidermal Eda-A1 over-expression results in continuous de novo embryonic hair follicle formation (Mustonen et al., 2003; Zhang et al., 2003), and expression of a constitutively active Edar receptor causes a 40% increase in hair placode numbers (Mou et al., 2006). These data raise the possibility that that forced Eda-A1/Edar signaling might override the requirement for Wnt/β-catenin activity in hair follicle induction, and lead to the suggestion that Edar signaling provides a fate-determining step in follicle development (Mou et al., 2006).
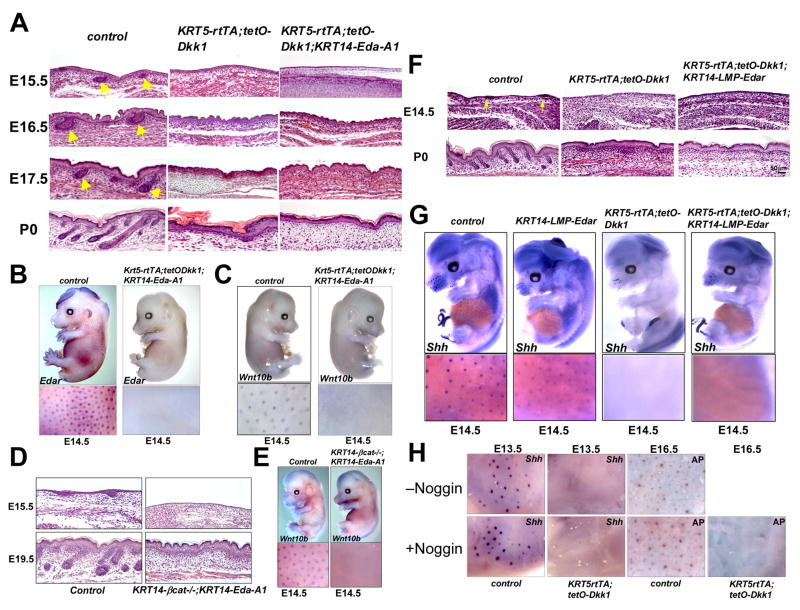
To test whether forced epidermal expression of Eda-A1 can rescue the effects of Wnt/β-catenin inhibition or loss of ectodermal β-catenin, we generated KRT5-rtTA;tetO-Dkk1 embryos and KRT14-β-cat−/− embryos that also carried a KRT14-Eda-A1 transgene (Mustonen et al., 2003). The placode marker Shh and NF-κB reporter expression were elevated in KRT14-Eda-A1 and KRT14-Eda-A1; κ-Gal embryos respectively (Supplementary Figure S4A, B). Over-expression of Eda-A1 was confirmed by whole mount in situ hybridization (Supplementary Figure S4C). Doxycycline treated KRT5-rtTA;tetO-Dkk1;KRT14-Eda-A1 embryos displayed a total absence of hair follicle development, similar to the phenotype of KRT5-rtTA;tetO-Dkk1 littermates (Figure 5A), and whole mount in situ hybridization revealed a lack of patterned upregulation of Edar and Wnt10b (Figure 5B,C). Expression of Shh, a target of Eda-A1/Edar/NF-κB (Pummila et al., 2007; Schmidt-Ullrich et al., 2006), was also absent in KRT5-rtTA;tetO-Dkk1;K14-Eda-A1 skin (Supplementary Figure S4B). Similarly, KRT14-β-cat−/−;KRT14-Eda-A1 embryos lacked all histological signs of hair follicle development (Figure 5D) and patterned expression of placode markers (Figure 5E, Supplementary Figure S4D). Thus, forced expression of Eda-A1 was not sufficient to rescue hair follicle development in embryos lacking ectodermal Wnt/β-catenin activity.
Figure 5. Eda-A1, activated Edar, or Noggin fail to rescue primary hair follicle development in Wnt-inhibited skin.
(A) Hematoxylin/eosin (H&E) stained sections of skin from doxycycline treated KRT5-rtTA;tetO-Dkk1, KRT5-rtTA;tetO-Dkk1;KRT14-Eda-A1 and control littermate embryos. Yellow arrows indicate developing hair follicles in controls. (B, C) In situ hybridization of doxycycline treated E14.5 KRT5-rtTA;tetO-Dkk1;KRT14-Eda-A1 and control embryos for Edar (B) and Wnt10b (C). (D) H&E stained sections of KRT14-Cre;Ctnnb1fl/fl;KRT14-Eda-A1 (KRT14-βcat−/−;KRT14-Eda-A1) and control littermate skin at E15.5 and E19.5. (E) In situ hybridization of E14.5 KRT14-βcat−/−;KRT14-Eda-A1 and control embryos for Wnt10b. (F) H&E stained E14.5 and P0 skin sections of the indicated genotypes. (G) Whole mount in situ hybridization of doxycycline treated E14.5 KRT14-LMP-Edar, KRT5-rtTA;tetO-Dkk1 and KRT5-rtTA;tetO-Dkk1;KRT14-LMP-Edar and control embryos, using Shh probe. (H) Treatment of skin explants from doxycycline treated E13.5 and E16.5 littermate control and KRT5-rtTA;tetO-Dkk1 embryos with or without Noggin for 24 hrs. Hair follicle induction was monitored by in situ hybridization for Shh (E13.5) or staining with alkaline phosphatase (AP) to reveal dermal condensates (E16.5).
Lack of Edar expression in the absence of Wnt/β-catenin signaling could explain the failure of Eda-A1 alone to rescue hair placode formation. To test this we utilized KRT14-LMP-Edar transgenic mice in which a ligand-independent LMP1-EDAR fusion protein is expressed at low levels in basal ectodermal cells (Tucker et al., 2004). In a wild-type background, KRT14-LMP1-Edar expression in this line increases the density of epidermal NF-κB-active spots at E14.5, and causes formation of enlarged, irregular placodes at E18.5 (Supplementary Figure S5A). In Eda-null (Ta/Ta) mutant animals and in downless-Sleek mice that carry a dominant negative mutation in Edar, expression of LMP1-EDAR rescues primary hair follicle and tooth development (Tucker et al., 2004; Supplementary Figure S5B, and data not shown), confirming that LMP1-EDAR acts as a constitutively active receptor. Patterned activation of NF-κB signaling, and formation of primary hair follicle placodes, assayed by histological analysis and whole mount in situ hybridization for Shh at E14.5, were completely absent in KRT5-rtTA;tetO-Dkk1;KRT14-Edar-LMP embryos doxycycline treated from E0.5, and hair follicles were absent at birth (Supplementary Figure S5C; Figure 5F, G). Thus, epithelial expression of a constitutively active EDAR receptor is not sufficient to generate patterned NF-κB signaling activity, or hair follicle placode development, in the absence of Wnt/β-catenin signaling.
Exogenous Noggin fails to rescue hair follicle formation in embryonic skin with impaired Wnt/β-catenin signaling
Eda-A1 signaling directs the expression of BMP antagonists that counteract the placode inhibitory effects of BMPs, and the BMP inhibitor Noggin can partially rescue primary hair follicle development in Ta mutant skin (Mou et al., 2006; Pummila et al., 2007). To test whether suppression of BMP antagonist expression might be responsible for absence of hair placode development in Wnt/β-catenin-inhibited skin, we treated skin explants from control and induced KRT5-rtTA;tetO-Dkk1 transgenic E13.5 and E16.5 embryos with recombinant Noggin for 24 hours. Exogenous Noggin increased hair follicle induction, monitored by in situ hybridization for Shh, and by an enzymatic assay for the dermal condensate marker alkaline phosphatase, in wild-type control skin (Botchkarev et al., 1999; Figure 5H) but was unable to restore primary (E13.5) or secondary (E16.5) placode development in skin from induced KRT5-rtTA;tetO-Dkk1 embryos (Figure 5H). Thus, in the absence of Wnt/β-catenin signaling, placode formation cannot be rescued by Eda-A1, or by a constitutively active Edar receptor, or by a BMP antagonist.
Maintenance of Wnt/β-catenin activity at later stages of primary hair follicle development requires Edar signaling
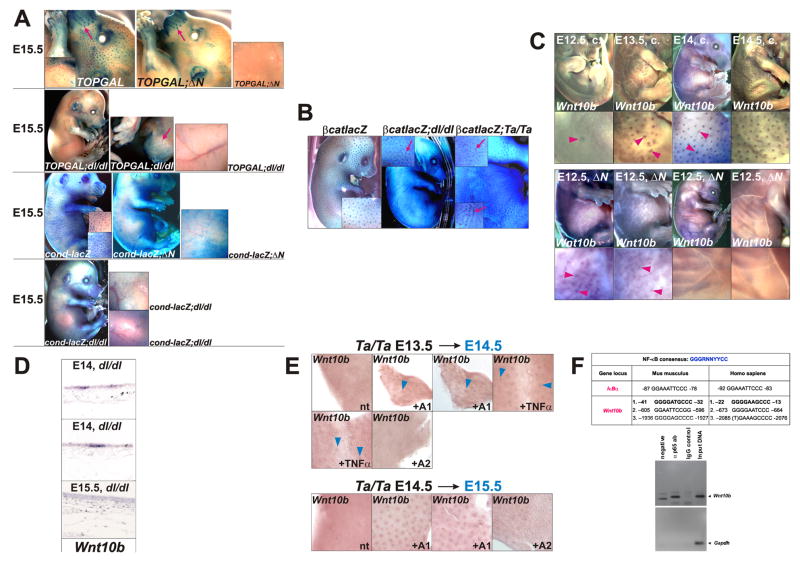
Sites of β-catenin and NF-κB signaling activity overlap after the initiation stage of hair follicle development, suggesting that these pathways may also interact later in morphogenesis. To determine whether Wnt/β-catenin activity is maintained at later stages in the absence of Edar signaling, we examined Wnt reporter gene expression in offspring of matings between TOPGAL and cond-lacZ Wnt reporter mice and ΔN, dl or Ta mice at E15.5 – P0. For both Wnt reporters, focal activity was lost by E15.5 in Edar pathway mutants (Figure 6A and data not shown). Thus, maintenance of canonical Wnt signaling in primary placodes requires Edar activity, suggesting that NF-κB may regulate the expression or activity of Wnts or components of the canonical Wnt pathway.
Figure 6. At later stages of primary hair follicle development Eda-A1/Edar/NF-κB signaling is required for maintenance of Wnt/β-catenin activity and Wnt10b expression, but not for patterned β-catenin mRNA upregulation, and Wnt10b is a potential direct NF-κB target.
(A) TOPGAL and cond-lacZ Wnt reporter expression analyzed by X-Gal staining in control, ΔN, and dl/dl backgrounds at E15.5. (B) Whole mount X-gal staining of E15.5 β-catenin+/lacZ (βcatlacZ), β-catenin+/lacZ;dl/dl (βcatlacZ;dl/dl), and β-catenin+/lacZ;Ta/Ta (βcatlacZ;Ta/Ta) embryos as indicated. Patches of β-catenin expression appear fused and form strings in downless and tabby mutant embryos (red arrows), as also observed in embryos of these genotypes at E13.5 and E14.5 (Figure 2C). (C) Whole mount in situ hybridization of control (c.) and ΔN embryos at the time points indicated using Wnt10b probe. Arrowheads indicate focal expression. (D) In situ hybridization for Wnt10b using sagittal sections of control and dl/dl skin at E14.0 and E15.5. (E) Whole mount in situ hybridization of E13.5 (upper panels) and E14.5 (lower panels) Ta/Ta skin explants using Wnt10b probe. Explants were treated for 24 hours with recombinant Fc-Eda-A1 (+ A1), Fc-Eda A2 (+A2), TNFα (+ TNFα), or were untreated (nt). (F) Upper panel: Conserved NF-κB binding sites in the human and murine Wnt10b promoters at the positions indicated. A verified NF-κB DNA binding site in the IκBα promoter is listed for comparison. Lower panels: ChIP using E14.5 dorsal skin extracts, primers that amplify a region encompassing the two proximal NF-κB consensus sequences in murine Wnt10b, and anti-p65 or IgG control antibodies. Primers amplifying a Gapdh promoter fragment were used as a negative control.
Wnt10b is a potential direct NF-κB target gene
In contrast to Wnt reporter gene expression, focal up-regulation of β-catenin transcription was maintained in the absence of Edar signaling at E15.5 (Figure 6B). We therefore asked whether Wnt ligand expression was affected by loss of Edar signaling. In situ hybridization for Wnt10b in E12.5 – E15.5 wild type embryos revealed that focal expression appeared first at E13.5 in an irregular pattern similar to that seen for Wnt reporter expression in some embryos at this stage. By E14.0, this pattern had resolved into a regular array (Figure 6C). By contrast, in ΔN embryos, Wnt10b expression was very weak at E14.0, with an irregular pattern. By E14.5 patterned Wnt10b expression was absent from ΔN skin (Figure 6C; Supplementary Figure S6A), and also disappeared from dl mutant skin after E14.0 (Figure 6D). These data suggest that the initial, irregular pattern of Wnt10b expression is NF-κB-independent, but that enhancement, refinement and maintenance of expression requires NF-κB activity. Similarly, patterned expression of Wnt10a and Lef1 was absent in trunk skin of ΔN embryos at E14.5 and E15.5 (Supplementary Figure S6B), consistent with previous identification of Wnt10a as a potential NF-κB target in murine B-cells (Krappmann et al., 2004). Development of secondary awl hair and vibrissae follicles is unaffected by loss of Edar signaling, and these displayed normal expression of Wnt10b, Wnt10a and Lef1 in ΔN embryos (Supplementary Figure S6A,B and data not shown).
To examine whether Eda-A1/NF-κB signaling can promote Wnt10b expression in primary hair follicle development, skin explants from E13.5 or E14.5 Ta/Ta embryos were treated for 24 hours with recombinant Fc-Eda-A1 or TNFα, that stimulate NF-κB transcriptional activity, or with Fc-Eda-A2 isoform, which is incapable of activating NF-κB in vivo (Schmidt-Ullrich et al., 2006). Fc-Eda-A1 and TNFα were able to maintain Wnt10b expression in hair placodes of Ta skin, while untreated and Fc-Eda-A2-treated explants lacked patterned Wnt10b expression (Figure 6E). Similarly, Wnt10b expression was expanded in E14.5 embryonic skin by forced expression of Eda-A1 from a KRT14-Eda-A1 transgene (Supplementary Figure S6C). We identified three conserved NF-κB DNA binding sites in the Wnt10b promoter region (Figure 6F). ChIP using extracts of E14.5 mouse embryo epidermis revealed binding of the p65 subunit of NF-κB to a region of chromatin encompassing the two sites most proximal to the transcription start site of murine Wnt10b (Figure 6F). These data identify Wnt10b as a potential direct target of NF-κB in primary hair follicle development. Our results further suggest that the requirement for NF-κB signaling in maintenance of Wnt/β-catenin signaling at later stages of primary hair follicle development may be due in part to NF-κB-dependent expression of Wnt10b and its close relative Wnt10a.
DISCUSSION
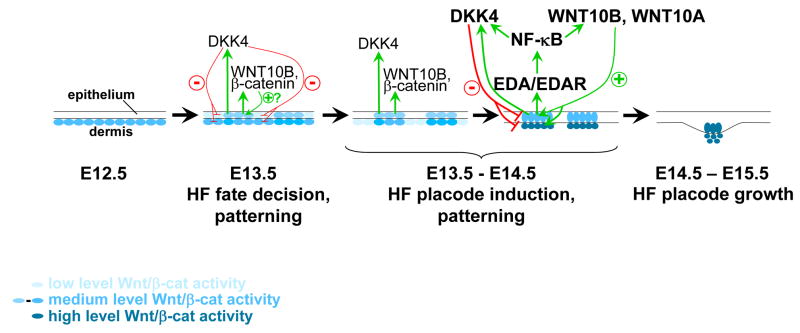
The Wnt/β-catenin and NF-κB signaling pathways play critical roles in development, homeostasis and cancer (Clevers, 2006; Courtois, 2005; Naugler and Karin, 2008). However, how Wnt and NF-κB pathway components interact in the complex network of biological communication that regulates these processes remains unclear. Here we have used an accessible and well-characterized developmental system to dissect the precise temporal relationship and molecular cross-talk by which these key signaling pathways intersect to initiate development of a complex mini-organ, the primary hair follicle. Our results reveal a mandatory role for Wnt/β-catenin signaling within the surface ectoderm in priming keratinocytes to become follicular keratinocytes. This finding is consistent with the observed induction of ubiquitous hair placode formation in embryos carrying an activating mutation in surface ectodermal β-catenin (Narhi et al., 2008; Zhang et al., 2008). Our data support a model in which Wnt/β-catenin and Eda/Edar signals engage in a complex interplay following the initial adoption of placode fate by surface ectodermal cells in response to activated Wnt signaling (Figure 7).
Figure 7. Model for interactions of Wnt/β-catenin and Edar signaling pathways in primary hair follicle development.
Wnt activity lies both upstream and downstream of Edar signaling. At early stages of hair follicle development an irregular pre-pattern of Wnt activity is established in the epithelium and is required for patterned inhibition of Wnt signaling in interplacodal regions, possibly via the actions of secreted Wnt-induced inhibitors such as DKK4. Reinforcement of signaling in pre-placodes involves elevation of β-catenin and Wnt10b transcription. Expression of Eda and Edar requires Wnt signaling. Maintenance of Wnt signaling and elevated Wnt10a, Wnt10b, and Dkk4 expression at later stages requires Eda-A1/Edar/NF-κB activity. HF, hair follicle.
Tissue recombination experiments suggest that a dermal signal initiates hair follicle placode development (Hardy, 1992); however, whether this signal is uniform, or is generated by clustered mesenchymal cells that form a pre-pattern, has been unclear. We identified cond-lacZ expression as a very early marker of skin patterning that is initially expressed broadly in the upper dermis and becomes patterned in both epithelium and dermis at least one day before the morphological appearance of placodes. Our finding that patterning of dermal cond-lacZ expression requires epithelial β-catenin is consistent with a model in which patterning first arises in the epithelium rather than the dermis. Specific inhibition and activation of the Wnt/β-catenin pathway in upper dermal cells will be required to determine the functional relevance of dermal β-catenin signaling for epithelial patterning and hair follicle development.
Irrespective of the contribution of dermal Wnt signaling, we show here that Wnt/β-catenin signaling within epithelial cells is required for activation of Eda/Edar/NF-κB signaling, and subsequent molecular and morphological events essential for hair follicle development. We find that, in addition to its known role in regulating Eda expression (Durmowicz et al., 2002; Laurikkala et al., 2001), Wnt/β-catenin also directly regulates expression of Edar. One role of Edar signaling is to suppress placode-inhibitory BMP signals (Mou et al., 2006; Pummila et al., 2007). However, we find that neither exogenous Noggin, nor forced activation of Eda-A1 or ligand-independent Edar, can rescue hair follicle development in the absence of Wnt signaling. These data indicate that expression of additional Wnt targets is required. As secondary (awl) hair follicle morphogenesis requires Wnt/β-catenin but is independent of NF-κB signaling, some of these additional targets may also be utilized in secondary hair follicle development. Our data further suggest that formation of the “messy” pre-pattern established by focal activation of β-catenin signaling at E13.5 is necessary to produce a molecular context in which downstream patterning events can proceed.
While focal Wnt/β-catenin signaling occurs in the absence of Edar signaling, we show that refinement of the pattern of Wnt/β-catenin activation is dependent on activity of the Edar pathway in primary placode induction. In the absence of NF-κB signaling, the borders of Wnt reporter-positive cell patches are irregular, and these patches sometimes appear to be fused, or occur in string-like shapes. Thus, consistent with previous suggestions (Mou et al., 2006), NF-κB signaling plays a critical role in refining the pattern of hair placode borders. Our data, and those from another group (Fliniaux et al., 2008), indicate that expression of the secreted Wnt inhibitor Dkk4 is regulated by NF-κB as well as by LEF/TCF/β-catenin, suggesting a possible mechanism by which NF-κB signaling indirectly limits Wnt activity and refines placode borders. Competition between short range or cell autonomous placode promoting signals (such as WNT10B and β-catenin) and longer range inhibitory signals (DKK4) acting downstream of initial irregular Wnt activation is consistent with a reaction-diffusion model for establishment of a regular array of placodes (Sick et al., 2006).
Although initially activated, Wnt signaling and focal expression of Wnt10b and its close relative Wnt10a are not maintained in the skin in the absence of NF-κB signaling. Our data implicate Wnt10b as a potential direct target of the Edar pathway in primary placode induction, and suggest that NF-κB-dependent maintenance of Wnt10b and Wnt10a expression could explain in part the eventual disappearance of Wnt pathway activity in the absence of NF-κB signaling. Wnt10a and Wnt10b display overlapping expression in developing hair follicles, suggesting partial functional redundancy (Reddy et al., 2001). Consistent with this, while the precise role of Wnt10b in hair follicle development is unclear, loss of function mutations in human WNT10A are associated with hair follicle defects but do not cause complete absence of hair follicle development (Adaimy et al., 2007).
Unlike Wnt/β-catenin signaling activity, upregulated β-catenin-lacZ expression is maintained in Eda/Edar/NF-κB pathway mutants at E15.5. Patterned upregulation of β-catenin mRNA expression is absent in embryos with forced epithelial expression of the Wnt inhibitor Dkk1, indicating that initiation of this transcriptional activity depends on Wnt signaling (Andl et al., 2002). However, our results suggest that, once established, upregulated β-catenin transcription may occur independently of both Wnt and Edar signaling.
The Wnt/β-catenin and Edar pathways are activated and have important functions in tooth and sweat gland development, suggesting that similar interacting mechanisms to those described here may be relevant to the development of other ectodermal appendages. While β-catenin signaling plays a critical role in diverse skin cancers (Malanchi et al., 2008; Yang et al., 2008), possible interactions of the Wnt/β-catenin and NF-κB signaling pathways in these conditions have not been fully explored and would be an interesting subject for future studies.
EXPERIMENTAL PROCEDURES
Generation of mouse lines
Mice or embryos were genotyped by PCR of genomic DNA. Mice mated into dl or Ta backgrounds were bred to homozygosity for the dl or Ta mutations. To induce Dkk1 expression in KRT5-rtTA tetO-Dkk1 double transgenic embryos, pregnant female mice were placed on doxycycline chow (1 mg/kg, Bio-serv, Laurel, MD) from E0.5. All aspects of animal care and experimental protocols were approved by the Berlin Animal Review Board (Reg. 0261/02) or the University of Pennsylvania IACUC Committee.
Histology, immunofluorescence, X-Gal staining and in situ hybridization
Immunofluorescence of paraffin-sectioned tissue, whole mount X-Gal staining for detection of β-galactosidase activity, and whole mount and section in situ hybridization were performed as described previously (Andl et al., 2006; Chu et al., 2004; Schmidt-Ullrich et al., 2001; Schmidt-Ullrich et al., 1996; Schmidt-Ullrich et al., 2006). Detailed methods and probe sequences are provided in Supplementary Methods.
Quantitative RT-PCR
Dissected dorsal skin was dispase treated (BD Bioscience, Sparks, MD) to separate epidermis and dermis. RNA was extracted using RNeasy Mini Kit (Qiagen, Inc, Valencia, CA). qRT-PCR primers are detailed in Supplementary Methods. Reactions were performed in triplicate using SYBR green on an MJ Opticon II thermocycler (Bio-Rad, Hercules, CA). Relative expression levels were standardized using β-actin as an internal control. Data were analyzed using the Opticon III program. Statistical significance was calculated using Student’s t-test.
Embryonic skin culture
Embryonic back skin explants were cultured for 24 hours on Millipore filters at 37°C in DMEM, 10% FCS, 1 mM sodium pyruvate, and 100 units/ml penicillin/streptomycin using Falcon center-well organ culture dishes and fine metal grids (Goodfellow), or on Transwell Permeable Supports in DMEM, 10% FBS, in Corning 12 well Transwell plates. Where indicated, Fc-Eda-A1 or Fc-Eda A2 (0.1 – 0.5 μg/ml) (Gaide and Schneider, 2003), TNFα (25 ng/ml), or recombinant mouse Noggin (1000 ng/ml) (R&D Systems, MN), were added to the culture medium.
Chromatin immunoprecipitation (ChIP) assays
The TRANSFAC programs “patch” and “Alibaba2” (http://www.gene-regulation.com/index.html) and Motif Search (http://motif.genome.jp/) were used for identification of LEF/TCF and NF-κB binding sites. Accession numbers are provided in Supplemental Methods. ChIP assays were performed using the Chromatin Immunoprecipitation kit (Upstate, Charlottesville, VA). Epidermal cells were dissociated, fixed in 1% formaldehyde for 15 minutes at RT, sonicated and incubated overnight at 4°C with anti-β-catenin antibody (clone 14, BD Bioscience, San Jose, CA), anti-p65 (C-20, SC-372, Santa Cruz Biotechnology, Santa Cruz, CA), or control IgG, followed by addition of Protein A agarose Beads. Purified DNA was subjected to semi-quantitative PCR with primers described in Supplementary Data.
Supplementary Material
Acknowledgments
We thank Juliette Bergemann, Karin Ganzel, Inge Krahn and Sarah Ugowski for excellent technical help; Pascal Schneider for recombinant Fc-Eda-A1 and Fc-Eda A2; Gregory Shackleford for Wnt10a and Wnt10b probes; Irma Thesleff for Ta mice; Jean Richa for transgenic mouse production; and Leroy Ash for histology. We thank Giulietta Roël for critical reading of the manuscript. This work was funded by NIH grants R01-AR47709-09 and RO1-DE015342 (SEM), National Foundation for Ectodermal Dysplasia (SEM), DFG grant SCHM 855/3-1 (RS.U), and a BMBF grant (CS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adaimy L, Chouery E, Megarbane H, Mroueh S, Delague V, Nicolas E, Belguith H, de Mazancourt P, Megarbane A. Mutation in WNT10A is associated with an autosomal recessive ectodermal dysplasia: the odonto-onycho-dermal dysplasia. Am J Hum Genet. 2007;81:821–828. doi: 10.1086/520064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Murchison EP, Liu F, Zhang Y, Yunta-Gonzalez M, Tobias JW, Andl CD, Seykora JT, Hannon GJ, Millar SE. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol . 2006;16:1041–1049. doi: 10.1016/j.cub.2006.04.005. Epub 2006 May 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Bazzi H, Fantauzzo KA, Richardson GD, Jahoda CA, Christiano AM. The Wnt inhibitor, Dickkopf 4, is induced by canonical Wnt signaling during ectodermal appendage morphogenesis. Dev Biol. 2007;305:498 – 507. doi: 10.1016/j.ydbio.2007.02.035. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1:158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- Chu EY, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, Glick A, Wysolmerski JJ, Millar SE. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development. 2004;131:4819–4829. doi: 10.1242/dev.01347. Epub 2004 Sep 4811. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Courtois G. NF-kappaB in skin homeostasis. Exp Dermatol. 2005;14:781–782. doi: 10.1111/j.1600-0625.2005.0355d.x. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Durmowicz MC, Cui CY, Schlessinger D. The EDA gene is a target of, but does not regulate Wnt signaling. Gene. 2002;285:203–211. doi: 10.1016/s0378-1119(02)00407-9. [DOI] [PubMed] [Google Scholar]
- Fliniaux I, Mikkola ML, Lefebvre S, Thesleff I. Identification of dkk4 as a target of Eda-A1/Edar pathway reveals an unexpected role of ectodysplasin as inhibitor of Wnt signalling in ectodermal placodes. Dev Biol. 2008 doi: 10.1016/j.ydbio.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaide O, Schneider P. Permanent correction of an inherited ectodermal dysplasia with recombinant EDA. Nat Med. 2003;9:614–618. doi: 10.1038/nm861. [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- Headon DJ, Overbeek PA. Involvement of a novel Tnf receptor homologue in hair follicle induction. Nat Genet. 1999;22:370–374. doi: 10.1038/11943. [DOI] [PubMed] [Google Scholar]
- Houghton L, Lindon C, Morgan BA. The ectodysplasin pathway in feather tract development. Development. 2005;132:863–872. doi: 10.1242/dev.01651. Epub 2005 Jan 2026. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang TX, Widelitz RB, Shen WM, Will P, Wu DY, Lin CM, Jung HS, Chuong CM. Integument pattern formation involves genetic and epigenetic controls: feather arrays simulated by digital hormone models. Int J Dev Biol. 2004;48:117–135. doi: 10.1387/ijdb.041788tj. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krappmann D, Wegener E, Sunami Y, Esen M, Thiel A, Mordmuller B, Scheidereit C. The IkappaB kinase complex and NF-kappaB act as master regulators of lipopolysaccharide-induced gene expression and control subordinate activation of AP-1. Mol Cell Biol. 2004;24:6488–6500. doi: 10.1128/MCB.24.14.6488-6500.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Eby MT, Sinha S, Jasmin A, Chaudhary PM. The ectodermal dysplasia receptor activates the nuclear factor-kappaB, JNK, and cell death pathways and binds to ectodysplasin A. J Biol Chem. 2001;276:2668–2677. doi: 10.1074/jbc.M008356200. [DOI] [PubMed] [Google Scholar]
- Laurikkala J, Mikkola M, Mustonen T, Aberg T, Koppinen P, Pispa J, Nieminen P, Galceran J, Grosschedl R, Thesleff I. TNF signaling via the ligand-receptor pair ectodysplasin and edar controls the function of epithelial signaling centers and is regulated by Wnt and activin during tooth organogenesis. Dev Biol. 2001;229:443–455. doi: 10.1006/dbio.2000.9955. [DOI] [PubMed] [Google Scholar]
- Laurikkala J, Pispa J, Jung HS, Nieminen P, Mikkola M, Wang X, Saarialho-Kere U, Galceran J, Grosschedl R, Thesleff I. Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Development. 2002;129:2541–2553. doi: 10.1242/dev.129.10.2541. [DOI] [PubMed] [Google Scholar]
- Liu F, Thirumangalathu S, Gallant NM, Yang SH, Stoick-Cooper CL, Reddy ST, Andl T, Taketo MM, Dlugosz AA, Moon RT, et al. Wnt-beta-catenin signaling initiates taste papilla development. Nat Genet. 2007;39:106–112. doi: 10.1038/ng1932. Epub 2006 Nov 2026. [DOI] [PubMed] [Google Scholar]
- Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W, Huelsken J. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. Epub 2003 Mar 3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola ML, Pispa J, Pekkanen M, Paulin L, Nieminen P, Kere J, Thesleff I. Ectodysplasin, a protein required for epithelial morphogenesis, is a novel TNF homologue and promotes cell-matrix adhesion. Mech Dev. 1999;88:133–146. doi: 10.1016/s0925-4773(99)00180-x. [DOI] [PubMed] [Google Scholar]
- Mou C, Jackson B, Schneider P, Overbeek PA, Headon DJ. Generation of the primary hair follicle pattern. Proc Natl Acad Sci U S A. 2006;103:9075–9080. doi: 10.1073/pnas.0600825103. Epub 2006 Jun 9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustonen T, Pispa J, Mikkola ML, Pummila M, Kangas AT, Pakkasjarvi L, Jaatinen R, Thesleff I. Stimulation of ectodermal organ development by Ectodysplasin-A1. Dev Biol. 2003;259:123–136. doi: 10.1016/s0012-1606(03)00157-x. [DOI] [PubMed] [Google Scholar]
- Narhi K, Jarvinen E, Birchmeier W, Taketo MM, Mikkola ML, Thesleff I. Sustained epithelial beta-catenin activity induces precocious hair development but disrupts hair follicle down-growth and hair shaft formation. Development. 2008;135:1019 – 1028. doi: 10.1242/dev.016550. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pummila M, Fliniaux I, Jaatinen R, James MJ, Laurikkala J, Schneider P, Thesleff I, Mikkola ML. Ectodysplasin has a dual role in ectodermal organogenesis: inhibition of Bmp activity and induction of Shh expression. Development. 2007;134:117–125. doi: 10.1242/dev.02708. [DOI] [PubMed] [Google Scholar]
- Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Aebischer T, Hulsken J, Birchmeier W, Klemm U, Scheidereit C. Requirement of NF-kappaB/Rel for the development of hair follicles and other epidermal appendices. Development. 2001;128:3843–3853. doi: 10.1242/dev.128.19.3843. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Memet S, Lilienbaum A, Feuillard J, Raphael M, Israel A. NF-kappaB activity in transgenic mice: developmental regulation and tissue specificity. Development. 1996;122:2117–2128. doi: 10.1242/dev.122.7.2117. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Tobin DJ, Lenhard D, Schneider P, Paus R, Scheidereit C. NF-kappaB transmits Eda A1/EdaR signalling to activate Shh and cyclin D1 expression, and controls post-initiation hair placode down growth. Development. 2006;133:1045–1057. doi: 10.1242/dev.02278. [DOI] [PubMed] [Google Scholar]
- Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science. 2006;314:1447–1450. doi: 10.1126/science.1130088. Epub 2006 Nov 1442. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Headon DJ, Courtney JM, Overbeek P, Sharpe PT. The activation level of the TNF family receptor, Edar, determines cusp number and tooth number during tooth development. Dev Biol. 2004;268:185–194. doi: 10.1016/j.ydbio.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Yan M, Wang LC, Hymowitz SG, Schilbach S, Lee J, Goddard A, de Vos AM, Gao WQ, Dixit VM. Two-amino acid molecular switch in an epithelial morphogen that regulates binding to two distinct receptors. Science. 2000;290:523–527. doi: 10.1126/science.290.5491.523. [DOI] [PubMed] [Google Scholar]
- Yang SH, Andl T, Grachtchouk V, Wang A, Liu J, Syu LJ, Ferris J, Wang TS, Glick AB, Millar SE, Dlugosz AA. Pathological responses to oncogenic Hedgehog signaling in skin are dependent on canonical Wnt/beta3-catenin signaling. Nat Genet. 2008;40:1130–1135. doi: 10.1038/ng.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, Birchmeier W, Hsu W. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132:1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Brancaccio A, Weiner L, Missero C, Brissette JL. Ectodysplasin regulates pattern formation in the mammalian hair coat. Genesis. 2003;37:30–37. doi: 10.1002/gene.10230. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, Tobias JW, Piccolo S, Schmidt-Ullrich R, Nagy A, et al. Activation of beta-catenin signaling programs embryonic epidermis to hair follicle fate. Development. 2008;135:2161 – 2172. doi: 10.1242/dev.017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.