Summary
Kalirin, one of the few Rho GDP/GTP exchange factors (GEFs) that includes spectrin-like repeats, plays a critical role in axon extension and maintenance of dendritic spines. PC12 cells were used to determine whether Cdk5, a critical participant in both processes, regulates the actions of Kalirin. Expression of Kalirin 7 in non-differentiated PC12 cells caused the GEF-activity dependent extension of broad cytoplasmic protrusions; co-expression of dominant negative Cdk5 largely eliminated this response. The spectrin-like repeat region of Kalirin plays an essential role in this response, which is not mimicked by the GEF domain alone. Thr1590, which follows the first GEF domain of Kalirin, is the only Cdk5 phosphorylation site in Kalirin 7. Although Kalirin 7/Ala1590 retains GEF activity, it is unable to cause extension of protrusions. Kalirin 7/Asp1590 has slightly increased GEF activity and DN-Cdk5 fails to block its ability to cause extension of protrusions. Phosphorylation of Thr1590 causes a slight increase in GEF activity and Kalirin 7 solubility. Dendritic spines formed by cortical neurons in response to the expression of Kalirin 7/Ala1590 differ in shape from those formed in response to Kalirin 7 or Kalirin 7/Asp1590. The presence of Thr1590 in each major Kalirin isoform would allow Cdk5 to regulate Kalirin function throughout development
Keywords: RhoGEF, PC12 cells, spectrin-like repeats, PP-1, phosphorylation, roscovitine, calyculin A
In mammals, flies and worms, members of the Trio/Kalirin family of Rho guanine nucleotide exchange factors (GEFs) play essential roles in neuronal process outgrowth (Awasaki et al., 2000; Newsome et al., 2000; Bateman et al., 2000; Steven et al., 1998; Ma et al., 2003; Debant et al., 1996). In the adult nervous system, Kalirin 7, the major Kalirin isoform expressed, is essential for the maintenance of dendritic spines and the dendritic arbor (Ma et al., 2003; Ma et al., 2008). Kalirin and Trio are unique in having two RhoGEF domains and multiple spectrin-like repeats (Rossman et al., 2005; Johnson et al., 2000; Debant et al., 1996). The spectrin-like repeat region of Kalirin is half the size of spectrin itself and affects cell morphology in a GEF-activity independent manner (Schiller et al., 2008). As for many other RhoGEFs, the factors that control the catalytic activity and actions of Kalirin are poorly understood (Rossman et al., 2005; Schiller et al., 2006). During development the interaction of Trio with Delected in Colorectal Cancer (DCC) allows it to mediate the effects of netrin-1 on axonal growth (Briancon-Marjollet et al., 2008). In mature neurons, receptor tyrosine kinases like EphB and signaling proteins like CamKIIα affect the ability of Kalirin 7 to participate in the formation of dendritic spines (Penzes et al., 2003; Xie et al., 2007).
Cdk5, a proline directed kinase, phosphorylates substrates which regulate axon guidance, cytoskeletal dynamics and the formation and function of dendritic spines (Bibb et al., 2001b; Cheung et al., 2006; Dhavan and Tsai, 2001; Humbert et al., 2002; Lee et al., 1996; Nikolic et al., 1998), the same events in which Kalirin plays a key role. Cdk5 is active only when bound to a non-cyclin cofactor, p35 or p39 (Dhavan and Tsai, 2001). Neuronal growth cones are enriched in Cdk5/p35 complex along with Kalirin, Trio and Rac1 (Nikolic et al., 1998; Awasaki et al., 2000; Johnson et al., 2000; Humbert et al., 2002; Estrach et al., 2002). Trio can be phosphorylated by Cdk5, and inhibition of Cdk5 results in a decrease in the ability of Trio to activate Rac (Xin et al., 2004). In cells that secrete neuropeptides, inhibition of Cdk5 results in the accumulation of filamentous actin beneath the plasma membrane and in a decrease in the regulated release of peptide-containing granules (Xin et al., 2004). Since Kalirin has several potential Cdk5 phosphorylation sites and Cdk5 and Kalirin are involved in many of the same physiological processes, we sought an experimental system in which we could determine whether Cdk5 plays a role in controlling Kalirin function.
We turned to rat PC12 pheochromocytoma cells to evaluate this possibility. Although NGF treatment of PC12 cells increases the expression of p35 and the activation of Cdk5 (Harada et al., 2001), non-differentiated PC12 express Cdk5, p35 and p39 (Harada et al., 2001; Sharma et al., 1999; Tang et al., 1995). Antisense-mediated reduction of Kalirin expression in PC12 cells demonstrated a role for Kalirin in neurite extension (Chakrabarti et al., 2005). We did not examine the actions of Kalirin in NGF-differentiated PC12 cells because levels of Trio protein increase rapidly in response to treatment with NGF (Estrach et al., 2002). The dose and timing of NGF application are critical determinants of the response observed and Kalirin interacts directly with TrkA, resulting in activation of TrkA in the absence of ligand (Chakrabarti et al., 2005).
We chose to explore the effects of Cdk5 on Kalirin 7 because it is the major isoform in the adult rodent brain, has a single RhoGEF domain and has a single potential Cdk5 phosphorylation site, Thr1590 (Johnson et al., 2000; Penzes et al., 2000). Thr1590 is situated in a 60 amino acid region that follows the GEF domain and precedes the 20 amino acid sequence unique to Kalirin 7 (Fig. 1A) (Penzes et al., 2000). The same site is found in the larger isoforms of Kalirin as well as the N-terminally truncated Δ-isoforms. The larger isoforms of Kalirin are expressed early in development (Johnson et al., 2000) and cause axon initiation and extension when over-expressed in sympathetic neurons (May et al., 2002). Expression of Kalirin 7 is first seen when synapses begin to form during post-natal development. Since antisense mediated reductions in Kalirin 7 expression in hippocampal pyramidal neurons and interneurons reduced the size of the dendritic tree (Ma et al., 2003; Ma et al., 2008; Ma et al., 2003), we were hopeful that exogenous Kalirin 7 would produce a morphological response that could be quantified in PC12 cells.
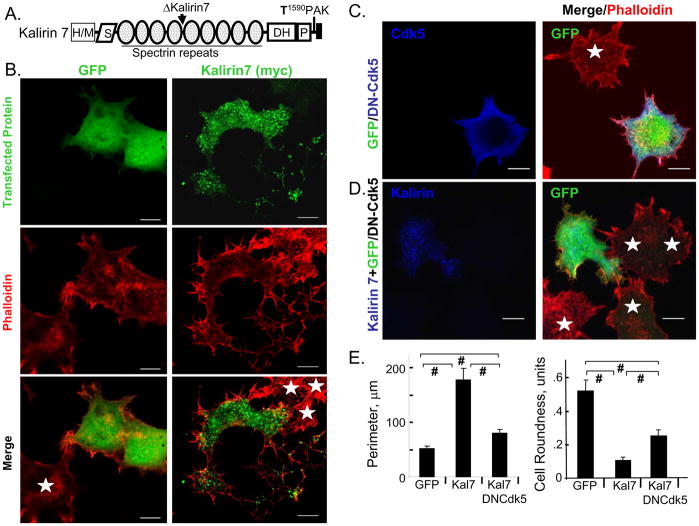
Fig. 1. Kalirin7 produces a Cdk5-dependent response in PC12 cells.
A. Domains of Kalirin7 and its N-terminal Δ-splice variant are shown; S, Sec14P- like; DH, Dbl homology; P, pleckstrin homology; T1590PAK, putative Cdk5 phosphorylation site. B. PC12 cells were fixed 24h after transfection with vectors encoding GFP or Kalirin7. Transfected GFP protein was visualized using fluorescence or Myc antibody (green); filamentous actin was visualized using TRITC-phalloidin (red). White arrowheads, protrusions; stars, non-transfected cells; arrow, nucleus. Scale, 10μm. C. PC12 cells transfected with dual promoter vector encoding GFP (green) and DN-Cdk5 (blue); TRITC-phalloidin (red). GFP-expressing cells always expressed DN-Cdk5. D. PC12 cells co-transfected with vectors encoding Kalirin7 (myc; blue) and GFP/DN-Cdk5 (GFP, green); TRITC-phalloidin (red). White stars, non-transfected; scale, 10μm. E. Using the GFP or myc signal to identify cell margins, perimeter and cell roundness were evaluated using SimplePCI software: control (GFP), n=18; Kalirin7 (Kal7), n=15; Kalirin7 with DN-Cdk5, n=24; p-values calculated using the Student’s t-test (two-tailed) and standard error of the mean plotted; #, p<0.001.
We expressed Kalirin 7 in non-differentiated PC12 cells and found that it causes a GEF-activity dependent decrease in cell roundness and formation of cytoplasmic protrusions. These effects are blocked by dominant negative Cdk5. We then demonstrated that Cdk5 phosphorylates Kalirin 7 on Thr1590, increasing its GEF activity slightly and changing its solubility properties. Inhibitors of protein phosphatase 1 (PP1) stabilize phosphorylation at this site. A Kalirin 7 mutant that cannot be phosphorylated by Cdk5 does not cause the formation of protrusions while the phosphomimetic mutant, Kalirin 7/Asp1590, does. The response of PC12 cells to Kalirin 7/Asp1590 is not blocked by dominant negative Cdk5, placing Kalirin 7 downstream of Cdk5. To verify the importance of this interaction in neurons, we expressed Kalirin 7/Ala1590 in cortical neurons; although dendritic spines formed, they exhibited aberrant morphology.
Materials and Methods
Construction of expression vectors
Inserts encoding His6-myc-tagged Kalirin7 and ΔKalirin7 were constructed in the pEAK vector (Ma et al., 2003; Johnson et al., 2000; Penzes et al., 2000). In pCMS.EGFP.His-myc-KGEF1→7end, His6-myc precedes KGEF1 and the protein extends to the C-terminus of Kalirin7 (Asp1250-V1654). After changing Thr1590 to Ala1590 or Asp1590 (Quickchange; Stratagene, La Jolla, CA), inserts were sequenced in their entirety and subcloned into pCMS.EGFP.His-myc-KGEF1→7end, pGEX.GST-KGEF1 and pEAK.His6-myc-Kalirin7. ΔKalirin7 was subcloned into pVL1393 (BD Biosciences) for expression in the Baculovirus system. Cdk5 and p35 were subcloned as described (Xin et al., 2004). A dominant negative Cdk5 mutant (Lys33 to Thr, DN-Cdk5) was generated as described above (Tan et al., 2003).
Purification of recombinant proteins
pGEX.GST-KGEF1, -KGEF1→7end and its mutants were purified as described (Alam et al., 1997; Penzes et al., 2001; Schiller et al., 2006). The pGEX-GST-Rac1 vector was a gift from Dr. Richard Cerione (Cornell University). pVL1393-ΔKalirin7 was co-transfected with Baculo-Gold linearized virus into Sf9 cells (BD Biosciences). Cells were extracted into TES-mannitol [TM] buffer containing protease inhibitor cocktail (Xin et al., 2004). ΔKalirin7 bound to Talon Superflow Metal Affinity Resin (BD Biosciences); was eluted with 50mM NaH2PO4, pH 7.0, 300mM NaCl, 1M imidazole, 0.05% Triton X-100 containing 1mM PMSF.
pEAK RAPID cells
Transfection and extraction was as described (Xin et al., 2004). Western blots used: myc monoclonal 9E10 1:10; Cdk5 polyclonal 1:500, p35 polyclonal 1:1000 and PP1 monoclonal 1:1000 antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); Rac monoclonal antibody 1:1000 (BD Biosciences); phospho-Thr/Pro polyclonal antibody 1:1000 (Cell Signaling Technology, Beverly, MA); Kalirin7 C-terminal specific polyclonal antibody JH2959 1:1000 (Penzes et al., 2000). Antibody to p39 was a gift from Dr. Amy Fu (Hong Kong University, Science/Technology, China). Kalirin phospho-Thr1590 antibody CT188 (P-T1590) was generated at Covance (Hazleton, PA). Specificity for P-T1590 was assessed by solid phase ELISA; no cross-reactivity with non-phosphorylated peptide was observed at >1:300 dilution.
In vitro, cell based Cdk5 assays
To determine whether phosphorylation of Kalirin by Cdk5 was proline-directed, wild type peptide was compared to mutant peptide with a Pro to Ala mutation: T1590PAK, P1584IQLPKTPAKLRNNSK; T1590AAK, PIQLPKTAAKLRNNSK. Kinase assay with recombinant Cdk5/p25 (Upstate) was performed based on the company protocol and cell derived immunoprecipitated Cdk5/p35 complex was assayed (Xin et al., 2004).
In vitro and cell based Rac activation assays
In vitro Rac activation assays using GST-KGEF1, GST-KGEF1→7end, GST-KGEF1→7end mutants, and GST-Rac1 were performed as described (Schiller et al., 2005). Data were analyzed using the formula d/dt(fluorescence) = −k*([Rac-GDP-MANT]−[EDTA-endpoint]); the initial rate gives the reaction velocity. The extent of GDP-MANT loading was calculated using ε356 = 5700 (Invitrogen Corp.). Cell based Rac activation assays were described (May et al., 2002; Xin et al., 2004).
PC12 cells
PC12 cells were maintained in DMEM-F12 (Mediatech, Inc., Herndon, VA) containing 10% fetal calf serum (Hyclone, Logan, UT) and 10% NuSerum (Collaborative Research, Waltham, MA) with 25mM HEPES, penicillin and streptomycin (Xin et al., 2004). Cells (60% confluent) plated onto poly-L-lysine coated cover slips were transfected and fixed for immunocytochemistry 24h later (Xin et al., 2004). Co-expression of EGFP and DN-Cdk5 from the dual promoter pCMS.EGFP vector was verified by staining cells with rabbit polyclonal antiserum to Cdk5 (1:250) (Santa Cruz Biotechnology, Inc.) followed by Alexa633-conjugated donkey anti-rabbit IgG (1:500) (Invitrogen Corp.) and TRITC-phalloidin; GFP positive cells always expressed Cdk5. Co-transfection of Kalirin7 and DN-Cdk5 was established using myc antibody with Alexa633-conjugated goat anti-mouse IgG and GFP fluorescence. Images were taken with a Zeiss LSM 510 Meta confocal microscope (CCAM, University of Connecticut Health Center).
Image quantification was performed with SimplePCI Imaging software (Compix Inc., Sewickley, PA). Transfected cells were identified based on co-expression of GFP or staining of epitope-tagged exogenous protein. The transfection rate for PC12 cells was 2–3%; at least 15 fields of cells were analyzed for each vector. Well separated transfected cells were identified using the SimplePCI thresholding software. Cell perimeter (horizontal pixels+vertical pixels+1.4142*diagonal pixels surrounding the object) and cell roundness [4*π*area/(perimeter)2] were measured based on this threshold; for a circle, roundness=1.0.
Striatal tissue, striatal and cortical cultures
Adult rat striatum extracted into SDS lysis buffer (Xin et al., 2004) was fractionated (20μg total protein) by SDS-PAGE and subjected to Western blot analysis. Striatal cultures were prepared from P1 rat pups after dissociating striata with 0.25% trypsin; 2–3×106 cells in DMEM-F12, 10% FCS, 2mM Glutamax, 0.5mg/ml gentamycin were plated per well of a 12-well plate. Medium was changed every 5 days. After 21d in vitro, cells treated with 10μM roscovitine for 4h or 25nM Calyculin for 30min were extracted into SDS-lysis buffer; 10% of each sample was subjected to Western blot analysis. Neonatal rat brain cortical cultures were prepared and nucleofected as described (Ma et al., 2003; Ma et al., 2008).
Kalirin7 solubility assay
PC12 cells were nucleofected (3μg plasmid DNA/2–3 × 106 cells) with the manufacturer’s protocol (AMAXA, Germany, Cat. #: VCA-1003). Cells were extracted in 20mM NaTES, 10mM mannitol, pH 7.4 containing protease and phosphatase inhibitor cocktails (Xin et al., 2004); centrifugation at 430,000×g for 15min was used to separate soluble from insoluble proteins, which were resuspended using SDS-lysis buffer. Aliquots of the soluble fraction and the resuspended pellet were subjected to Western blot analysis using antibody to myc.
Results
PC12 cells provide a test system for actions of Kalirin 7
Endogenous Kalirin plays an essential role in the ability of PC12 cells to produce neurites in response to stimulation with NGF (Chakrabarti et al., 2005). A direct interaction between TrkA and the PH domain of Kalirin 7 can lead to down-regulation of TrkA, complicating the study of exogenous Kalirin in differentiated PC12 cells (Chakrabarti et al., 2005). PC12 cells expressing exogenous Kalirin 7 are unable to produce neurites in response to NGF (50 ng/ml; not shown); however, in the absence of NGF, exogenous Kalirin 7 causes extension of broad, thin cytoplasmic protrusions (Fig. 1B). Control cells (expressing GFP) are compact polygons, with filamentous actin concentrated at the periphery; filopodia are found at the vertices and lamellipodia are rare (Fig. 1B). The cytoplasmic protrusions extended in response to Kalirin 7 are decorated with small lamellipodia; filamentous actin accumulates at the margins of the cell, often forming spokes within the lamellipodia. Punctate staining for Kalirin 7 is apparent in the perinuclear region and near the tips of the protrusions. Staining is also sometimes apparent in the nucleus; the reasons for this are not clear.
The protrusions formed in response to Kalirin 7 are easily distinguished from neurites formed in response to NGF (Black et al., 1986) (Fig. 1B). To quantify the morphological effects of Kalirin 7, cell perimeter and cell roundness (see Methods) were measured (Fig. 1D). Because of the large protrusions, cell perimeter is increased 3- to 4-fold in PC12 cells expressing Kalirin 7. A perfectly round cell has a roundness indicator of 1.0; cells expressing Kalirin 7 adopt a more polarized morphology, resulting in an approximately 5-fold decrease in cell roundness (Fig. 1D). Although this unusual response to Kalirin 7 resembles neither axonal extension nor spine formation, the fact that it is quantifiable allowed us to use it to test the role of Cdk5.
Expression of DN-Cdk5 alters the ability of PC12 cells to respond to Kalirin 7
Like Kalirin, Cdk5 is involved in both neurite outgrowth and the formation of dendritic spines (Aoki et al., 2004; Harada et al., 2001; Dhavan and Tsai, 2001; Tan et al., 2003; Xin et al., 2004). Kalirin 7 contains a single consensus Cdk5 phosphorylation site, -Thr1590PAK-, which is highly conserved (Fig. 1A). To test the role of Cdk5, which is active in non-differentiated PC12 cells (Harada et al., 2001), in the morphological response of PC12 cells to Kalirin 7, we evaluated the ability of DN-Cdk5 to block the response. We first expressed DN-Cdk5 alone, which should inhibit endogenous Cdk5 (Tan et al., 2003); no significant morphological changes are observed in non-differentiated PC12 cells (Fig. 1C). Co-expression of DN-Cdk5 and Kalirin 7 reduces protrusion formation by Kalirin 7 (Fig. 1D). Cell perimeter increases less than two-fold compared to the GFP control and is significantly less than observed in cells expressing Kalirin 7 alone (Fig. 1E). Cells expressing Kalirin 7 and DN-Cdk5 are more round than cells expressing Kalirin 7 alone, but are not as round as cells expressing GFP. Since DN-Cdk5 inhibits Cdk5 without affecting the activity of Cdk1 or Cdk2 (Meikrantz and Schlege, 1996), our data suggest that Cdk5 plays a role in the morphological effects of Kalirin 7 on non-differentiated PC12 cells.
Kalirin7 is a Cdk5 substrate
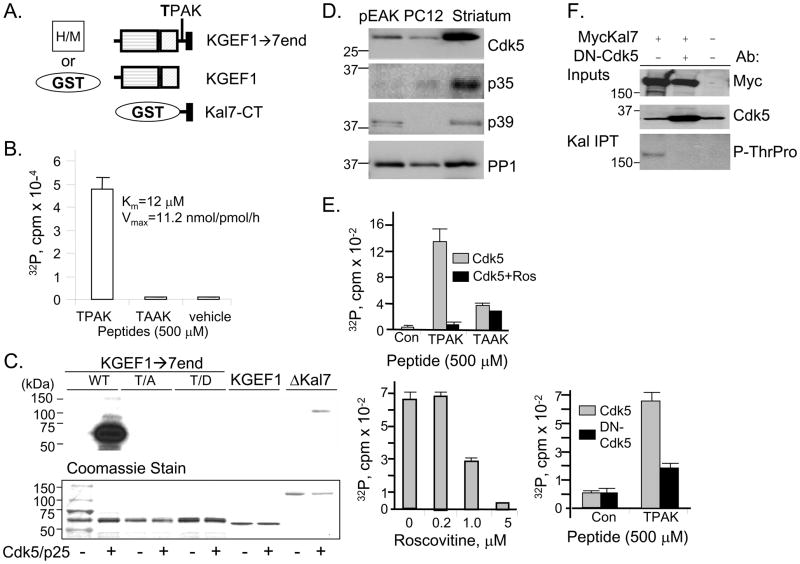
In addition to His-myc-tagged Kalirin7 and ΔKalirin7 (Fig. 1A), we generated mammalian and bacterial expression vectors encoding the DH/PH (GEF) domain followed by the COOH-terminus of Kalirin7 (KGEF1→7end) (Fig. 2A). Vectors encoding the GEF domain (KGEF1) and the region unique to Kalirin7 (Kal7-CT), which both lack Thr1590, were generated previously (Penzes et al., 2001; Schiller et al., 2006; Schiller et al., 2005). We first used synthetic peptides and recombinant Cdk5/p25 to explore the hypothesis that Thr1590 can be phosphorylated by Cdk5 (Fig. 2B). Cdk5/p25 dependent phosphorylation of the T1590PAK peptide was observed. To verify the importance of Pro1591 in the ability of Cdk5/p25 to recognize this site, it was changed to Ala (TAAK); no Cdk5/p25 mediated phosphorylation was detected with the TAAK peptide. The Km and Vmax values determined for the TPAK peptide are comparable to those reported for the Cdk5 site in histone H1 (Qi et al., 1995). We next asked whether Cdk5/p25 can phosphorylate GST-KGEF1→7end (Fig. 2C). Robust phosphorylation was observed; no signal was detected without the addition of Cdk5/p25. As controls, and to verify the phosphorylation site, GST fusion proteins with point mutations of Thr1590 to Ala or Asp, and a control, GST-KGEF1 (which terminates at Arg1573, before the T1590PAK site) were incubated with Cdk5/p25. None of these proteins served as a Cdk5/p25 substrate. The smallest naturally occurring variant of Kalirin, ΔKalirin7, which includes Thr1590, was also phosphorylated by Cdk5/p25. Despite the presence of similar amounts of KGEF1→7end and ΔKal7, KGEF1→7end is phosphorylated much more extensively, suggesting that access to Thr1590 is restricted by the presence of spectrin-like repeats 5 through 9.
Fig. 2. Kalirin-Thr1590 is phosphorylated by Cdk5.
A. GST and His/myc tagged proteins used in these studies are illustrated. B. The Kalirin peptides were incubated with 4ng recombinant Cdk5/p25 complex and 2μCi γ32P-ATP for 30min at 30C; cpm incorporated into peptide is on the y-axis. Km and Vmax were determined by varying the concentration of peptide from 3.7 to 100μM. C. Recombinant GST-KGEF1→7end and ΔKalirin7 are Cdk5/p25 substrates. Recombinant proteins (2μg) were incubated without or with 4ng recombinant Cdk5/p25 complex and 2μCi γ32P-ATP for 30min at 30C. Following fractionation by SDS-PAGE and transfer to a PVDF membrane, samples were visualized by autoradiography (16h) or by Coomassie Brilliant Blue staining; the first sample was loaded with the molecular weight marker. D. Lysates of pEAK RAPID cells, PC12 cells and mouse striatum (20μg) were fractionated by SDS-PAGE; endogenous Cdk5, p35, p39 and PP-1 were visualized. E. Top, synthetic Kalirin peptides were incubated with γ32P-ATP in the presence of Cdk5/p35 complex immunoprecipitated from pEAK RAPID cells expressing exogenous Cdk5 and p35 using a p35 antibody; 10μM roscovitine was included in the indicated assays (black bar). Middle, indicated doses of roscovitine were incubated with TPAK peptide. Bottom, TPAK peptide was incubated with γ32P-ATP in the presence of Cdk5/p35 or DN-Cdk5/p35 complex immunoprecipitated (p35 antibody) from pEAK RAPID cells. Incorporation of 32P into peptide was quantified by Cerenkov counting. F. pEAK RAPID cells expressing myc-Kalirin7 or parent vector (−) and DN-Cdk5 were extracted for immunoprecipitation using spectrin repeat region antibody. Inputs were analyzed using antibody to myc or Cdk5.
Immunoprecipitated Kalirin7 was visualized using a phospho-ThrPro antibody. Expression of DN-Cdk5 eliminated the band detected by the P-ThrPro antibody.
Roscovitine and DN-Cdk5 can be used to manipulate Kalirin phosphorylation in cells
We sought a cell system in which we could readily study the phosphorylation of Kalirin by Cdk5. Like PC12 cells, pEAK Rapid cells express Cdk5 (Fig. 2D); while PC12 cells express p35, pEAK Rapid cells express p39. Cdk5 and p35 are expressed at substantially higher levels in the adult rat striatum. To increase the amount of enzyme available, Cdk5 and p35 were co-expressed in pEAK RAPID cells (Xin et al., 2004); antibody to p35 was used to isolate the Cdk5/p35 complex. In agreement with the results obtained using recombinant Cdk5/p25 complex, the TPAK peptide, but not the TAAK peptide, was phosphorylated by immunoprecipitated Cdk5/p35 complex (Fig. 2E, top). Phosphorylation was inhibited when roscovitine, an inhibitor of Cdk1, Cdk2 and Cdk5, was added to the immunoprecipitate (Zhang et al., 2006; Canduri et al., 2004); since immunoprecipitation was carried out using antibody to p35, which does not interact with Cdk1 or Cdk2 (Dhavan and Tsai, 2001; Lee et al., 1996), we can conclude that roscovitine acts by inhibiting Cdk5 in the complex (Fig. 2E, bottom left). Consistent with this conclusion, when co-expressed with DN-Cdk5, phosphorylation of the TPAK peptide by the p35/Cdk5 complex was inhibited (Fig. 2E, bottom right).
We next sought evidence that Kalirin7 is phosphorylated by Cdk5 in cells. Expression of exogenous Kalirin7 yielded a 191kDa band detected by antibody to Myc and by antibody specific for the dipeptide P-ThrPro (Fig. 2F); this band was not detected in GFP transfected control cells. There are three ThrPro sequences in Kalirin7, only one of which is a potential Cdk5 site. Co-transfection of vectors encoding DN-Cdk5 and Kalirin7 yielded similar levels of MycKalirin7, but the 191kDa band was no longer detected by the P-ThrPro antibody, suggesting that endogenous Cdk5/p39 present in pEAK RAPID cells phosphorylates Thr1590 in Kalirin7.
Thr1590 plays a critical role in the ability of Kalirin7 to alter PC12 cell morphology
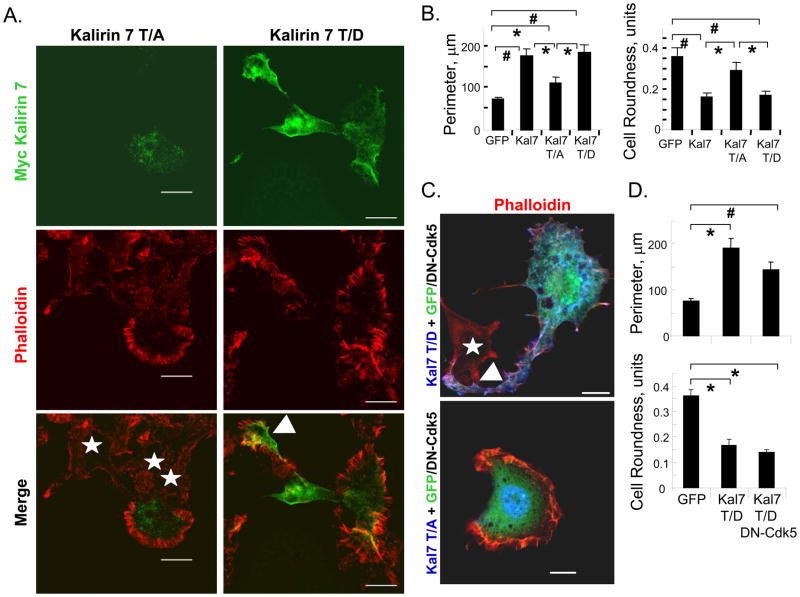
To explore the role of Cdk5-mediated phosphorylation of Kalirin7, we generated vectors encoding Kalirin7 in which Thr1590 was mutated to Ala1590 or Asp1590; if phosphorylation of Thr1590 plays a critical role in its morphological effects, Kalirin7-T/A, which cannot be phosphorylated, and Kalirin7-T/D, which might mimic phosphorylated Kalirin7, would be expected to affect PC12 cell morphology differently. When expressed in non-differentiated PC12 cells, Kalirin7-T/D mimicked the effects of Kalirin7 while Kalirin7-T/A did not (Fig. 3A). Expression of Kalirin7-T/A was not without effect; PC12 cells expressing Kalirin7-T/A were typically surrounded by extensive lamellipodia filled with spokes of filamentous actin; cytoplasmic protrusions were not observed (Fig. 3A, left). In cells expressing Kalirin7-T/D, broad, flat cytoplasmic protrusions decorated with lamellipodia were prominent (Fig. 3A, right, arrowhead). Filamentous actin was localized near the plasma membrane, especially in lamellipodial spokes, often overlapping sites of Kalirin7-T/D expression. Nuclear staining for both Kalirin7-T/A and Kalirin7-T/D was often apparent, although its significance is not known.
Fig. 3. Thr1590 plays an essential role in the effects of Kalirin7 on PC12 cell morphology.
A. PC12 cells expressing Kalirin7-T/A or Kalirin7-T/D were examined 24h post-transfection. Kalirin7 was visualized using antibody to Myc (green) and filamentous actin was visualized using TRITC-phalloidin (red). White arrow head, protrusion; white stars, non-transfected cells; White arrow, nucleus; scale bar, 10μm. B. Cell perimeter and roundness were quantified as described in Fig. 1: GFP, n=27; Kalirin7 (Kal7), n=24; Kalirin7-T/A (Kal7 T/A), n=15; Kalirin7-T/D (Kal7 T/D), n=24. C. PC12 cells co-transfected with vectors encoding GFP/DN-Cdk5 and either Kalirin7-T/A or Kalirin7-T/D were fixed after 24h. Exogenous Kalirin was visualized with myc antibody (blue); DN-Cdk5 was identified based on co-expression of GFP; filamentous actin was visualized with TRITC-phalloidin (red). White arrow head, protrusion; white stars, non-transfected cells; White arrow, nucleus; scale bar, 10μm. D. Cell perimeter and cell roundness were evaluated as described in Fig. 1: control GFP, n = 18; Kalirin7-T/D, n=15; Kalirin7-T/D with DN-Cdk5, n=18. p-values were calculated using Student’s t-test (two-tailed) and standard error of the mean plotted; #, p<0.001; *, p<0.05.
To compare the morphological effects of the Kalirin mutants, cell perimeter and roundness were quantified (Fig. 3B). Kalirin7-T/D increased cell perimeter as much as Kalirin7. Kalirin7-T/A, which caused extensive lamellipodial formation, caused a lesser, but still significant, increase in cell perimeter. Kalirin7 and Kalirin7-T/D caused a similar decrease in cell roundness; in contrast, Kalirin7-T/A did not alter cell roundness. Mutation of the single Cdk5 phosphorylation site in Kalirin7 alters the ability of Kalirin7 to affect PC12 cell morphology.
Since co-expression of DN-Cdk5 diminished the ability of Kalirin7 to increase cell perimeter and decrease cell roundness, we asked whether DN-Cdk5 altered the response of PC12 cells to Kalirin7-T/A or Kalirin7-T/D. Co-expression of DN-Cdk5 did not alter the response of PC12 cells to either Kalirin mutant (Fig. 3C, D). The fact that Kalirin7-T/D alters cell roundness and perimeter in the presence of DN-Cdk5 supports the conclusion that Cdk5 functions upstream of Kalirin7. The fact that DN-Cdk5 is without effect on the morphological response of PC12 cells to Kalirin7-T/D or T/A supports the hypothesis that it is acting by blocking Cdk5 mediated phosphorylation of Thr1590 in Kalirin7.
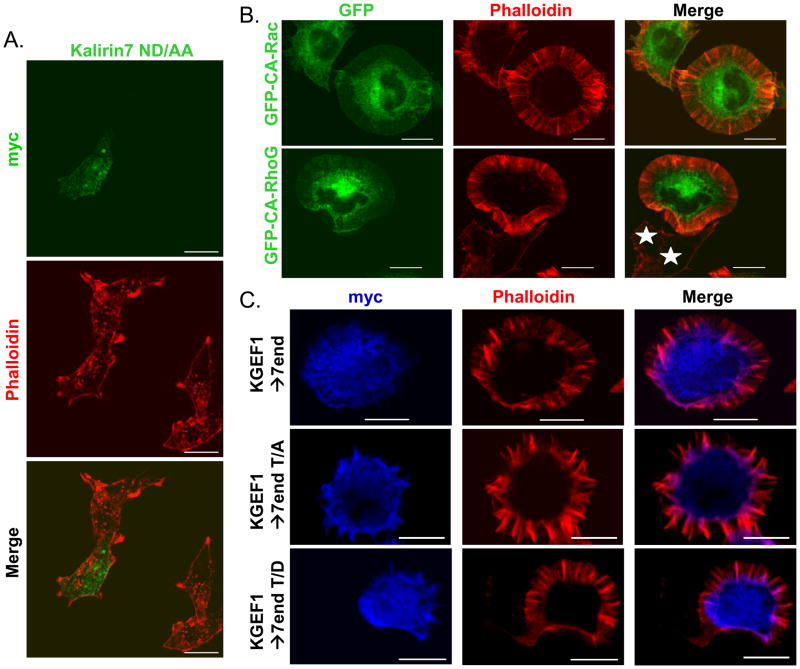
The GEF activity and spectrin-like repeat region of Kalirin7 are necessary for protrusion formation by PC12 cells
We next assessed the role of GEF activity in these changes in cell perimeter and roundness. Kalirin7 mutated at two sites near the C-terminus of its DH domain (N1443D1444 to AA; Kalirin7-ND/AA) has greatly diminished GEF activity (Penzes et al., 2003; Chakrabarti et al., 2005; Schiller et al., 2006). Expression of Kalirin7 ND/AA in PC12 cells failed to generate any distinguishable morphological change; cytoplasmic protrusions were not extended and lamellipodia were not formed (Fig. 4A). Kalirin7-ND/AA was localized along the plasma membrane and staining was again apparent in the nucleus. Quantitative analysis indicated that expression of the GEF-inactive mutant of Kalirin7 had no significant effect on cell perimeter or roundness (data not shown).
Fig. 4. The GEF activity and spectrin repeat region of Kalirin7 are required for protrusion formation.
A. PC12 cells transfected 24h earlier with vector encoding inactive Kalirin7-ND/AA were fixed; Kalirin7 was visualized using myc antibody (green); filamentous actin visualized using TRITC-phalloidin (red). No morphological differences were detected in cells expressing Kalirin7-ND/AA. B. PC12 cells expressing GFP fusion proteins of constitutively active (CA)-Rac or CA-RhoG were fixed after 24h. Fusion proteins visualized based on GFP fluorescence (green); filamentous actin visualized with TRITC-phalloidin (red); white stars, non-transfected cells. C. PC12 cells expressing KGEF1→7end or the T/A and T/D mutants were visualized with myc antibody (blue), filamentous actin was visualized simultaneously. Scale: 10μm.
Since the GEF activity of Kalirin7 is required for the induction of cytoplasmic protrusions in non-differentiated PC12 cells, we evaluated the morphological response of non-differentiated PC12 cells to expression of constitutively active Rac or RhoG (Fig. 4B). The effects of these two Kalirin substrates were similar; cells exhibited an approximately two-fold increase in cell perimeter (not shown). Unlike cells expressing Kalirin7, cells expressing constitutively active Rac or RhoG produced massive lamellipodial structures filled with spokes of filamentous actin. Cell roundness was decreased slightly in cells expressing constitutively active Rac but was unchanged by expression of constitutively active Rho G (data not shown).
Since KGEF1→7end is an active GEF, includes Thr1590 and is phosphorylated by Cdk5, we next asked whether it produced a morphological response like that of Kalirin7 or constitutively active Rac and RhoG when expressed in non-differentiated PC12 cells (Fig. 4C). PC12 cells expressing KGEF1→7end adopted a round shape, with massive lamellipodial structures surrounding the entire cell, as observed in cells expressing constitutively active Rac; protrusions like those extended in response to Kalirin 7 were never observed. KalGEF1 produced a similar response (data not shown). We next asked whether mutation of Thr1590 to Asp or Ala altered the morphological response. Non-differentiated PC12 cells responded to KGEF1→7end-T/D and KGEF1→7end-T/A in the same manner; protrusions like those formed in response to Kalirin7 were not observed. Our data indicate that GEF activity, the spectrin-like repeat region and the Cdk5 site in Kalirin7 each play essential roles in the morphological response observed.
Generation of antibody specific for Kalirin phosphorylated at Thr1590
Since Kalirin is a Cdk5 substrate both in cells and in vitro, we generated an antibody against the phospho-T1590PAK peptide (P-T1590 Ab) (Fig. 5). The specificity of this antibody for the phosphopeptide was first established by ELISA (Fig. 5A). Plates were coated with equal amounts of phospho-TPAK or TPAK peptide; only the phospho-peptide yielded a detectable signal. Western blotting was used to further characterize the P-T1590 antibody. Equal amounts of GST-KGEF1→7end and GST-KGEF1→7endT/A were analyzed directly or after incubation with 2 or 10ng recombinant Cdk5/p25 (Fig. 5B). Coomassie Brilliant Blue staining demonstrated that a similar amount of each protein was present (Fig. 5B, lower panel). The P-T1590 antibody detected GST-KGEF1→7end only after phosphorylation by Cdk5/p25 and failed to detect the T/A mutant protein even after exposure to Cdk5/p25 (Fig. 5B, upper panel).
Fig. 5. A phospho-T1590PAK Kalirin antibody (P-T1590 Ab) detects Cdk5 phosphorylation of Kalirin7 and ΔKalirin7.
A. A 96-well plate was coated with 50ng non-phosphorylated peptide (open squares) or 50ng QLPK-phospho-T1590PAKLRNNSK (solid diamonds). Bound antibody was visualized using alkaline phosphatase-conjugated antibody to rabbit IgG and p-nitrophenylphosphate. B. The specificity of the P-T1590 Ab was tested using purified GST-KGEF1→7end and GST-KGEF1→7endT/A. Aliquots of each fusion protein (2μg) were analyzed directly (0) or after incubation with recombinant Cdk5/p25 complex (2 or 10ng) and 1mM ATP. Upper panel: Western blot analysis with P-T1590 antibody. Lower panel: Coomassie Brilliant Blue stained membrane. C. pEAK RAPID cells transfected with vectors encoding Kalirin7 or parent vector were treated with or without 10μM roscovitine (Ros) for 4h before extraction into SDS lysis buffer. Duplicate samples visualized using antibody to P-T1590 (upper panel) or myc (lower panel).
To determine the ability of the P-T1590 antibody to detect phosphorylation of Kalirin in a cellular environment, duplicate wells of pEAK RAPID cells were transfected with vectors encoding Kalirin7 or its parent vector as control; one well was treated with roscovitine for 4h before harvest and the other was not (Fig. 5C). Lysates were visualized with antibody to myc or to P-T1590. Expression of Kalirin7 was not affected by roscovitine. Under basal conditions, phosphorylated Kalirin7 was detected; signal intensity was reduced substantially after treating the cells with 10μM roscovitine (Fig. 5C, upper panel), supporting the hypothesis that phosphorylation of Thr1590 is catalyzed by endogenous Cdk5/p39, Cdk1 or Cdk2 under basal conditions in these cells. Normalizing the intensity of the P-T1590 signal to the myc signal, roscovitine treatment reduced phosphorylation of Thr1590 to 30% of control (Fig. 5D).
Protein phosphatase 1 rapidly dephosphorylates P-T1590
Since a basal level of Kalirin phosphorylation at T1590 was detectable, we assessed the ability of a panel of protein phosphatase inhibitors to stabilize this modification. pEAK RAPID cells transiently expressing Kalirin7 were incubated with inhibitors of protein phosphatase 1 (PP1), protein phosphatase-2A (PP2A), and protein phosphatase-2B (PP2B or Calcineurin) and extracted for analysis of Thr1590 phosphorylation (Fig. 6A). Incubation with Calyculin A, an inhibitor of PP1 and PP2A, resulted in a substantial increase in the amount of Kalirin7 phosphorylated at T1590. None of the more selective PP2A inhibitors (cantharidin, endothall) resulted in a similar increase in phosphorylation at this site. Thus PP1, which plays a critical role in synaptic plasticity (Morishita et al., 2001), is likely to play a major role in the dephosphorylation of Kalirin Thr1590. Inhibitors of calcineurin were without effect on the level of Thr1590 phosphorylation.
Fig. 6. Protein phosphatase 1 rapidly dephosphorylates Kalirin-P-T1590.
A. pEAK RAPID cells transiently transfected with vector encoding Kalirin7 were treated with phosphatase inhibitors for 30min before extraction in SDS lysis buffer. Duplicate aliquots of cell extract were visualized with antibody to P-T1590 (upper panel) or myc (lower panel). B. pEAK RAPID cells transiently transfected with parent vector or vectors encoding Kalirin7, ΔKalirin7 or KGEF1 were treated with or without 25 nM Calyculin A for 30min before extraction into SDS lysis buffer. Duplicates of cell extract were visualized with antibody to P-T1590 (upper panel) or myc (lower panel). C. PC12 cells expressing exogenous myc Kalirin7 and striatal cultures expressing endogenous Kalirin7 were exposed to medium containing 10μM roscovitine for 4h or 25nM Calyculin A for 30min (CA); control cells (Con) received vehicle.
To verify the ability of Calyculin A to increase phosphorylation of Kalirin at Thr1590, Kalirin7, ΔKalirin7, and KGEF1 were transiently expressed in duplicate wells of pEAK RAPID cells; one set of cells was treated with Calyculin A before extraction. Western blot analysis with the P-T1590 antibody (Fig. 6B, upper panel) and the myc antibody (Fig. 6B, lower panel) showed that Calyculin A treatment of cells expressing Kalirin7 or ΔKalirin7 substantially enhanced the signal detected by the P-T1590 antibody. No signal was detected for KGEF1, which does not include T1590. The substantial increase in the signal detected by the P-T1590 antibody after 30 min of Calyculin A treatment indicates that phosphorylation-dephosphorylation of T1590 occurs rapidly under basal conditions.
As shown previously, levels of Cdk5, p35/p39 and PP1 vary widely in pEAK Rapid cells, PC12 cells and striatum. The ability of roscovitine and Calyculin A to affect Thr1590 phosphorylation of exogenous Kalirin7 in PC12 cells and endogenous Kalirin7 in primary cultures of neonatal rat striatum was assessed (Fig. 6C). Basal phosphorylation of Thr1590 was not detected when exogenous Kalirin7 was expressed in PC12 cells; after pretreatment with Calyculin A, P-Thr1590 could be detected (Fig. 6C, upper). Basal phosphorylation of endogenous Kalirin7 on Thr1590 was detected in mature cultures of striatal neurons (Fig. 6C, lower). Pretreatment with roscovitine, which should only be affecting Cdk5 in these non-dividing cells (Dhavan and Tsai, 2001), caused a slight decrease in Thr1590 phosphorylation while pretreatment with Calyculin A caused a substantial increase in Thr1590 phosphorylation. Steady state phosphorylation of Thr1590 by Cdk5 followed by rapid PP1-mediated dephosphorylation suggests a role for this site in regulating Kalirin function.
Kalirin GEF1 activity is increased by phosphorylation of Thr1590
Since the GEF activity of Kalirin is critical for its effect on PC12 cell morphology as well as its role in the maintenance of dendritic spines, the dendritic arbor and axonal elongation (Ma et al., 2003; May et al., 2002), we asked whether Thr1590 phosphorylation altered GEF activity. We used both in-cell assays based on the binding of GTP-Rac to the CRIB domain of PAK (May et al., 2002; Schiller et al., 2006) and assays with purified KGEF1→7end to address this issue.
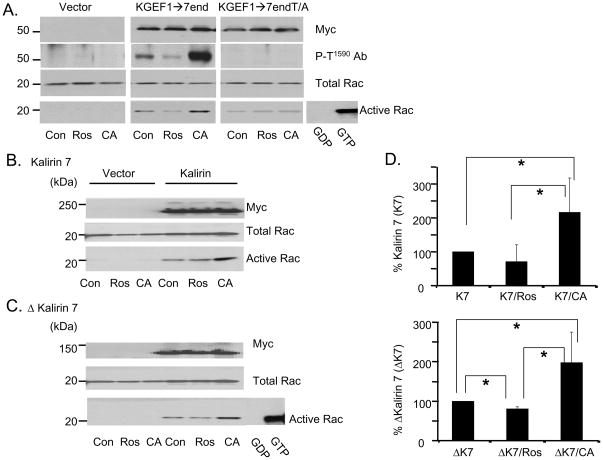
We first utilized the Pak-CRIB pull-down assay to determine whether the ability of cells transiently expressing various Kalirin constructs to activate Rac was altered following pre-incubation with roscovitine or Calyculin A (Fig. 7). Cells expressing GFP, KGEF1→7end or KGEF1→endT/A, were treated with roscovitine or Calyculin A and harvested for analysis of active Rac (Fig. 7A). Active Rac was not detectable in extracts of control cells (Fig. 7A, left). Pre-incubation of KGEF1→7end expressing cells with roscovitine reduced levels of active Rac and preincubation with Calyculin A increased levels of active Rac (Fig. 7A, middle). While expression of KGEF1→7end and Rac was not altered by drug treatment, levels of P-T1590 were decreased by roscovitine and increased by Calyculin A. Expression of KGEF1→7endT/A activated Rac, but neither drug affected the level of Rac activation (Fig. A, right). Since the effects of roscovitine and Calyculin A on GEF activity were abrogated when Thr1590 was mutated to Ala, we can conclude that GEF activity is enhanced by Thr1590 phosphorylation. Although it cannot be activated by phosphorylation of Thr1590, KGEF1→7end T/A retains significant GEF activity.
Fig. 7. The GEF activity of Kalirin is increased following Calyculin A treatment of cells.
Activation of Rac was assessed using GST-Pak-Crib. A. pEAK RAPID cells transfected with parent vector or vectors encoding KGEF1→7end or KEGF1→7end-Thr1590/Ala1590 were untreated (Con) or treated with 10μM roscovitine (Ros) for 4h or 25nM Calyculin A (CA) for 30min. Transfected proteins were detected with myc antibody (upper); phosphorylated protein was detected with P-T1590 Ab (second), and total Rac (third) was visualized with Rac antibody. GTP-bound Rac was isolated using GST-Pak-Crib resin and quantified by Western blot analysis with Rac antibody (bottom). B and C. pEAK RAPID cells transfected with vectors encoding Kalirin7 (B) or μKalirin7 (C) were analyzed as described in A. Control cells transfected with parent vector. Pooled cell extract incubated with GDP or GTPγS, negative and positive controls. D. Data from three independent assays quantified; activated Rac in control cells expressing Kalirin7 (upper) or μKalirin7 (lower) set to 100%; activated Rac in roscovitine or Calyculin A treated cells expressed as a percentage of relevant control; p values calculated using Student’s T test; *, p<0.05.
Similar results were obtained in cells expressing Kalirin7 or ΔKalirin7 (Figs.7B, C). Expression of Kalirin and Rac was unaltered by roscovitine or Calyculin A treatment. For both Kalirin7 and ΔKalirin7, levels of active Rac were increased approximately two-fold in the presence of Calyculin A. To quantify the effect, Rac activation in cells expressing Kalirin7 or ΔKalirin7 was set to 100%. In cells pre-incubated with Calyculin A, the same amount of Kalirin7 orΔKalirin7 produced significantly more Rac-GTP (Fig. 7D). Pre-incubation with roscovitine produced a slight decrease in Rac activation by ΔKalirin7; its effect on Rac activation by Kalirin7 was not significant. These data suggest that phosphorylation of Thr1590 increases the catalytic activity of the GEF domain in KGEF1→end, ΔKalirin7 and Kalirin7 or increases their access to Rac in this cell-based assay.
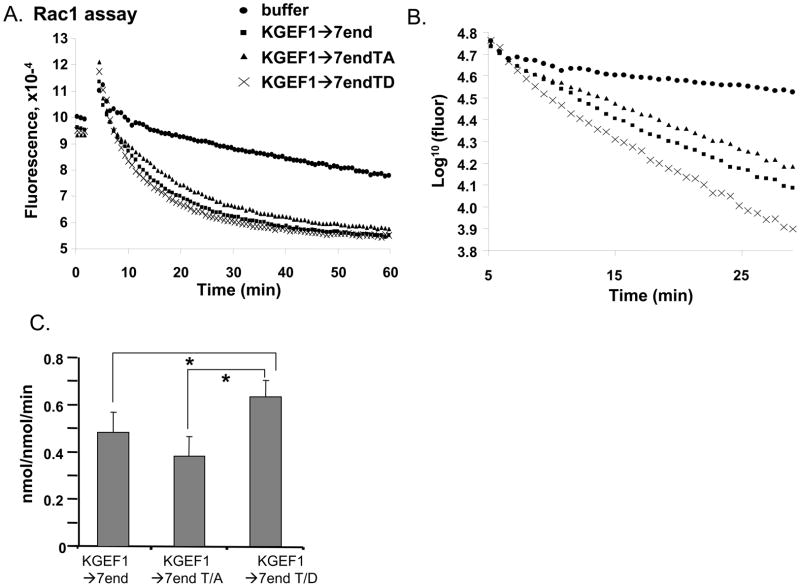
To distinguish between these possibilities, we compared the catalytic activities of purified GST-KGEF1→7end, GST-KGEF1→7endT/A, and GST-KGEF1→7endT/D using GST-Rac1 loaded with GDP-Mant. Representative data from one assay are shown in Fig. 8A; initial rates were calculated from data obtained over the first 10 to 15 min using a semi-logarithmic plot (Fig. 8B). Data from at least three independent assays for each enzyme/substrate combination are summarized in Fig. 8C. Although Thr1590 lies beyond the COOH-terminus of the PH domain, mutation of this residue to Asp affects GEF activity. With Rac1 as the substrate, KGEF1→7endT/D is significantly more active than KGEF1→7endT/A or KGEF1→7end. If replacing Thr1590 with Asp somewhat mimics phosphorylation of Thr1590, this result is consistent with the ability of Calyculin A to increase both Thr1590 phosphorylation and Rac activation by Kalirin in cell-based assays. Substitution of Ala for Thr1590 prohibits phosphorylation and an effect on catalytic activity was not anticipated.
Fig. 8. GEF activity of Kalirin is increased by mutation of Thr1590 to Asp.
GEF activity was assessed in vitro using GDP-Mant-loaded Rac1 (2μM). A. Change in fluorescence with time is shown; buffer or the proteins indicated (final concentration 20μM) were added at t=3min. B. Data from the first 20min converted to a semi-logarithmic plot enabled initial rate calculation. C. Data from three assays for GEF activities of GST-KGEF1→K7end, KGEF1→K7endT/A and KGEF1→K7endT/D averaged. Significant differences indicated; *, p<0.05.
Thr1590 to Asp mutation increases Kalirin7 solubility in PC 12 cells
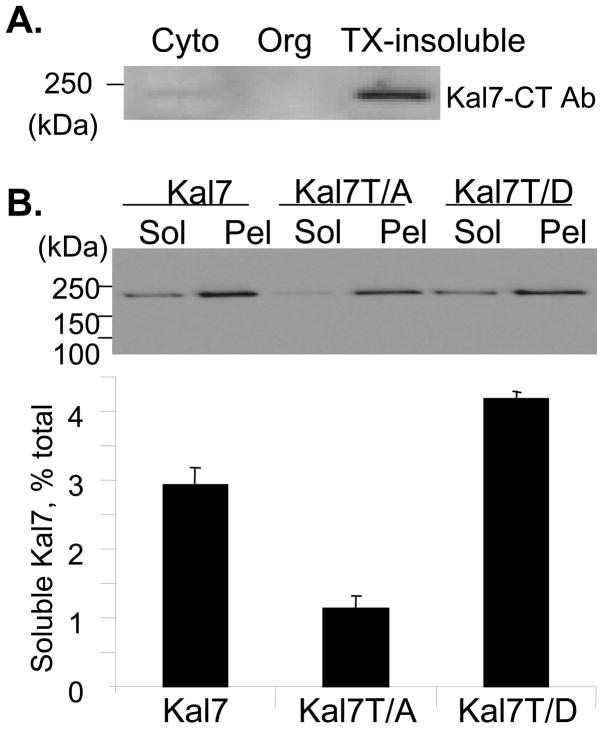
Since phosphorylation of Thr1590 produced only a modest increase in GEF activity, we sought evidence for other ways in which phosphorylation at this site might alter Kalirin7 function. Protein phosphorylation is well recognized as one means of targeting proteins to specific sites (Gulli et al., 2000). Endogenous Kalirin7 in adult rat cortex is largely insoluble (Penzes et al., 2001). When rat brain cytosol and organelle-enriched fractions were separated from the remaining Triton X-100 insoluble material, almost all of the Kalirin7 was recovered from the TX-100 insoluble pellet (Fig. 9A). Because of the many indirect effects of treating cells with roscovitine or Calyculin A, we utilized Kalirin7-T/A and Kalirin7-T/D to assess solubility in PC12 cells. While the three proteins were expressed at similar levels, a greater percentage of the Kalirin7-T/D was recovered from the soluble fraction; Kalirin7-T/A was less soluble than wildtype Kalirin7 (Fig. 9B). Access of RhoGEFs to their Rho GTPase substrates is critical and could be altered by a solubility change of this type.
Fig. 9. Solubility of Kalirin7-T/D is increased.
A. Cortex from adult rat brain was separated into cytosol, organelle and TX-100 insoluble fractions; 20μg of protein from each fraction was subjected to Western blot analysis using antibody specific for Kalirin7. B. PC12 cells expressing wildtype Kalirin7, Kalirin7-T/A and Kalirin7-T/D were separated into soluble and insoluble fractions as described in Methods; 10% of the soluble fraction (Sol) and 1% of the resuspended pellet fraction (Pel) was subjected to Western blot analysis for myc. Bar graph: quantification of data from two independent experiments; error bar gives range.
Thr1590 affects the formation of dendritic spines in primary cortical neurons
Thr1590 is present in each of the major isoforms of Kalirin, suggesting regulatory roles for Cdk5 as Kalirins 9 and 12 participate in process outgrowth (May et al., 2002) and as Kalirin 7 participates in spine formation (Ma et al., 2003; Ma et al., 2008). Since methods for assessing the effects of Kalirin 7 on spine formation have been optimized, we explored the possibility that phosphorylation of Thr1590 affects this response. Rat cortical neurons were nucleofected simultaneously with vectors encoding GFP and Kalirin7 or its T/A and T/D mutants (Fig. 10). GFP fills each transfected cell, making it possible to visualize dendritic processes and spines (Fig. 10); exogenous Kalirin 7 was visualized using myc antibody. After 16 days in vitro, the dendritic processes shown in Panel A are covered with spines, most of which have a readily discernible spine head. Myc staining is not detectable in these GFP-expressing neurons and the spine density observed is typical of neurons of this age (Ma et al., 2003; Ma et al., 2008). As expected, spine density is increased substantially in cortical neurons expressing exogenous Kalirin 7 (Panel B). Although dendritic spines are more numerous in neurons expressing Kalirin7-T/A (Panel C) than in neurons expressing GFP, very few of the spines have a clear spine head. Kalirin7-T/A is localized to the tips of the spine-like structures that form, but the clusters are noticeably smaller than those formed by Kalirin 7 and the spines are shorter and thinner than normal.
Fig. 10. Thr1590 of Kalirin7 plays an essential role determining the shape of dendritic spines.
Cortical neurons from P1 rats were nucleofected on DIV1 with vector encoding GFP alone (A) or co-transfected with vectors encoding GFP and Kalirin7 (B), Kalirin7-T/A (C) or Kalirin7-T/D (D). Fixed neurons were immunostained for GFP (left) or Myc (middle) (DIV16). Merged images shown in right panels. Scale, 10 μm.
Expression of Kalirin7-T/D also caused an increase in spine number (Panel D); many of the spines formed in response to this protein, which may mimic Kalirin 7 phosphorylated at Thr1590, had large spine heads. Thr1590 clearly plays a critical role in the pathway through which Kalirin 7 affects spine structure. Further studies will be required to determine whether Cdk5 and PP1 are the key factors controlling the phosphorylation state of Kalirin 7 in dendritic spines and how phosphorylation of Thr1590 alters the actions of Kalirins 9 and 12.
Discussion
Kalirin is a Cdk5 substrate
Cdk5 plays important roles during neuronal development and in mature neurons (Dhavan and Tsai, 2001; Hahn et al., 2005; Humbert et al., 2002). To date, over two dozen proteins with diverse functions have been identified as Cdk5 substrates (Hahn et al., 2005; Humbert et al., 2002; Nikolic, 2002; Takashima et al., 2001; Dhavan and Tsai, 2001; Castillo-Liuva et al., 2007; Fu et al., 2007; Kesavapany et al., 2006). Four GEFs, Kalirin, Trio, RasGRF1, and RasGRF2 are phosphorylated by Cdk5 (Kesavapany et al., 2004; Xin et al., 2004; Kesavapany et al., 2006). The Cdk5 site in Kalirin 7 (T1590PAK) lies just beyond the first PH domain; since it precedes the 20 amino acid sequence unique to Kalirin 7, this site also occurs in Kalirins 8, 9 and 12, the Kalirin isoforms most prevalent early in development. This site is conserved in mammals and chickens and Ser substitutes for Thr in Drosophila Trio; in contrast, the C. elegans and Takifugu rubripes homologues lack a (T/S)PAK motif. Recombinant Cdk5/p25 phosphorylates Thr1590 more efficiently in GST-KGEF1→7end than in ΔKalirin7. Although access of the kinase complex to Thr1590 may be restricted, exogenous Kalirin7 expressed in pEAK RAPID cells and endogenous Kalirin7 in striatal neurons is phosphorylated at this site, based on our antibody staining.
Thr1590 plays an essential role in the actions of Kalirin
Since expression of exogenous Kalirin7 disrupted NGF signaling in PC12 cells (Chakrabarti et al., 2005), we used non-differentiated cells to establish a role for Cdk5 in regulating the effects of Kalirin on the cytoskeleton. Expression of full-length Kalirin7 in non-differentiated PC12 cells caused the extension of broad cytoplasmic protrusions. Kalirin7 in which Thr1590 was mutated to Ala to prevent phosphorylation failed to cause this response; lamellipodia continued to form, yielding a phenotype similar to that produced by expression of the isolated GEF domain of Kalirin 7 or constitutively active Rac or RhoG. Kalirin7 in which Thr1590 was mutated to Asp in an attempt to mimic phosphorylation caused the formation of protrusions. Unlike Kalirin7, the morphological effects of Kalirin7-T/D were not blocked by DN-Cdk5. The ability of Cdk5, which is ubiquitously expressed, to phosphorylate Kalirin7 may be controlled by activation/localization of Cdk5/p35/p39, localization of Kalirin7 or a conformational change in Kalirin7 that unmasks the Cdk5 site (Nikolic, 2002; Dhavan and Tsai, 2002).
Although the protrusions formed in undifferentiated PC12 cells are distinctly different from the neurites formed in NGF-differentiated PC12 cells, their formation indicates that the cells have adopted a somewhat polarized morphology. Cdk5 plays an essential role in establishing cell polarity in organisms ranging from yeast to human. For example, Rac1 and laminin form an autocrine pathway that orients the apical pole during cyst development by polarized epithelial cells (O’Brien et al., 2001). Cdk5 plays an essential role in localizing Cdc24 and activated Rac1 in Ustilago maydis cells infected by the corn smut fungus; without active Cdk5, these cells are unable to sustain the dramatic polar growth required for formation of infective structures (Castillo-Liuva et al., 2007). Inhibition of endogenous Cdk5 by DN-Cdk5 blocked the ability of Kalirin7 to cause the formation of protrusions, identifying Cdk5 as an upstream regulator of Kalirin.
Kalirin 7 is highly expressed only in mature neurons (Ma et al., 2003; Ma et al., 2008). Cdk5 plays an important role in spine formation and synaptic plasticity (Bibb et al., 2001a; Dhavan and Tsai, 2001; Humbert et al., 2002; Lee et al., 1996; Nikolic et al., 1998; Cheung et al., 2006). Primary cortical neurons respond to exogenous Kalirin7 by producing additional dendritic spines (Ma et al., 2003; Ma et al., 2008); mutation of Thr1590 to Ala or to Asp does not eliminate the ability of Kalirin 7 to promote spine formation. The fact that exogenous Kalirin7-T/A causes the appearance of short, thin spine-like structures that lack a clear spine head strongly suggests that Kalirin7 is one of the Cdk5 substrates that plays an essential role in spine morphogenesis.
Formation of protrusions requires full-length, active Kalirin 7
Trio, a Kalirin homologue (McPherson et al., 2004), causes neurite outgrowth in NGF-treated PC12 cells; its GEF activity is required for this effect (Estrach et al., 2002). Likewise, the GEF activity of Kalirin7 is required for its effect on protrusion formation. Expression of constitutively active Rac or RhoG causes the formation of lamellipodia, but does not cause the formation of protrusions or produce polarity. Expression of the isolated GEF1 domain of Kalirin, KalGEF1→7end or ΔKalirin7 mimics expression of constitutively active Rac; protrusions are not formed. The fact that protrusion formation requires the presence of the spectrin repeat region of Kalirin7 suggests a special role for this non-catalytic region. Expression of a fragment of Kalirin 7 that contains only its Sec14p domain followed by its nine spectrin-like repeats completely disrupts normal cytoskeletal organization in non-neuronal cells; the exogenous protein and filamentous actin accumulate under the plasma membrane (Schiller et al., 2008). Both the ability of the Sec14p domain to bind specific phosphoinositides and the ability of the spectrin repeat region to oligomerize are essential to this response. In addition, proteins such as HAP1, DISC1, iNOS and PAM, which are known to interact with the spectrin repeat region of Kalirin (Alam et al., 1997; Colomer et al., 1997; Ratovitski et al., 1999; Takaki et al., 2005), may play a role in the response. A yeast two-hybrid study identified the NH2-terminal region of Kalirin as an interactor with the tetramerization domain of αII-spectrin (Oh and Fung, 2007); this interaction has not yet been verified in tissue.
Phospho-T1590 is dephosphorylated by PP1
Phosphorylation and dephosphorylation are dynamic processes. A survey of phosphatase inhibitors indicates that protein phosphatase 1 (PP1) is largely responsible for the dephosphorylation of phospho-Thr1590 in pEAK RAPID cells. Phosphorylation of Thr1590 in exogenous Kalirin7 expressed in PC12 cells and in endogenous Kalirin7 in striatal neurons is increased following a 30min treatment with Calyculin A. PP1, with its multiple substrates (Cohen, 2002), regulates the phosphorylation state of many receptors, activates Na+ channels (Greengard et al., 1999) and plays an essential role in long term depression (Morishita et al., 2001). PP1 is found in a complex with another RhoGEF, Lfc. This complex, which regulates Rho-dependent organization of F-actin in spines, involves the interaction of Lfc with neurabin and spinophilin (Ryan et al., 2005). Both neurabin and spinophilin were identified as interactors with the C-terminal PDZ binding motif of Kalirin7 (Penzes et al., 2001). Phosphorylation state often regulates protein localization and specific protein/protein interactions (Gulli et al., 2000). The fact that Kalirin7-T/D is more soluble than Kalirin7 suggests a similar role in this case.
Phosphorylation of T1590 enhances the GEF activity of Kalirin 7
The first GEF domain of Kalirin has specificity for Rac and RhoG (Penzes et al., 2001; May et al., 2002; Schiller et al., 2005). With a cell based assay, we demonstrate that treatment with Calyculin A, which increases phosphorylation of Thr1590, increases the ability of KalGEF1→7end, Kalirin7 and ΔKalirin7 to activate Rac. Calyculin A has no effect on Rac activation in cells expressing GFP or KGEF1→7endT/A, supporting the conclusion that Calyculin A acts by stabilizing phospho-Thr1590. Differences in the level of expression of KGEF1→7end and KGEF→7endT/A or T/D make a direct comparison of their GEF activities using the cell based assay difficult. Whether the increase in activity upon phosphorylation of Thr1590 is caused by a change in Km or Vmax or a change in subcellular localization requires further study. The magnitude of the Calyculin A effect on Thr1590 phosphorylation (5- to 10-fold increase, 30min) suggests a high turnover rate for phosphorylation at this site, allowing control by activation of Cdk5 or inhibition of PP1. Cdk5/p35 mediated phosphorylation of Ras guanine nucleotide releasing factor (RasGRF2), a widely expressed RhoGEF that includes a Ras exchanger motif, reduces its ability to activate Rac in cell based assays (Kesavapany et al., 2004). RasGRF1 also interacts with and is phosphorylated by Cdk5 on Ser731. Phosphorylation on this site leads to RasGRF1 degradation through a calpain-dependent mechanism. A reduction of RasGRF1 levels leads to nuclear condensation in neurons (Kesavapany et al., 2006). In agreement with the cell-based assays, in vitro assessment of catalytic activity demonstrated that KGEF1→7end T/D was twice as active as KGEF1→7end T/A using Rac1 as the substrate. In a cellular environment, differences of this magnitude are likely to be of functional significance.
Acknowledgments
We thank Dr. Martin Schiller for sharing data, Jaqueline Sobota for assistance with imaging, Atul Deshpande for initiating phosphatase inhibitor studies and Darlene D’Amato for expert technical assistance. Support: DA-015464.
Abbreviations
- DH
Dbl homology
- PH
pleckstrin homology
- GEF
guanine nucleotide exchange factor
- PP-1
protein phosphatase 1
- Cdk5
cyclin dependent kinase 5
- PSD
postsynaptic density
References
- Alam MR, Johnson RC, Darlington DN, Hand TA, Mains RE, Eipper BA. Kalirin, a cytosolic protein with spectrin-like and GDP/GTP exchange factor-like domains that interacts with peptidylglycine α-amidating monooxygenase, an integral membrane peptide-processing enzyme. J Biol Chem. 1997;272:12667–12675. doi: 10.1074/jbc.272.19.12667. [DOI] [PubMed] [Google Scholar]
- Aoki K, Nakamura T, Matsuda M. Spatio-temporal regulation of Rac1 and Cdc42 activity during nerve growth factor-induced neurite outgrowth in PC12 cells. J Biol Chem. 2004;279:713–719. doi: 10.1074/jbc.M306382200. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Saito M, Sone M, Suzuki E, Sakai R, Ito K, Hama C. The Drosophila Trio plays an essential role in patterning of axons by regulating their directional extension. Neuron. 2000;26:119–131. doi: 10.1016/s0896-6273(00)81143-5. [DOI] [PubMed] [Google Scholar]
- Bateman J, Shu H, Van Vactor D. The GEF Trio mediates axonal development in the Drosophila embryo. Neuron. 2000;26:93–106. doi: 10.1016/s0896-6273(00)81141-1. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001a;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Nishi A, O’Callaghan JP, Ule J, Lan MS, Snyder GL, Horiuchi A, Saito T, Hisanaga SI, Czernik AJ, Nairn AC, Greengard P. Phosphorylation of protein phosphatase inhibitor-1 by cdk5. J Biol Chem. 2001b;276:14490–14497. doi: 10.1074/jbc.M007197200. [DOI] [PubMed] [Google Scholar]
- Black MM, Aletta JM, Greene LA. Regulation of microtubule composition and stability during nerve growth factor-promoted neurite outgrowth. J Cell Biol. 1986;103:545–557. doi: 10.1083/jcb.103.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briancon-Marjollet A, Ghogha A, Nawabi H, Triki I, Auziol C, Fromont S, Piche C, Enslen H, Chebli K, Cloutier JF, Castellani V, Debant A, Lamarche-Vane N. Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance. Mol Cell Biol. 2008;28:2314–2323. doi: 10.1128/MCB.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canduri F, Uchoa HB, de Azevedo WFJ. Molecular models of cyclin-dependent kinase 1 complexed with inhibitors. Biochem Biophys Res Commun. 2004;324:661–666. doi: 10.1016/j.bbrc.2004.09.109. [DOI] [PubMed] [Google Scholar]
- Castillo-Liuva S, Alvarez-Tabares I, Weber I, Steinberg G, Perez-Martin J. Sustained cell polarity and virulence in the phytopathogenic fungus Ustilago maydis depends on an essential cyclin-dependent kinase from the Cdk5/Pho85 family. J Cell Sci. 2007;120:1584–1595. doi: 10.1242/jcs.005314. [DOI] [PubMed] [Google Scholar]
- Chakrabarti K, Lin R, Schiller NI, Wang Y, Koubi D, Fan YX, Rudkin BB, Johnson GR, Schiller MR. Critical role for Kalirin in nerve growth factor signaling through TrkA. Mol Cell Biol. 2005;25:5106–5118. doi: 10.1128/MCB.25.12.5106-5118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung Zh, Fu AK, Ip NY. Synaptic roles of Cdk5: implications in higher cognitive functions and neurodegenerative diseases. Neuron. 2006;50:13–18. doi: 10.1016/j.neuron.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Cohen PTW. Protein phosphatase 1 - targeted in many directions. J Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- Colomer V, Engelender S, Sharp AH, Duan K, Cooper JK, Lanahan A, Lyford G, Worley P, Ross CA. Huntingtin-associated protein 1 (HAP1) binds to a Trio-like polypeptide, with a rac1 guanine nucleotide exchange factor domain. Hum Mol Genet. 1997;6:1519–1525. doi: 10.1093/hmg/6.9.1519. [DOI] [PubMed] [Google Scholar]
- Debant A, Serra-Pages C, Seipel K, O’Brien S, Tang M, Park SH, Streuli M. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci U S A. 1996;93:5466–5471. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH. A decade of Cdk5. Nature Reviews. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Estrach S, Schmidt S, Diriong S, Penna A, Blangy A, Fort P, Debant A. The Human Rho-GEF Trio and Its Target GTPase RhoG Are Involved in the NGF Pathway, Leading to Neurite Outgrowth. Curr Biol. 2002;12:307–312. doi: 10.1016/s0960-9822(02)00658-9. [DOI] [PubMed] [Google Scholar]
- Fu WY, Chen Y, Sahin M, Zhao XS, Shi L, Bikoff JB, Lai KO, Yung WH, Fu AK, Greenberg ME, Ip NY. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nature Neurosci. 2007;10:67–76. doi: 10.1038/nn1811. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Gulli MP, Jaquenoud M, Shimada Y, Niederhäuser G, Wiget P, Peter M. Phosphorylation of the Cdc42 exchange factor Cdc24 by the PAK-like kinase Cla4 may regulate polarized growth in yeast. Mol Cell. 2000;6:1155–1167. doi: 10.1016/s1097-2765(00)00113-1. [DOI] [PubMed] [Google Scholar]
- Hahn CM, Kleinholz H, Koester MP, Grieser S, Thelen K, Pollerberg GE. Role of Cyclin-Dependent Kinase and Its Activator P35 in Local Axon and Growth Cone Stabilization. Neuroscience. 2005;134:449–465. doi: 10.1016/j.neuroscience.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Harada T, Morooka T, Ogawa S, Nishida E. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nature Cell Biol. 2001;3:453–459. doi: 10.1038/35074516. [DOI] [PubMed] [Google Scholar]
- Humbert S, Dhavan R, Tsai LH. p39 activates Cdk5 in neurons, and is associated with the actin cytoskeleton. J Cell Sci. 2002;113:975–983. doi: 10.1242/jcs.113.6.975. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Penzes P, Eipper BA, Mains RE. Isoforms of kalirin, a neuronal Dbl family member, generated through use of different 5′- and 3′-ends along with an internal translational initiation site. J Biol Chem. 2000;275:19324–19333. doi: 10.1074/jbc.M000676200. [DOI] [PubMed] [Google Scholar]
- Kesavapany S, Amin N, Zheng Y, Nijhara R, Jaffe H, Sihag R, Gutkind JS, Takahashi S, Kulkarni A, Grant Ph, Pant HC. p35/cyclin-dependent kinase 5 phosphorylation of ras guanine nucleotide releasing factor 2 (RasGRF2) mediates Rac-dependent Extracellular Signal-regulated kinase 1/2 activity, altering RasGRF2 and microtubule-associated protein 1b distribution in neurons. J Neurosci. 2004;24:4421–4431. doi: 10.1523/JNEUROSCI.0690-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavapany S, Pareek TK, Zheng YL, Amin N, Gutkind JS, Ma W, Kulkarni AB, Grant Ph, Pant HC. Neuronal nuclear organization is controlled by cyclin-dependent kinase 5 phosphorylation of Ras Guanine nucleotide releasing factor-1. Neurosignals. 2006;15:157–173. doi: 10.1159/000095130. [DOI] [PubMed] [Google Scholar]
- Lee KY, Rosales JL, Tang D, Wang JH. Interaction of cyclin-dependent kinase 5 (Cdk5) and neuronal Cdk5 activator in bovine brain. J Biol Chem. 1996;271:1538–1543. doi: 10.1074/jbc.271.3.1538. [DOI] [PubMed] [Google Scholar]
- Ma XM, Huang J, Wang YP, Eipper BA, Mains RE. Kalirin, a Multifunctional Rho Guanine Nucleotide Exchange Fector, Is Necessary for Maintenance of Hippocampal Pyramidal Neuron Dendrites and Dendritic Spines. J Neurosci. 2003;23:10593–10603. doi: 10.1523/JNEUROSCI.23-33-10593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Wang Y, Ferraro F, Mains RE, Eipper BA. Kalirin-7 is an Essential Component of both Shaft and Spine Excitatory Synapses in Hippocampal Interneurons. J Neurosci. 2008;28:711–724. doi: 10.1523/JNEUROSCI.5283-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V, Schiller MR, Eipper BA, Mains RE. Kalirin Dbl-homology GEF1 domain initiates new axon outgrowths through RhoG-mediated mechanisms. J Neurosci. 2002;22:6980–6990. doi: 10.1523/JNEUROSCI.22-16-06980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson CE, Eipper BA, Mains RE. Multiple novel isoforms of Trio are expressed in the developing rat brain. Gene. 2004;347:125–135. doi: 10.1016/j.gene.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Meikrantz W, Schlege R. Suppression of appoptosis by dominant negative mutants of cyclin-dependant protein kinases. J Biol Chem. 1996;271:10205–10209. doi: 10.1074/jbc.271.17.10205. [DOI] [PubMed] [Google Scholar]
- Morishita W, Connor JH, Xia H, Quinlan EM, Shenolikar S, Malenka RC. Regulation of synaptic strength by protein phosphatase 1. Neuron. 2001;32:1133–1148. doi: 10.1016/s0896-6273(01)00554-2. [DOI] [PubMed] [Google Scholar]
- Newsome TP, Schmidt S, Dietzl G, Keleman K, Asling B, Debant A, Dickson BJ. Trio combines with Dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell. 2000;101:283–294. doi: 10.1016/s0092-8674(00)80838-7. [DOI] [PubMed] [Google Scholar]
- Nikolic M. The role of Rho GTPases and associated kinases in regulating neurite outgrowth. Int J Biochem Cell Biol. 2002;34:731–745. doi: 10.1016/s1357-2725(01)00167-4. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–198. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Jou TzSh, Pollack AL, Zhang Q, Hansen StH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nature Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- Oh Y, Fung LWM. Brain proteins interacting with the tetramerization region of non-erythroid alpha spectrin. Cell Mol Biol letters. 2007;12:604–620. doi: 10.2478/s11658-007-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Alam MR, Kambampati V, Mains RE, Eipper BA. An Isoform of Kalirin, a Brain-Specific GDP/GTP Exchange Factor, Is Enriched in the Postsynaptic Density Fraction. J Biol Chem. 2000;275:6395–6403. doi: 10.1074/jbc.275.9.6395. [DOI] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, Kambampati V, Mains RE, Eipper BA. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–242. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Qi Z, Huang QQ, Lee KY, Lew J, Wang JH. Reconstitution of neuronal Cdc2-like kinase from bacteria-expressed Cdk5 and an active fragment of the brain-specific activator. J Biol Chem. 1995;270:10847–10854. doi: 10.1074/jbc.270.18.10847. [DOI] [PubMed] [Google Scholar]
- Ratovitski EA, Alam MR, Quick RA, McMillan A, Bao C, Hand TA, Johnson RC, Mains RE, Eipper BA, Lowenstein CJ. Kalirin inhibition of inducible nitric oxide synthase. J Biol Chem. 1999;274:993–999. doi: 10.1074/jbc.274.2.993. [DOI] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J. GEF Means Go: Turning on Rho GTPases with Guanine Nucleotide-Exchange Factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Ryan XP, Alldritt J, Svenningsson P, Allen PB, Wu GY, Nairn AC, Greengard P. The Rho-Specific GEF Lfc Interacts with Neurabin and Spinophilin to regulate Dendritic Spine Morphology. Neuron. 2005;47:85–100. doi: 10.1016/j.neuron.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Schiller MR, Blangy A, Huang J, Mains RE, Eipper BA. Induction of lamellipodia by Kalirin does not require its guanine nucleotide exchange factor activity. Exp Cell Res. 2005;307:402–417. doi: 10.1016/j.yexcr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Schiller MR, Chakrabarti K, King G, Schiller N, Eipper B, Maciejewski M. Regulation of RhoGEF activity by intramolecular and intermolecular SH3 interactions. J Biol Chem. 2006;281:18774–18786. doi: 10.1074/jbc.M512482200. [DOI] [PubMed] [Google Scholar]
- Schiller MR, Ferraro F, Wang Y, Ma X-M, McPherson CE, Sobota JA, Schiller NI, Mains RE, Eipper BA. Autonomous functions for the Sec14p/spectrin-repeat region of Kalirin. Exp Cell Res. 2008 doi: 10.1016/j.yexcr.2008.05.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Sharma M, Amin ND, Albers RW, Pant HC. Regulation of cyclin-dependent kinase 5 catalytic activity by phosphorylation. Proc Natl Acad Sci U S A. 1999;96:11156–11160. doi: 10.1073/pnas.96.20.11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven R, Kubiseski TJ, Zheng H, Kulkarni S, Mancillas J, Morales AR, Hogue CWV, Pawson T, Culotti JG. Unc-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell. 1998;92:785–795. doi: 10.1016/s0092-8674(00)81406-3. [DOI] [PubMed] [Google Scholar]
- Takaki M, Paek M, Kamiya A, Balkissoon R, Tomoda T, Penzes P, Sawa A. Disrupted-in-schizophrenia-1 (DISC1) binds to Kalirin-7 and modulates its function as a GDP/GTP exchange factor. Soc Neurosci Abst. 2005 program No. 674.9. [Google Scholar]
- Takashima A, Murayama M, Yasutake K, Takahashi H, Yokoyama M, Ishiguro K. Involvement of cdk5 activator p25 on tau phosphorylation in mouse brain. Neurosci Lett. 2001;22:37–40. doi: 10.1016/s0304-3940(01)01864-x. [DOI] [PubMed] [Google Scholar]
- Tan TC, Valova VA, Malladi CHS, Graham ME, Robinson PhJ. Cdk5 is essential for synaptic vesicle endocytosis. Nature Cell Biol. 2003;5:701–710. doi: 10.1038/ncb1020. [DOI] [PubMed] [Google Scholar]
- Tang A, Yeung J, Lee KY, Matsushita M, Matsui H, Tomizawa K, Hatase O, Wang JH. An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activity. J Biol Chem. 1995;270:26897–26903. doi: 10.1074/jbc.270.45.26897. [DOI] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeirer DJ, Penzes P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Ferraro F, Back N, Eipper BA, Mains RE. Cdk5 and Trio modulate endocrine cell exocytosis. J Cell Sci. 2004;117:4739–4748. doi: 10.1242/jcs.01333. [DOI] [PubMed] [Google Scholar]
- Zhang B, V, Tan B, Lim KM, Tay TE, Zhuang S. Study of the inhibition of cyclin-dependent kinases with roscovitine and indirubin-3′-oxime from molecular dynamics simulations. J Mol Model. 2006;13:78–89. doi: 10.1007/s00894-006-0127-x. [DOI] [PubMed] [Google Scholar]