Abstract
The oral bacterium, Aggregatibacter actinomycetemcomitans, produces a leukotoxin (LtxA) that is specific for white blood cells (WBCs) from humans and Old World primates by interacting with lymphocyte function antigen-1 (LFA-1) on susceptible cells. To determine if LtxA could be used as a therapeutic agent for the treatment of WBC diseases, we tested the in vitro and in vivo anti-leukemia activity of the toxin. LtxA kills human malignant WBC lines and primary leukemia cells from acute myeloid leukemia patients, but healthy peripheral blood mononuclear cells (PBMCs) are relatively resistant to LtxA-mediated cytotoxicity. Levels of LFA-1 on cell lines correlated with killing by LtxA and the toxin preferentially killed cells expressing the activated form of LFA-1. In a SCID mouse model for human leukemia, LtxA had potent therapeutic value resulting in long-term survival in LtxA-treated mice. Intravenous infusion of LtxA into a rhesus macaque resulted in a drop in WBC counts at early times post-infusion; however, red blood cells, platelets, hemoglobin and blood chemistry values remained unaffected. Thus, LtxA may be an effective and safe novel therapeutic agent for the treatment of hematologic malignancies.
Keywords: Acute myeloid leukemia, lymphoma, immunotoxin, targeted therapy
Introduction
Since the 1970s, bacteria and their toxins have been investigated for anticancer activities [1, 2]. Bacterial toxins are not only toxic, but can be engineered to be specific by fusing the toxin to other molecules such as immunoglobulins and interleukins. Interaction between toxins and their host cells represents extensive evolution that has resulted in exquisite activity and specificity that is difficult to replicate in silico. Indeed, pathogenic bacteria have evolved toxins that are highly effective at manipulating host cell machinery. Because of these properties, several bacterial toxins, including Clostridium botulinum neurotoxin, Pseudomonas aeruginosa exotoxin A (PE), and Corynebacterium diphtheriae diphtheria toxin (DT) have been used as therapeutic agents [2]. C. botulinum neurotoxin (BOTOX) is used to treat neurological and muscular disorders [3] while C. diphtheriae DT has been used in the treatment of T-cell lymphoma (ONTAK) [4, 5] and investigated for a variety of other hematologic malignancies [6–8].
Aggregatibacter actinomycetemcomitans is an opportunistic Gram negative bacterium that is the etiological agent of localized aggressive periodontitis (LAP) and is also part of the normal oral flora in many healthy individuals [9, 10]. A. actinomycetemcomitans produces a 113 kDa RTX (repeats in toxin) leukotoxin (LtxA) that kills specifically leukocytes of humans and Old World primates [11, 12] through perturbation of host cell membranes. The toxin is part of the family of membrane-active toxins that includes E. coli α-hemolysin (HlyA) and Bordetella pertussis adenylate cyclase (CyaA) [13, 14]. At the N-terminus are amphipathic helices that are believed to interact with the host cell membrane receptor and at the C-terminal half are nonapeptide glycine-rich repeats that are involved in calcium binding [13]. RTX toxins are secreted via an uncleaved C-terminal signal sequence by a type-I secretion mechanism [15] and we have recently characterized the components of this system in A. actinomycetemcomitans [16, 17]. Like HlyA and CyaA [18], LtxA is post-translationally modified at internal lysine residues with fatty acid moieties that are required for activity [19].
LtxA binds lymphocyte function antigen-1 (LFA-1) [20], a β2 integrin on the surface of white blood cells composed of CD11a and CD18 and involved in immune cell migration and signaling. During infection, cells become activated and LFA-1 changes conformation, allowing it to bind ICAM-1,-2,-3 [21, 22]. Interaction between LFA-1 and the ICAMs results in migration of activated cells to the site of insult [21, 22]. LFA-1 is expressed only on cells of hematopoietic origin, which helps to explain the specificity of the LtxA. Several years ago, we made the novel discovery that A. actinomycetemcomitans secretes LtxA into culture supernatants [23]. We have since developed a strategy for the purification of a large quantity of active, soluble LtxA from both laboratory and clinical isolates of A. actinomycetemcomitans [24, 25].
At relatively high concentrations of LtxA, cells undergo necrosis while at low concentrations, cell death results from apoptosis. Fong et al. [26] has recently shown that the first step to cellular intoxication by LtxA is an increase in intracellular calcium levels even before interaction with the LFA-1 receptor. The mechanism by which this occurs is unknown. LtxA binding to LFA-1 then causes clustering of LFA-1 into lipid rafts and this interaction may then stimulate an integrin signaling pathway. Insertion of LtxA into the host cell membrane perturbs membrane structure and this event ultimately leads to cell death [13, 27–29]. LtxA is considered to be a pore-forming toxin; but at low doses, it likely activates certain pathways that subvert host cell defenses, although enzymatic domains of the toxin have not yet been identified.
Interestingly, the receptor for LtxA, LFA-1, is over-expressed and activated on several leukemias and lymphomas [30–32], indicating that malignant blast cells would be more susceptible to killing by LtxA than normal WBCs. Because of this targeted potential and known specificity of A. actinomycetemcomitans LtxA, we investigated the therapeutic utility of the native toxin for the treatment of hematologic malignancies.
Materials and Methods
Human cells
Human cell lines were obtained from ATCC (Manassas, VA) and maintained in RPMI 1640 medium with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) at 37° C, 5% CO2. Cells were grown for several days until the concentration of cells reached approximately 1.0 × 106 cells/ml. The Jurkat cell lines used for LFA-1 experiments were J-β2.7/LFA-1 wt, J-β2.7/LFA-1 Δ, J-β2.7/mock and have been previously described [33, 34]. Frozen primary human leukemia cells were purchased from AllCells, LLC. (Emeryville, CA). Viability of these primary cells was >90%.
Isolation of healthy human peripheral blood mononuclear cells (PBMCs)
Peripheral blood was collected from four healthy human volunteers into BD Vacutainer Cell Preparation Tubes (CPT) containing sodium citrate (Becton-Dickinson, Franklin Lakes, NJ). Tubes were processed immediately after collection according to the manufacturer's instructions to isolate the layer of mononuclear cells. Experiments involving blood from human subjects were approved by the UMDNJ Institutional Review Board (IRB). All human subjects gave informed consent to participate.
Purification of LtxA
Leukotoxin (LtxA) was purified from culture supernatants of A. actinomycetemcomitans strain NJ4500 as previously described [24, 25]. The storage buffer for the purified toxin was 20 mM Tris-HCl, pH 6.8, 250 mM NaCl, and 0.2 mM CaCl2. The typical yield was 0.5 mg/100 ml starting culture. For long-term storage (greater than one month), protein was lyophilized in sterile glass vials and stored at −80 °C. Samples were reconstituted in sterile distilled water prior to use and we found that when stored in this manner, LtxA was stable for at least 6 months. All toxin preparations were filtered through a 0.22 μm filter prior to use. A sample preparation of purified LtxA is shown in Figure 1.
Figure 1. Purification of LtxA from A. actinomycetemcomitans.

Coomassie Blue-stained SDS-PAGE gel of purified LtxA (lane 2). Twenty-five μl of sample were loaded into the well. The sizes of the molecular weight markers (lane 1) are shown at the left.
LtxA cytotoxicity assay
To determine IC50 values, human cells (~106 cells/ml) were mixed with purified LtxA at various concentrations. The mixture was incubated at 37° C, 5% CO2 for 24 hours. Cellular viability (ATP production) was then determined using the CellTiter-Glo luminescent cell viability assay (Promega, Madison, WI) according to the manufacturer's instructions. Plates were read in a Synergy HT plate reader in the luminescence mode (Bio-Tek, Winooski, VT). Cytotoxicity assays were performed at least three different times.
Flow cytometry analysis
Cells were stained with phycoerythrin (PE) or Fluorescein isothiocyanate (FITC)-labeled monoclonal antibody to CD11a or CD18 as recommended by the manufacturer (Biolegend, San Diego, CA). Samples (at least 10,000 cells/run) were analyzed with a FACSCalibur instrument (BD Biosciences, Franklin Lakes, NJ) and data was analyzed using FlowJo software (Ashland, OR).
SCID mouse studies
SCID mice (36–42 days old) were purchased from Charles River Laboratories (Wilmington, MA) and maintained in the UMDNJ Cancer Center barrier facility. Mice were injected with PBS-washed 4×106 bioluminescent HL-60luc cells [35] (in 100 μl) i.p. on day 0 and then treated with LtxA (in 200 μl) i.p. on days 3, 4, and 5 with 40 μg/day. For bioluminescence imaging, mice were anesthetized with isofluorane and then transferred directly to the IVIS 200 in vivo imaging system (Caliper Life Sciences, Hopkinton, MA), and maintained under anesthesia. Mice were injected i.p. with luciferin (150 mg/kg) and photons were collected over a 3-minute period. Following imaging, mice were immediately returned to their cages. When mice became terminally ill, they were euthanized by CO2 asphyxiation according to UMDNJ Institutional Animal Care guidelines. Images from whole body imaging were analyzed with the LivingImage software.
At 30 days, necropsy was performed. Mice were euthanized with CO2 and then the tumor was excised, measured and sections were cut and tissues were fixed in 10% formalin. Paraffin embedded samples of the tissues were then stained with hematoxylin and eosin. All experiments involving mice were approved by the UMDNJ Institutional Animal Care and Use Committee (IACUC).
Studies on LtxA in a rhesus monkey
Primate experiments were carried out at The University of Wisconsin National Primate Research Center (WNPRC) and approved by The University of Wisconsin-Madison Animal Care and Use Committee. A 13.6 kg rhesus monkey (Macaca mulatta) was administered LtxA (22 μg/kg) by first anesthetizing the monkey with ketamine (5 mg/kg) and medetomidine (30 μg/kg). The monkey was then fitted with a saphenous vein catheter. LtxA was diluted in 15 mls of 0.9 % NaCl and infused i.v. at 1.0 ml/min and then increased to 3.0 mls/min after ten minutes when the animal showed no adverse reactions. Following infusion, the effects of the medetomidine were reversed with 250 μg/kg atipamezole. Blood draws were carried out by first restraining the monkeys in a table-top restraint device according to WNPRC standard protocol. Blood was obtained from the saphenous vein using a vacutainer system. Another monkey (19 kg) served as a control and received saline infusion without LtxA but otherwise identical treatment. The monkeys were evaluated twice daily for signs of pain, illness, and stress by observing appetite, stool, behavior, and physical conditions.
Statistical analyses
Bioluminescence was analyzed by the 2-sample t-test. The Kaplan-Meier survival plot was analyzed using the log-rank test and numbers were compared with a chi-squared test. A p value of 0.05 or less was considered statistically significant.
Results
Susceptibility of human leukemia cells to LtxA
Previous studies on A. actinomycetemcomitans LtxA and host cell susceptibility utilized cell-associated LtxA isolated from bacterial cells or sonic extracts [12, 36–38]. In contrast, we purify secreted LtxA from cell-free culture supernatants (Figure 1), which represents the most mature and active form of the toxin [23–25]. Thus, to determine the in vitro specificity and activity of our purified preparation of LtxA, we determined the concentration of LtxA required to kill 50% of the cells (IC50 values) against several human cell lines and PBMCs after a 24-hour incubation. We also calculated the fraction of dead cells (FDC) remaining after a 24-hour treatment with 2.0 μg/ml of the toxin. Table 1 shows representative IC50 and FDC values obtained from three independent experiments. We found that LtxA is able to kill numerous hematological malignant cell lines but does not affect non-WBC lines, as previously reported [12, 36]. Furthermore, LtxA did not target MEG-01 or CMK cells, which are megakaryoblasts. Most of the malignant cell lines were highly sensitive to LtxA, with more than 90% cell death after 24 hours of exposure to the toxin (HL-60, THP-1, GDM-1, Jurkat, Molt-4, KU812, RL, U937).
Table 1.
LtxA induces cell death in various human hematologic malignant cell lines.
| Disease/Cell line | IC50 value | FDC1 | CD11a2 | CD182 |
|---|---|---|---|---|
| Acute myeloid leukemia | ||||
| HL-60 | 200 ng/ml | >99 | 90 | 90 |
| THP-1 | 8 ng/ml | >99 | 96 | 95 |
| GDM-1 | 200 ng/ml | 98 | ND3 | ND |
| CMK | >10 μg/ml | ND | ND | ND |
| Acute lymphoblastic leukemia | ||||
| Jurkat | 200 ng/ml | >99 | ND | ND |
| Loucy | 300 ng/ml | 67 | 32 | 89 |
| Molt-4 | 30 ng/ml | 93 | ND | ND |
| Chronic myelogenous leukemia | ||||
| KU812 | 300 ng/ml | 96 | ND | ND |
| K562 | >10 μg/ml | ND | <1 | <1 |
| MEG-01 | >10 μg/ml | ND | ND | ND |
| Non-Hodgkin's lymphoma | ||||
| RL | 50 ng/ml | >99 | ND | ND |
| Toledo | 5 ng/ml | 81 | 85 | 86 |
| Burkitt's lymphoma | ||||
| Daudi | ND | 25 | 17 | 20 |
| CA46 | ND | 29 | 2 | 2 |
| Histiocytic lymphoma | ||||
| U937 | 80 ng/ml | 97 | ND | ND |
| Multiple myeloma | ||||
| RPMI 8226 | ND | 34 | ND | ND |
| Non-WBC lines | ||||
| Hep G2 (liver) | >10 μg/ml | ND | ND | ND |
| MCF7 (mammary) | >10 μg/ml | ND | ND | ND |
| A549 (lung) | >10 μg/ml | ND | ND | ND |
| ECV304 (bladder) | >10 μg/ml | ND | ND | ND |
| HGF-1 (gingiva) | >10 μg/ml | ND | ND | ND |
FDC-Fraction of dead cells after 24-treatment with 2.0 μg/ml LtxA, percent dead
Percent positive
ND - Not determined
We also tested the activity of LtxA against primary PBMCs from AML patients. Table 2 shows that all of the samples were highly sensitive to LtxA after 24 hours and the response was dose-dependent (data not shown). One patient (sample 4) had relapsed AML and exhibited only a partial response to Idarubicin/Ara-C treatment.
Table 2.
LtxA induces cell death in primary cells from patients with AML.
| Sample | Disease diagnosis | FDC1, 2 μg/ml, % |
|---|---|---|
| 1 (154 L/TS) | AML-newly diagnosed | >99 |
| 2 (159527) | AML-newly diagnosed | 80 |
| 3 (07–164) | AML-newly diagnosed | >99 |
| 4 (PB0048) | AML-Relapsed | >99 |
Fraction of dead cells after 24-hour incubation; see Table 1
Expression levels of LFA-1
We wished to determine why some cells are more sensitive to LtxA than others. Given that LFA-1 is the receptor for LtxA, we considered the possibility that LFA-1 is over-expressed on cells that are most sensitive to LtxA. To test this hypothesis, we examined the levels of LFA-1 (CD11a and CD18) on the surfaces of both resistant and sensitive cells using flow cytometry (Figure 2A and Table 1). K562 is a CML cell line that is known to lack LFA-1 expression and be resistant to LtxA [20]. As expected, K562 did not show expression of either CD11a or CD18. In contrast, THP-1 cells are some of the most LtxA-sensitive cells that we tested (see Table 1) and also expressed very high levels of both CD11a and CD18 (LFA-1hi). HL-60 cells are also sensitive to LtxA and the levels of CD11a and CD18 were high, but less than THP-1. Loucy cells showed a broad, variable expression of CD11a but high levels of CD18. This cell line is partially sensitive to LtxA with a relative high IC50 value and only approximately two-thirds of the cells being killed after 24 hours (Table 1). Toledo cells have a more broad distribution of LFA-1 expression, and while the IC50 value is low (5 ng/ml), about 20% of the cells appear to be viable after 24-hour treatment (Table 1). Daudi cells show low levels of LFA-1 expression and are also resistant to LtxA. CA46 cells do not display expression of either CD11a or CD18 and are also relatively resistant to LtxA-mediated cytotoxicity.
Figure 2. Analysis of LFA-1 levels by flow cytometry.
Human cell lines (A) or primary PBMCs from acute myeloid leukemia patients (B) were stained with PE-labeled antibody to either CD11a or CD18 and measured with flow cytometry.
LFA-1 levels on primary cells from leukemia patients demonstrated expression of both CD11a and CD18 (Figure 2B). Sample AM154 showed high, yet variable, levels of expression while the other primary samples contained distinct populations of LFA-1hi cells. The AML159527 sample contained two populations, cells with no LFA-1 and LFA-1hi and approximately 20% of the cells were not affected by LtxA (Table 2).
Effects of LtxA on normal PBMCs
Ideally, a therapeutic agent should preferentially target the diseased cell type while minimally affecting normal cells. To determine the effects of LtxA on normal PBMCs, we tested LtxA on PBMCs from four healthy human subjects and found in every case that the cells were generally resistant to killing by LtxA (Figure 3). There was a drop in viability at higher concentrations of LtxA, but it never exceeded 30–40% cell death even at the highest doses. Flow cytometric analysis showed that normal PBMCs express CD11a at relatively high levels (Figure 4A). Results for CD18 paralleled those for CD11a (data not shown). We next treated these cells with LtxA to determine the subpopulation of cells that are affected. We found that after treatment with LtxA, only the large LFA-1hi cells were depleted (Figure 4B, C). These results show that LtxA is able to selectively kill the small fraction of large LFA-1hi WBCs in a mixture of PBMCs.
Figure 3. Sensitivity of WBCs to LtxA-mediated cytotoxicity.
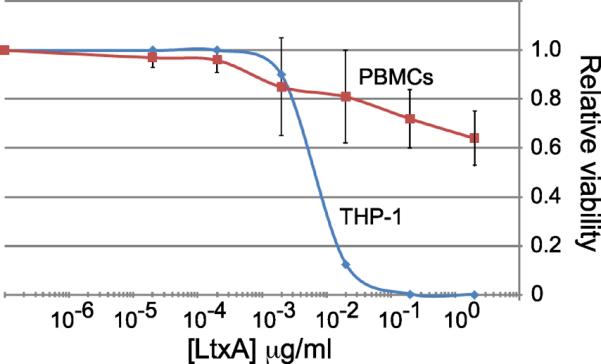
A) The malignant human monocyte cell line, THP-1, and PBMCs from four healthy adults were treated with LtxA at different concentrations for 24 hours. Cell viability was determined by measurement of cellular ATP. Untreated samples represent a relative viability of 1.0. The curve for PBMCs represents the average of the four human PBMC samples performed in quadruplicate. The vertical bars represent standard deviation.
Figure 4. Flow cytometry of healthy human PBMCs.
A) PBMCs from healthy individuals were stained with CD11a antibody and analyzed by flow cytometry. B) Analysis of cell size (forward scatter) vs. CD11a expression after 24-hour buffer treatment (−LtxA). C) Analysis of cell size (forward scatter) vs. CD11a expression after 24-hour treatment with LtxA (+LtxA).
LtxA preferentially targets cells with activated LFA-1
The large size of the cells targeted by LtxA suggests that these cells are activated and differentiating. Thus, a possibility is that LtxA recognizes the activated form of LFA-1 [39, 40] better than LFA-1 in the resting state. To test this idea, we used a Jurkat T-cell line that expresses a high level of constitutively activated LFA-1 (J-β2.7/LFA-1 Δ) [33]. As controls, we also used isogenic cell lines that either express the wild type form of LFA-1 (J-β2.7/LFA-1 wt) or lack LFA-1 expression completely (J-β2.7/mock) [34]. We found that cells with activated LFA-1 were ten times more sensitive to LtxA-mediated toxicity than cells with resting state LFA-1 and LFA-1-deficient cells were not affected by the toxin (Figure 5). Thus, LtxA is more toxic towards WBCs expressing the activated form of LFA-1.
Figure 5.

Sensitivity of Jurkat-derived T-cells to LtxA expressing wild type LFA-1, activated LFA-1, or no LFA-1. Results shown are representative of biological duplicates.
Biological activity of LtxA in SCID mice
Given the specificity of LtxA for malignant WBCs, we wished to determine if the toxin has in vivo bioactivity. To demonstrate therapeutic proof-of-concept, we tested the toxin in the SCID mouse xenograft model of human HL-60 myeloid leukemia [41, 42]. SCID mice were inoculated intraperitoneally with 4 × 106 HL-60luc cells [35] on day 0 and then either treated i.p. with LtxA (2 mg/kg/dose) on days 1, 2, and 3 or remained untreated and served as our negative control (leukemic mice). Another group of four mice that was given neither HL-60luc cells nor LtxA served as our control to determine normal life expectancy of the mice (data not shown). After approximately 25 days, leukemic mice that were not treated with LtxA began to form large masses in the peritoneal cavity and showed signs of illness such as lethargy and un-groomed fur (Figure 6A, top). In contrast, the majority (7/8) of mice that received LtxA treatment did not develop peritoneal tumor masses and appeared healthy (Figure 6A, bottom). In vivo bioluminescent imaging (BLI) [43] of HL-60luc cells [35] allowed us to observe and quantify tumor burden over time (Figure 6B). BLI revealed a significantly greater signal in untreated leukemic mice versus LtxA-treated animals (Figure 6C). Tumor burden correlated with survival of the animals. Kaplan-Meier survival plots show that untreated leukemic mice began to die on day 43 and all mice were dead by day 75 (Figure 6D). In contrast, only one mouse died on day 61 in the group that was treated with LtxA and the remaining survivors lived to at least day 300 disease-free. These survivors remained healthy and never showed signs of peritoneal tumors. The mean survival time for the untreated leukemic mice was 56 days versus 153 days for LtxA-treated mice calculated on day 166. All control mice that were not given leukemia cells were still alive at the end of the study (data not shown).
Figure 6. Bioactivity of LtxA in the SCID mouse xenograft model of human HL-60 myeloid leukemia.
Bioluminescent HL-60luc cells were injected into SCID mice i.p. A) Mice that were not treated with LtxA (top) developed peritoneal tumors while LtxA-treated mice (bottom) remained tumor-free and healthy. Mice were photographed on day 29. B) Bioluminescent imaging of leukemic mice. Mice were imaged with the IVIS 200 imaging system after injecting with luciferin. Numbers denote the day post-injection of leukemia cells. C) Bioluminescence from mice on days 8 and 22 was quantified using the LivingImage software. D) Kaplan-Meier survival plots of experimental mice. P≤0.05 was considered significant.
On day 30, one animal from each group was sacrificed for histopathological examination. The peritoneal tumor from the leukemic untreated animal measured approximately 2000 mm3 in volume (Figure 7A) and H&E staining of tumor sections revealed mononuclear HL-60 cells (Figure 7B). The mouse that was treated with LtxA showed no gross signs of tumor formation or nodules and was anatomically indistinguishable from the control mouse that was not injected with HL-60luc cells (data not shown). While the i.p. route of administration may have limited predictability for effective clinical doses, these results show that LtxA has potent anti-leukemia activity in vivo.
Figure 7. Histopathological analysis of HL-60 tumor.
A) A peritoneal tumor removed from an untreated leukemic mouse on day 30. The large intestine is to the left of the tumor. B) H&E staining of a tumor section at 400 × magnification.
Biological activity of LtxA in a non-human primate
Because murine cells are otherwise resistant to LtxA-mediated cytotoxicity, we wished to determine the effects of systemic administration of LtxA in the rhesus macaque (Macaca mulatta). Two Rhesus monkeys were used in the study. One monkey received a single intravenous (i.v.) infusion of 300 μg LtxA (22 μg/kg) while the second animal served as a control and was given only saline. Blood was drawn from the animals immediately prior to infusion and at various times post-infusion. Complete blood counts and differentials were performed as well as blood chemistry analysis.
Throughout the 3-week study, red blood cell (RBC), platelet (PLT), and hemoglobin (HGB) values remained unchanged in the LtxA-treated monkey and were similar to the control animal (Figure 8). In contrast, in the treated monkey, the total white blood cell (WBC) count dropped during the first several hours post-infusion and then increased (Figure 8). Differential hematology revealed an initial drop in both neutrophils and lymphocytes. After dropping during the first hour, the neutrophil count increased to almost four times the starting value by ten hours and then slowly declined before returning to the starting value. Lymphocyte values dropped initially and remained low for 12 hours before increasing.
Figure 8. Effect of i.v. administration of LtxA into a nonhuman primate.
Rhesus macaques were given LtxA (22 μg/kg) or saline (control) followed by sampling of blood. The pre-infusion sample was collected immediately prior to infusion with LtxA or saline. Time notes hours post-infusion. RBC, red blood cells; WBC, white blood cells; Neu, neutrophils; Lym, lymphocytes; PLT, platelets; HGB, hemoglobin.
Blood chemistry values for the LtxA-treated monkey revealed no change in markers of either liver toxicity (AST, bilirubin, alkaline phosphatase) or kidney function (BUN, creatinine) during the course of the experiment and the animal appeared in good health as evaluated twice daily by veterinary staff (data not shown). Taken together, these results indicate that intravenously-administered LtxA is active and specific in a non-human primate at a non-toxic dose.
Discussion
Each year, more than 53,000 people die of hematologic malignancies (leukemia, lymphoma, myeloma) with more than 135,000 new annual diagnoses in the US alone. The median survival of patients with acute myeloid leukemia (AML) is approximately one year with a 5-year survival rate of just 22%. And for older adults (>55 years of age) with AML, over the last 30 years, there have been essentially no improvements in the survival rate. Furthermore, leukemia causes more deaths than any other cancer among children and young adults under the age of 20. Current treatment for these cancers includes the use of compounds that target cellular processes of nearly all cells of the body, not just the cancerous ones, resulting in serious side effects characteristic of traditional chemotherapy. A significant fraction of patients eventually show resistance to many of the drugs, rendering treatment largely ineffective. These facts further emphasize the need to identify, study, and ultimately introduce novel anti-leukemia agents into the clinic [7]. A new class of cancer therapeutics includes targeted agents, such as monoclonal antibody and other biological agents [2, 44]. These agents are more specific than traditional chemotherapy often resulting in fewer adverse reactions in patients.
Herein, we show that a native, secreted bacterial toxin has specificity and significant in vitro and in vivo bioactivity against malignant WBCs. Analysis of malignant cell lines revealed variability in the sensitivity to LtxA and this susceptibility was directly related to cell-surface levels of LFA-1 rather than the nature of the disease (Table 1 and Figure 2). Cell lines that did not express both CD11a and CD18 were resistant LtxA-mediated killing. While CD18 has been shown to be the functional receptor for LtxA and confers species specificity [45], CD11a is also required as demonstrated by the Loucy cell line and shown previously [20]. Loucy cells expressed high levels of CD18 but low levels of CD11a and were only partially killed by LtxA. Primary AML cells also showed significant sensitivity to LtxA and flow cytometry revealed high levels of LFA-1 expression on cells. Cells from sample 1 were variable for LFA-1 expression but were highly susceptible to LtxA. One explanation is that expression of CC11a and CD18 is erratic, but at some point during the 24-hour incubation, all cells express LFA-1 and become sensitive to LtxA. Interestingly, sample 2 exhibited 80% cell death (Table 2) which corresponded roughly to the percent of cells that were LFAhi (Figure 2). Numerous studies have shown increased levels of LFA-1 expression on malignant cells from leukemia and lymphoma patients [30, 46–49] and our results are in agreement with these reports.
In addition to levels of CD18 and CD11a on the cell surface, the sensitivity of a WBC to LtxA also depends on the activation state of LFA-1. We showed here that a T-cell line that expresses an activated form of LFA-1 was ten times more sensitive to LtxA than an isogenic line that expresses the resting state form of LFA-1. Just as interaction between LFA-1 and the ICAMs requires transition of LFA-1 to an active conformation [39, 40], LtxA also appears to prefer the activated state of LFA-1. In light of the natural role of the bacterial toxin in host immune evasion, this strategy makes sense since activated cells are those with the most potent anti-bacterial activity. Hence, instead of affecting a large proportion of host WBCs and causing significant morbidity, the bacterium is able to successfully persist in the host by targeting the most relevant subpopulations of WBCs.
We also observed that human PBMCs from healthy individuals were relatively resistant to LtxA-mediated cytotoxicity even though they express LFA-1. Taichman et al. also noted that normal PBMCs were not efficiently killed by LtxA [50]. Thus, while LFA-1 is required for LtxA killing, it is not sufficient. It is possible that the state of LFA-1 on resting, normal PBMCs is unavailable for binding to LtxA and our studies with the J-β2.7 Jurkat cells expressing activated (ie. exposed) LFA-1 support this notion. Indeed, this mechanism is similar to ONTAK, a diphtheria toxin-IL-2 fusion drug that binds to cells with activated IL-2 receptor such as activated T-cells, B-cells, and macrophages [51]. In addition, it is also possible that other differences exist to render malignant cells more sensitive to LtxA, perhaps in a pathway downstream from the initial toxin-cell interaction. Indeed, rapidly-dividing cells may be most sensitive to LtxA due to the loss of checkpoint control and repair mechanisms during apoptosis. A third possibility is that, while cells express both CD11a and CD18, these molecules are not assembled as dimers to form the LFA-1 molecule that is recognized by LtxA.
Of the normal PBMCs that were affected by LtxA, the large LFA-1hi WBCs were selectively depleted. These targeted cells may represent the small fraction of activated cells present in a healthy person at any one time. Given the results we obtained with normal PBMCs and those from leukemia patients, the potential implication is that normal WBCs in a patient might be less susceptible to LtxA treatment than their malignant counterparts.
In the SCID mouse xenograft model for human leukemia, LtxA was highly effective at treating leukemia and prolonging survival. The concentration we used (2 mg/kg/dose) is similar to doses used in analogous studies with other biological agents, such as monoclonal antibody therapy [52–55] and bacterial immunotoxins [56]. Of significance, in 7/8 LtxA-treated animals, we were able to achieve complete elimination of tumor growth, which correlated with event-free long-term-survival.
Throughout the SCID mouse experiment, we failed to observe any adverse reactions to LtxA administration. While mouse cells are otherwise resistant to killing by LtxA, the fact that generalized, non-specific toxicity, such as liver or kidney dysfunction, did not occur with relatively high, effective doses of LtxA is noteworthy. To assess effectiveness and potential toxicity of LtxA in a non-human primate, the toxin was infused i.v. into a rhesus monkey. The in vivo effect appeared to parallel the in vitro specificity of the toxin. Only WBCs were affected, but over time, WBC counts increased to pre-infusion values. These results are in stark contrast to treatment with traditional chemotherapy where near complete killing of RBCs, WBCs, and platelets is observed for prolonged periods of time resulting in anemia and thrombocytopenia. The spike in neutrophil count after an initial decrease is a phenomenon that has been observed for other protein toxins [8, 57] and may be the result of an early agonistic effect on the distribution of mature neutrophils in the body. Platelets do not express LFA-1 [58, 59], and we found that the platelet counts were unaffected in the LtxA-treated monkey. Hence, our results demonstrate the correlation between LtxA in vitro and in vivo specificity.
During ex vivo studies, we observed that normal human PBMCs were minimally affected while in the monkey, normal cells also appeared to be affected. However, it is important to note that the WBC counts never dropped by more than 50–60%, in contrast to complete killing of malignant cells. Nonetheless, two possible explanations could account for the depletion of WBCs in the monkey. First, it is possible that the number of activated and/or LFA-1hi cells in the animal is much higher than in humans. Second, the toxin may be more active in blood than under non-physiological in vitro assay conditions. Indeed, other toxins can become activated by mammalian components [60] and the natural environment for LtxA is the oral cavity and saliva. The fact that we were able to administer a lower dose to the monkey with an observable effect compared to the mice supports this latter hypothesis. The dose of LtxA administered (22 μg/kg) into the monkey achieved a desired effect, and we failed to note any drug-related toxicities. This suggests that a maximum tolerated dose was not reached, and future studies should identify these parameters.
In conclusion, we have shown proof-of-concept that LtxA has targeted activity in vitro and in vivo with no gross side effects at effective doses in mice and a non-human primate. We have also identified a possible mechanism by which LtxA distinguishes between normal and malignant WBCs. To our knowledge, LtxA represents the first non-recombinant bacterial toxin shown to possess targeted in vivo anti-cancer activity. Given that LFA-1 also plays a critical role in the etiology of many autoimmune diseases, it would be of significant interest to determine whether LtxA has therapeutic benefit for other diseases as well.
Acknowledgements
We thank David Lagunoff for expert pathology services, Roger Strair and Joseph Bertino for helpful discussions, Chris Berghout and Amy Le for technical assistance, and Laura Weinstein for assistance with statistical analyses. This work was generously supported by grants from the National Institute of Dental and Craniofacial Research (R01 DE16133 to SCK, 1F32DE017828-01 to NVB) and a grant from the New Jersey Commission of Science and Technology and the Foundation of UMDNJ (to SCK). The WNPRC, University of Wisconsin-Madison was supported by grant number P51 RR000167 from the National center for Research Resources (NCRR), a component of the NIH. Research at the WNPRC was conducted at a facility constructed with the support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01.
Footnotes
Conflict of Interest statement S.C.K. owns stock in a company that has licensed the patent from UMDNJ for the clinical use of leukotoxin. Ownership of stock occurred following the completion of studies described in this manuscript.
References
- [1].Jain KK. Use of bacteria as anticancer agents. Expert Opin Biol Ther. 2001;1:291–300. doi: 10.1517/14712598.1.2.291. [DOI] [PubMed] [Google Scholar]
- [2].Kreitman RJ. Immunotoxins for targeted cancer therapy. The AAPS journal. 2006;8:E532–551. doi: 10.1208/aapsj080363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004;75:951–957. doi: 10.1136/jnnp.2003.034702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Duvic M, Kuzel TM, Olsen EA, et al. Quality-of-life improvements in cutaneous T-cell lymphoma patients treated with denileukin diftitox (ONTAK) Clin Lymphoma. 2002;2:222–228. doi: 10.3816/clm.2002.n.003. [DOI] [PubMed] [Google Scholar]
- [5].Olsen E, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19:376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- [6].Kreitman RJ, Pastan I. Immunotoxins in the treatment of hematologic malignancies. Current drug targets. 2006;7:1301–1311. doi: 10.2174/138945006778559139. [DOI] [PubMed] [Google Scholar]
- [7].Liu TF, Urieto JO, Moore JE, et al. Diphtheria toxin fused to variant interleukin-3 provides enhanced binding to the interleukin-3 receptor and more potent leukemia cell cytotoxicity. Exp Hematol. 2004;32:277–281. doi: 10.1016/j.exphem.2003.11.010. [DOI] [PubMed] [Google Scholar]
- [8].Perentesis JP, Gunther R, Waurzyniak B, et al. In vivo biotherapy of HL-60 myeloid leukemia with a genetically engineered recombinant fusion toxin directed against the human granulocyte macrophage colony-stimulating factor receptor. Clin Cancer Res. 1997;3:2217–2227. [PubMed] [Google Scholar]
- [9].Fine DH, Kaplan JB, Kachlany SC, Schreiner HC. How we got attached to Actinobacillus actinomycetemcomitans: a model for infectious diseases. Periodontol 2000. 2006;42:114–157. doi: 10.1111/j.1600-0757.2006.00189.x. [DOI] [PubMed] [Google Scholar]
- [10].Slots J, Ting M. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontol 2000. 1999;20:82–121. doi: 10.1111/j.1600-0757.1999.tb00159.x. [DOI] [PubMed] [Google Scholar]
- [11].Taichman NS, Dean RT, Sanderson CJ. Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infect Immun. 1980;28:258–268. doi: 10.1128/iai.28.1.258-268.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Taichman NS, Simpson DL, Sakurada S, Cranfield M, DiRienzo J, Slots J. Comparative studies on the biology of Actinobacillus actinomycetemcomitans leukotoxin in primates. Oral Microbiol Immunol. 1987;2:97–104. doi: 10.1111/j.1399-302x.1987.tb00270.x. [DOI] [PubMed] [Google Scholar]
- [13].Lally ET, Hill RB, Kieba IR, Korostoff J. The interaction between RTX toxins and target cells. Trends Microbiol. 1999;7:356–361. doi: 10.1016/s0966-842x(99)01530-9. [DOI] [PubMed] [Google Scholar]
- [14].Welch RA. RTX toxin structure and function: a story of numerous anomalies and few analogies in toxin biology. Curr Top Microbiol Immunol. 2001;257:85–111. doi: 10.1007/978-3-642-56508-3_5. [DOI] [PubMed] [Google Scholar]
- [15].Delepelaire P. Type I secretion in gram-negative bacteria. Biochim Biophys Acta. 2004;1694:149–161. doi: 10.1016/j.bbamcr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- [16].Crosby JA, Kachlany SC. TdeA, a TolC-like protein required for toxin and drug export in Aggregatibacter (Actinobacillus) actinomycetemcomitans. Gene. 2007;388:83–92. doi: 10.1016/j.gene.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Isaza MP, Duncan MS, Kaplan JB, Kachlany SC. Screen for leukotoxin mutants in Aggregatibacter actinomycetemcomitans: genes of the phosphotransferase system are required for leukotoxin biosynthesis. Infect Immun. 2008;76:3561–3568. doi: 10.1128/IAI.01687-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stanley P, Koronakis V, Hughes C. Acylation of Escherichia coli hemolysin: a unique protein lipidation mechanism underlying toxin function. Microbiol Mol Biol Rev. 1998;62:309–333. doi: 10.1128/mmbr.62.2.309-333.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Balashova NV, Shah C, Patel JK, Megalla S, Kachlany SC. Aggregatibacter actinomycetemcomitans LtxC is required for leukotoxin activity and initial interaction between toxin and host cells. Gene. 2009;443:42–47. doi: 10.1016/j.gene.2009.05.002. [DOI] [PubMed] [Google Scholar]
- [20].Lally ET, Kieba IR, Sato A, et al. RTX toxins recognize a beta2 integrin on the surface of human target cells. J Biol Chem. 1997;272:30463–30469. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- [21].Hogg N, Smith A, McDowall A, et al. How T cells use LFA-1 to attach and migrate. Immunology letters. 2004;92:51–54. doi: 10.1016/j.imlet.2003.10.014. [DOI] [PubMed] [Google Scholar]
- [22].Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- [23].Kachlany SC, Fine DH, Figurski DH. Secretion of RTX leukotoxin by Actinobacillus actinomycetemcomitans. Infect Immun. 2000;68:6094–6100. doi: 10.1128/iai.68.11.6094-6100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kachlany SC, Fine DH, Figurski DH. Purification of secreted leukotoxin (LtxA) from Actinobacillus actinomycetemcomitans. Protein Expr Purif. 2002;25:465–471. doi: 10.1016/s1046-5928(02)00037-2. [DOI] [PubMed] [Google Scholar]
- [25].Diaz R, Ghofaily LA, Patel J, et al. Characterization of leukotoxin from a clinical strain of Actinobacillus actinomycetemcomitans. Microb Pathog. 2006;40:48–55. doi: 10.1016/j.micpath.2005.10.005. [DOI] [PubMed] [Google Scholar]
- [26].Fong KP, Pacheco CM, Otis LL, et al. Actinobacillus actinomycetemcomitans leukotoxin requires lipid microdomains for target cell cytotoxicity. Cell Microbiol. 2006;8:1753–1767. doi: 10.1111/j.1462-5822.2006.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yamaguchi N, Kieba IR, Korostoff J, Howard PS, Shenker BJ, Lally ET. Maintenance of oxidative phosphorylation protects cells from Actinobacillus actinomycetemcomitans leukotoxin-induced apoptosis. Cell Microbiol. 2001;3:811–823. doi: 10.1046/j.1462-5822.2001.00161.x. [DOI] [PubMed] [Google Scholar]
- [28].Mangan DF, Taichman NS, Lally ET, Wahl SM. Lethal effects of Actinobacillus actinomycetemcomitans leukotoxin on human T lymphocytes. Infect Immun. 1991;59:3267–3272. doi: 10.1128/iai.59.9.3267-3272.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Korostoff J, Wang JF, Kieba I, Miller M, Shenker BJ, Lally ET. Actinobacillus actinomycetemcomitans leukotoxin induces apoptosis in HL-60 cells. Infect Immun. 1998;66:4474–4483. doi: 10.1128/iai.66.9.4474-4483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bechter OE, Eisterer W, Dirnhofer S, et al. Expression of LFA-1 identifies different prognostic subgroups in patients with advanced follicle center lymphoma (FCL) Leuk Res. 1999;23:483–488. doi: 10.1016/s0145-2126(99)00036-3. [DOI] [PubMed] [Google Scholar]
- [31].Horst E, Radaszkiewicz T, Hooftman-den Otter A, et al. Expression of the leucocyte integrin LFA-1 (CD11a/CD18) and its ligand ICAM-1 (CD54) in lymphoid malignancies is related to lineage derivation and stage of differentiation but not to tumor grade. Leukemia. 1991;5:848–853. [PubMed] [Google Scholar]
- [32].Inghirami G, Wieczorek R, Zhu BY, Silber R, Dalla-Favera R, Knowles DM. Differential expression of LFA-1 molecules in non-Hodgkin's lymphoma and lymphoid leukemia. Blood. 1988;72:1431–1434. [PubMed] [Google Scholar]
- [33].Lu CF, Springer TA. The alpha subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin lymphocyte function-associated antigen-1. J Immunol. 1997;159:268–278. [PubMed] [Google Scholar]
- [34].Weber KS, York MR, Springer TA, Klickstein LB. Characterization of lymphocyte function-associated antigen 1 (LFA-1)-deficient T cell lines: the alphaL and beta2 subunits are interdependent for cell surface expression. J Immunol. 1997;158:273–279. [PubMed] [Google Scholar]
- [35].Isaza MP, Chau JT, Le A, et al. A bioluminescent HL-60 cell line to assay anti-leukaemia therapeutics under physiological conditions. Luminescence. 2008;23:17–21. doi: 10.1002/bio.1010. [DOI] [PubMed] [Google Scholar]
- [36].Simpson DL, Berthold P, Taichman NS. Killing of human myelomonocytic leukemia and lymphocytic cell lines by Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun. 1988;56:1162–1166. doi: 10.1128/iai.56.5.1162-1166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Taichman NS, Wilton JM. Leukotoxicity of an extract from Actinobacillus actinomycetemcomitans for human gingival polymorphonuclear leukocytes. Inflammation. 1981;5:1–12. doi: 10.1007/BF00910774. [DOI] [PubMed] [Google Scholar]
- [38].Tsai CC, Shenker BJ, DiRienzo JM, Malamud D, Taichman NS. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect Immun. 1984;43:700–705. doi: 10.1128/iai.43.2.700-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- [40].van Kooyk Y, van de Wiel-van Kemenade P, Weder P, Kuijpers TW, Figdor CG. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989;342:811–813. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]
- [41].McCormack E, Bruserud O, Gjertsen BT. Animal models of acute myelogenous leukaemia - development, application and future perspectives. Leukemia. 2005;19:687–706. doi: 10.1038/sj.leu.2403670. [DOI] [PubMed] [Google Scholar]
- [42].Uckun FM. Severe combined immunodeficient mouse models of human leukemia. Blood. 1996;88:1135–1146. [PubMed] [Google Scholar]
- [43].Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng. 2002;4:235–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- [44].Dalle S, Thieblemont C, Thomas L, Dumontet C. Monoclonal antibodies in clinical oncology. Anti-cancer agents in medicinal chemistry. 2008;8:523–532. doi: 10.2174/187152008784533071. [DOI] [PubMed] [Google Scholar]
- [45].Dileepan T, Kachlany SC, Balashova NV, Patel J, Maheswaran SK. Human CD18 is the functional receptor for Aggregatibacter actinomycetemcomitans leukotoxin. Infect Immun. 2007 doi: 10.1128/IAI.00314-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Angelopoulou MK, Kontopidou FN, Pangalis GA. Adhesion molecules in B-chronic lymphoproliferative disorders. Seminars in hematology. 1999;36:178–197. [PubMed] [Google Scholar]
- [47].Mengarelli A, Zarcone D, Caruso R, et al. Adhesion molecule expression, clinical features and therapy outcome in childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2001;40:625–630. doi: 10.3109/10428190109097660. [DOI] [PubMed] [Google Scholar]
- [48].Pinto A, Carbone A, Gloghini A, Marotta G, Volpe R, Zagonel V. Differential expression of cell adhesion molecules in B-zone small lymphocytic lymphoma and other well-differentiated lymphocytic disorders. Cancer. 1993;72:894–904. doi: 10.1002/1097-0142(19930801)72:3<894::aid-cncr2820720339>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [49].Reuss-Borst MA, Klein G, Waller HD, Muller CA. Differential expression of adhesion molecules in acute leukemia. Leukemia. 1995;9:869–874. [PubMed] [Google Scholar]
- [50].Taichman NS, McArthur WP, Tsai CC, et al. Leukocidal mechanisms of Actinobacillus actinomycetemcomitans. In: Genco RJ, Mergenhagen SE, editors. Host-parasite interactions in periodontal diseases. American Society for Microbiology; Washington, D.C.: 1982. pp. 261–269. [Google Scholar]
- [51].Bousvaros A, Stevens AC, Strom TB, Murphy J, Lamont JT. Interleukin-2 fusion protein (DAB389IL-2) selectively targets activated human peripheral blood and lamina propria lymphocytes. Digestive diseases and sciences. 1997;42:1542–1548. doi: 10.1023/a:1018891432581. [DOI] [PubMed] [Google Scholar]
- [52].Bleeker WK, Munk ME, Mackus WJ, et al. Estimation of dose requirements for sustained in vivo activity of a therapeutic human anti-CD20 antibody. British journal of haematology. 2008;140:303–312. doi: 10.1111/j.1365-2141.2007.06916.x. [DOI] [PubMed] [Google Scholar]
- [53].Drake AS, Brady MT, Wang XH, et al. Targeting 11q23 positive acute leukemia cells with high molecular weight-melanoma associated antigen-specific monoclonal antibodies. Cancer Immunol Immunother. 2008 doi: 10.1007/s00262-008-0567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhong RK, Donnenberg AD, Shultz LD, et al. Evaluation of monoclonal antibody-mediated anti-acute myeloid leukemia immunotherapy in a SCID/hu model. Leuk Res. 1996;20:581–589. doi: 10.1016/0145-2126(96)00004-5. [DOI] [PubMed] [Google Scholar]
- [55].Zhou Y, Du W, Koretsky T, Bagby GC, Pang Q. TAT-mediated intracellular delivery of NPM-derived peptide induces apoptosis in leukemic cells and suppresses leukemogenesis in mice. Blood. 2008;112:2474–2483. doi: 10.1182/blood-2007-12-130211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Weldon JE, Xiang L, Chertov O, et al. A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood. 2009;113:3792–3800. doi: 10.1182/blood-2008-08-173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Walz G, Zanker B, Brand K, et al. Sequential effects of interleukin 2-diphtheria toxin fusion protein on T-cell activation. Proc Natl Acad Sci U S A. 1989;86:9485–9488. doi: 10.1073/pnas.86.23.9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Diacovo TG, deFougerolles AR, Bainton DF, Springer TA. A functional integrin ligand on the surface of platelets: intercellular adhesion molecule-2. The Journal of clinical investigation. 1994;94:1243–1251. doi: 10.1172/JCI117442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Soligo D, Cattoretti G, Colombi M, et al. Bone marrow and tissue expression of gpIIb/IIIa, LFA-1, Mac-1 and gp150,95 glycoproteins. European journal of haematology. 1989;42:173–181. doi: 10.1111/j.1600-0609.1989.tb01207.x. [DOI] [PubMed] [Google Scholar]
- [60].Abrami L, Fivaz M, Decroly E, et al. The pore-forming toxin proaerolysin is activated by furin. J Biol Chem. 1998;273:32656–32661. doi: 10.1074/jbc.273.49.32656. [DOI] [PubMed] [Google Scholar]







