Abstract
PTEN (phosphatase and tensin homologue deleted on chromosome 10) is a lipid phosphatase that functions as a negative regulator of the phosphoinositide-3-kinase (PI3K) pathway. The present study sought to examine in depth the interaction between PTEN and eNOS activity. Co-expression of eNOS and PTEN in COS-7 cells significantly decreased NO production compared to eNOS alone, while co-expression of eNOS and the dominant negative mutant PTEN(C124A) significantly increased NO production. Upon examination of the putative eNOS phosphorylation sites, phosphorylation of S116, T497, S617, S635 and S1179 was decreased by PTEN co-expression, while the dominant negative PTEN(C124A) produced an increase in phosphorylation of all sites except S116 and S635. A myristoylation-deficient eNOS construct with little dependence on phosphorylation state (G2AeNOS) was not significantly affected by co-expression with either PTEN or PTEN(C124A). Likewise, an eNOS construct with a triple phospho-null mutation (S617A, S635A & S1179A) was also unaffected by co-expression with either PTEN or PTEN(C124A). Purified PTEN or PTEN(C124A) failed to interact with purified eNOS in vitro, arguing against a direct interaction between PTEN and eNOS. When the PTEN constructs were expressed in human aortic endothelial cells (HAECs), PTEN significantly decreased NO production and PTEN(C124A) increased it, and both S617 and S1179 were altered by co-expression with the PTEN constructs. Increased expression of PTEN in endothelial cells did not influence superoxide production. We conclude that PTEN is a regulator of eNOS function both when expressed in COS-7 cells and in human endothelial cells, and does so via its effects on the PI3K/Akt pathway.
Introduction
PTEN (phosphatase and tensin homologue deleted on chromosome 10) is a tumor-suppressor gene that was originally characterized as a dual specificity phosphatase that could dephosphorylate serine, threonine and tyrosine residues (25). In addition to proteins, PTEN was later shown to dephosphorylate acidic phospholipids, in particular PtdIns(3,4,5)P3, with high efficiency (21). These actions directly oppose those of the lipid kinase, phosphatidyl inositol 3-kinase (PI3-K) and downstream signaling molecules such as Akt (30). Mutations in PTEN are commonly found in a number of cancers, particularly in advanced stages (19) and alterations in PTEN function can dramatically influence the architecture of the vasculature and in particular, modulate the process of angiogenesis (22, 32, 35). Recently, pharmacological inhibition of PTEN and conditional knockout of PTEN in lung epithelial have been shown to protect against acute lung injury or ALI (17, 31). However the mechanisms by which PTEN evokes changes in vascular and lung function are poorly understood.
The protein kinase Akt (also referred to as PKB) is a well-known activator of endothelial nitric oxide synthase (eNOS) (5, 13) and PTEN therefore represents a potentially important negative regulator of NO production and cardiovascular function. Indeed, recent studies have suggested that PTEN, when upregulated via activation of the p38MAPK in response to exposure to human cytomegalovirus (29), palmitic acid (33) or resistin (28), inhibits eNOS activity. Although these studies suggest that PTEN can indeed inhibit eNOS activity, no study has yet examined the mechanisms by which PTEN inhibits eNOS in depth.
In addition to calcium-calmodulin, eNOS activity is regulated by a number of post-translational mechanisms that include its subcellular location, protein:protein interactions and the phosphorylation of serine, threonine and more recently, tyrosine residues (6, 12, 14, 24). In particular, the phosphorylation of Serines 1179, 635 and 617 and tyrosine 83 correlate with increased eNOS activity and the phosphorylation of S116 and T497 are inhibitory (note: amino acid numbers refer to bovine eNOS). The ability of eNOS to generate NO can also be influenced by factors influencing the fidelity of synthesis or state of “uncoupling” these include the binding of hsp90, changes in phosphorylation and intracellular levels of tetrahydrobiopterin (9).
Currently the mechanisms by which PTEN influences eNOS activity are not established. In this study we investigate whether PTEN influences the phosphorylation of eNOS at specific serine or threonine residues and determine whether the modification of these sites is of functional significance. We also determine whether PTEN influences the phosphorylation of tyrosine 83 on eNOS and the generation of superoxide in endothelial cells. This is achieved in both a heterologous expression system and in physiologically relevant endothelial cells.
Materials and Methods
Cell Culture and Transfection
COS-7 cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen) containing L-glutamine, penicillin, streptomycin and 10% (v/v) fetal bovine serum. Cells were transfected with Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen). Human aortic endothelial cells (HAECs) were obtained from Cascade Biologics, and cultured on gelatin-coated plates in EBM®-2 Endothelial Cell Basal Medium (Clonetics). HAECs were transduced with GFP, PTEN or PTEN(C124A) constructs that had been inserted into the AdEasy adenoviral expression system (Invitrogen).
Construction of PTEN and eNOS constructs
GFP-tagged PTEN was obtained from Dr. K. Yamada. A GFP-tagged dominant-negative PTEN construct (PTEN(C124A)) was created by point mutation of the cysteine at position 124 to alanine (21) using the following primers: Sense: 5’-GTTGCAGCAATTCACGCCAAAGCTGGAAAGGGA-3’, Antisense 5’-TCCCTTTCCAGCTTTGGCGTGAATTGCTGCAAC-3’. A cytosolic bovine eNOS construct (G2AeNOS) was constructed by mutating the alanine at position 2 of wild type bovine eNOS (WTeNOS) to a glycine, preventing fatty acylation of the enzyme, as previously described (27). A phospho-null eNOS mutant (3SAeNOS) was generated by mutating serines 617, 635 and 1179 to alanine on bovine eNOS using sequential PCR mutagenesis with primers previously described (1, 13), and then sub-cloning the various fragments into the WTeNOS construct (4).
NO release from intact cells
COS-7 cells were transfected with the constructs of interest and studied 48 hr later. HAECs were transduced with adenovirus and studied 24 hr later. In both cases cells, when stimulated, were challenged with the Ca2+ ionophore ionomycin (1 µM) for 30 min. Media containing NO (primarily NO2−) was ethanol-precipitated to remove proteins and was refluxed in sodium iodide/glacial acetic acid to convert NO2− to NO. NO was then quantified via specific chemiluminescence following reaction with ozone (Sievers). All NO measurements were corrected by subtracting the signal detected from cells transfected with empty vector (pcDNA3).
Immunoprecipitation
Cells were washed twice with phosphate-buffered saline and lysed in homogenization buffer of the following composition: 50 mM Tris-HCl, 0.1 mM EGTA, 0.1 mM EDTA, 0.1% SDS, 1% NP-40, 0.1% deoxycholic acid. Lysates were homogenized and cleared by centrifugation. After determining protein concentration (DC protein assay kit, Pierce), equal amounts of protein were incubated with an excess of GFP antibody (Abcam) for 2 hr at 4°C. Protein A/G sepharose beads were then added and the mixture incubated for another 1 hr at 4°C. The immune complexes were washed 3x with lysis buffer and resuspended in phosphatase buffer (see below) in preparation for the in vitro phosphatase assay.
ADP-sepharose isolation of eNOS constructs and in vitro phosphatase assay
Cells were washed twice with phosphate-buffered saline and homogenized in buffer of the following composition: 50 mM Tris-HCl, pH 7.5, 0.1 mM EGTA, 0.1 mM EDTA, 10 mM NaF, 1 mM orthovanadate and 1x protease inhibitor cocktail (Sigma). Pre-hydrated 2,5-ADP-sepharose beads (Amersham) were then added to the fractions and rotated at 4°C for 3 hr. Beads were then washed 3 times in homogenization buffer, before being resuspended in phosphatase assay buffer of the following composition: 50 mM HEPES, 10 mM MgCl2 and 10 mM DTT. The 2,5-ADP-sepharose beads bound to eNOS were then mixed with Protein A/G beads bound to either GFP, PTEN or PTEN(C124A), or mixed with an equal volume of phosphatase buffer containing the alkaline phosphatase CIP as a control, and incubated at 37°C for 30 min. Reactions were terminated after 30 min by boiling samples in SDS loading buffer.
Immuno-Blotting
Cells were washed with phosphate-buffered saline and lysed in homogenization buffer of the following composition: 50 mM Tris-HCl, 0.1 mM EGTA, 0.1 mM EDTA, 0.1% SDS, 1% NP-40, 0.1% deoxycholic acid. Lysates were homogenized and cleared by centrifugation, then boiled in 5x Laemmli buffer and size fractionated by SDS-PAGE. Blots were transferred to nitrocellulose membrane in standard transfer buffer for 18 hr at 4°C and 40V, and then blocked in non-fat milk for 1h. After blocking, membranes were incubated with phospho-specific antibodies raised to the individual eNOS phosphorylation sites (phospho-S116 & phospho-S617, Upstate; phospho-T497, Cell Signaling Technologies; phospho-S635 & phospho-S1179, BD Transduction Laboratories), with anti-eNOS monoclonal antibody (9D10, Zymed Laboratories), or with anti-PTEN (Cell Signaling) for 2 hr, washed, incubated with secondary antibody conjugated to horseradish peroxidase (anti-mouse or anti-rabbit, Amersham), washed again, and then developed using chemiluminescence (Amersham).
Measurement of eNOS derived O2−
Endothelial cells expressing control virus or PTEN were incubated with 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine·HCl (CMH, 20µM, Alexis Biochemicals, San Diego, CA) in DPBS along with 25 µM desferrioxamine (Calbiochem, La Jolla, CA) and 5 µM diethyldithiocarbamate (Alexis Biochemicals, Lausen, Switzerland). Adherent cells were detached, centrifuged at 500 g and the cell pellet was washed and suspended in a final volume of 35 µl and loaded into a 50-µl capillary tube and analyzed with a MiniScope MS200 EPR (Magnettech, Berlin, Germany) at a microwave power of 40 mW, modulation amplitude of 3,000 mG, and modulation frequency of 100 kHz. EPR spectra were analyzed using ANALYSIS software (version 2.02; Magnettech).
Statistical Analysis
NO release data are expressed as mean ± S.E.M. All analyses were performed using GraphPad InStat© software (GraphPad Software, Inc.) and were made using a two-tailed Student’s t-test. Differences were considered as significant at P < 0.05 (*).
Results
PTEN modulates eNOS activity
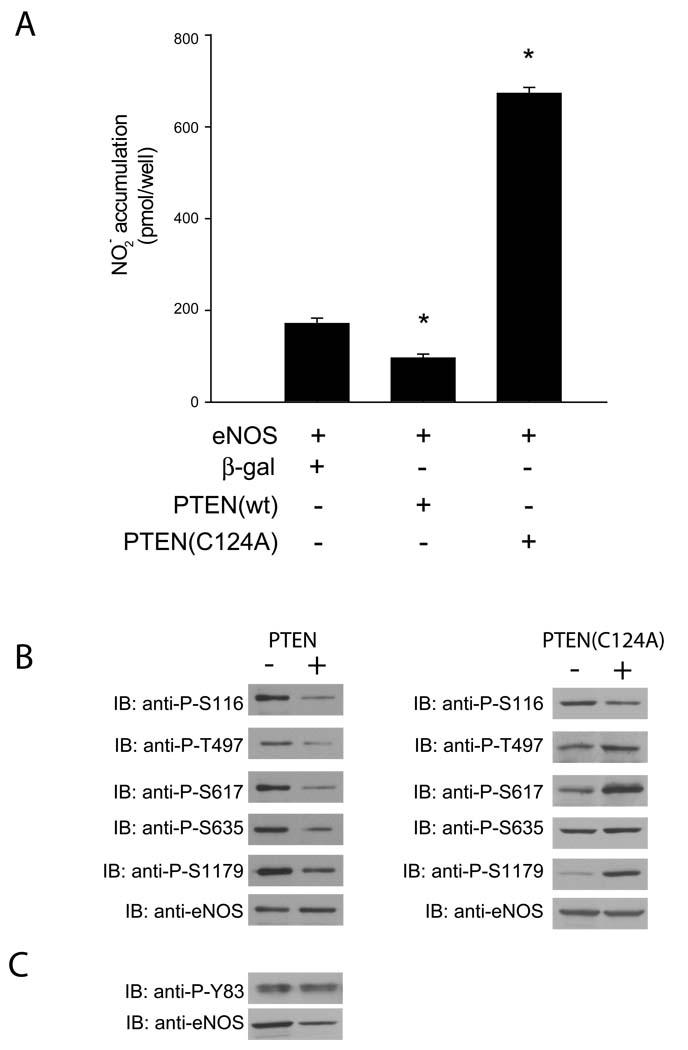
To examine the effect of PTEN on eNOS function, COS-7 cells were transfected with a combination of bovine eNOS and either PTEN, PTEN(C124A) or β-galactosidase. NO production was significantly reduced in cells expressing PTEN as opposed to those expressing β-galactosidase (Figure 1a). Conversely, cells expressing the dominant negative PTEN(C124A) showed greatly increased NO production compared to those expressing eNOS and β-galactosidase. When lysates from cells expressing eNOS and either PTEN, PTEN(C124A) or β-galactosidase were immunoblotted and probed with antibodies raised against the putative eNOS phosphorylation sites, eNOS from cells co-expressing PTEN showed decreased phosphorylation of the S116, T497, S617, S635 and S1179 residues when compared to eNOS from cells co-expressing β-galactosidase (Figure 1b). In contrast, cells expressing PTEN(C124A) showed increased phosphorylation of all sites except S116 and S635 in comparison to eNOS from cells expressing β-galactosidase. The phosphorylation state of Y83 was unaffected by PTEN (Figure 1c).
Figure 1.
(A) NO release measured from cells expressing eNOS and either β-galactosidase, PTEN or PTEN(C124A). COS-7 cells were transfected with the constructs indicated, and after 48 hr were stimulated with ionomycin (1 µM, 30 min). Data are presented as mean ± S.E.M. for 5 experiments (*, P<0.05 versus NO production in cells expressing eNOS and β-galactosidase, unpaired t-test). (B) Immuno-blot of eNOS phosphorylation sites. COS-7 cells were transfected with the above constructs, and 48 hr later cells were lysed, run on SDS-PAGE, immunoblotted and then probed with phospho-specific antibodies (upper panel) to the residues listed versus total eNOS (lower panel). (C) Immunoblot of Y83 phosphorylated eNOS (upper panel) versus total eNOS (lower panel)
PTEN has no effect on an acylation-deficient eNOS construct
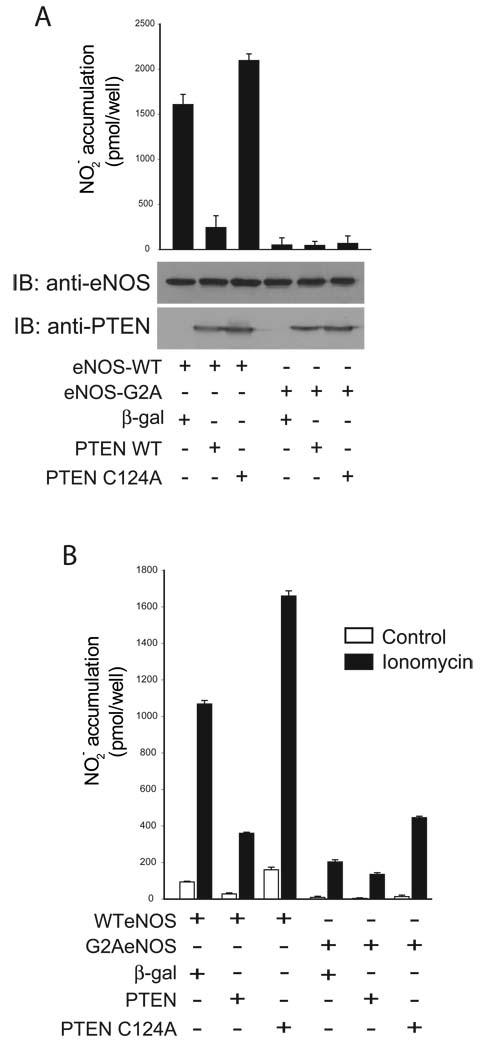
We have previously shown that a myristoylation-deficient eNOS construct (G2AeNOS) has greatly reduced activity as a consequence of reduced phosphorylation compared to wild-type eNOS (4). It was therefore of interest to determine if the activity of this largely unphosphorylated G2AeNOS construct was affected by co-expression with PTEN. When COS-7 cells were co-transfected with G2AeNOS and the PTEN constructs, neither PTEN nor PTEN(C124A) affected basal (unstimulated) NO production (Figure 2a). In cells exposed to ionomycin to stimulate calcium-dependent eNOS activity, PTEN induced significant changes in the activity of both WT and G2AeNOS, however, the effects on the latter were greatly diminished (Figure 2b).
Figure 2.
NO release measured from cells expressing either eNOS or G2AeNOS, and co-expressing either β-galactosidase, PTEN or PTEN(C124A). COS-7 cells were transfected with the constructs indicated 48 hr before the experiment. Samples were taken immediately preceeding the experiment to measure release from the previous 24 hr (A), after 30 min of basal release, and after 30 minutes of stimulation with 1 µM ionomycin (B). Data are presented as mean ± S.E.M. for 5 experiments (*, P<0.05 versus NO production in cells expressing eNOS and β-galactosidase, unpaired t-test). After transfection with the above constructs cells were lysed, run on SDS-PAGE, immunoblotted and probed with the antibodies shown.
A phospho-null eNOS mutant is also unaffected by PTEN
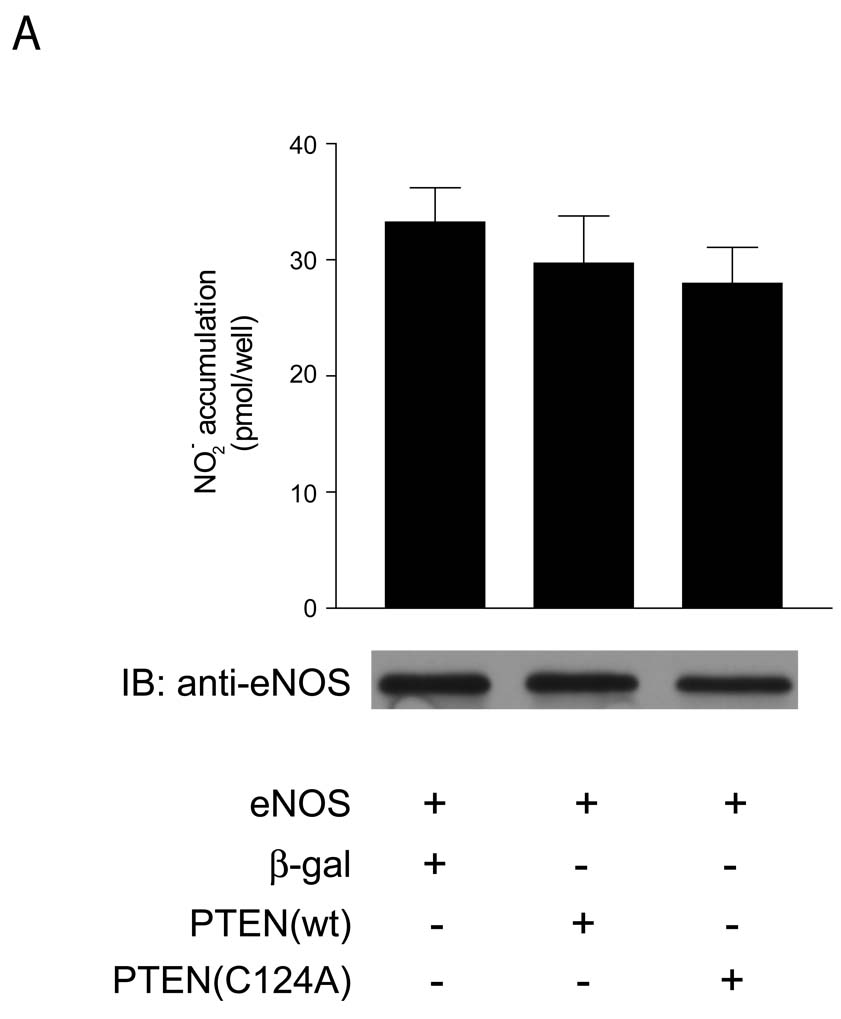
As the inability of PTEN to influence the activity of G2AeNOS suggested a prominent role for eNOS phosphorylation in mediating the effect of PTEN on eNOS activity we then examined the effect of PTEN on a phospho-null eNOS construct. We have previously created a triple-mutant eNOS construct in which serines 617, 635 and 1179 were mutated to alanine (3SAeNOS) to create a mutant which was phospho-null at these three resides and showed greatly reduced activity (4). When COS-7 cells were co-transfected with 3SAeNOS and the PTEN constructs, neither active PTEN nor dominant negative PTEN(C124A) affected NO production (Figure 3).
Figure 3.
NO release measured from cells expressing 3SAeNOS and either β-galactosidase, PTEN or PTEN(C124A). COS-7 cells were transfected with the constructs indicated, and after 48 hr were stimulated with ionomycin (1 µM, 30 min). Data are presented as mean ± S.E.M. for 6 experiments (*, P<0.05 versus NO production in cells expressing eNOS and β-galactosidase, unpaired t-test). After transfection with the above constructs cells were lysed, run on SDS-PAGE, immunoblotted and probed with the antibodies shown.
PTEN does not dephosphorylate eNOS directly
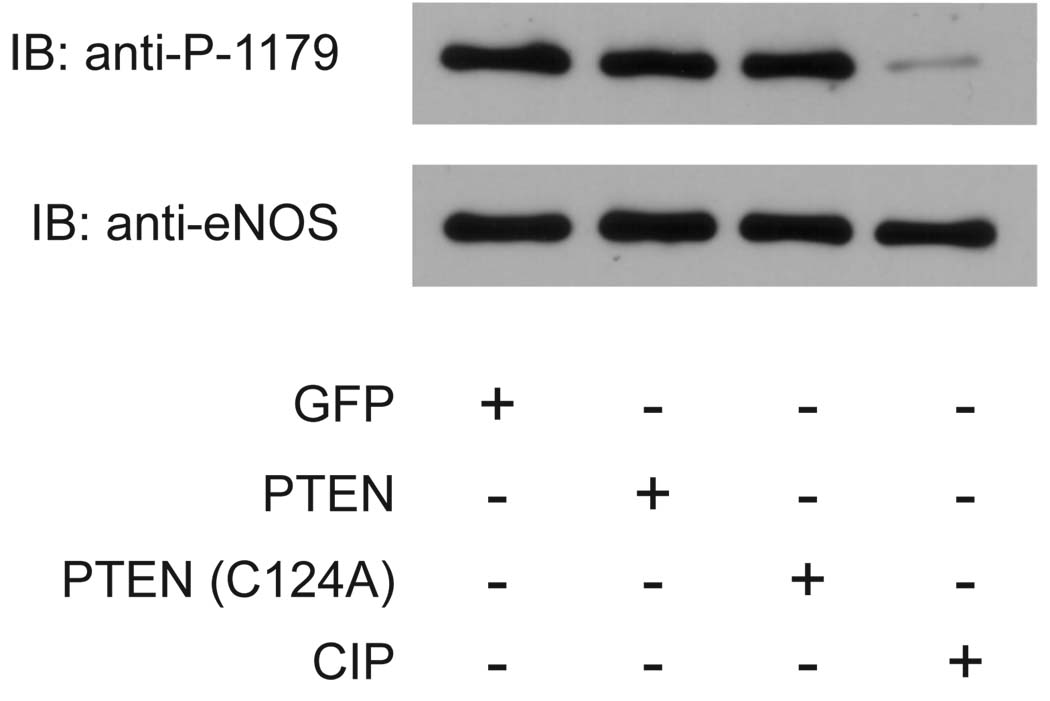
In order to rule out the possibility of a direct interaction between PTEN and eNOS, we performed an in vitro phosphatase assay in which we separately isolated both eNOS and either PTEN or PTEN(C124A) and then examined the ability of each construct to affect the phosphorylation state of eNOS (as determined by phosphorylation of the S1179 site). Neither PTEN nor PTEN(C124A) had any effect on the phosphorylation state of S1179, although treatment with the alkaline phosphatase CIP almost completely abolished the phosphorylation of the residue (Figure 4).
Figure 4.
Immunoblot of eNOS after in vitro phosphatase assay. COS-7 cells were transfected with the constructs shown and 48hrs later cells were lysed. eNOS was isolated using 2,5-ADP-sepharose beads, and GFP, PTEN or PTEN(C124A) were immunoprecipitated by virtue of their GFP tag. eNOS beads were then mixed with either the immunoprecipitated proteins or the alkaline phosphatase CIP as a positive control in vitro for 30 min at 37°C. Reactions were terminated by boiling in SDS sample buffer, run on SDS-PAGE, immunoblotted and then probed with antibody for either phospho-S1179 or total eNOS.
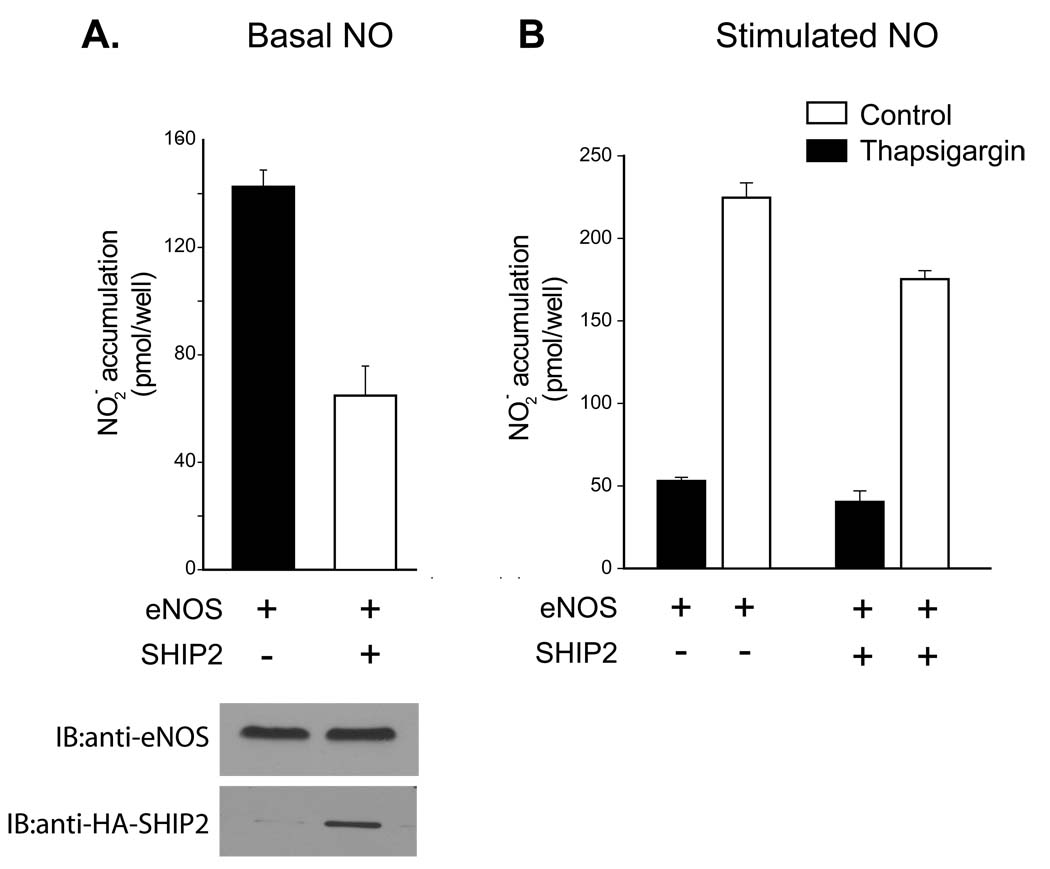
The 5-phosphatase SHIP2 also inhibits eNOS activity
To determine whether 5-phosphatases such as SHIP2 also inhibit eNOS activity, COS-7 cells were transfected with eNOS with and without SHIP2. Basal (Figure 5A) and thapsigargin stimulated (Figure 5B) NO production was significantly reduced in cells expressing SHIP2 as compared to those expressing the control plasmid β-galactosidase. Co-expression of SHIP2 did not modify the level of eNOS expressed (Figure 5A, lower panel).
Figure 5.
NO release measured from cells expressing eNOS with and without SHIP2. COS-7 cells were transfected with the constructs indicated, and after 48 hr were stimulated with thapsigargin (100nM, 30 min). Data are presented as mean ± S.E.M. for 6–8 experiments (*, P<0.05 versus NO production in cells expressing eNOS and β-galactosidase, unpaired t-test).
PTEN influences eNOS activity in human aortic endothelial cells
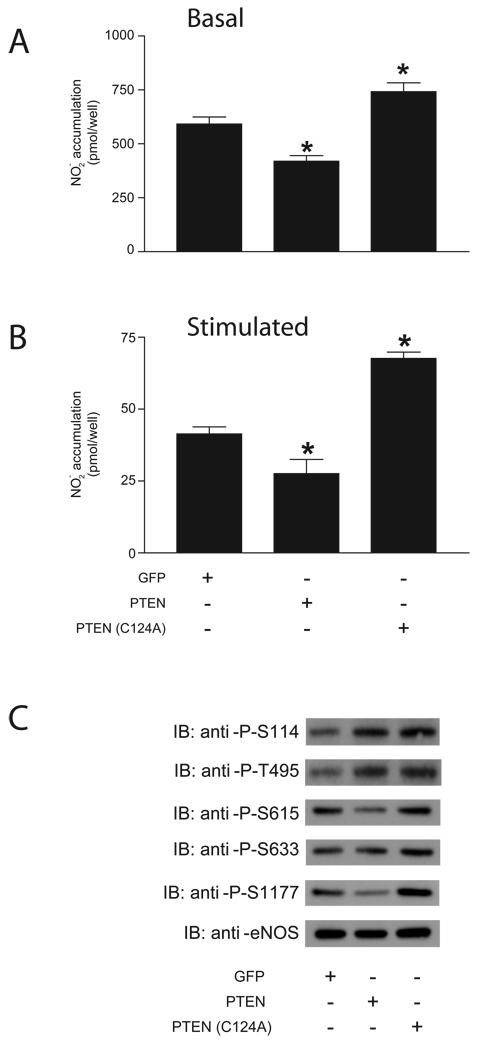
After demonstrating that PTEN decreases eNOS activity in COS-7 cells by altering the phosphorylation state of the enzyme, we then sought to examine the effect of PTEN on eNOS activity in a more relevant physiological setting. We therefore transduced HAECs with GFP, PTEN or PTEN(C124A). Over-expression of PTEN significantly decreased NO production over 24 hr in unstimulated HAECs as well as NO production by HAECs stimulated with ionomycin (Figure 6A–B). Conversely, PTEN(C124A) significantly increased NO production under the same treatment conditions.
Figure 6.
NO release measured from HAECs transduced with GFP, PTEN or PTEN(C124A). Cells were transfected with the constructs indicated 24 hr before the experiment. Samples were taken immediately prior to the experiment in order to measure NO release from the previous 24 hr (A) and after 30 minutes of stimulation with 1 µM ionomycin (B). Data are presented as mean ± S.E.M. for 6 experiments (*, P<0.05 versus NO production in cells expressing GFP, unpaired t-test). (C) Immuno-blot of eNOS phosphorylation sites. HAECs were transduced with the above constructs, and 24 hr later cells were lysed, run on SDS-PAGE, immunoblotted and then probed with phospho-specific antibodies listed.
When lysates from HAECs expressing GFP, PTEN or PTEN(C124A) were probed with phospho-specific antibodies, eNOS from cells expressing PTEN showed a slight increase in phosphorylation of S116 and T497, and a decrease in phosphorylation of S617, S635 and S1179. eNOS from cells expressing PTEN(C124A) showed an increase in phosphorylation of S116 and T497, and a slight increase in phosphorylation of S617, S635 and S1179 (Figure 6C).
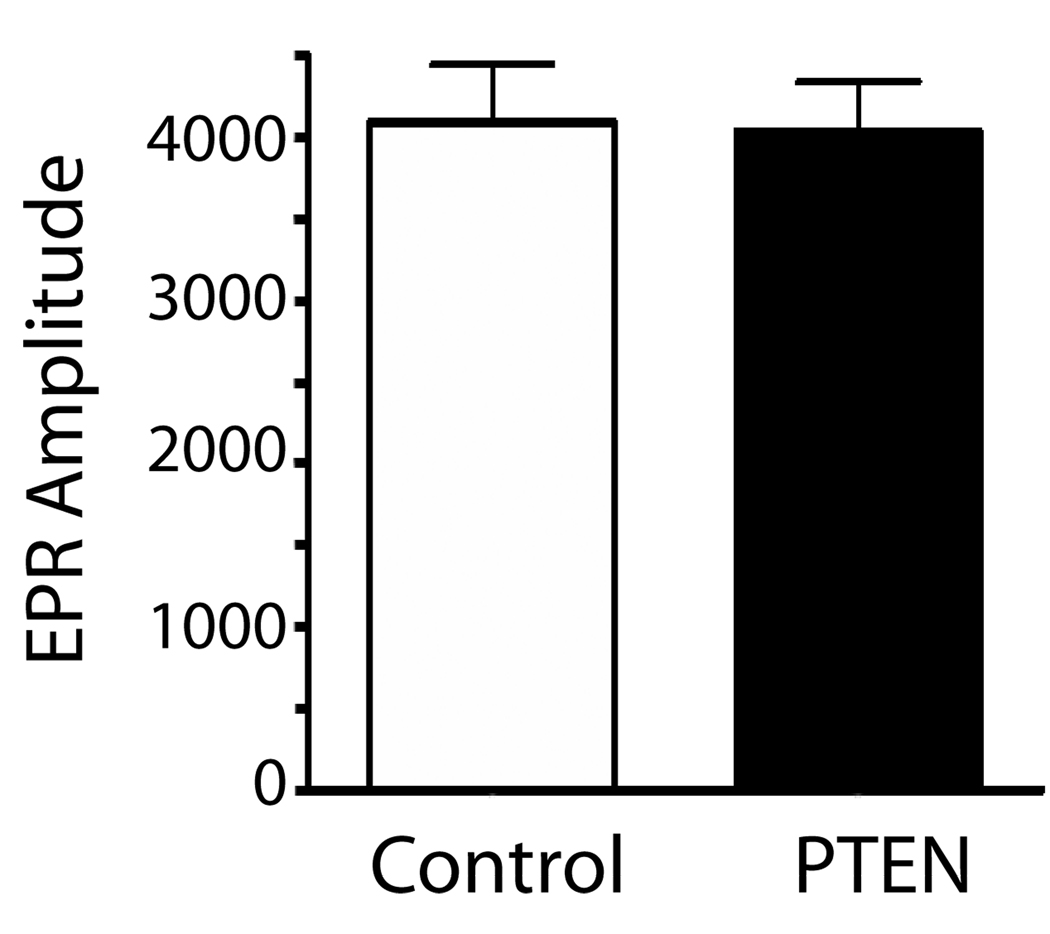
PTEN does not modify eNOS-derived superoxide production in endothelial cells
To assess whether the ability of PTEN to modify NO release involves the generation of reactive oxygen species such as superoxide, BAEC transduced with control adenovirus (GFP) or PTEN and superoxide levels monitored by EPR. As shown in Figure 7, PTEN does not modify the amount of superoxide generated in endothelial cells.
Figure 7.
eNOS-dependent superoxide production was estimated in endothelial cells transduced with control (GFP) or PTEN adenovirus by electron paramagnetic resonance (EPR) assay using the spin-trap compound 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine·HCl (CMH), n=4.
Discussion
PTEN (phosphatase and tensin homologue deleted on chromosome 10) has been implicated in a number of cardiovascular pathologies, including cardiac hypertrophy, heart failure, cardiac preconditioning and hypertension (26). Many of these effects on the cardiovascular system involve modulation of NO production, yet the interaction between PTEN and eNOS remains to be fully elucidated. While recent studies have provided evidence that PTEN signaling plays a role in eNOS inhibition due to the actions of human cytomegalovirus (29), palmitic acid (33) or resistin (28), the studies in question have primarily determined eNOS activity by the measurement of a surrogate marker, the phosphorylation state of S1177 on human eNOS (the residue corresponding to S1179 on bovine eNOS) and only one study (33) has attempted to directly measure eNOS activity by use of an L-[3H] arginine conversion in cell lysates which has inherent limitations. To the best of our knowledge this study is the first to comprehensively address the effect of PTEN on the multi-site phosphorylation of eNOS, to establish the functional significance of phosphorylation and to measure NO release from intact cells. In addition we show that the ability of PTEN to modulate eNOS activity is not due to effects on superoxide production or direct de-phosphorylation.
The co-expression of PTEN and eNOS in COS-7 cells resulted in significantly less NO production compared to cells expressing a control plasmid (β-galactosidase) and eNOS. Furthermore, expression of the dominant negative mutant of PTEN, PTEN(C124A) greatly increased the NO production when compared to control, suggesting that endogenous PTEN is a significant restraint on eNOS activity. As PTEN directly opposes the actions of PI3-K and therefore inhibits Akt/PKB, the effect of PTEN expression on the phosphorylation of all putative eNOS regulatory sites was examined. PTEN expression resulted in a decrease in phosphorylation of S116, T497, S617, S635 and S1179 while the dominant negative PTEN(C124A) produced an increase in phosphorylation of all sites except S116 and S635. There was no effect of PTEN on Y83 phosphorylation. The decrease in phosphorylation of S617 and S1179 in response to co-expression with PTEN, and the increase in phosphorylation of these sites when co-expressed with PTEN(C124A) could be predicted due to their ability to be phosphorylated by Akt (13, 23) and indeed, reduced phosphorylation of S1179 due to PTEN has been previously reported (28, 29, 33). In contrast, the influence of PTEN co-expression on the phosphorylation state of S116, T497 and S635 was unexpected, as S635 is thought to be phosphorylated primarily via protein kinase A (PKA) (23), T497 is thought to be phosphorylated primarily by protein kinase C (PKC) (8), and S116, while being far less well characterized than the other two residues, has been reported to be insensitive to PI3K inhibition (16).
One potential explanation for the decreased phosphorylation of T497 in response to PTEN expression, and subsequent increased phosphorylation in response to PTEN(C124A) expression, may be previous studies which have reported that the activity of PKC is enhanced by the protein kinase PDK-1, which is in turn activated by PI3-K (7, 18). Thus the decrease in PI3-K activity due to PTEN expression would also have the downstream effect of reducing PKC activity and reducing phosphorylation at T497, while the expression of PTEN(C124A) would increase T497 phosphorylation. While PKC has been reported to be involved in S116 phosphorylation (16), the same study also reported that PI3-K inhibition with wortmannin had no effect on S116 phosphorylation. However the study of Kou et al. was performed in bovine aortic endothelial cells whereas the present results were obtained in COS-7 cells expressing eNOS. Therefore the possibility of different cell types utilizing different signaling pathways cannot be discounted.
The decreased phosphorylation of S635 in response to PTEN expression, however, is difficult to reconcile with the literature. Previous studies have reported that PI3K can directly inhibit PKA (10, 15). In this case, PTEN expression would be expected to inhibit PI3K which would then have the effect of increasing PKA activity and S635 phosphorylation, the opposite of what was observed. Adding to the complexity of this response is the lack of effect of PTEN(C124A) on phosphorylation state of S635. This may indicate that although overexpression of PTEN exerts some effects on the phosphorylation of this site, PTEN has no significant role in the phosphorylation of this site in the normal physiological state, and thus the mechanisms behind the decreased phosphorylation of S635 in response to PTEN co-expression remain enigmatic.
The ability of PTEN to modulate the phosphorylation states of PI3-K-independent sites raised the possibility that PTEN may act to directly dephosphorylate eNOS. We therefore performed an in vitro phosphatase assay using purified eNOS and PTEN. Neither PTEN nor PTEN(C124A) was able to directly alter the phosphorylation state of eNOS, even though the alkaline phosphatase, CIP almost abolished S1179 phosphorylation. These findings are consistent with the poor efficacy of PTEN against protein substrates (25). Furthermore, SHIP2, which dephosphorylates PtdIns(3,4,5)P3 at the 5 position, in contrast to the 3 position catalyzed by PTEN, also inhibited eNOS activity and lends further support to an indirect action of PTEN on eNOS activity via the PI3K/Akt pathway.
As PTEN appeared to be exerting its effects on eNOS via modulation of enzyme phosphorylation, we then sought to examine the effect of PTEN on G2AeNOS, a myristoylation-deficient enzyme which has markedly reduced activity due to a lack of phosphorylation (4). Neither PTEN nor PTEN(C124A) co-expression significantly altered basal or unstimulated NO production by the G2AeNOS construct. This was most likely due to the absence of phosphorylation on the G2AeNOS, effectively negating PTEN from suppressing Akt-dependent phosphorylation. Next, we sought to further confirm that the eNOS phosphorylation state plays a pivotal role in the interaction between PTEN and eNOS. We have previously constructed an eNOS phosphonull mutant (3SAeNOS) in which S617 and S1179 (the two sites regulated primarily by Akt), as well as S635, have been mutated to alanine to prevent phosphorylation (4). NO production by this 3SA mutant was completely unaffected by co-expression of PTEN or PTEN(C124A), once more suggesting that it is through modulation eNOS phosphorylation that PTEN exerts its effects on NO production. Interestingly, although 3SAeNOS retains both S116 and T497, PTEN no longer had any affect on eNOS function, suggesting that perhaps the changes in the phosphorylation state of these sites by PTEN expression do not play a large functional role.
Decreased eNOS activity in cardiovascular disease is frequently attributed to increased superoxide generation at the expense of nitric oxide synthesis in a process termed “eNOS uncoupling” (9). Alterations in the phosphorylation state of eNOS have also been shown to influence superoxide production (3, 20) so therefore we investigated whether PTEN could modify superoxide generation from eNOS. Despite significantly attenuating the phosphorylation of S1179, PTEN did not modify superoxide production in endothelial cells. However, this may be due to the ability of PTEN to reduce the phosphorylation of other eNOS sites including T497 which could ameliorate the effects on S1179.
Finally, we sought to examine the interaction between PTEN and eNOS in a more physiologically relevant context, human aortic endothelial cells (HAECs). Increased expression of PTEN in HAECs significantly reduced NO production, and the expression of PTEN(C124A) significantly increased NO production. These findings mirrored those obtained in COS-7 cells and suggest that endogenous PTEN suppresses eNOS activity in human endothelial cells. Likewise, PTEN decreased, and dominant negative PTEN(C124A) increased the phosphorylation of eNOS at S615 and S1177 in HAECs (equivalent to S617 and S1179 on the bovine isoform).
Inhibition of PTEN provides protection against acute lung injury (ALI) (17, 31). However, the molecular target(s) of PTEN that afford protection in ALI are not yet established. eNOS-derived NO is known to regulate microvascular and epithelial permeability (2, 11) and breakdown of the alveolar-capillary barrier underlies the pathogenesis of ALI(34). It is therefore possible that PTEN by modulating eNOS activity could influence the amount of NO produced and thus alter the changes in permeability seen in ALI.
In conclusion, the present study sought a detailed examination of the interaction between PTEN and eNOS. We find that PTEN negatively regulates eNOS activity, primarily through altering the phosphorylation state of S617 and S1179. Additionally, we show for the first time that there is no direct interaction between PTEN and eNOS, and confirm that PTEN exerts its effects on eNOS via the PI3K/Akt pathway.
Acknowledgments
This work was supported by grants from the NIH (HL085827, HL092446) and the AHA (EIA to DF) and also by funding from the Cardiovascular Discovery Institute (CVDI) of the Medical College of Georgia.
Abbreviations
- PTEN
Phosphatase and tensin homologue deleted on chromosome 10
- HAECs
human aortic endothelial cells
- eNOS
endothelial nitric oxide synthase
- NO
nitric oxide
References
- 1.Bauer PM, Fulton D, Boo YC, Sorescu GP, Kemp BE, Jo H, Sessa WC. Compensatory phosphorylation and protein-protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J Biol Chem. 2003;278:14841–14849. doi: 10.1074/jbc.M211926200. [DOI] [PubMed] [Google Scholar]
- 2.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 3.Chen CA, Druhan LJ, Varadharaj S, Chen YR, Zweier JL. Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. J Biol Chem. 2008;283:27038–27047. doi: 10.1074/jbc.M802269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Church JE, Fulton D. Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J Biol Chem. 2006;281:1477–1488. doi: 10.1074/jbc.M505968200. [DOI] [PubMed] [Google Scholar]
- 5.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 6.Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutil EM, Toker A, Newton AC. Regulation of conventional protein kinase Cisozymes by phosphoinositide-dependent kinase 1 (PDK-1) Current Biology. 1998;8:1366–1375. doi: 10.1016/s0960-9822(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 8.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:E68–E75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 9.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 10.Fujino H, Salvi S, Regan JW. Differential Regulation of Phosphorylation of the cAMP Response Element-Binding Protein after Activation of EP2 and EP4 Prostanoid Receptors by Prostaglandin E2 10.1124/mol.105.011833. Mol Pharmacol. 2005;68:251–259. doi: 10.1124/mol.105.011833. [DOI] [PubMed] [Google Scholar]
- 11.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fulton D, Church JE, Ruan L, Li C, Sood SG, Kemp BE, Jennings IG, Venema RC. Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr-83. J Biol Chem. 2005;280:35943–35952. doi: 10.1074/jbc.M504606200. [DOI] [PubMed] [Google Scholar]
- 13.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulton D, Ruan L, Sood SG, Li C, Zhang Q, Venema RC. Agonist-stimulated endothelial nitric oxide synthase activation and vascular relaxation. Role of eNOS phosphorylation at Tyr83. Circ Res. 2008;102:497–504. doi: 10.1161/CIRCRESAHA.107.162933. [DOI] [PubMed] [Google Scholar]
- 15.Jo S-H, Leblais V, Wang PH, Crow MT, Xiao R-P. Phosphatidylinositol 3-Kinase Functionally Compartmentalizes the Concurrent Gs Signaling During {beta}2-Adrenergic Stimulation 10.1161/01.RES.0000024115.67561.54. Circ Res. 2002;91:46–53. doi: 10.1161/01.res.0000024115.67561.54. [DOI] [PubMed] [Google Scholar]
- 16.Kou R, Greif D, Michel T. Dephosphorylation of Endothelial Nitric-oxide Synthase by Vascular Endothelial Growth Factor. IMPLICATIONS FOR THE VASCULAR RESPONSES TO CYCLOSPORIN A 10.1074/jbc.M204519200. J Biol Chem. 2002;277:29669–29673. doi: 10.1074/jbc.M204519200. [DOI] [PubMed] [Google Scholar]
- 17.Lai JP, Bao S, Davis IC, Knoell DL. Inhibition of the phosphatase PTEN protects mice against oleic acid-induced acute lung injury. Br J Pharmacol. 2009;156:189–200. doi: 10.1111/j.1476-5381.2008.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein Kinase C Isotypes Controlled by Phosphoinositide 3-Kinase Through the Protein Kinase PDK1 10.1126/science.281.5385.2042. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 20.Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA, Jr, Sessa WC. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J Biol Chem. 2003;278:44719–44726. doi: 10.1074/jbc.M302836200. [DOI] [PubMed] [Google Scholar]
- 21.Maehama T, Dixon JE. he tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 22.Marsh DJ, Kum JB, Lunetta KL, Bennett MJ, Gorlin RJ, Ahmed SF, Bodurtha J, Crowe C, Curtis MA, Dasouki M, Dunn T, Feit H, Geraghty MT, Graham JM, Jr, Hodgson SV, Hunter A, Korf BR, Manchester D, Miesfeldt S, Murday VA, Nathanson KL, Parisi M, Pober B, Romano C, Eng C, et al. PTEN mutation spectrum and genotypephenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet. 1999;8:1461–1472. doi: 10.1093/hmg/8.8.1461. [DOI] [PubMed] [Google Scholar]
- 23.Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA, Huang W, Venema RC, Kemp BE. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem. 2002;277:42344–42351. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- 24.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42:271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, Parsons R, Tonks NK. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci U S A. 1997;94:9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Sessa WC, Barber CM, Lynch KR. Mutation of N-myristoylation site converts endothelial cell nitric oxide synthase from a membrane to a cytosolic protein. Circ Res. 1993;72:921–924. doi: 10.1161/01.res.72.4.921. [DOI] [PubMed] [Google Scholar]
- 28.Shen YH, Zhang L, Gan Y, Wang X, Wang J, LeMaire SA, Coselli JS, Wang XL. Up-regulation of PTEN (phosphatase and tensin homolog deleted on chromosome ten) mediates p38 MAPK stress signal-induced inhibition of insulin signaling. A cross-talk betweenstress signaling and insulin signaling in resistin-treated human endothelial cells. J Biol Chem. 2006;281:7727–7736. doi: 10.1074/jbc.M511105200. [DOI] [PubMed] [Google Scholar]
- 29.Shen YH, Zhang L, Utama B, Wang J, Gan Y, Wang X, Chen L, Vercellotti GM, Coselli JS, Mehta JL, Wang XL. Human cytomegalovirus inhibits Akt-mediated eNOS activation through upregulating PTEN (phosphatase and tensin homolog deleted on chromosome 10) Cardiovasc Res. 2006;69:502–511. doi: 10.1016/j.cardiores.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 31.Tiozzo C, De Langhe S, Yu M, Londhe VA, Carraro G, Li M, Li C, Xing Y, Anderson S, Borok Z, Bellusci S, Minoo P. Deletion of Pten Expands Lung Epithelial Progenitor Pools and Confers Resistance to Airway Injury. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.200901-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsigkos S, Zhou Z, Kotanidou A, Fulton D, Zakynthinos S, Roussos C, Papapetropoulos A. Regulation of Ang2 release by PTEN/PI3-kinase/Akt in lung microvascular endothelial cells. J Cell Physiol. 2006;207:506–511. doi: 10.1002/jcp.20592. [DOI] [PubMed] [Google Scholar]
- 33.Wang XL, Zhang L, Youker K, Zhang MX, Wang J, LeMaire SA, Coselli JS, Shen YH. Free fatty acids inhibit insulin signaling-stimulated endothelial nitric oxide synthase activation through upregulating PTEN or inhibiting Akt kinase. Diabetes. 2006;55:2301–2310. doi: 10.2337/db05-1574. [DOI] [PubMed] [Google Scholar]
- 34.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 35.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in humanprostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]